Abstract
Identifying relevant mediators responsible for the pathogenesis during sepsis may lead to finding novel diagnostic and therapeutic targets. Recent studies indicate programmed cell death receptor (PD)-1 plays a significant role in the development of immune suppression associated with sepsis. Here we determine if B7-H1, the primary ligand of PD-1, contributes to the pathogenesis of sepsis. We report that B7-H1 is up-regulated extensively on various immune cells during sepsis and B7-H1 gene deficiency protects mice from the lethality of sepsis. In terms of the histological development of multiple organ damage and inflammatory cytokine levels in circulation or at infectious site, B7-H1 deficient mice showed a remarkable reduction in these indices when compared with wild type (WT) mice. However, B7-H1 gene deficient mice did not exhibit a lower bacterial burden when compared to WT mice, although they recruited more macrophages and neutrophils into infectious site. In addition, we found that, during sepsis, while there were no marked differences affecting ex vivo macrophage cytokine productive capacity between PD-1 and B7-H1 gene deficient mice; preservation of ex vivo macrophage phagocytic function was only seen in septic PD-1 knockout mouse cells. Finally, higher percentage B7-H1+ neutrophils in peripheral blood correlated not only with higher levels of pro- and anti-inflammatory cytokines/chemokines (CCL2, IL-6, CXCL2, KC, TNF-α, and IL-10), but with lethal outcome as well. Together, these results indicate B7-H1 contributes to septic morbidity in fashion distinct from PD-1 and suggest B7-H1 expression on neutrophils could be used as a biomarker of septic severity.
INTRODUCTION
Sepsis affects more than 750,000 patients annually in the US and remains a leading cause of death worldwide. It is a major healthcare problem causing significant morbidity, mortality and costs. In addition, sepsis is likely to remain relevant as its incidence continues to rise because of an aging population with increasing numbers of patients infected with antibiotic-resistant organisms, patients with compromised immune systems, and patients who undergo prolonged, high-risk surgery (1, 2). Currently, there are no specific therapeutic interventions that are FDA-approved for treatment of sepsis, which implies in part that the pathophysiology of sepsis, its accompanying systemic inflammatory response syndrome (SIRS) and the events that lead to multiple organ failure (MOF) and death are still poorly understood (3).
Sepsis describes a complex clinical syndrome that develops from the host response to infectious pathogens. Inflammation arises primarily as a response to infectious challenge. This immune response to infection must be controlled to ensure it is optimal for defense, while avoiding the consequences of excessive inflammation, which is often more dangerous than the original pathogenic insult. A fundamental pathologic feature of sepsis is the failure to maintain an appropriate balance between excessive and inadequate inflammation (4). Current evidence supports the concept that sepsis is an overwhelming inflammatory response triggered by major pathological stimuli, driven and modulated by a multitude of endogenous mediators activated in cascade, resulting in profound immune suppression (3). Clinically, the development of an overwhelming inflammatory response, i.e., SIRS, suggests an inability to regulate and confine the inflammatory response; the results of which are manifested by septic shock, MOF, and death. However, all the clinical trials of agents during the past two decades that were designed to block the activity of such likely biochemical triggers and mediators (such as LPS, TNF-α, IL-1, NO, and coagulation factors) have not shown a benefit, implying that we don't have adequate knowledge of mechanisms associated with the development of SIRS and sepsis (5).
The innate immune response is the first-line of host defense that rapidly operates to limit infection, in which infiltrating leukocytes such as macrophages and neutrophils are essential. After being triggered, these cells release cytokines, chemokines, and other mediators, rendering an inflammatory response. During sepsis, these inflammatory components are pleiotropic, redundant, and interwoven, illustrating a highly sophisticated, non-linear dynamic system with great variability, connectivity, and cross-regulation. It has been proposed that excessive generation of these mediators sets the stage for development of SIRS, MOF and lethality (6). Thus, the negative results of clinical trials and the intrinsic complexity of innate immunity suggest that targeting a single cytokine or mediator may not be sufficient to affect the balance between effectiveness and harmfulness of the inflammatory response during sepsis.
Antigen-independent signals provided by pathways from B7:CD28 family, whether stimulatory or inhibitory, are critical to a balanced immune response (7). The receptor PD-1 and its ligands, B7-H1 (a.k.a., PD-L1) and B7-DC (a.k.a., PD-L2), members of the B7:CD28 family, have been demonstrated to be widely expressed in tissues/organs and participates in a large spectrum of immune responses. Most studies on B7-H1/B7-DC:PD-1 pathway has focused on T cell related immunity. This pathway has been found to exert critical inhibitory functions in the setting of persistent antigenic stimulation such as chronic viral infections, tumors, and encountering of self-antigens (8, 9). PD-1 activation contributes directly to T cell exhaustion and the resulting chronic viral infection as well as tumor aggression (10, 11). It also controls multiple tolerance checkpoints that prevent autoimmunity (12). However, its functions during acute microbial infections are much less clear (13). In our own hands, PD-1 has been demonstrated to play a crucial role in regulating the balance between effective pathogen clearance and by-stander tissue damage by the antimicrobial immune response (14). It is not clear what the role of B7-H1 and/or B7-DC are to septic immunopathology.
Here we determine if B7-H1 contributes to the pathogenesis of sepsis. The results indicate that B7-H1 plays a significant role in the development of septic morbidity/mortality, which appears to associate with the capacity to attenuate the overwhelming inflammatory response seen during sepsis. In addition, differential B7-H1 expression on neutrophils appears to correlate with the development of septic mortality.
MATERIALS AND METHODS
Mice and Cecal ligation and puncture (CLP)
Male C57BL/6J mice were purchased from The Jackson Laboratory (Bar Harbor, ME). B7-H1−/− mice (15) were obtained as a generous gift from Dr. Lieping Chen (Yale University, New Haven, CT) and maintained in our animal facility. PD-1−/− mice (16) were kindly provided by Dr. Tasuku Honjo (Kyoto University, Kyoto, Japan) via Dr. Megan Sykes (Massachusetts General Hospital, Transplantation Biology Research Center, Boston, MA) and maintained in our animal facility. All mice used in this study were male and aged 11 to 13 weeks. All protocols carried out with animals are in accordance with the NIH Guide for Animal Use and Care and were approved by the animal welfare committee of Rhode Island Hospital (Providence, RI).
CLP
Mice were anesthetized using Isoflurane, a midline incision (~1 cm) was made below the diaphragm, exposing the cecum, and then the cecum was ligated distally and punctured twice with a 22-gauge needle. In the control animals (sham), the cecum was located and mobilized but was neither ligated nor punctured. The abdominal incision was then closed in layers with an Ethilon 6.0 suture, and the animals were resuscitated with 1.0 ml lactated Ringer's solution by subcutaneous injection. Animals were subsequently allowed food and water ad lib (14).
Flow cytometry
To determine the changes in cell surface expression of B7-H1 on mouse blood leukocytes, 50 μl of whole blood was mixed with staining antibody and Fc blocker followed by an incubation on ice for 45 min. RBCs were lysed with Ammonium-Chloride-Potassium lysing buffer and samples were washed and analyzed on a FACSarray flow cytometry (BD Bioscicence, San Jose, CA). Peritoneal leukocytes and splenocytes were isolated and assayed as described (14). Fluorochrome conjugated anti-F4/80 (clone BM8), anti-CD115 (clone AFS98), anti-Ly-6G (clone 1A8), anti-NK1.1 (clone PK136), anti-CD3 (clone 145-2C11) and their corresponding isotype controls were purchased from BioLegend (San Diego, CA). Fluorochrome conjugated anti-CD4 (clone GK1.5), anti-CD8 (clone 53–6.7), anti-B7-H1 (clone MIH5), anti-B7-DC (clone TY25) antibodies along with the appropriate isotype controls were purchased from eBioscience (San Diego, CA).
Evaluation of systemic and local bacterial burden
Blood samples were collected and spread on Trypticase Soy Agar with 5% Sheep Blood (TSA II) agar plate (BD Bioscicence) with dilutions of 1 μl, 10 μl or 100 μl in 100 μl saline aliquots. Peritoneal lavage fluid was harvested after injecting 2 ml of PBS into the peritoneum and serially diluted in sterile saline. A 100 μl aliquot of each dilution was spread on a TSA II blood agar plate. All plates were incubated at 37°C for 24–48h. Colonies were counted and expressed as CFU/100 μl for blood samples or CFU/ml for peritoneal lavage samples.
Cytokine and chemokine determinations
Concentrations of murine TNF-α, IL-6, IL-1β, IFN-γ, IL-10, KC, CXCL2, and CCL2 were measured in cell-free peritoneal lavage fluid, plasma, and cell culture supernatants by using sandwich ELISA (BioLegend, BD biosciences and R&D systems, Minneapolis, MN).
Peritoneal macrophage isolation and in vitro culture
Peritoneal lavages were performed using 10 ml of PBS. Lavages containing peritoneal exudate cells from individual mice were processed separately. Cells were pelleted and resuspended at a concentration of 106 macrophages/ml in RPMI 1640 containing 10% FCS, 2 mM L-glutamine and 5 μg/ml gentamicin (Life Technologies, Grand Island, NY). Macrophages were plated in 24-well tissue culture plates at 1 ml/well and incubated at 37°C in 5% CO2 for 90min. Adhered macrophages were cultured with media only, media containing LTA (1 μg/ml) (Sigma-Aldrich, St. Louis, MO) or LPS (200 ng/ml) (Sigma-Aldrich) for 24h. Supernatants from these in vitro cultures were collected and the concentrations of cytokines were determined by ELISA. Macrophages left in wells were quantified by a CyQuant proliferation assay kit (Life Technologies) and cytokine concentrations were adjusted by the ratio of cell numbers.
Phagocytosis assay
Peritoneal leukocytes were plated in 6-well tissue culture plates at 4×106/ml/well and incubated in complete RPMI 1640 medium at 37 °C. Ninety minutes later, non-adherent cells were washed off with PBS. Adherent macrophages were then co-cultured with ~ 60 μg of pHrodo - conjugated E. coli (Life Technologies) in PBS at 37 °C for 1h and then were washed completely with PBS. Cells were harvested by scraping and detected by flow cytometry. The percentages of pHrodo positive cells were used as indicators of the capacity of phagocytosis.
Statistical analysis
For survival studies, the log rank test was used to determine significance. All other data are shown as the mean ± S.E.M and analyzed with the Mann-Whitney Rank Sum test or ANOVA on ranks if they were frequency/intensity data. Parametric data was analyzed with the unpaired Student's t test as indicated. Correlation was determined by Pearson product moment correlation. A p value <0.05 was considered statistically significant, p values <0.01 and <0.001 are also indicated. Graphs were plotted and statistics assessed using SigmaPlot & Statistics Software (SYSTAT, Chicago, IL).
RESULTS
The expression of B7-H1 is upregulated on a variety of immune cells during sepsis
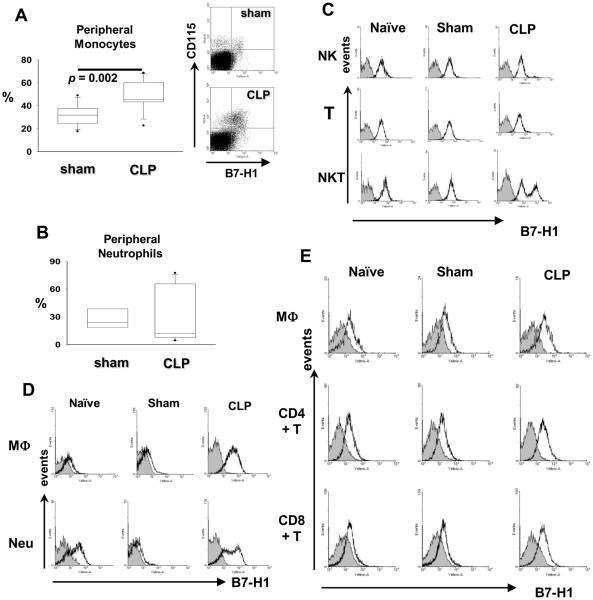
B7-H1 is expressed on numerous cell types, including myeloid cells, lymphoid cells, and many parenchymal cells. It is constitutively expressed in murine myeloid and lymphoid cells and is upregulated in response to pro-inflammatory cytokines (9). To know how experimental sepsis affects the expression of B7-H1, we examined the expression of B7-H1 on myeloid and lymphoid cells from peripheral blood (Fig 1A–C), peritoneum (Fig 1D), and spleen (Fig 1E) at 24hr post CLP surgery. More peripheral blood monocytes express B7-H1 during sepsis (Fig 1A); whereas peripheral blood neutrophils display a polarized expression pattern of B7-H1, i.e., in some mice, the percentage of B7-H1+ neutrophils increased, while in others, decreased (Fig 1B, 7B). For NK cells, NKT cells and T cells, it appeared that almost all these cells constitutively express B7-H1 in naïve, sham control, and septic mice; and only NKT cells express augmented B7-H1 during sepsis (Fig 1C). B cells did not express B7-H1. B7-DC was not detected on any of the above cells (data not shown).
Figure 1. The expression of B7-H1 in myeloid and lymphoid cells is augmented by sepsis.
(A) The percentage of B7-H1+ monocytes increases in peripheral blood during sepsis. B7-H1 expression on peripheral monocytes (gated as CD115+Ly-6G−) was determined by flow cytometry (n = 12 for sham, n = 15 for CLP, pooled from three experiments). p value as shown by Mann-Whitney test, CLP vs. sham treated WT mice. (B) The percentage of B7-H1+ peripheral neutrophils either increases or decreases during sepsis. Peripheral neutrophils are defined as CD115−Ly-6G+ cells (n = 6 for sham, n = 12 for CLP, pooled from two experiments). (C) Peripheral NKT cells express augmented B7-H1 during sepsis, while peripheral NK cells and T cells do not. Representative histograms of B7-H1 expression on NK (gated as NK1.1+CD3−), T (gated as NK1.1−CD3+), and NKT (gated as NK1.1+CD3+) cells from three experiments. (D) Macrophages and neutrophils from infectious sites express augmented B7-H1. Representative histograms of B7-H1 expression on peritoneal macrophages (gated as F4/80+Ly-6G−) and neutrophils (gated as F4/80−Ly-6G+) from two experiments. (E) Spleen macrophages and T cells from septic mice express higher levels of B7-H1 than that from non-septic mice. Representative histograms of B7-H1 expression on splenic macrophages (gated as F4/80+Ly-6G−), CD4+T cells (gated as CD3+CD4+), and CD8+T cells (gated as CD3+CD8+) from two experiments. (C – E) B7-H1 expression (black lines) is overlayed on isotype control (gray filled).
Figure 7. Higher percentage of B7-H1+ peripheral blood neutrophils is correlated with lethal outcomes in CLP induced sepsis.
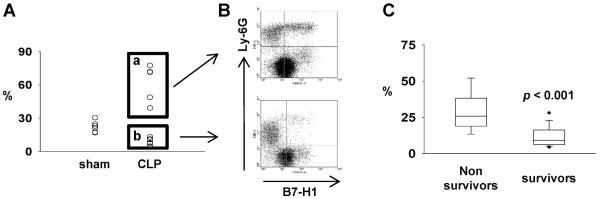
(A) The expression of B7-H1 on peripheral neutrophils either increases (box a) or decreases (box b) during sepsis. Peripheral neutrophils are defined as CD115−Ly-6G+ cells. (B) Representative flow chart (gated on CD115−Ly-6G+) of increased (upper) or decreased (lower) B7-H1 expression on neutrophils. (C) The percentage of B7-H1 expressing peripheral neutrophils from non-survived mice is significantly higher than that from survived mice. At 24 hours post CLP treatment, small amount of blood was obtained by cheek puncture and used for measure the expression. Then, mice survival was monitored for 14 days. p < 0.001 for survivors vs non-survivors by Mann-Whitney test (n = 41 pooled from 4 experiments). Mice that didn't survived 24 hours were not included.
At the infectious site, the peritoneum, macrophages and neutrophils are the dominant cell types (17–19); therefore, the expression of B7-H1 on these two cell types was studied in more detail. The expression of B7-H1 on both macrophages and neutrophils was enhanced during sepsis (Fig 1D). Again, as noted above, B7-DC was not expressed on these two cells from naïve, sham treated or CLP treated mice (data not shown).
With respect to immune cells obtained from spleen, another important tertiary lymphoid organ (Fig 1E), we found that macrophages expressed elevated levels of B7-H1 during septic response, however, neutrophils did not express B7-H1 under any circumstances we examined here. In addition, the expression of B7-H1 on CD4+ T cells and CD8+ T cells was upregulated during sepsis.
B7-H1−/− mice are less susceptible to CLP-induced lethality than WT mice and show reduced organ damage
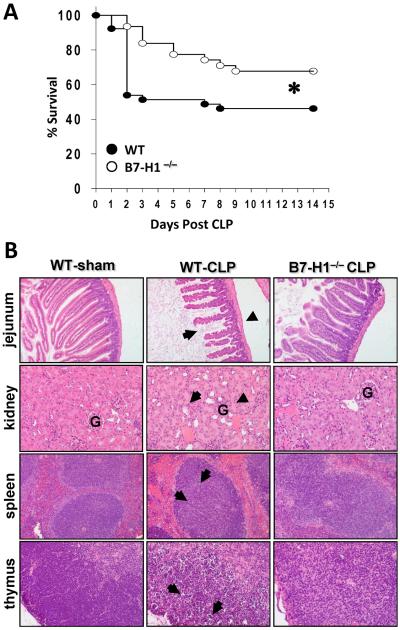
To establish the role of B7-H1 in a murine model of experimental sepsis, both WT C57BL/6J (B7-H1+/+) and B7-H1−/− mice were subjected to CLP surgery and mortality was monitored. We observed that in WT mice, CLP caused mortality of 54% (21 of 39 mice dead) at 14 days post CLP; while B7-H1−/− mice were markedly protected against CLP-induced lethality, as their mortality at 14 days post CLP was 32% (20 of 31 mice survived) (Fig. 2A). These data demonstrate that B7-H1 gene deficiency can protect mice from death caused by experimental polymicrobial sepsis.
Figure 2. B7-H1 gene deficiency protects mice from sepsis-induced lethality, and reduces organ damage.
(A) B7-H1−/− mice are resistant to CLP induced leathlity as compared with WT mice. WT (n = 39) and B7-H1−/− mice (n = 31) were subjected to CLP, and survival was monitored for 14 days. *, p < 0.05, WT vs B7-H1−/− mice by log-rank test. (B) B7-H1−/− mice show less histological evidence of tissue destruction than WT mice do during severe sepsis. B7-H1−/− mice and WT were subjected to CLP surgery or sham control surgery. Mice were euthanized 40 h after surgery. Tissues were stained with hematoxylin and eosin. Original magnification: × 200 for jejunum, × 400 for kidney, spleen and thymus. (jejunum) Black arrow and arrowheads point to examples of villus shortening and mucosal wall thinning in WT septic mice, respectively, while B7-H1−/− septic mice do not show this pathology. (kidney) Normal kidney from WT-sham group shows cortex with the glomerulus (G). Black arrow and arrowheads indicate acute tubular necrosis and congested glomerulus (G), respectively, in a kidney from a WT septic mouse. Kidney from B7-H1−/− septic mice showes rare tubular necrosis and much less congestion. (spleen & thymus) Black arrows illustrate areas of kayrorrhectic, pyknotic, apoptotic cell bodies, which are absent in B7-H1−/− septic mice. Data are representative of 4–8 mice per group.
The development of MSOF is believed to contribute to septic mortality (20). To determine the extent to which B7-H1 gene deficiency could lessen the detrimental effects of sepsis on various organ systems, we examined the general pathology of several organs following CLP (Fig. 2B). With respect to gut injury, we observed villus shortening, epithelial cell loss, mucosal cell sloughing, and mucosal wall thinning in WT mice following CLP. In the kidney, while evidence of tubular necrosis could be seen, these changes in the jejunum and kidney were largely absent in septic B7-H1−/− mice. Looking at two lymphoid organs, such as the spleen and thymus, we have previously documented (14, 21) and again here observed not only marked disrupted tissue architecture and loss of cellularity, but also substantial evidence of karyorrhectic/apoptotic cell bodies in septic WT mice. In contrast, septic B7-H1−/− mouse splenic histology appears relatively normal; and the thymus shows few signs of karyorrhectic/apoptitic cell bodies, although the loss of lymphocytes was not obviously prevented. These results show that B7-H1 gene deficiency reduces the pathological damage seen in multiple organs in response to severe sepsis, which likely improves the survival of B7-H1−/− mice.
B7-H1 gene deficiency lessens inflammatory response but not bacterial burden in septic mice
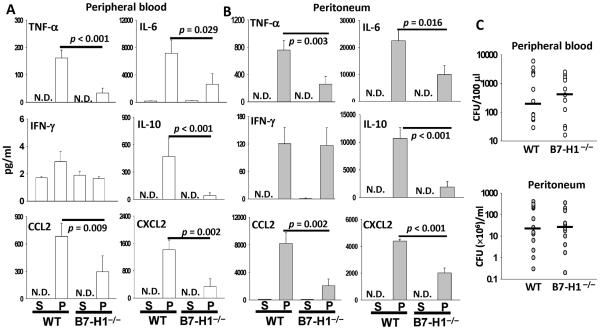
Then, we investigated the effect of B7-H1 gene deficiency on systemic blood inflammatory cytokine/chemokine levels after CLP. The systemic peripheral blood cytokine profiles of sham- or CLP-treated WT and B7-H1−/− mice were measured at 24 h after surgery. Sham surgery did not cause a significant rise in cytokine production. Pro-inflammatory cytokines (IL-6 and TNF-α) and the anti-inflammatory cytokine (IL-10) were markedly higher in septic WT mice than in septic B7-H1−/− mice. Two chemokines, CXCL2 (a.k.a., MIP-2) and CCL2 (a.k.a., MCP-1), were significantly lower in septic B7-H1−/− mice than that in septic WT mice (Fig. 3A). Similar to the systemic blood cytokine profile, expression of local peritoneal lavage inflammatory cytokines (TNF-α, IL-6, and IL-10) and chemokines (CXCL2 and CCL2) was attenuated in septic B7-H1−/− mice when compared to septic WT mice. There was no difference between WT and B7-H1−/− mice for IFN-γ production during sepsis; nevertheless, CLP surgery only induced a small amount of IFN-γ in both mouse strains (Fig. 3A, B). Overall, these results indicate that the inflammatory response to sepsis in B7-H1 deficient mice is less vigorous than that in WT mice. Subsequently, the effect of B7-H1 gene deficiency on bacterial burden was evaluated. Surprisingly, at 24 h post CLP, we observed that WT and B7-H1−/− mice had similar bacterial burden in both systemic circulation and infectious site (Fig 3C).
Figure 3. B7-H1 gene deficiency lessens inflammatory responses but not bacterial burden.
B7-H1−/− mice manifest significantly lower systemical (A) and local (B) levels of inflammatory cytokines at 24 h post-CLP than WT mice do. The graphs depict data (mean ± s.e.m of S: sham n=7–8 sham, P: CLP n=13) pooled from three independent experiments (N.D., not detected). p value as shown by Mann-Whitney test. (C) B7-H1−/− mice have almost equal bacterial burden to WT mice have after CLP. Levels of aerobic bacteria are expressed as CFU per 100 μl for blood and CFU per ml for peritoneal lavage. The graphs depict data pooled from three independent studies showing similar results. (n = 12–13) The horizontal bar indicates the median for each group.
B7-H1 gene deficiency promotes recruitment of macrophages and neutrophils into the infectious site of peritoneum
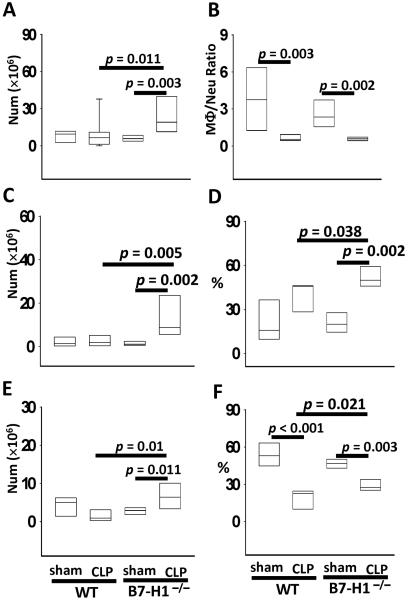
Macrophages and neutrophils are prominent bacteria-killing cells and constitute major cell populations in peritoneum at 24 h when mice are subjected to CLP surgery. Inasmuch, we examined whether B7-H1 gene deficiency affected macrophage and neutrophil recruitment to peritoneum. As shown in Fig 4A, sham and CLP treated WT mice recruited similar numbers of leukocytes, while CLP treated B7-H1−/− mice recruited more leukocytes than WT mice did; therefore, B7-H1 gene deficiency appears to promote sepsis induced leukocyte recruitment. In addition, in WT mice that were subjected to sham surgery, there were more macrophages than neutrophils in peritoneum at 24 h post surgery; otherwise, CLP treated mice manifested the opposite, more neutrophils than macrophages, which results in a significantly decreased macrophage:neutrophil ratio. However, this macrophage:neutrophil ratio shift was not affected by B7-H1 deficiency (Fig 4B). Looking at the neutrophil recruitment in WT mice, sepsis did not promote their recruitment into peritoneum in increasing numbers (Fig 4C), although there was a trend toward a frequency increase (Fig 4D). Alternatively, B7-H1 gene deficiency did enhance sepsis induced neutrophil recruitment, both in number (Fig 4C) and in percentage (Fig 4D). Further, B7-H1 gene deficiency also enabled more macrophages to be recruited to the site of infection (Fig 4E, F). There were more macrophages present in the septic B7-H1 gene deficient mice's peritoneum than in either sham treated B7-H1−/− mice or septic WT mice's peritoneum (Fig 4E). While the proportion of macrophages in peritoneum decreased sharply during sepsis; B7-H1 gene deficiency lessens this decline since more macrophages were recruited than in the WT mice (Fig 4F). Together, B7-H1 gene deficiency appears to promote the recruitment of macrophages and neutrophils into the site of infection in response to CLP-induced sepsis.
Figure 4. B7-H1 gene deficiency enhances neutrophils and macrophages recruitment into infectious site.
(A) B7-H1 deficiency increases total leukocytes recruitment during sepsis. (B) Macrophage:neutrophil ratio in peritoneum decreases during sepsis, which is not affected by B7-H1 gene deficiency. (C–D) Septic B7-H1 deficient mice recruit more neutrophils (gated as F4/80−Ly-6G+) into peritoneum than septic WT mice do, in numbers (C) and in percentage (D). (E–F) Septic B7-H1 gene deficient mice recruit more macrophages (gated as F4/80+Ly-6G−) into peritoneum than septic WT mice do, in numbers (E) and percentage (F). The graphs depict data pooled from four independent experiments (sham n = 7 – 8, CLP n = 10). p value from Mann-Whitney test.
B7-H1 gene deficiency partially reverses mouse macrophages' altered cytokine productive capacity, but does not restore their ability to engulf bacteria during sepsis
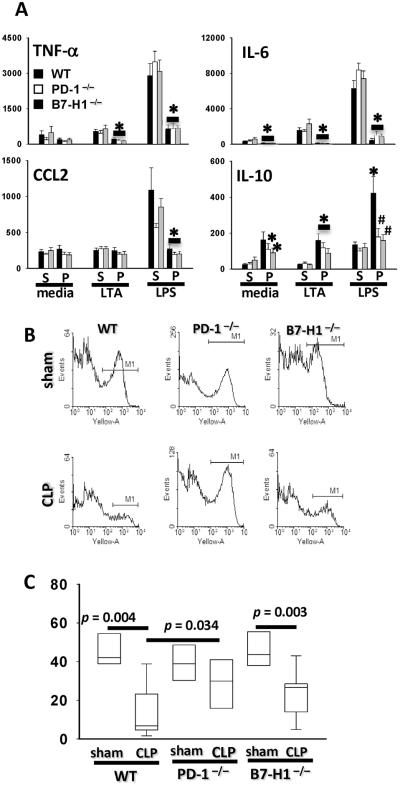
Severe sepsis often associates with the development of monocyte/macrophage dysfunction (14, 22), which is characterized by reduced, even diminished ability to phagocytose bacteria and to produce cytokines ex vivo. Conversely, the production of certain anti-inflammatory cytokines, including IL-10 is enhanced in monocytes/macrophages isolated from septic patients and mice. Thus, whether B7-H1 gene deficiency affects these dysfunctional developments during sepsis was assessed. As shown in Fig 5A, peritoneal macrophages from septic WT mice produced less TNF-α, IL-6, CCL2 and greater amount of IL-10 than macrophages from sham control WT mice did. The reduced capacity to produce TNF-α and CCL2 was not restored by either B7-H1 or PD-1 gene deficiency. Both B7-H1 and PD-1 gene deficiency partially reversed the changes of IL-6 and IL-10 production, however, only the effect on IL-10 production was statistically significant. There was no difference between B7-H1 and PD-1 gene deficiency on affecting the septic mouse macrophages' ability to produce cytokines mentioned above.
Figure 5. B7-H1 gene deficiency partially prevents macrophages from developing sepsis induced dysfunction.
(A) Peritoneal macrophages from septic B7-H1−/− mice or PD-1−/− mice produce lower IL-10 when compared with peritoneal macrophages from septic WT mice. Peritoneal macrophages from sham (S) or CLP (P) surgery treated WT, B7-H1−/− or PD-1−/− mice were stimulated with LTA or LPS; the cell supernatant was collected and secreted cytokines were measured by ELISA. *, p < 0.05, CLP treated vs sham treated mice; #, p < 0.05, gene deficiency mice vs WT mice by Mann-Whitney test. The graphs depict data (mean ± s.e.m) pooled from four independent experiments (sham n = 8 – 10, CLP n = 10 – 12). (B–C) B7-H1 deficiency does not prevent peritoneal macrophages from phagocytic ability diminishing. (B) Representative flow chart of phagocytosis analysis of peritoneal macrophages from WT, B7-H1−/− or PD-1−/− mice. Peritoneal macrophages were enriched by adhering to plastic plate and then fed with pHrodo - conjugated E. coli. The phagocytic capacity was measured by frequency of pHrodo fluorescence positive cells (M1). (C) Quantitative analysis of phagocytosis. Each group includes 4 – 10 mice pooled from three experiments. Outliers are shown at 5th/95th percentiles. p value as shown by Mann-Whitney test.
The capacity of peritoneal macrophages to phagocytose bacteria plummets during sepsis in WT mice (Fig 5B, C) (14). This decline appears to be only slightly (but not significantly) altered in B7-H1 gene deficient mice; while peritoneal macrophages from PD-1 gene deficiency septic mice retained their ability to phagocytose, resulting in a significantly higher phagocytic ability for peritoneal macrophages derived from PD-1 gene deficient septic mice as compared to that from septic WT mice (Fig 5C). These results show that B7-H1 does not contribute to reserving the diminished capacity of peritoneal macrophages to phagocytose bacteria during sepsis, but partially reserves their altered ability to produce cytokines.
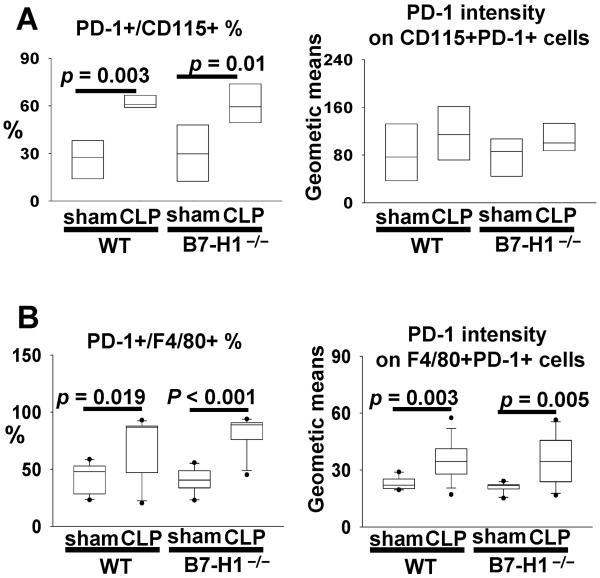
B7-H1 gene deficiency does not affect sepsis-induced upregulation of PD-1 expression on macrophages
We have demonstrated that PD-1 expression on macrophages is upregulated in response to CLP and this upregulation plays an important role in the development of macrophage dysfunction (14). In light of the well established notion that B7-H1 is the most important ligand for inducing PD-1 signaling (8) and the results mentioned above, it becomes important to determine whether B7-H1 gene deficiency affects the development of macrophage dysfunction in septic mice through regulating the expression of PD-1. As shown in Fig 6, both peripheral monocytes (A) and peritoneal macrophages (B) express higher frequency and increased intensity of PD-1 expression during sepsis; however, these changes were not affected by B7-H1 gene deficiency. Thus, the upregulation of PD-1 on macrophages during sepsis is independent of the presence of B7-H1 gene product.
Figure 6. B7-H1 gene deficiency does not affect the upregulation of PD-1 expression on macrophages during sepsis.
(A) The expression of PD-1 on peripheral monocytes from sham or CLP WT/ B7-H1−/− mice (n = 5 for sham, n = 7 for CLP pooled from two experiments). (B) The expression of PD-1 on peritoneal macrophages from sham/CLP WT/B7-H1−/− mice (n = 10 for sham, n = 13 for CLP pooled from three experiments). p value as shown by Mann-Whitney test.
High expression of B7-H1 on neutrophils associates with worse outcome/reduced survival of sepsis
While initial examination of peripheral blood neutrophils in septic mice showed great variation (a trend towards an increase that was not significant) in the frequency of B7-H1+ cells (Fig 1B), we subsequently observed that peripheral blood neutrophils displayed a polarized expression pattern of B7-H1 during sepsis (Fig 7A, B). In some mice, the percentage of B7-H1+ neutrophils increased (Fig 7A box a, B upper chart), while in others, it decreased (Fig 7A box b, B lower chart). This was also not the case with any other cell populations we examined in this study. To evaluate significance of these two sub-populations we attempted to assess whether the neutrophil expression of B7-H1 was associated with the outcomes of septic mice. To do this, the cheek puncture technique was adopted to obtain a small amount of peripheral blood without having to kill the mouse as done for the other experiments. B7-H1 expression by peripheral blood neutrophils was assessed 24 h after CLP surgery and the survival of these mice was then observed for 14 days. As shown in Fig 7C, we found that neutrophils from mice that went on to succumb to the morbid effects of sepsis consistently expressed a markedly higher level B7-H1 than neutrophils from mice that subsequently survived.
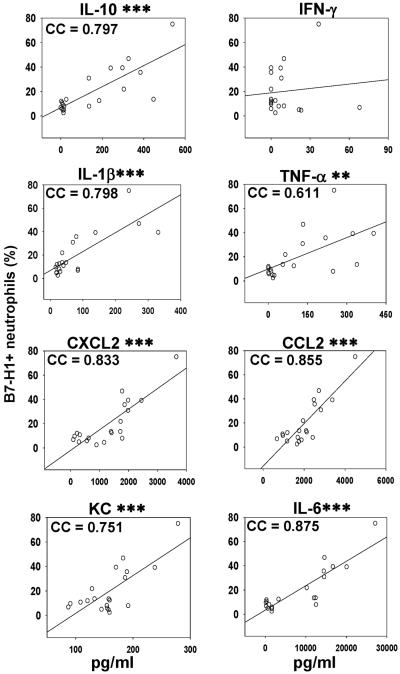
Elevated circulating inflammatory cytokine levels are correlated with upregulated B7-H1 expression on neutrophils during sepsis
Inflammatory cytokines are implicated to be major contributors of the pathophysiology of sepsis. To determine whether the expression of B7-H1 on peripheral neutrophils was associated with the intensity of inflammatory response, the correlation between the frequency of B7-H1+ peripheral blood neutrophils and the circulating levels of IL-10, IFN-γ, IL-1β, TNF-α, CXCL2, CCL2, KC, and IL-6 was analyzed. As shown in Fig 8, except for IFN-γ, a significant correlation appears to exist between the rising levels of any of the other cytokines/chemokines with the increasing expression of B7-H1 on neutrophils. It is noteworthy that IL-6 and CCL2 exhibited the strongest correlation with the increasing B7-H1 expression on neutrophils. Collectively, the increased frequency of B7-H1+ neutrophils in circulation is correlated not only with the systemic blood cytokines/chemokines level, but the outcome as well.
Figure 8. Higher percentage of B7-H1+ peripheral blood neutrophils is correlated with higher levels of inflammatory cytokines.
The correlation between peripheral neutrophils' B7-H1 expression frequency and systemic inflammatory cytokines levels was assessed. At 24 hours post CLP treatment, mice were sacrificed and bled. The expressions of B7-H1 on peripheral neutrophils were determined by flow cytometry. The levels of IL-10, IFN-γ, IL-1β, TNF-α, CXCL2, CCL2, KC, and IL-6 in plasma were measured by ELISA. The graphs depict data pooled from four experiments (n = 20) and correlation was assessed by Pearson product moment correlation analysis (significance is marked as **, p < 0.01 or ***, p < 0.001). Every dot represents data from one mouse. Correlation cofactor (CC) is shown.
DISCUSSION
B7-H1:PD-1 pathway has been implicated in controlling antigenic/T cell tolerance, autoimmunity, and immune responses to a number of, largely viral, infectious diseases (8). We have demonstrated that PD-1 played a pivotal role in the innate immune response of sepsis (14). Here we report on some of our initial studies looking at the role of B7-H1 in the immune response of this acute polymicrobial infection. B7-H1 is one of the most widely expressed molecules among the B7:CD28 family members (9). It is expressed on hematopoietic, endothelial as well as epithelial cells and is upregulated in response to inflammatory cytokines (11), suggesting B7-H1 may have a role in inflammatory response.
In our study here we initially detected the changes in B7-H1 expression on myeloid and lymphoid cells in response to polymicrobial septic challenge. The expression of B7-H1 was upregulated on monocytes and NKT cells from peripheral blood, on macrophages and neutrophils from infectious site, the peritoneum, and on macrophages and T cells from the spleen, a tertiary lymphoid bed. Almost 100% of peripheral NK cells and T cells express B7-H1; and the expression was virtually unchanged in response to septic challenge. Notably, the expression of B7-H1 on peripheral blood neutrophils was not consistently upregulated in all mice; there was also a tendency of declined neutrophil B7-H1+ frequency in some septic mice. This enhanced expression of B7-H1 on myeloid and lymphoid cells suggests that B7-H1 may participate in both the innate and adaptive immune response to sepsis. However, more convincing evidence to supporting B7-H1's role in sepsis comes from the observation that B7-H1 gene deficiency significantly reduced the CLP induced mortality. In agreement with this protective effect, the pathological damage that is typically seen in jejunum, kidney, spleen and thymus (23–25) was largely absent; and the levels of pro- and anti-inflammatory cytokines and chemokines in circulation as well as at the infectious site were profoundly reduced. Nevertheless, unlike what we have seen in PD-1−/− mice, the bacterial burden in septic WT and B7-H1−/− mice were similar, both in blood and in peritoneal fluid, indicating that the protective effect of B7-H1 gene deficiency was not dependent on clearing bacteria more efficiently. Although it is believed that failure to remove the invading pathogens would initiate hyperactive pro-inflammatory responses/SIRS (26), the above data suggest that B7-H1 gene deficiency may down regulate the inflammatory response irrespective of the microbial stimulation. The results also support a role for SIRS in the pathophysiology of sepsis and implying that B7-H1:PD-1 pathway plays an important role in the development of inflammatory response.
To further explore how B7-H1 participates in regulating the inflammatory response to septic challenge, we chose to look at how B7-H1 gene deficiency affects the migration of macrophages and neutrophils into the infectious site. Here we found that B7-H1 gene deficiency actually enhanced the recruitment of macrophages and neutrophils into the peritoneum of CLP mice. This is not totally surprising since mice deficient in these inhibitory molecules usually have greater numbers of leukocytes than WT mice have, which serves as source for more recruitable cells (7). Nonetheless, this increase of professional phagocytes in the peritoneum did not improve bacterial clearance, suggesting that cell functions, other than phagocytosis, may be more relevant. It has been demonstrated by others and us that there is a significant reduction in the ex vivo production of inflammatory cytokines (including IL-1β, IL-6 and TNF-α) by monocytes/macrophages from patients and mice with sepsis. Conversely, the production of certain anti-inflammatory cytokines, including IL-10, is enhanced (14, 22). We found that B7-H1 gene deficiency partially reversed this alteration. Notably, macrophages from septic B7-H1−/− mice produced less IL-10 than macrophages from septic WT mice did. In addition, upon the stimulation with LPS, they essentially secreted the same amount of IL-10 as the macrophages from derived sham control mice. Similar changes were observed in PD-1−/− mice (14). On the other hand, B7-H1 gene deficiency did not reverse the diminished phagocytic capacity seen in the WT CLP mice, while PD-1 gene deficiency did (14). This difference might explain why bacterial burden in B7-H1−/− and PD-1−/− CLP mice are so distinct. Since upregulated PD-1 expression is associated with the development of various leukocyte dysfunctions and B7-H1 is the primary ligand for stimulating PD-1 ligation/signaling (9), we thought it was important to investigate whether B7-H1 gene deficiency affected the upregulation of PD-1 on macrophages during sepsis that we had previously reported (14). In this respect we found there were no differences in the sepsis-induced upregulation of PD-1 expression between macrophages derived from WT as opposed to B7-H1−/− mice, indicating that the regulation of macrophage expression of PD-1 appears to be independent from the presence of B7-H1. From above observations, the expression of B7-H1 appears to be an essential element in the innate immune response during sepsis; and the similarity in B7-H1−/− and PD-1−/− mice may reside in their impact on the inflammatory response. Indeed, B7-H1 seems to be less involved in the regulation of dysfunctional macrophages, e.g., sepsis-induced phagocytic capacity decline, than PD-1. Together, it is suggested that B7-H1 and PD-1 affect different aspects of innate immunity, which possibly relate to their diverse expression patterns, different functional characters (B7-H1 activation vs. PD-1 signaling), and/or other ligand:receptor interactions (e.g., B7-H1: B7-1) (9). Also, the impact of B7-H1 gene deficiency may include actions mediated through immune: non-immune cells (27). We have recently reported that the increased expression of B7-H1 on mouse liver sinusoidal endothelial cell contributes to the development of liver injury following the onset of experimental sepsis (28).
PD-1 has been well established as an exhaustion marker of T cells relative to chronic viral infection (10, 29, 30) and also been suggested as a potential marker of myeloid cell dysfunction in acute bacterial infection (14). An increased B7-H1 expression on hematopoietic and non-hematopoietic cells during immune responses has been demonstrated to deliver inhibitory signals to activated T cells that expressed PD-1. It has also been reported that inflammatory stimulants, such as pro-inflammatory cytokines, can elevate the B7-H1 expression by myeloid and lymphoid cells (31). In this respect, we noticed a distinctive expression pattern for B7-H1 on peripheral neutrophils during sepsis, i.e., where some mice had more B7-H1 expressing neutrophils while others had less B7-H1 expressing neutrophils when compared with non septic (Sham) mice. This phenomenon made us speculate that this divergence in expression might be related with outcomes (survival) of sepsis. Interestingly, we confirm that the emergence of a high B7-H1 expressing neutrophil population in peripheral blood correlates with unfavorable outcome (death). Moreover, we observed a correlation between the higher percentage B7-H1+ expression on neutrophils and higher levels of circulating inflammatory cytokines, notably IL-10, IL-1β, CXCL2, CCL2, and IL-6. It has been well documented that levels of pro-inflammatory cytokines IL-6, IL-8, and CCL2, as well as the immunosuppressive cytokine IL-10 are higher in those patients with fatal outcome (32–38). Thus, this observation further supports that an increased frequency of B7-H1 expression might be used as an indicator to predict lethal outcome of sepsis. The nature or changing biological function of these cells in sepsis remains to be explored.
The B7-H1:PD-1 pathway has been demonstrated to play a critical role in regulating the delicate balance between protective immunity and tolerance (27). While the role of this pathway in adaptive immunity during chronic infections and cancer is well established, its functions in innate immunity during acute bacterial/fungal infections are much less clear. The increasing knowledge of the complexity of activation of innate immune response ensures that it is tightly regulated and finely tuned. Early studies suggest that the B7-H1:PD-1 pathway was important in limiting the developing effector T cell responses and in maintaining a state of antigenic tolerance so as to curb by-stander tissue damage. However, in view of recent studies, the role of this pathway during acute infections appears to be more complex (8–10). In agreement with this, here we demonstrate the pivotal role of B7-H1 in the inflammatory response during acute polymicrobial sepsis, in that B7-H1 gene deficiency effectively decreased the production of inflammatory cytokines/chemokines, thus, reducing tissue damage and mortality. Along with our former finding that PD-1 gene deficiency has similar effects on these aspects, we conclude that B7-H1:PD-1 pathway may be a key mediator in inflammatory response during acute polymicrobial infection. Therefore, it is possible in the setting of sepsis that undesirable inflammatory responses that lead to SIRS as well as to organ damage could be manipulated through intervening B7-H1:PD-1 pathway. On the other hand, identification of patients at increased risk of death is clearly important in severe sepsis. In this respect, while the coexistence of several clinical scores for sepsis (SOFA, SAPS II, SAPS III, and LOD) reflects the fact that none of them produces enough accuracy to predict outcome with a good sensitivity and specificity (39), it has been suggested that concomitant evaluation of multiple cytokines or proposed mediators of the pathological processes leading to sepsis may give us a better predictive insight (33). The correlation of elevated frequency of B7-H1+ neutrophils with higher inflammatory cytokine levels and increased risk of septic death not only supports the role of B7-H1 as a mediator in the inflammatory axis, but also suggests B7-H1 might be such a candidate molecule for predicting the risk of death, possibly in combination with injury severity scores and other circulating diagnostic molecules.
In conclusion, these findings extend our understanding of the implications of B7-H1:PD-1 ligation, from their regulatory role in maintaining antigenic tolerance, autoimmunity, tumorgenesis, chronic viral diseases, to having roles as mediators in the innate inflammatory response generated in response to a septic insult. Along with our previous report of PD-1's role in macrophage dysfunction during sepsis, both B7-H1 and PD-1 might impact inflammation through affecting innate immune cell functions. Since all of the clinical trials designed to neutralize single inflammatory cytokines, thus far, have failed, some have suggested that normalization of a number of components of the inflammatory cytokine storm through surface receptors in a “global” fashion might represent a potential alternative approach to improve overall prognosis (40). To this end, our observations may assist in the design of appropriate therapeutic interventions for treatment of sepsis.
Acknowledgments
The authors acknowledge Dr. Lieping Chen (Yale University, New Haven, CT) for providing B7-H1−/− mice, Dr. Tasuku Honjo (Kyoto University, Kyoto, Japan) and Dr. Megan Sykes (Massachusetts General Hospital, Transplantation Biology Research Center, Boston, MA) for providing PD-1−/− mice. We also thank Paul Monfils for technical assistance with the histologic specimens.
Abbreviations
- CLP
cecal ligation and puncture
- MOF
multiple organ failure
- PD-1
programmed cell death receptor-1
- SIRS
systemic inflammatory response syndrome
- WT
wild type
REFERENCES
- 1.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N. Engl. J. Med. 2003;348:1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 2.Angus DC. The lingering consequences of sepsis: a hidden public health disaster? JAMA. 2010;304:1833–1834. doi: 10.1001/jama.2010.1546. [DOI] [PubMed] [Google Scholar]
- 3.Bosmann M, Ward PA. The inflammatory response in sepsis. Trends. Immunol. 2013;34:129–136. doi: 10.1016/j.it.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen J. The immunopathogenesis of sepsis. Nature. 2002;420:885–891. doi: 10.1038/nature01326. [DOI] [PubMed] [Google Scholar]
- 5.Wenzel RP, Edmond MB. Septic shock--evaluating another failed treatment. N. Engl. J. Med. 2012;366:2122–2124. doi: 10.1056/NEJMe1203412. [DOI] [PubMed] [Google Scholar]
- 6.Cai B, Deitch EA, Ulloa L. Novel insights for systemic inflammation in sepsis and hemorrhage. Mediators. Inflamm. 2010:642462. doi: 10.1155/2010/642462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu. Rev. Immunol. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 8.Sharpe AH, Wherry EJ, Ahmed R, Freeman GJ. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat. Immunol. 2007;8:239–245. doi: 10.1038/ni1443. [DOI] [PubMed] [Google Scholar]
- 9.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu. Rev. Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 11.Topalian SL, Drake CG, Pardoll DM. Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr. Opin. Immunol. 2012;24:207–212. doi: 10.1016/j.coi.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Francisco LM, Sage PT, Sharpe AH. The PD-1 pathway in tolerance and autoimmunity. Immunol. Rev. 2010;236:219–242. doi: 10.1111/j.1600-065X.2010.00923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown KE, Freeman GJ, Wherry EJ, Sharpe AH. Role of PD-1 in regulating acute infections. Curr. Opin. Immunol. 2010;22:397–401. doi: 10.1016/j.coi.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang X, Venet F, Wang YL, Lepape A, Yuan Z, Chen Y, Swan R, Kherouf H, Monneret G, Chung CS, Ayala A. PD-1 expression by macrophages plays a pathologic role in altering microbial clearance and the innate inflammatory response to sepsis. Proc. Natl. Acad. Sci. U. S. A. 2009;106:6303–6308. doi: 10.1073/pnas.0809422106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dong H, Zhu G, Tamada K, Flies DB, van Deursen JM, Chen L. B7-H1 determines accumulation and deletion of intrahepatic CD8(+) T lymphocytes. Immunity. 2004;20:327–336. doi: 10.1016/s1074-7613(04)00050-0. [DOI] [PubMed] [Google Scholar]
- 16.Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11:141–151. doi: 10.1016/s1074-7613(00)80089-8. [DOI] [PubMed] [Google Scholar]
- 17.Ayala A, Karr SM, Evans TA, Chaudry IH. Factors responsible for peritoneal granulocyte apoptosis during sepsis. J. Surg. Res. 1997;69:67–75. doi: 10.1006/jsre.1997.5027. [DOI] [PubMed] [Google Scholar]
- 18.Ebong SJ, Call DR, Bolgos G, Newcomb DE, Granger JI, O'Reilly M, Remick DG. Immunopathologic responses to non-lethal sepsis. Shock. 1999;12:118–126. doi: 10.1097/00024382-199908000-00005. [DOI] [PubMed] [Google Scholar]
- 19.Craciun FL, Schuller ER, Remick DG. Early enhanced local neutrophils recruitment in peritonitis-induced sepsis improves bacterial clearance and survival. J. Immunol. 2010;185:6930–6938. doi: 10.4049/jimmunol.1002300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Semeraro N, Ammollo CT, Semeraro F, Colucci M. Sepsis, thrombosis and organ dysfunction. Thromb. Res. 2012;129:290–295. doi: 10.1016/j.thromres.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 21.Hotchkiss RS, Tinsley KW, Swanson PE, Schmieg RE, Jr, Hui JJ, Chang KC, Osborne DF, Freeman BD, Cobb JP, Buchman TG, Karl IE. Sepsis-induced apoptosis causes progressive profound depletion of B and CD4+ T lymphocytes in humans. J. Immunol. 2001;166:6952–6963. doi: 10.4049/jimmunol.166.11.6952. [DOI] [PubMed] [Google Scholar]
- 22.Lyn-Kew K, Standiford TJ. Immunosuppression in sepsis. Curr. Pharm. Des. 2008;14:1870–1881. doi: 10.2174/138161208784980545. [DOI] [PubMed] [Google Scholar]
- 23.Coopersmith CM, Stromberg PE, Dunne WM, Davis CG, Amiot DM, 2nd, Buchman TG, Karl IE, Hotchkiss RS. Inhibition of intestinal epithelial apoptosis and survival in a murine model of pneumonia-induced sepsis. J. A. M. A. 2002;287:1716–1721. doi: 10.1001/jama.287.13.1716. [DOI] [PubMed] [Google Scholar]
- 24.Hotchkiss RS, Nicholson DW. Apoptosis and caspases regulate death and inflammation in sepsis. Nat. Rev. Immunol. 2006;6:813–822. doi: 10.1038/nri1943. [DOI] [PubMed] [Google Scholar]
- 25.Gupta A, Berg DT, Gerlitz B, Sharma GR, Syed S, Richardson MA, Sandusky G, Heuer JG, Galbreath EJ, Grinnell BW. Role of protein C in renal dysfunction after polymicrobial sepsis. J. Am. Soc. Nephrol. 2007;18:860–867. doi: 10.1681/ASN.2006101167. [DOI] [PubMed] [Google Scholar]
- 26.Bone RC. Toward a theory regarding the pathogenesis of the systemic inflammatory response syndrome: what we do and do not know about cytokine regulation. Crit. Care. Med. 1996;24:163–172. doi: 10.1097/00003246-199601000-00026. [DOI] [PubMed] [Google Scholar]
- 27.Riella LV, Paterson AM, Sharpe AH, Chandraker A. Role of the PD-1 pathway in the immune response. Am. J. Transplant. 2012;12:2575–2587. doi: 10.1111/j.1600-6143.2012.04224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hutchins NA, Wang F, Wang Y, Chung CS, Ayala A. Kupffer cells potentiate liver sinusoidal endothelial cell injury in sepsis by ligating programmed cell death ligand-1. J. Leukoc. Biol. 2013;94:963–970. doi: 10.1189/jlb.0113051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crawford A, Wherry EJ. The diversity of costimulatory and inhibitory receptor pathways and the regulation of antiviral T cell responses. Curr. Opin. Immunol. 2009;21:179–186. doi: 10.1016/j.coi.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaufmann DE, Walker BD. PD-1 and CTLA-4 inhibitory cosignaling pathways in HIV infection and the potential for therapeutic intervention. J. Immunol. 2009;182:5891–5897. doi: 10.4049/jimmunol.0803771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamazaki T, Akiba H, Iwai H, Matsuda H, Aoki M, Tanno Y, Shin T, Tsuchiya H, Pardoll DM, Okumura K, Azuma M, Yagita H. Expression of programmed death 1 ligands by murine T cells and APC. J. Immunol. 2002;169:5538–5545. doi: 10.4049/jimmunol.169.10.5538. [DOI] [PubMed] [Google Scholar]
- 32.Lichtenstern C, Brenner T, Bardenheuer HJ, Weigand MA. Predictors of survival in sepsis: what is the best inflammatory marker to measure? Curr. Opin. Infect. Dis. 2012;25:328–336. doi: 10.1097/QCO.0b013e3283522038. [DOI] [PubMed] [Google Scholar]
- 33.Andaluz-Ojeda D, Bobillo F, Iglesias V, Almansa R, Rico L, Gandía F, Resino S, Tamayo E, de Lejarazu RO, Bermejo-Martin JF. A combined score of pro- and anti-inflammatory interleukins improves mortality prediction in severe sepsis. Cytokine. 2012;57:332–336. doi: 10.1016/j.cyto.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 34.Mera S, Tatulescu D, Cismaru C, Bondor C, Slavcovici A, Zanc V, Carstina D, Oltean M. Multiplex cytokine profiling in patients with sepsis. A. P. M. I. S. 2011;119:155–163. doi: 10.1111/j.1600-0463.2010.02705.x. [DOI] [PubMed] [Google Scholar]
- 35.Panacek EA, Marshall JC, Albertson TE, Johnson DH, Johnson S, MacArthur RD, Miller M, Barchuk WT, Fischkoff S, Kaul M, Teoh L, Van Meter L, Daum L, Lemeshow S, Hicklin G, Doig C. Monoclonal Anti-TNF: a Randomized Controlled Sepsis Study Investigators. 2004. Efficacy and safety of the monoclonal anti-tumor necrosis factor antibody F(ab')2 fragment afelimomab in patients with severe sepsis and elevated interleukin-6 levels. Crit. Care. Med. 32:2173–2182. doi: 10.1097/01.ccm.0000145229.59014.6c. [DOI] [PubMed] [Google Scholar]
- 36.El-Maghraby SM, Moneer MM, Ismail MM, Shalaby LM, El-Mahallawy HA. The diagnostic value of C-reactive protein, interleukin-8, and monocyte chemotactic protein in risk stratification of febrile neutropenic children with hematologic malignancies. J. Pediatr. Hematol. Oncol. 2007;29:131–136. doi: 10.1097/MPH.0b013e3180308770. [DOI] [PubMed] [Google Scholar]
- 37.Bozza FA, Salluh JI, Japiassu AM, Soares M, Assis EF, Gomes RN, Bozza MT, Castro-Faria-Neto HC, Bozza PT. Cytokine profiles as markers of disease severity in sepsis: a multiplex analysis. Crit. Care. 2007;11:R49. doi: 10.1186/cc5783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heper Y, Akalin EH, Mistik R, Akgöz S, Töre O, Göral G, Oral B, Budak F, Helvaci S. Evaluation of serum C-reactive protein, procalcitonin, tumor necrosis factor alpha, and interleukin-10 levels as diagnostic and prognostic parameters in patients with community-acquired sepsis, severe sepsis, and septic shock. Eur. J. Clin. Microbiol. Infect. Dis. 2006;25:481–491. doi: 10.1007/s10096-006-0168-1. [DOI] [PubMed] [Google Scholar]
- 39.Pierrakos C, Vincent JL. Sepsis biomarkers: a review. Crit. Care. 2010;14:R15. doi: 10.1186/cc8872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Clatworthy MR, Smith KG. FcgammaRIIb balances efficient pathogen clearance and the cytokine-mediated consequences of sepsis. J. Exp. Med. 2004;199:717–723. doi: 10.1084/jem.20032197. [DOI] [PMC free article] [PubMed] [Google Scholar]