Abstract
Peripheral axons can re-extend robustly after nerve injury. Soon after a nerve crush regenerating axons grow through the nerve segment distal to the lesion in close proximity to distal axons that are still morphologically and molecularly preserved. Hence, following the progress of regenerating axons necessitates markers that can distinguish between regenerating and degenerating axons. Here, we show that axonal levels of superior cervical ganglion 10 (SCG10) is dynamically regulated after axonal injury and provides an efficient method to label regenerating axons. In contrast to the rapid loss of SCG10 in distal axons (Shin et al., 2012b), we report that SCG10 accumulates in the proximal axons within an hour after injury, leading to a rapid identification of the lesion site. The increase in SCG10 levels is maintained during axon regeneration after nerve crush or nerve repair and allows for more selective labeling of regenerating axons than the commonly used markers growth-associated protein 43 (GAP43) and YFP. SCG10 is preferentially expressed in regenerating sensory axons rather than motor axons in the sciatic nerve. In a mouse model of slow Wallerian degeneration, SCG10 labeling remains selective for regenerating axons and allows for a quantitative analysis of delayed regeneration in this mutant. Taken together, these data demonstrate the utility of SCG10 as an efficient and selective marker of sensory axon regeneration.
Keywords: Axon regeneration, Dorsal root ganglia (DRG), STMN2, NMNAT, Marker of regenerating axon, Nerve repair
Introduction
The peripheral nervous system can often recover from mechanical, chemical, and pathological insults that injure axonal projections. Impaired sensory and motor function as a result of the destruction of damaged axons can be restored if the axons re-grow across the injured area and successfully reinnervate their target cells (Allodi et al., 2012). However, efficient recovery of neural function depends on the speed of reinnervation– if the target cell is denervated for a prolonged period, it may lose function even if the neural connection is eventually restored (Gordon et al., 2011). Therefore, there is much interest in identifying molecular mechanisms regulating axon regeneration and developing methods that facilitate the regenerative responses (Blackmore, 2012; Bradke et al., 2012; Liu et al., 2011; Patodia and Raivich, 2012). The need for such studies has encouraged the development of in vivo assays for axon regeneration that can be used in genetic and pharmacological models. Such anatomical assays require methods to selectively label regenerating axons, distinguishing them from the distal axons undergoing Wallerian degeneration. Since it takes ~40 h for distal axons to fragment following axotomy (Beirowski et al., 2005), the need for selective regeneration markers is particularly acute in the early phase of the injury response.
Transgenic expression of neuronal YFP is a method to visualize axon regeneration and degeneration as well as normal axon morphology. However, YFP remains in distal axon fragments even during axonal degeneration, so regenerating axons are obscured by the YFP-positive degenerative particles (Bareyre et al., 2005; Pan et al., 2003) unless a YFP-negative nerve graft is transplanted to avoid the background signal (Witzel et al., 2005). Neuronal tracers such as the lipophilic DiI (Honig and Hume, 1989) and BDA (biotinylated dextran amine) can be injected proximal to a lesion and will selectively label regenerating axons (Liu et al., 2010). However, these methods are much more technically difficult and time consuming than immunocytochemistry. Indeed, antibody staining for proteins that are selectively localized to regenerating rather than degenerating axons would be a powerful and simple method for labeling re-growing axons. One popular target is growth-associated protein 43 (GAP43), whose transcription is upregulated days after axon injury (Bisby and Tetzlaff, 1992; Skene and Willard, 1981a) leading eventually to intense GAP43 immunolabeling in regenerating axons (Abe et al., 2010; Ackermann et al., 2002).
Superior cervical ganglion 10 (SCG10), which is also known as stathmin 2 (STMN2), is a neuronally expressed stathmin family protein that regulates microtubule dynamics and protein trafficking (Ozon et al., 1997; Riederer et al., 1997; Wang et al., 2013). SCG10 is highly expressed during development and plays an important role in axonal outgrowth by modulating microtubule stability (Morii et al., 2006; Sugiura and Mori, 1995; Tararuk et al., 2006). Interestingly, axonal injury leads to an increase in SCG10 expression in adult sensory neurons (Mason et al., 2002; Voria et al., 2006). In contrast, we recently demonstrated that SCG10 is rapidly lost from distal axons within hours of an axonal injury (Shin et al., 2012b). The differential regulation of SCG10 in regenerating cell bodies and the distal axon segments led us to test the hypothesis that SCG10 may be an efficient and selective marker for re-growing axons in the early stage of axon regeneration. In the current study, we show that SCG10 levels are increased in the axon segments proximal to a lesion in vitro and in vivo within an hour after the injury. After nerve crush or nerve repair, the rise in the proximal SCG10 expression persists while the axons re-grow through the distal nerve segment, which is nearly devoid of SCG10. We demonstrate that the SCG10 immunolabeling is more selective for regenerating axons than either GAP43 or YFP, especially in the early stage of regeneration and in conditions where Wallerian degeneration is delayed. We show that SCG10 is preferentially expressed in sensory fibers, and demonstrate that axonal regeneration can be quantified using SCG10 labeling in a genetic model with slowed axon regeneration. Hence, axonal SCG10 is dynamically regulated upon nerve injury and is a selective marker for regenerating sensory axons, thereby providing a useful new method to assess regeneration after nerve injury and repair.
Material and Methods
Mice
Adult C57BL6 mice were purchased from Jackson Laboratory or Harlan Laboratories and used for in vivo analysis of protein levels and regeneration assays. YFP 16 mice (Feng et al., 2000) were kindly provided by Dr. Joshua Sanes (Harvard University, Cambridge). Advillin-Cre mice (Zhou et al., 2010) or Chat-Cre mice (MMRRC, #017259) were crossed to Thy1-STOP-YFP mice (Bareyre et al., 2005) to label sensory or motor neurons, respectively. Cyt-NMNAT1 mice (gifted by Dr. Jeffrey Milbrandt, Washington University, St. Louis) (Sasaki et al., 2009) and the wild type littermates were used to assess SCG10 expression patterns and axon regeneration. Mouse husbandry and surgeries were performed under the supervision of Division of Comparative Medicine at Washington University.
Antibodies
Anti-SCG10 rabbit antiserum recognizes a C-terminal peptide (Ozon et al., 1997) and is available from Novus Biologicals (STMN2 NBP1-49461). We purified the antiserum using SulfoLink Kit (Thermo). The following antibodies were purchased: rabbit anti-GAP43 (Millipore), Alexa Fluor 488-conjugated mouse anti-β3 tubulin (Tuj1; Covance), Alexa Fluor 488-conjugated anti-mouse secondary antibody (Invitrogen), Cy3-conjugated anti-rabbit secondary antibody (Jackson ImmunoResearch) and Alexa Fluor 647-conjugated anti-rabbit secondary antibody (Invitrogen). Actin was labeled by rhodamine phalloidin (Invitrogen).
DRG culture preparation
In order to locate axonal segments proximal or distal to axotomy, embryonic dorsal root ganglion (DRG) neurons were plated in a small discrete area, so we could transect axons emanating from the cell bodies (Miller et al., 2009). Briefly, 4-well chamber slides were coated with poly-D-lysine (Sigma) and laminin (Sigma). DRGs were collected from E13.5 CD1 embryos (Charles River), treated with trypsin-EDTA for 20 minutes at 37 °C and then followed by trituration into single cells. The cell suspension was plated as a spot at a density of approximately two DRGs per well in 2 μl of medium. The plates were incubated for 20 minutes at 37 °C in order to allow the cells to attach to the plastic before medium was added. The Neurobasal medium (Invitrogen) was supplemented with 2 % B27 (Invitrogen), 25 ng/ml nerve growth factor (Harlan), and 1 μM 5-fluoro-2′-deoxyuridine (Sigma) and 1 μM uridine (Sigma) that block the mitosis of non-neuronal cells. Cultures were maintained for 8–9 d before axotomy. We axotomized cultures with a microscalpel.
In vitro analysis of SCG10 levels
Embryonic DRG cultures were fixed in 4 % paraformaldehyde for 20 minutes and then immunostained. Briefly, cultures were blocked in blocking solution (5 % normal goat serum and 0.1 % Triton X-100 in PBS) for 1 h and then incubated with anti-SCG10 antibody (1:5000) and anti-β3 tubulin antibody (1:500) over night at 4 °C. Samples were washed twice with 0.1 % Triton X-100 in PBS (PBS-T), incubated with secondary antibodies for 1 h at room temperature, washed three times in PBS-T and then mounted in VectaShield (Vector Laboratories). Immunofluorescence images were taken at a Nikon D-Eclipse C1 confocal microscope platform using a 20X oil objective. Confocal stacks were rendered as z projection images. The average SCG10 and β3 tubulin intensities were measured in proximal or distal regions that span 250 μm from the cut site using NIH ImageJ. The SCG10 intensity was first normalized to the β3 tubulin intensity to take axonal density into account and then expressed as a percent changed from the SCG10 level in uncut axons.
Sciatic nerve surgery
For nerve crush, mice were anesthetized with isoflurane and the sciatic nerve was exposed by a small incision on the skin. The nerve was crushed with fine forceps for 20 seconds. Then the incision was sutured by nylon suture. Nerve repair was performed as described by Whitlock et al. (2010). Mice were anesthetized with a cocktail of ketamine and medetomidine. The sciatic nerve was first transected by surgical scissors and then rejoined by 10-0 nylon microepineurial sutures. Four sutures were made along the junction of proximal and distal stumps. Mice were later euthanized by CO2 and sciatic nerves were obtained for analysis.
Immunofluorescence of sciatic nerves
Sciatic nerves were fixed in 4 % paraformaldehyde for 1.5 h. The nerves then were washed with PBS, immersed in 30 % sucrose in PBS, cryopreserved in OCT compound (Tissue-Tek) and cryosectioned at 20 μm-thickness for nerve repair experiment and 10 μm-thickness for crushed nerves. Samples were immunostained with the same procedure described above for immunolabeling of DRG cultures except for secondary antibodies incubated for 2 h. Primary antibodies used were anti-SCG10 (1:5000), anti-GAP43 (1:500) and Alexa Fluor 488-conjugated anti-β3 tubulin (1:500). Multiple confocal pictures were taken with a 10X air objective along the nerve and montaged into a single image using Photoshop (Adobe).
In vivo analysis of SCG10 and GAP43 levels
SCG10 or GAP43 intensity was measured in proximal or distal region spanning 500 μm from the sciatic nerve crush site. The average intensity was then compared to the intensity in uncut nerve and then expressed as a percent changed from the uncut control.
Regeneration assay
SCG10 or GAP43 fluorescence intensity was assessed from the confocal images of sciatic nerves subjected to crush injury. The crush site was determined via β3 tubulin labeling by which deformation of the nerve and disruption of axons are identified. SCG10 or GAP43 intensity was measured along the length of the nerve using a line scan macro written in ImageJ (Shin et al., 2012a). Averaging the intensities at 50 neighboring pixels reduces noise in fluorescence images. Then, regeneration index was determined by measuring the distance from the crush site to the location where SCG10 or GAP43 level is half of the level at the crush site.
Statistical tests
ANOVA or a t test was used as described in results. When ANOVA was used, a Tukey post hoc test was performed for means comparison. P values greater than 0.05 were considered not significant.
Results
SCG10 levels rapidly increase in the proximal stump of injured axons
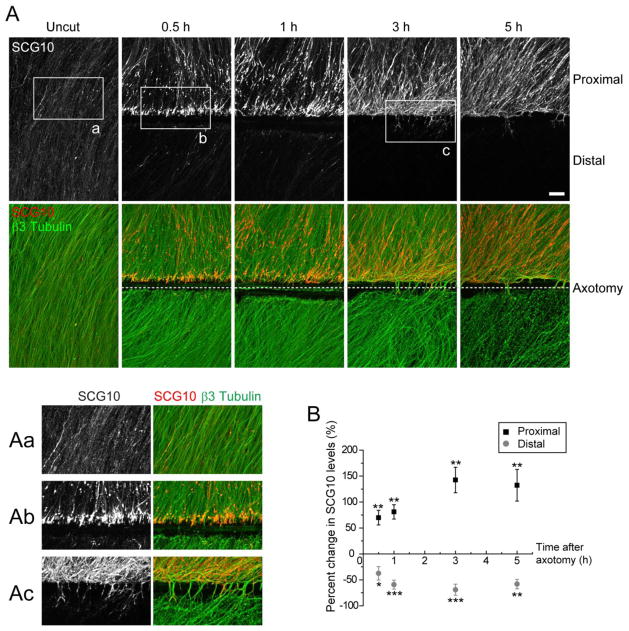
We previously demonstrated that SCG10 levels are dynamically regulated in response to axonal injury, with a rapid loss of SCG10 from axon segments distal to an axotomy (Shin et al., 2012b). Here we study the temporal regulation of SCG10 protein in proximal axons following injury. We transected axons of cultured murine embryonic DRG neurons and performed immunolabeling for SCG10. In uncut axons, SCG10 is localized throughout the processes in a punctate pattern (Figure 1A, boxed area magnified in Figure 1Aa). After axotomy, there is a significant increase in SCG10 levels in the proximal axon stumps within half an hour of injury; the intensity of SCG10 immunostaining is elevated by ~70 % (p < 0.01) in the region spanning 250 μm from the cut site (Figure 1B). The proximal levels of SCG10 continue to increase for 3 h after axotomy and then plateau. The rise in SCG10 levels in the proximal axon stumps is coincident with the rapid loss of SCG10 from distal axons (Figures 1A and 1B; p < 0.001 at 1 h) (Shin et al., 2012b).
Figure 1. SCG10 accumulates in the proximal axons but is lost in the distal axons rapidly after axotomy in vitro.
(A) Axonal SCG10 is examined in cultured DRG neurons by immunostaining without injury or 0.5, 1, 3 and 5 h after transecting axons. After the axotomy SCG10 levels increase proximal but decrease distal to the injury. β3 tubulin labels microtubules. Boxed regions are magnified in (Aa), (Ab), and (Ac). Note formation of growth cones shown in (Ac). Scale bar = 50 μm.
B) Quantification of the proximal and distal SCG10 levels shown in (A). Changes in the SCG10 levels after injury are quantified as percent increase or decrease from the level in uncut axons. n = 6 for each condition except for n = 5 for distal at 5 h; *p < 0.05, **p < 0.01 and ***p < 0.001 vs uncut by t test. Error bars represent SEM.
The pattern of SCG10 localization in proximal axons changes in the hours after injury. At 0.5 h after cut, SCG10 preferentially accumulates at the distal tips of transected proximal axons (Figure 1A, magnified in Figure 1Ab). This localization pattern suggests that the anterograde flux of SCG10 leads to accumulation of the protein at the axon terminal. Indeed, we have previously demonstrated that SCG10 is transported predominantly in the anterograde direction (Shin et al., 2012b). As time progresses, SCG10 accumulation spreads more proximally, and by 5 h after axotomy SCG10 strongly labels much of the proximal axon. During this time some axons reassemble growth cones and extend beyond the axotomy site (Figure 1A, magnified in Figure 1Ac). We find that SCG10 localizes to these de novo growth cones (Figure 2). Immunolabeling of SCG10, tubulin and actin reveals that SCG10 is enriched in the central domain of the growth cones. These findings demonstrate that SCG10 is a selective marker for injured proximal axons and reassembled growth cones. Hence, accumulation of SCG10 highlights the axonal compartment in which axon regeneration will occur.
Figure 2. SCG10 localizes to growth cones of regenerating axons.
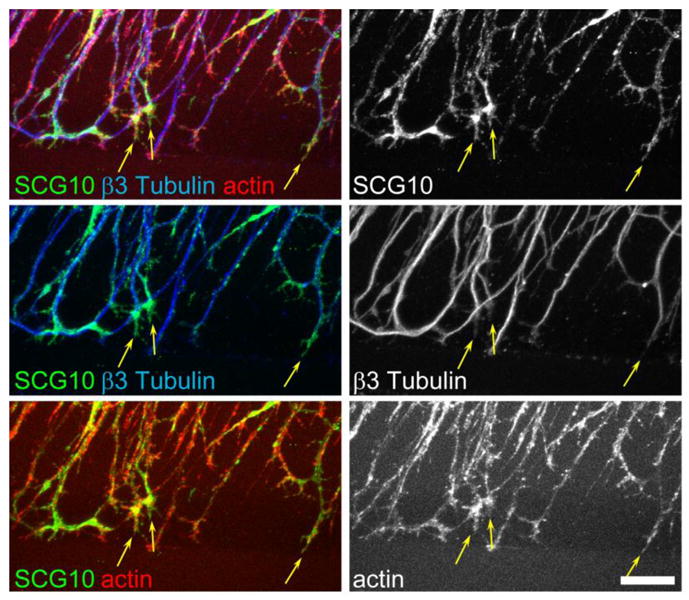
Immunostaining of transected axons in cultured DRG neurons at 5 h after axotomy. SCG10 is enriched in the central domain of the growth cones of regenerating axons and colocalizes with tubulin and actin. Representative growth cones are indicated by arrows. Scale bar = 20 μm.
Nerve injury induces a dynamic change in the SCG10 levels in vivo
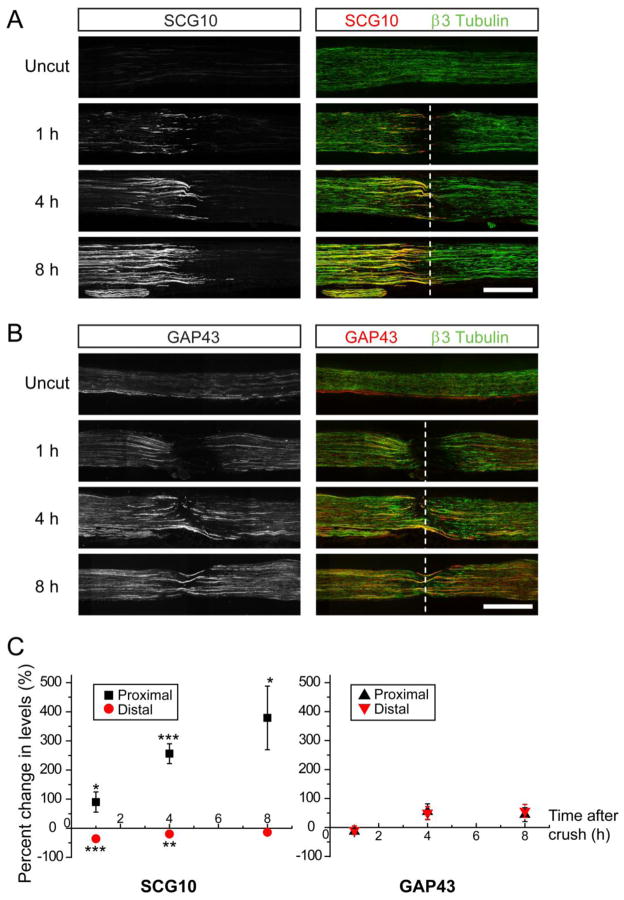
A nerve lesion in the peripheral nervous system leads to degeneration of distal axon segments and re-growth of proximal axons to their targets. These two contrasting events become distinguishable by microscopy only days after the injury is given. After transection or crush lesion to the sciatic nerve, distal axons display a latent period longer than 40 h before they undergo fragmentation (Beirowski et al., 2005). Therefore, axons regenerating beyond the site of injury are morphologically indistinguishable from injured distal axons that remain intact during the latent period. Furthermore, common axonal markers such as β3 tubulin and neurofilaments are still present in degenerating axonal fragments, rendering it difficult to discriminate regenerating fibers and degenerating axons by immunostaining of nerve sections for those markers. Because SCG10 selectively labels the proximal axons even before axon regeneration takes place in our in vitro setting, it may be a useful marker for labeling regenerating axons. To investigate this possibility, we assessed SCG10 expression in proximal axons promptly after an in vivo nerve lesion. To monitor the SCG10 levels following injury, we performed nerve crush on adult mouse sciatic nerves and immunostained for SCG10 in longitudinal nerve sections. In intact control nerves, SCG10 is weakly expressed in axons where it partially colocalizes with neuronal β3 tubulin (Figure 3A). Within one hour of a crush injury, proximal SCG10 levels show a marked increase compared to baseline levels in the control nerve (90 % increase, p < 0.05), when measured within 500 μm of the crush site (Figure 3A and 3C). By 8 h after the lesion, the proximal SCG10 intensity increases to approximately 5-fold that of the baseline (p < 0.05) (Figure 3A and 3C), revealing a dramatic accumulation of SCG10 in injured proximal axons in vivo.
Figure 3. SCG10 levels are increased in the proximal nerve segment promptly following sciatic nerve crush.
(A) and (B) Immunostaining of longitudinal sciatic nerve sections without a crush injury or at 1, 4, and 8 h after crush. Proximal is to the left. Whereas SCG10 levels rapidly increase in the proximal axons after the lesion (A), GAP43 levels are not significantly altered during the first 8 h after injury (B). The neuron-specific β3 tubulin labels axons. Dotted line indicates the crush site. Scale bar = 500 μm.
(C) Left, The SCG10 levels shown in (A) are quantified as percent change from the level in the uncut nerve. n = 6 for uncut and 4 h and n = 5 for 1 and 8 h. Right, The GAP43 levels shown in (B) are quantified as percent change from the level in uncut nerve. n = 5 for uncut and n = 4 for 1, 4 and 8 h. *p < 0.05, **p < 0.01, and ***p < 0.001 vs uncut by t test. Error bars represent SEM.
Similar to the in vitro localization of SCG10, SCG10 in the sciatic nerve first accumulates at the terminal of the proximal nerve stump and later accumulates away from the lesion (Figure 3A). Unlike the dramatic increase in the proximal SCG10 levels, SCG10 levels in the distal segment were slightly decreased (Figure 3A and 3C). The difference in the SCG10 staining intensities creates a great contrast between proximal and distal axons during the early phase of injury. Therefore, our results demonstrate that immunostaining for SCG10 discriminates between the proximal and distal axon segments and allows for robust labeling of the proximal axon compartment.
To test whether the proximal increase is a common feature of axonally transported proteins, we examined levels of the axonal protein GAP43 following sciatic nerve crush (Skene and Willard, 1981b). GAP43 is a well-characterized marker that is often used to label regenerating axons after nerve injury (Abe et al., 2010; Ackermann et al., 2002; Kaneda et al., 2010). However, in the early hours after injury (i. e. 1, 4, and 8 h), GAP43 levels were not significantly changed (Figure 3B and 3C). Unlike SCG10, the GAP43 levels were indistinguishable between proximal and distal axons, making GAP43 unsuitable for selective labeling of either compartment.
Taken together, these finding show that SCG10 is differentially regulated in injured proximal and distal axons in vivo; a dramatic increase in SCG10 is a very early marker of the injured proximal nerve where axon regeneration will subsequently initiate.
SCG10 labels regenerating axons in the sciatic nerve after nerve crush
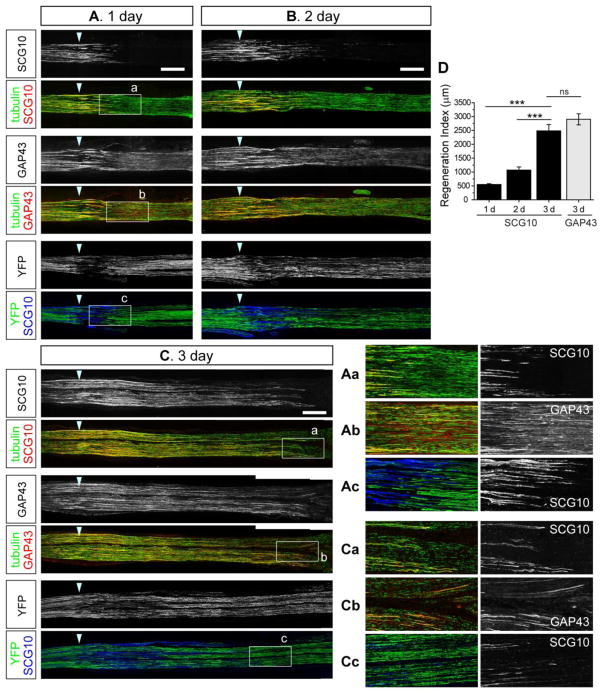
Regenerating axons extend beyond the lesion and grow across the area where distal axons are beginning to undergo degeneration. Hence, labeling methods specific for regenerating axons are required for investigating axon regeneration soon after injury. Because SCG10 protein is enriched in the proximal stump in the early phase of axon injury, we tested whether the SCG10 protein increase persists in the later phase while the proximal axons regenerate through the distal nerve segment. We crushed sciatic nerve and allowed axon regeneration to proceed for 1, 2, and 3 d, and assessed immunolabeling of SCG10. In the nerve sections, the site of crush was determined by immunostaining for β3 tubulin, which reveals a deformation of the nerve at the lesion site and highlights fragmentation of distal axons. At day 1, regenerating axons are intensely labeled with SCG10 while SCG10 immunoreactivity is undetectable in distal axons (Figure 4A, magnified in Figure 4Aa). β3 tubulin staining shows that there are still continuous axon segments in the distal area, demonstrating that the absence of SCG10 is not due to loss of distal axons. Instead, SCG10 specifically labels regenerating axons in the nerve.
Figure 4. SCG10 selectively labels regenerating axons and displays higher specificity than GAP43 or YFP in the early stage of axon regeneration after nerve crush.
(A), (B) and (C) Confocal images of longitudinal sciatic nerve sections at 1, 2 and 3 d after crush lesion. Cryosections obtained from the same nerves are immunostained for SCG10 and GAP43 at each time point. Co-staining for the neuron-specific β3 tubulin visualizes microtubules in axons. The YFP expression pattern is examined in the YFP 16 transgenic mice. At day 1 (A), SCG10 expression is specific to regenerating axons whereas GAP43 and YFP also labels distal axons that will eventually degenerate. Boxed regions are magnified in (Aa), (Ab) and (Ac). At day 3 shown in (C), SCG10 and GAP43 selectively label regenerating axons but YFP still labels degenerating particles robustly. Boxed regions are magnified in (Ca), (Cb) and (Cc). Arrowhead indicates the crush site. Scale bar = 500 μm.
(D) Axon regeneration during 1, 2 and 3 d after crush is quantified by a regeneration index that is the distance from the crush to the location at which the level of SCG10 is half of that at the crush site. Regeneration at day 3 is also quantified with the same method using the level of GAP43. n = 4 for 1 d, 7 for 2 d, 4 for 3 d with SCG10 staining, n = 3 for 3 d with GAP43 staining; ***p < 0.001 by ANOVA. Error bars represent SEM.
By 3 d after nerve crush surgery, regenerating axons have extended up to ~4.5 mm from the crush site, as assessed by co-staining for SCG10 and β3 tubulin (Figure 4C). Axonal disintegration has also progressed, with tubulin-positive fragments apparent in the very distal axons (magnified in Figure 4Ca). Staining for SCG10 does not label these degenerating particles, instead marking only the regenerating axons. Hence, SCG10 labeling can be used to measure the extent of regeneration. We have developed a regeneration index that measures the distance from the lesion to the location at which the levels of SCG10 are half of their levels at the crush site (see methods) (Shin et al., 2012a). This index effectively quantifies the progression of regeneration through time (Figure 4D).
SCG10 is a more selective marker of regenerating axons than GAP43 or YFP
To explore the utility of SCG10 as a regeneration marker, we compared it to markers previously used to assess regeneration: GAP43 immunostaining and genetically expressed YFP (Abe et al., 2010; Ackermann et al., 2002; Pan et al., 2003). To compare directly the utility of SCG10 and GAP43 staining, additional cryosections from the same nerves that were subjected to SCG10 staining were used for GAP43 staining (Figure 4). GAP43 intensity was the highest in the area where regenerating axons were labeled by SCG10. However, the distal segments were also strongly labeled by GAP43 at one day after injury, making it difficult to unambiguously identify the regenerating axons (Figure 4A, magnified in Figure 4Ab). This distal staining pattern for GAP43 disappears by three days after crush when axonal destruction has progressed in the distal stump (Figure 4C, magnified in Figure 4Cb). At this later stage when GAP43 has been cleared from the distal area, SCG10 and GAP43 labeling methods generate comparable regeneration indices (Figure 4D).
In contrast, the YFP transgene (YFP 16) (Feng et al., 2000) is a poor marker of regenerating axons even three days after injury (Figure 4C). At this time point, distal fragments were still robustly labeled with YFP (magnified in Figure 4Cc), and therefore it is extremely difficult to distinguish regenerating axons from the degenerating distal segments. In addition, YFP is not detectable at the tips of growing axons, which were intensely labeled by staining for SCG10 (Figure 4Ac and 4Cc). Collectively, our data suggests that SCG10 is a more selective marker of regenerating peripheral axons than either GAP43 or YFP in the first three days after nerve injury.
SCG10 selectively labels regenerating axons after nerve repair
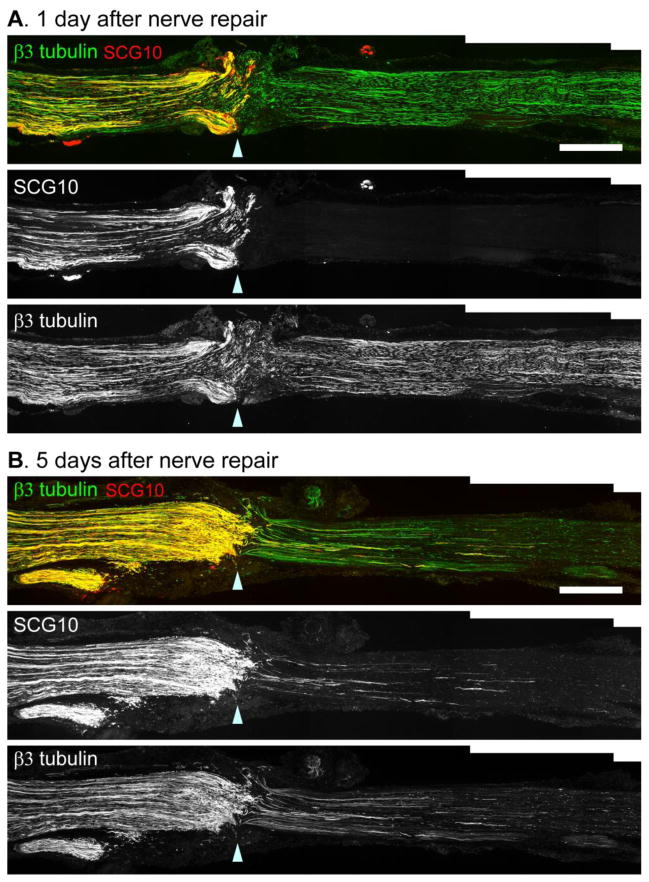
Peripheral axons can regrow after nerve transection and repair across the junction of the rejoined nerve ends (Witzel et al., 2005). We next tested the utility of SCG10 as a selective regeneration marker in the nerve repair model, which better reflects the clinical setting of nerve resection and repair after injury. Transected sciatic nerves were repaired by sutures on the proximal and distal stumps and examined for SCG10 immunostaining. To investigate background levels of SCG10 in the distal stump of the repaired nerve, we first assessed the nerve at 1 d after repair. Consistent with the crush model, SCG10 was undetectable in the distal axon segments that are labeled by β3 tubulin (Figure 5A). At 5 d after repair, regenerating axons visualized by SCG10 immunostaining had grown beyond the repair site (Figure 5B). The selective SCG10 immunoreactivity in regenerating axons demonstrates the utility of SCG10 labeling for analysis of axon regeneration in the nerve repair model.
Figure 5. SCG10 immunostaining selectively labels regenerating axons after nerve transection and repair.
(A) Immunostaining for SCG10 and β3 tubulin at 1 d after sciatic nerve transection and repair. The distal nerve segment is devoid of SCG10 immunoreactivity while β3 tubulin staining reveals that the distal axons are largely intact.
(B) At 5 d after nerve transection and repair, regenerating axons penetrate the distal nerve segment. Axon regeneration is apparent with both SCG10 and β3 tubulin immunostaining.
Arrowhead indicates the repair site. Scale bar = 500 μm.
SCG10 preferentially labels regenerating sensory axons in the sciatic nerve
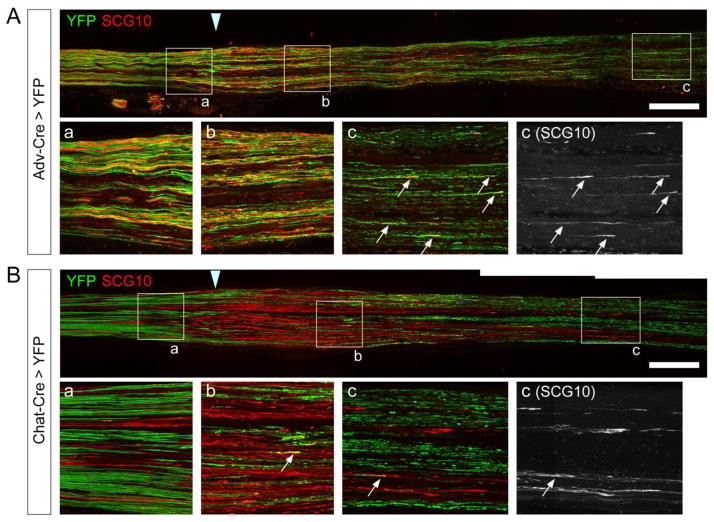
Although SCG10 is highly and selectively expressed in regenerating axons in the sciatic nerve, we noted that the intense SCG10 labeling is not present in all proximal axons that are positive for β3 tubulin (Figure 4). Because β3 tubulin is a pan-neuronal marker, we tested the possibility that SCG10 expression is specific for either the sensory or motor axons that populate the sciatic nerve. To label sensory or motor axons selectively, we utilized a Cre-inducible YFP line in combination with a sensory neuronal Cre driver, Advillin-Cre (Hasegawa et al., 2007; Zhou et al., 2010), or a motor neuronal Cre driver, Chat-Cre (Gong et al., 2007). The Thy1-STOP-YFP transgene includes a loxP-flanked STOP cassette, allowing for YFP expression only in the cells where the STOP cassette is excised via Cre activity (Bareyre et al., 2005). We performed sciatic nerve crush lesion on Advillin-Cre; Thy1-STOP-YFP (Adv-Cre > YFP) mice and Chat-Cre; Thy1-STOP-YFP (Chat-Cre > YFP) mice in order to induce a clear SCG10 expression pattern. SCG10 immunostaining was assessed 3 d after injury. In Adv-Cre > YFP nerves, we found that most of the YFP-positive axons are labeled by SCG10 in the regions near the crush site (both proximal and distal to the injury) (Figure 6A, magnified in a and b). This result demonstrates that SCG10 expression is enriched in the regenerating sensory axons. In the most distal area where individual regenerating axons are observed, the sensory axons were co-labeled with YFP and SCG10 (magnified in Figure 6Ac, arrows) while degenerating YFP-positive fragments were devoid of SCG10. In contrast, SCG10 immunoreactivity is largely excluded from motor axons labeled with Chat-Cre > YFP. Very few YFP-positive motor axons were labeled with SCG10 in the proximal and distal segments of the injured nerve (Figure 6B). Taken together, SCG10 is preferentially expressed in regenerating sensory rather than in motor axons. These results suggest that SCG10 can serve as an effective regeneration marker for sensory axons.
Figure 6. SCG10 preferentially labels regenerating sensory rather than motor axons.
(A) Sensory axons are visualized by YFP expression in the Adv-Cre >YFP mice. A longitudinal section of sciatic nerve was immunostained for SCG10 3 d after crush. Note that SCG10 stains most of YFP-positive regenerating axons. Boxed regions are magnified in (a), (b) and (c). Arrows indicate regenerating sensory fibers.
B) Motor axons are visualized by YFP in the Chat-Cre > YFP mice at 3 d after crush. SCG immunoreactivity rarely colocalizes with the YFP-positive motor fibers (arrows). Boxed regions are magnified in (a), (b) and (c).
Scale bar = 500 μm.
A mouse model of slow axon degeneration highlights the utility of SCG10 as a regeneration marker
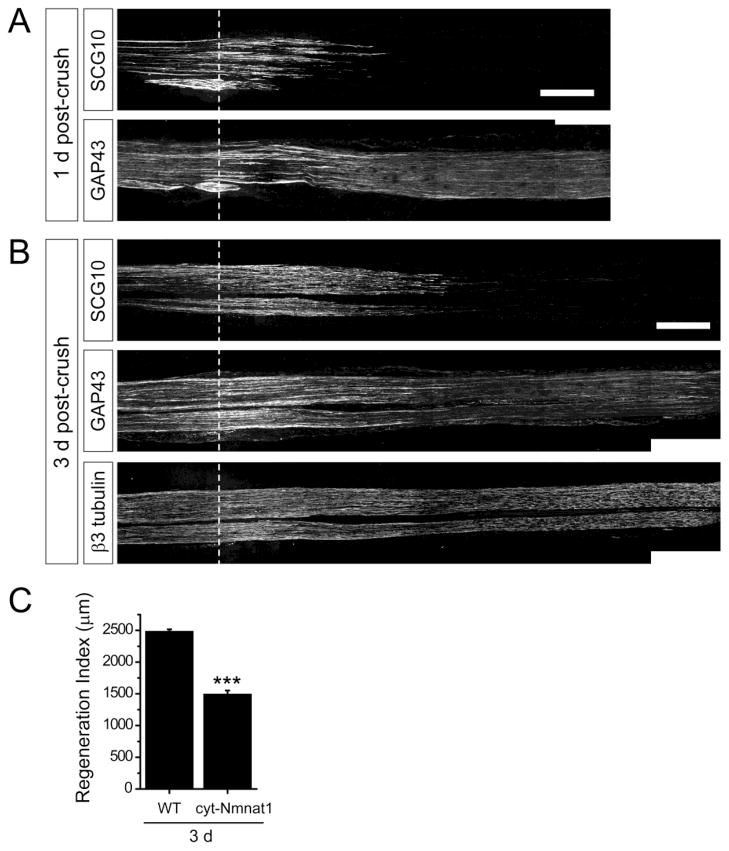
Our data indicate that differential expression of a protein in proximal versus distal axons is a crucial determinant of its specificity as a regeneration marker. Hence, the utility of a regeneration marker could be affected in a situation where degeneration of the distal axons is delayed. For instance, removal of the marker protein might be slowed from distal axons that are protected from destruction, impairing the ability to distinguish regenerating axons from persistent distal axons. To investigate this scenario, we examined SCG10 and GAP43 staining patterns in a transgenic mouse model overexpressing cytoplasmically targeted NMNAT1 (cyt-NMNAT1), the active moiety of the Wallerian degeneration slow protein (Araki et al., 2004; Avery et al., 2009; Conforti et al., 2009). In this line axon degeneration is delayed for more than seven days (Sasaki et al., 2009). At one and three days following nerve crush, SCG10 labeling was only apparent within regenerating axons in the nerve expressing cyt-NMNAT1 (Figure 7A and 7B). Thus, the selectivity of SCG10 labeling was not noticeably impaired by delayed degeneration in cyt-NMNAT1 mice. In contrast, GAP43 staining was dramatically affected in the sciatic nerves of cyt-NMNAT1 mice; GAP43 labeling remained in the distal axons even three days after the crush injury, making it very difficult to distinguish the regenerating fibers (Figure 7B). Thus, the cyt-NMNAT1 mice highlight the power of SCG10 labeling to selectively mark re-growing axons after injury even in mutants with dramatically delayed axon degeneration.
Figure 7. Selective SCG10 labeling reveals slowed axon regeneration in cyt-NMNAT1 mice.
(A) Distribution of SCG10 and GAP43 are compared at 1 d after crush in cyt-NMNAT1 mice, a model of delayed Wallerian degeneration. The distal axons are devoid of SCG10 despite of the slowed axon degeneration program. GAP43 robustly stains the distal axons. Scale bar = 500 μm.
(B) At 3 d after crush, the sciatic nerve of cyt-NMNAT1 is immunostained for SCG10, GAP43 and β3 tubulin. SCG10 staining shows that neurons from cyt-NMNAT1 expressing mice display slowed axon regeneration (quantified in (C)). GAP43 labeling persists in the distal axon segments. β3 tubulin shows that the distal cyt-NMNAT1 axons are intact at 3 d after injury. Scale bar = 500 μm.
(C) Axon regeneration in cyt-NMNAT1 mice are quantified by regeneration indices obtained from SCG10 immunostaining at 3 d after crush injury. n = 3 for each condition; ***p < 0.001 by t test. Error bars represent SEM.
The mouse models overexpressing NMNAT1 manifest significantly delayed axon regeneration (Bisby and Chen, 1990; Brown et al., 1994). The deficit may be caused by the hindrance from the distal axon stumps that slowly degenerate, reduced recruitment of macrophages to axons and cell bodies, and/or delayed dedifferentiation of Schwann cells (Benavides and Alvarez, 1998; Brown et al., 1992; Niemi et al., 2013). The previous studies on the NMNAT1-overexpressing mice have assessed axon regeneration by electrophysiological measurements or by electron microscopic analysis (Brown et al., 1994, 1992, 1991; Chen and Bisby, 1993). Using the SCG10 labeling method, we directly visualize regenerating axons in the longitudinal sections of the cyt-NMNAT1 sciatic nerve, and show that axon regeneration is markedly delayed by three days after nerve crush (p < 0.001) (Figure 7C). Hence, this result exemplifies that defects in the regeneration speed can be effectively detected by the SCG10 labeling. Collectively, the cyt-NMNAT1 model highlights the efficiency of SCG10 as a specific marker for regenerating axons.
Taken together, our data demonstrate that axonal injury induces a dynamic regulation of SCG10 protein levels with selective accumulation in proximal axon segments. SCG10 is highly expressed in regenerating axons, preferentially in sensory fibers. We show that SCG10 is a more selective marker of regenerating axons than the commonly used markers GAP43 and YFP. These attributes make SCG10 immunostaining a particularly powerful in vivo marker of peripheral axon regeneration soon after injury and in mutants with delayed axonal degeneration.
Discussion
Axonal regeneration is essential for the recovery of function after neuronal injury. As such, there is much interest in dissecting the molecular mechanisms controlling the regenerative response, as well as identifying novel therapeutic agents to improve regeneration. Such analysis requires efficient assays of in vivo regeneration, however current methods are particularly poor for assessing the earliest stages of regeneration. Here we demonstrate that immunolabeling for SCG10 is a powerful method to label regenerating sensory axons soon after injury, as well as to distinguish regenerating proximal axons from degenerating distal axons, even when such degeneration is delayed.
We have recently demonstrated that SCG10, a regulator of microtubule dynamics, is rapidly lost after traumatic axon injury selectively in the axon segments distal to the lesion (Shin et al., 2012b). We hypothesized that the loss of SCG10 in the distal axons would facilitate the identification of regenerating axons, where SCG10 protein persists. Here we find that SCG10 immunostaining is not only selective for regenerating axons, but that SCG10 levels are dynamically changed in the proximal stump of severed axons. Within an hour after nerve crush in vivo or axonal transection in vitro, SCG10 staining is promptly increased at the tip of cut axons. The proximal accumulation of SCG10 becomes more prominent during the next hours, leading to a dramatic contrast in the SCG10 labeling intensity between the proximal and distal segments. These results are consistent with our previous finding that SCG10 is transported predominantly in the anterograde direction (Shin et al., 2012b). We suggest that after acute injury, SCG10 builds up at the proximal axon stump as transport vesicles cannot move past the cut site. Since the increase in SCG10 is quite dramatic, additional mechanisms may promote the accumulation of SCG10 in proximal axons. For example, SCG10 mRNA may reside in axons and its local translation could be induced by axonal injury. Consistent with this model, axonal translation of some injury-responsive proteins, such as importin β1 and RanBP1, is facilitated by nerve injury (Gumy et al., 2010; Yudin et al., 2008). In addition, the levels of axonal SCG10 mRNA may be increased after injury by improved translocation of the mRNA from the cell body. On the other hand, transcriptional upregulation in the cell body could contribute to the increase in the axonal protein levels. Indeed, SCG10 transcript levels do increase in DRG cell bodies after axonal injury to the sciatic nerve (Mason et al., 2002). However, transcriptional regulation normally occurs hours to days after injury, so is unlikely to account for the extremely rapid rise in SCG10 protein levels at the proximal nerve stump, which is approximately 2 cm away from the cell body. Finally, the stability of SCG10 may be enhanced locally by injury. We have previously demonstrated that axonal SCG10 has a very short half-life due to JNK-dependent proteolysis (Shin et al., 2012b). Injury may alter signaling such that SCG10 degradation is reduced in proximal axons. Future studies will explore these candidate mechanisms for controlling SCG10 accumulation at proximal axon tips.
During development SCG10 is enriched in growth cones as well as in the axon shaft (Lutjens et al., 2000; Stein et al., 1988). SCG10 controls developmental axon growth by modulating the dynamics of microtubule assembly and disassembly. By sequestering tubulin dimers or severing microtubules, SCG10 shifts microtubule regulation toward destabilization (Antonsson, 1998; Gavet et al., 1998; Riederer et al., 1997). In the current study we found that SCG10 is highly expressed in regenerating axons with enrichment at the growth cone. This expression pattern suggests that SCG10 might be functionally involved in the re-growth of injured axons by promoting microtubule dynamics. Consistent with this hypothesis, nerve injury induces a decrease in the levels of acetylated tubulin, a marker of stable microtubules, in the proximal stump, suggesting that microtubule destabilization might be important for axon re-growth (Cho and Cavalli, 2012). It is likely that axonal regeneration will require spatially regulated domains of stable and unstable microtubules, and SCG10 is a candidate to regulate microtubules at the injury site.
In contrast to proximal SCG10 accumulation, distal axons lose SCG10 within the first few hours after injury in vitro and in vivo (Shin et al., 2012b). Our previous immunoblot analysis of the sciatic nerve showed that the distal levels of SCG10 are reduced by approximately 70% at 3 h after the nerve transection. However, by immunofluorescence in the current study we only detected a minor decrease of SCG10 levels in the distal segments up to 8 h. The apparent discrepancy between these results may reflect the location and size of the nerves that were analyzed. Whereas we assessed lysates from approximately 1 cm-long nerve segments in the immunoblot analysis, with the immunostaining method only 500 μm-long regions immediately distal to the crush were analyzed. The dynamics of SCG10 loss could be different in such close proximity to the crush, since, for example, ~15% of SCG10 is retrogradely transported (Shin et al., 2012b) and this would be expected to accumulate at the distal injury site, obscuring the loss of protein throughout the distal nerve. Despite the relative maintenance of distal SCG10 protein close to the lesion, the remarkable rise in the SCG10 levels is highly selective to the proximal segment, allowing for identification of the proximal axons by immunostaining for SCG10 at an early stage of nerve injury.
While SCG10 is an excellent marker of regenerating axons, it is not a universal marker, because not all axons that are positive for β3 tubulin co-stain with SCG10. Although SCG10 immunoreactivity is very strong near the crush site, fewer proximal axons are stained with SCG10 away from the lesion or in uninjured nerve. Consistent with previous studies (Mason et al., 2002), we find that SCG10 is prominently expressed in sensory axons that are injured and regenerating. In contrast, SCG10 protein and motor neuronal expression of YFP rarely colocalize, indicating that motoneurons do not strongly express SCG10 even after injury. Hence, SCG10 will serve as a better marker for sensory than motor axon regeneration. In addition to cell type specificity, SCG10 localization may differ along the axon. Indeed, others and we have shown that the stress kinase JNK regulates the localization and abundance of SCG10 within the axon (Shin et al., 2012b; Tararuk et al., 2006), which may in turn regulate microtubule stability in axonal microdomains.
Our study demonstrates that SCG10 has important advantages over GAP43 and transgenic YFP as a marker of regenerating peripheral axons. After a nerve crush, SCG10 immunolabeling is selectively increased in the axon stump proximal to a lesion, where axonal re-growth will occur. One day after injury, SCG10 labels regenerating axons in the distal nerve stump but not in the neighboring degenerating axons. Neither GAP43 nor YFP exclusively labels regenerating axons at this stage. GAP43 does selectively label regenerating axon by approximately 3 d after injury in wild type axons. YFP labels both regenerating and degenerating axons at 3 d after injury, rendering it extremely difficult to distinguish regenerating fibers in longitudinal sections. These findings with the transgenic YFP are consistent with a prior regeneration study that resorted to a double nerve crush spaced 3 days apart in order to allow enough time for the clearance of YFP from degenerating axons (Pan et al., 2003). The challenge of distinguishing degenerating from regenerating axons is highlighted in a genetic model of slow axon degeneration—in transgenic mice overexpressing cytoplasmically targeted NMNAT1, SCG10 selectively labels regenerating axons as early as 1 d after nerve crush whereas GAP43 still robustly labels distal axons at 3 d post-injury. Since injury response pathways can coordinately regulate axon regeneration and degeneration (Babetto et al., 2013; Shin et al., 2012a, 2012b; Xiong et al., 2010), genetic and pharmacological studies of molecular mechanisms regulating regeneration in vivo will require methods to distinguish between regenerating and degenerating axons. We suggest that SCG10 can serve as such a regeneration marker by selectively labeling regenerating axon fibers.
In conclusion, our data indicate that the rapid increase in SCG10 protein levels in injured proximal axons allow SCG10 to selectively label regenerating sensory axons from the earliest stages of the injury response in the peripheral nervous system. Utilizing SCG10 as a marker for axonal re-growth may facilitate investigations of the mechanisms underlying axon regeneration.
Highlights.
SCG10 levels are increased in proximal axons promptly after axonal injury
SCG10 is a selective marker for injured regenerating axons
SCG10 is a more selective marker of regenerating axons than GAP43 or YFP
SCG10 is expressed preferentially in regenerating sensory axons
SCG10 labeling detects delayed regeneration in a model of slow axon degeneration
Acknowledgments
We thank Dr. Jeffrey Milbrandt and Dr. Joshua Sanes for sharing transgenic mouse lines. We appreciate Ying Yan and Matt Wood in the Susan Mackinnon lab at Washington University for teaching us the nerve repair technique. We are grateful to the members of the DiAntonio and Milbrandt laboratories for helpful discussions. We thank Sylvia Johnson for her technical assistance. This work was supported by NIH grant NS065053 (to A.D.) and Wings for Life fellowship (to J.E.S.). A.D., J.E.S., and Washington University may receive income based on a license by the University to Novus Biologicals.
Footnotes
Conflict of interest: A.D., J.E.S., and Washington University may receive income based on a license by the University to Novus Biologicals.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jung Eun Shin, Email: jeshin@wustl.edu.
Stefanie Geisler, Email: geislers@neuro.wustl.edu.
Aaron DiAntonio, Email: diantonio@wustl.edu.
References
- Abe N, Borson SH, Gambello MJ, Wang F, Cavalli V. Mammalian target of rapamycin (mTOR) activation increases axonal growth capacity of injured peripheral nerves. The Journal of Biological Chemistry. 2010;285:28034–28043. doi: 10.1074/jbc.M110.125336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackermann PW, Ahmed M, Kreicbergs A. Early nerve regeneration after achilles tendon rupture--a prerequisite for healing? A study in the rat. Journal of orthopaedic research: official publication of the Orthopaedic Research Society. 2002;20:849–56. doi: 10.1016/S0736-0266(01)00159-0. [DOI] [PubMed] [Google Scholar]
- Allodi I, Udina E, Navarro X. Specificity of peripheral nerve regeneration: interactions at the axon level. Progress in neurobiology. 2012;98:16–37. doi: 10.1016/j.pneurobio.2012.05.005. [DOI] [PubMed] [Google Scholar]
- Antonsson B. Identification of in Vitro Phosphorylation Sites in the Growth Cone Protein SCG10. EFFECT OF PHOSPHORYLATION SITE MUTANTS ON MICROTUBULE-DESTABILIZING ACTIVITY. Journal of Biological Chemistry. 1998;273:8439–8446. doi: 10.1074/jbc.273.14.8439. [DOI] [PubMed] [Google Scholar]
- Araki T, Sasaki Y, Milbrandt J. Increased nuclear NAD biosynthesis and SIRT1 activation prevent axonal degeneration. Science (New York, NY) 2004;305:1010–3. doi: 10.1126/science.1098014. [DOI] [PubMed] [Google Scholar]
- Avery Ma, Sheehan AE, Kerr KS, Wang J, Freeman MR. Wld S requires Nmnat1 enzymatic activity and N16-VCP interactions to suppress Wallerian degeneration. The Journal of cell biology. 2009;184:501–13. doi: 10.1083/jcb.200808042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babetto E, Beirowski B, Russler EV, Milbrandt J, DiAntonio A. The Phr1 ubiquitin ligase promotes injury-induced axon self-destruction. Cell reports. 2013;3:1422–9. doi: 10.1016/j.celrep.2013.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bareyre FM, Kerschensteiner M, Misgeld T, Sanes JR. Transgenic labeling of the corticospinal tract for monitoring axonal responses to spinal cord injury. Nature Medicine. 2005;11:1355–1360. doi: 10.1038/nm1331. [DOI] [PubMed] [Google Scholar]
- Beirowski B, Adalbert R, Wagner D, Grumme DS, Addicks K, Ribchester RR, Coleman MP. The progressive nature of Wallerian degeneration in wild-type and slow Wallerian degeneration (WldS) nerves. BMC neuroscience. 2005;6:6. doi: 10.1186/1471-2202-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benavides E, Alvarez J. Peripheral axons of Wlds mice, which regenerate after a delay of several weeks, do so readily when transcription is inhibited in the distal stump. Neuroscience letters. 1998;258:77–80. doi: 10.1016/s0304-3940(98)00746-0. [DOI] [PubMed] [Google Scholar]
- Bisby MA, Chen S. Delayed wallerian degeneration in sciatic nerves of C57BL/Ola mice is associated with impaired regeneration of sensory axons. Brain research. 1990;530:117–20. doi: 10.1016/0006-8993(90)90666-y. [DOI] [PubMed] [Google Scholar]
- Bisby MA, Tetzlaff W. Changes in cytoskeletal protein synthesis following axon injury and during axon regeneration. Molecular neurobiology. 1992;6:107–23. doi: 10.1007/BF02780547. [DOI] [PubMed] [Google Scholar]
- Blackmore MG. Molecular control of axon growth: insights from comparative gene profiling and high-throughput screening. International review of neurobiology. 2012;105:39–70. doi: 10.1016/B978-0-12-398309-1.00004-4. [DOI] [PubMed] [Google Scholar]
- Bradke F, Fawcett JW, Spira ME. Assembly of a new growth cone after axotomy: the precursor to axon regeneration. Nature Reviews Neuroscience. 2012;13:183–93. doi: 10.1038/nrn3176. [DOI] [PubMed] [Google Scholar]
- Brown MC, Lunn ER, Perry VH. Consequences of slow Wallerian degeneration for regenerating motor and sensory axons. Journal of neurobiology. 1992;23:521–36. doi: 10.1002/neu.480230507. [DOI] [PubMed] [Google Scholar]
- Brown MC, Perry VH, Hunt SP, Lapper SR. Further studies on motor and sensory nerve regeneration in mice with delayed Wallerian degeneration. The European journal of neuroscience. 1994;6:420–8. doi: 10.1111/j.1460-9568.1994.tb00285.x. [DOI] [PubMed] [Google Scholar]
- Brown MC, Perry VH, Lunn ER, Gordon S, Heumann R. Macrophage dependence of peripheral sensory nerve regeneration: possible involvement of nerve growth factor. Neuron. 1991;6:359–70. doi: 10.1016/0896-6273(91)90245-u. [DOI] [PubMed] [Google Scholar]
- Chen S, Bisby MA. Impaired motor axon regeneration in the C57BL/Ola mouse. The Journal of comparative neurology. 1993;333:449–54. doi: 10.1002/cne.903330310. [DOI] [PubMed] [Google Scholar]
- Cho Y, Cavalli V. HDAC5 is a novel injury-regulated tubulin deacetylase controlling axon regeneration. The EMBO Journal. 2012;31:3063–3078. doi: 10.1038/emboj.2012.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conforti L, Wilbrey A, Morreale G, Janeckova L, Beirowski B, Adalbert R, Mazzola F, Di Stefano M, Hartley R, Babetto E, Smith T, Gilley J, Billington RA, Genazzani AA, Ribchester RR, Magni G, Coleman M. Wld S protein requires Nmnat activity and a short N-terminal sequence to protect axons in mice. The Journal of cell biology. 2009;184:491–500. doi: 10.1083/jcb.200807175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng G, Mellor RH, Bernstein M, Keller-Peck C, Nguyen QT, Wallace M, Nerbonne JM, Lichtman JW, Sanes JR. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron. 2000;28:41–51. doi: 10.1016/s0896-6273(00)00084-2. [DOI] [PubMed] [Google Scholar]
- Gavet O, Ozon S, Manceau V, Lawler S, Curmi P, Sobel A. The stathmin phosphoprotein family: intracellular localization and effects on the microtubule network. Journal of cell science. 1998;111(Pt 2):3333–46. doi: 10.1242/jcs.111.22.3333. [DOI] [PubMed] [Google Scholar]
- Gong S, Doughty M, Harbaugh CR, Cummins A, Hatten ME, Heintz N, Gerfen CR. Targeting Cre recombinase to specific neuron populations with bacterial artificial chromosome constructs. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2007;27:9817–23. doi: 10.1523/JNEUROSCI.2707-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon T, Tyreman N, Raji MA. The basis for diminished functional recovery after delayed peripheral nerve repair. Journal of Neuroscience. 2011;31:5325–5334. doi: 10.1523/JNEUROSCI.6156-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumy LF, Tan CL, Fawcett JW. The role of local protein synthesis and degradation in axon regeneration. Experimental Neurology. 2010;223:28–37. doi: 10.1016/j.expneurol.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa H, Abbott S, Han BX, Qi Y, Wang F. Analyzing somatosensory axon projections with the sensory neuron-specific Advillin gene. Journal of Neuroscience. 2007;27:14404–14414. doi: 10.1523/JNEUROSCI.4908-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honig MG, Hume RI. Dil and diO: versatile fluorescent dyes for neuronal labelling and pathway tracing. Trends in neurosciences. 1989;12:333–5. 340–1. [PubMed] [Google Scholar]
- Kaneda M, Nagashima M, Mawatari K, Nunome T, Muramoto K, Sugitani K, Kato S. Growth-associated protein43 (GAP43) is a biochemical marker for the whole period of fish optic nerve regeneration. Advances in experimental medicine and biology. 2010;664:97–104. doi: 10.1007/978-1-4419-1399-9_12. [DOI] [PubMed] [Google Scholar]
- Liu K, Lu Y, Lee JK, Samara R, Willenberg R, Sears-Kraxberger I, Tedeschi A, Park KK, Jin D, Cai B, Xu B, Connolly L, Steward O, Zheng B, He Z. PTEN deletion enhances the regenerative ability of adult corticospinal neurons. Nature Neuroscience. 2010;13:1075–1081. doi: 10.1038/nn.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K, Tedeschi A, Park KK, He Z. Neuronal intrinsic mechanisms of axon regeneration. Annual Review of Neuroscience. 2011;34:131–152. doi: 10.1146/annurev-neuro-061010-113723. [DOI] [PubMed] [Google Scholar]
- Lutjens R, Igarashi M, Pellier V, Blasey H, Di Paolo G, Ruchti E, Pfulg C, Staple JK, Catsicas S, Grenningloh G. Localization and targeting of SCG10 to the trans-Golgi apparatus and growth cone vesicles. The European journal of neuroscience. 2000;12:2224–34. doi: 10.1046/j.1460-9568.2000.00112.x. [DOI] [PubMed] [Google Scholar]
- Mason MRJ, Lieberman aR, Grenningloh G, Anderson PN. Transcriptional upregulation of SCG10 and CAP-23 is correlated with regeneration of the axons of peripheral and central neurons in vivo. Molecular And Cellular Neurosciences. 2002;20:595–615. doi: 10.1006/mcne.2002.1140. [DOI] [PubMed] [Google Scholar]
- Miller BR, Press C, Daniels RW, Sasaki Y, Milbrandt J, DiAntonio A. A dual leucine kinase-dependent axon self-destruction program promotes Wallerian degeneration. Nature Neuroscience. 2009;12:387–389. doi: 10.1038/nn.2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morii H, Shiraishi-Yamaguchi Y, Mori N. SCG10, a microtubule destabilizing factor, stimulates the neurite outgrowth by modulating microtubule dynamics in rat hippocampal primary cultured neurons. Journal of Neurobiology. 2006;66:1101–1114. doi: 10.1002/neu.20295. [DOI] [PubMed] [Google Scholar]
- Niemi JP, DeFrancesco-Lisowitz A, Roldán-Hernández L, Lindborg JA, Mandell D, Zigmond RE. A critical role for macrophages near axotomized neuronal cell bodies in stimulating nerve regeneration. Journal of Neurosicence. 2013;33:16236–16248. doi: 10.1523/JNEUROSCI.3319-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozon S, Maucuer A, Sobel A. The stathmin family -- molecular and biological characterization of novel mammalian proteins expressed in the nervous system. The Federation of European Biochemical Societies Journal. 1997;248:794–806. doi: 10.1111/j.1432-1033.1997.t01-2-00794.x. [DOI] [PubMed] [Google Scholar]
- Pan YA, Misgeld T, Lichtman JW, Sanes JR. Effects of neurotoxic and neuroprotective agents on peripheral nerve regeneration assayed by time-lapse imaging in vivo. Journal of Neuroscience. 2003;23:11479–11488. doi: 10.1523/JNEUROSCI.23-36-11479.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patodia S, Raivich G. Role of transcription factors in peripheral nerve regeneration. Frontiers in molecular neuroscience. 2012;5:8. doi: 10.3389/fnmol.2012.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riederer BM, Pellier V, Antonsson B, Di Paolo G, Stimpson Sa, Lütjens R, Catsicas S, Grenningloh G. Regulation of microtubule dynamics by the neuronal growth-associated protein SCG10. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:741–5. doi: 10.1073/pnas.94.2.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki Y, Vohra BPS, Baloh RH, Milbrandt J. Transgenic mice expressing the Nmnat1 protein manifest robust delay in axonal degeneration in vivo. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2009;29:6526–34. doi: 10.1523/JNEUROSCI.1429-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin JE, Cho Y, Beirowski B, Milbrandt J, Cavalli V, DiAntonio A. Dual Leucine Zipper Kinase Is Required for Retrograde Injury Signaling and Axonal Regeneration. Neuron. 2012a;74:1015–1022. doi: 10.1016/j.neuron.2012.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin JE, Miller BR, Babetto E, Cho Y, Sasaki Y, Qayum S, Russler EV, Cavalli V, Milbrandt J, DiAntonio A. SCG10 is a JNK target in the axonal degeneration pathway. Proceedings of the National Academy of Sciences of the United States of America. 2012b;109:E3696–705. doi: 10.1073/pnas.1216204109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skene JH, Willard M. Changes in axonally transported proteins during axon regeneration in toad retinal ganglion cells. The Journal of cell biology. 1981a;89:86–95. doi: 10.1083/jcb.89.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skene JH, Willard M. Axonally transported proteins associated with axon growth in rabbit central and peripheral nervous systems. The Journal of cell biology. 1981b;89:96–103. doi: 10.1083/jcb.89.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein R, Mori N, Matthews K, Lo LC, Anderson DJ. The NGF-inducible SCG10 mRNA encodes a novel membrane-bound protein present in growth cones and abundant in developing neurons. Neuron. 1988;1:463–76. doi: 10.1016/0896-6273(88)90177-8. [DOI] [PubMed] [Google Scholar]
- Sugiura Y, Mori N. SCG10 expresses growth-associated manner in developing rat brain, but shows a different pattern to p19/stathmin or GAP-43. Brain research. Developmental brain research. 1995;90:73–91. doi: 10.1016/0165-3806(96)83488-2. [DOI] [PubMed] [Google Scholar]
- Tararuk T, Östman N, Li W, Björkblom B, Padzik A, Zdrojewska J, Hongisto V, Herdegen T, Konopka W, Courtney MJ, Coffey ET. JNK1 phosphorylation of SCG10 determines microtubule dynamics and axodendritic length. The Journal of Cell Biology. 2006;173:265–277. doi: 10.1083/jcb.200511055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voria I, Hauser J, Axis a, Schenker M, Bichet S, Kuntzer T, Grenningloh G, Barakat-Walter I. Improved sciatic nerve regeneration by local thyroid hormone treatment in adult rat is accompanied by increased expression of SCG10. Experimental Neurology. 2006;197:258–267. doi: 10.1016/j.expneurol.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Wang J, Shan C, Cao W, Zhang C, Teng J, Chen J. SCG10 promotes non-amyloidogenic processing of amyloid precursor protein by facilitating its trafficking to the cell surface. Human molecular genetics. 2013 doi: 10.1093/hmg/ddt339. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Whitlock EL, Kasukurthi R, Yan Y, Tung TH, Hunter DA, Mackinnon SE. Fibrin glue mitigates the learning curve of microneurosurgical repair. Microsurgery. 2010;30:218–222. doi: 10.1002/micr.20754. [DOI] [PubMed] [Google Scholar]
- Witzel C, Rohde C, Brushart TM. Pathway sampling by regenerating peripheral axons. The Journal of Comparative Neurology. 2005;485:183–190. doi: 10.1002/cne.20436. [DOI] [PubMed] [Google Scholar]
- Xiong X, Wang X, Ewanek R, Bhat P, DiAntonio A, Collins CA. Protein turnover of the Wallenda/DLK kinase regulates a retrograde response to axonal injury. The Journal of Cell Biology. 2010;191:211–223. doi: 10.1083/jcb.201006039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yudin D, Hanz S, Yoo S, Iavnilovitch E, Willis D, Gradus T, Vuppalanchi D, Segal-Ruder Y, Ben-Yaakov K, Hieda M, Yoneda Y, Twiss JL, Fainzilber M. Localized regulation of axonal RanGTPase controls retrograde injury signaling in peripheral nerve. Neuron. 2008;59:241–252. doi: 10.1016/j.neuron.2008.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Wang L, Hasegawa H, Amin P, Han B, Kaneko S, He Y, Wang F. Deletion of PIK3C3/Vps34 in sensory neurons causes rapid neurodegeneration by disrupting the endosomal but not the autophagic pathway. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:9424–9. doi: 10.1073/pnas.0914725107. [DOI] [PMC free article] [PubMed] [Google Scholar]