Abstract
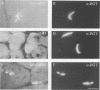
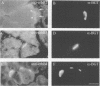
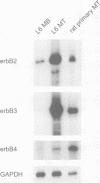
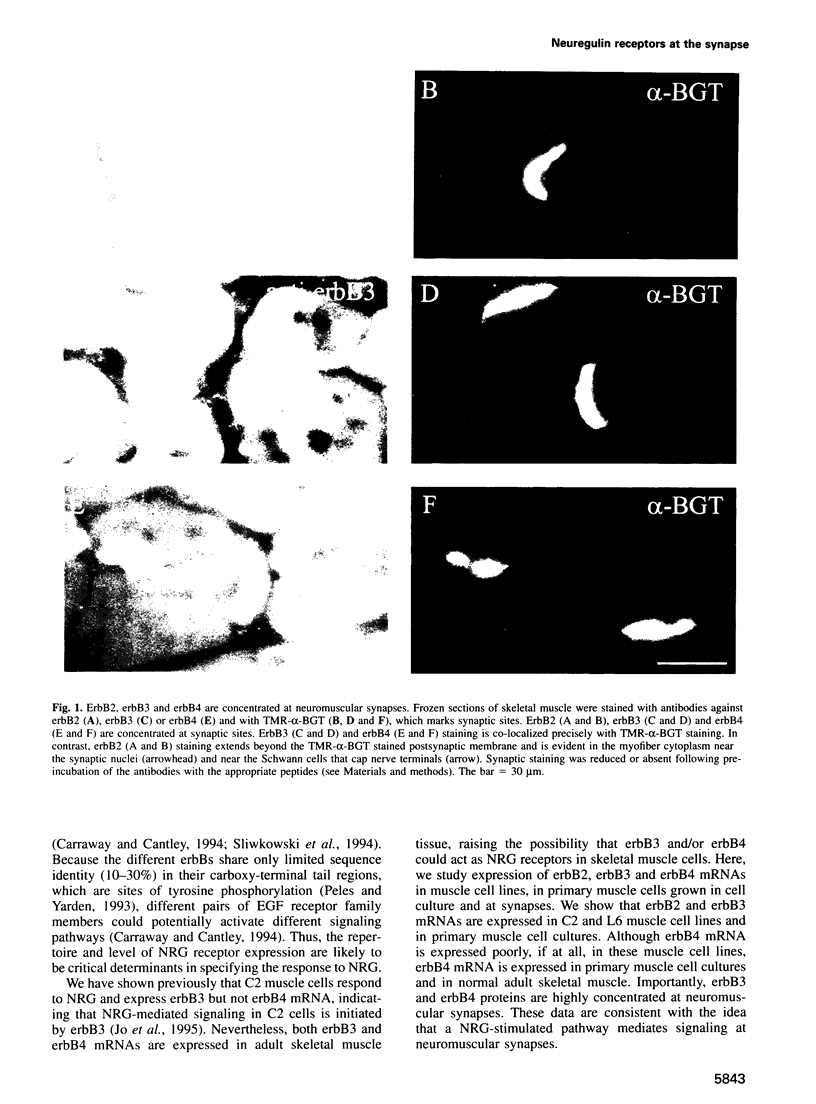
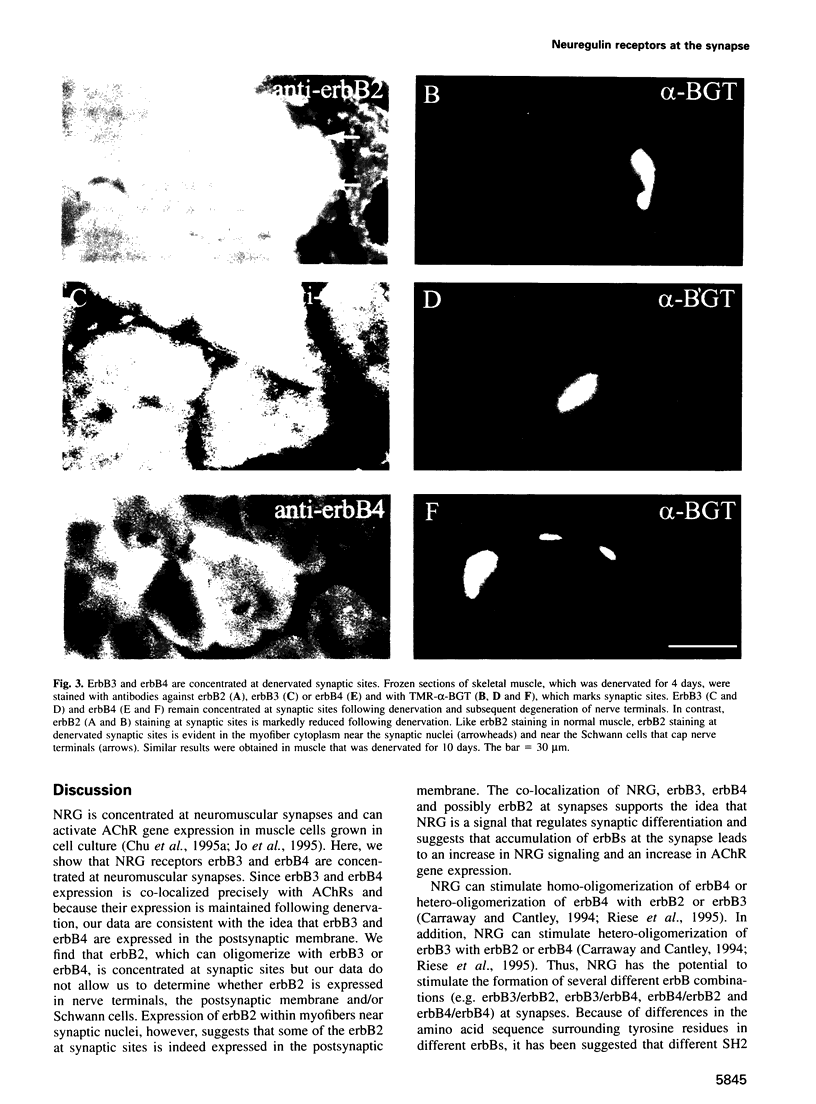
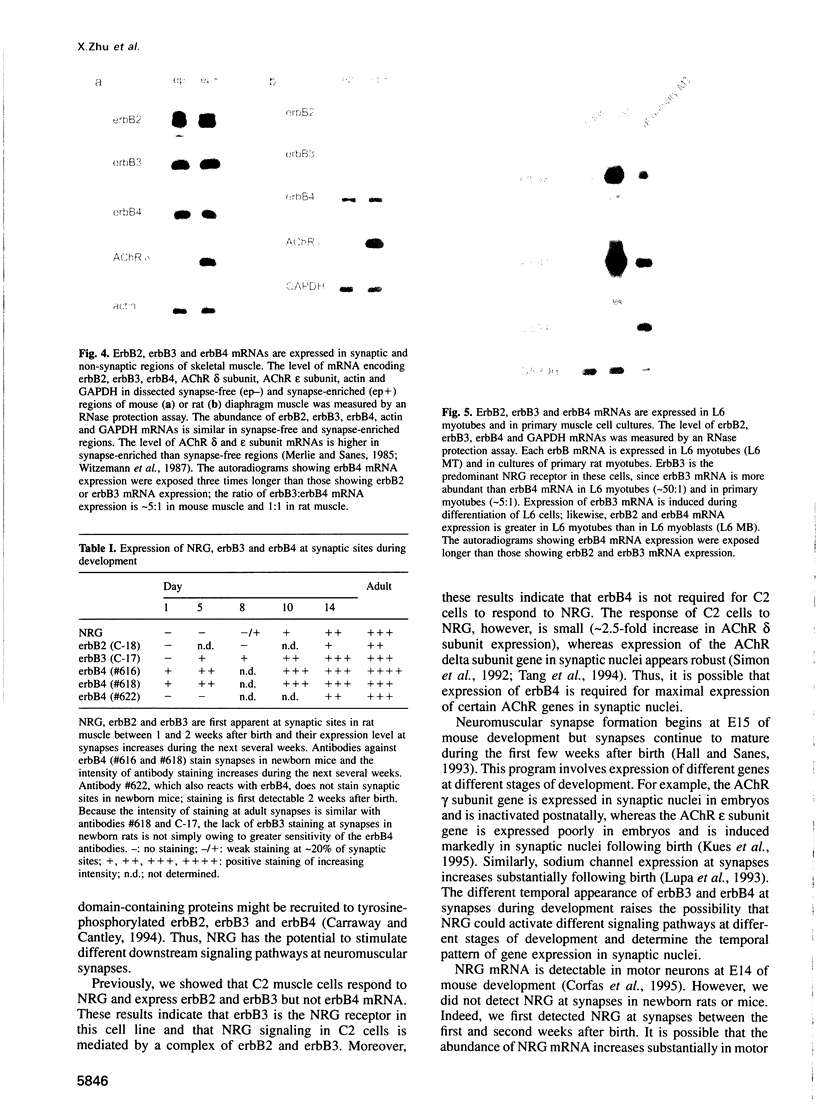
Neuregulin (NRG) is concentrated at synaptic sites and stimulates expression of acetylcholine receptor (AChR) genes in muscle cells grown in cell culture. These results raise the possibility that NRG is a synaptic signal that activates AChR gene expression in synaptic nuclei. Stimulation of NRG receptors, erbB3 and erbB4 initiates oligomerization between these receptors or between these receptors and other members of the epidermal growth factor (EGF) receptor family, resulting in stimulation of their associated tyrosine kinase activities. To determine which erbBs might mediate synapse-specific gene expression, we used antibodies against each erbB to study their expression in rodent skeletal muscle by immunohistochemistry. We show that erbB2, erbB3 and erbB4 are concentrated at synaptic sites in adult skeletal muscle. ErbB3 and erbB4 remain concentrated at synaptic sites following denervation, indicating that erbB3 and erbB4 are expressed in the postsynaptic membrane. In addition, we show that expression of NRG and erbBs, like AChR gene expression, increases at synaptic sites during postnatal development. The localization of erbB3 and erbB4 at synaptic sites is consistent with the idea that a NRG-stimulated signaling pathway is important for synapse-specific gene expression.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowe M. A., Fallon J. R. The role of agrin in synapse formation. Annu Rev Neurosci. 1995;18:443–462. doi: 10.1146/annurev.ne.18.030195.002303. [DOI] [PubMed] [Google Scholar]
- Burden S. Identification of an intracellular postsynaptic antigen at the frog neuromuscular junction. J Cell Biol. 1982 Sep;94(3):521–530. doi: 10.1083/jcb.94.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carraway K. L., 3rd, Sliwkowski M. X., Akita R., Platko J. V., Guy P. M., Nuijens A., Diamonti A. J., Vandlen R. L., Cantley L. C., Cerione R. A. The erbB3 gene product is a receptor for heregulin. J Biol Chem. 1994 May 13;269(19):14303–14306. [PubMed] [Google Scholar]
- Chu G. C., Moscoso L. M., Sliwkowski M. X., Merlie J. P. Regulation of the acetylcholine receptor epsilon subunit gene by recombinant ARIA: an in vitro model for transynaptic gene regulation. Neuron. 1995 Feb;14(2):329–339. doi: 10.1016/0896-6273(95)90289-9. [DOI] [PubMed] [Google Scholar]
- Corfas G., Rosen K. M., Aratake H., Krauss R., Fischbach G. D. Differential expression of ARIA isoforms in the rat brain. Neuron. 1995 Jan;14(1):103–115. doi: 10.1016/0896-6273(95)90244-9. [DOI] [PubMed] [Google Scholar]
- Fallon J. R., Hall Z. W. Building synapses: agrin and dystroglycan stick together. Trends Neurosci. 1994 Nov;17(11):469–473. doi: 10.1016/0166-2236(94)90135-x. [DOI] [PubMed] [Google Scholar]
- Falls D. L., Rosen K. M., Corfas G., Lane W. S., Fischbach G. D. ARIA, a protein that stimulates acetylcholine receptor synthesis, is a member of the neu ligand family. Cell. 1993 Mar 12;72(5):801–815. doi: 10.1016/0092-8674(93)90407-h. [DOI] [PubMed] [Google Scholar]
- Hall Z. W., Sanes J. R. Synaptic structure and development: the neuromuscular junction. Cell. 1993 Jan;72 (Suppl):99–121. doi: 10.1016/s0092-8674(05)80031-5. [DOI] [PubMed] [Google Scholar]
- Harris D. A., Falls D. L., Dill-Devor R. M., Fischbach G. D. Acetylcholine receptor-inducing factor from chicken brain increases the level of mRNA encoding the receptor alpha subunit. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1983–1987. doi: 10.1073/pnas.85.6.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo S. A., Zhu X., Marchionni M. A., Burden S. J. Neuregulins are concentrated at nerve-muscle synapses and activate ACh-receptor gene expression. Nature. 1995 Jan 12;373(6510):158–161. doi: 10.1038/373158a0. [DOI] [PubMed] [Google Scholar]
- Kues W. A., Sakmann B., Witzemann V. Differential expression patterns of five acetylcholine receptor subunit genes in rat muscle during development. Eur J Neurosci. 1995 Jun 1;7(6):1376–1385. doi: 10.1111/j.1460-9568.1995.tb01129.x. [DOI] [PubMed] [Google Scholar]
- Lupa M. T., Krzemien D. M., Schaller K. L., Caldwell J. H. Aggregation of sodium channels during development and maturation of the neuromuscular junction. J Neurosci. 1993 Mar;13(3):1326–1336. doi: 10.1523/JNEUROSCI.13-03-01326.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchionni M. A., Goodearl A. D., Chen M. S., Bermingham-McDonogh O., Kirk C., Hendricks M., Danehy F., Misumi D., Sudhalter J., Kobayashi K. Glial growth factors are alternatively spliced erbB2 ligands expressed in the nervous system. Nature. 1993 Mar 25;362(6418):312–318. doi: 10.1038/362312a0. [DOI] [PubMed] [Google Scholar]
- McMahan U. J. The agrin hypothesis. Cold Spring Harb Symp Quant Biol. 1990;55:407–418. doi: 10.1101/sqb.1990.055.01.041. [DOI] [PubMed] [Google Scholar]
- Merlie J. P., Sanes J. R. Concentration of acetylcholine receptor mRNA in synaptic regions of adult muscle fibres. Nature. 1985 Sep 5;317(6032):66–68. doi: 10.1038/317066a0. [DOI] [PubMed] [Google Scholar]
- Morrissey T. K., Levi A. D., Nuijens A., Sliwkowski M. X., Bunge R. P. Axon-induced mitogenesis of human Schwann cells involves heregulin and p185erbB2. Proc Natl Acad Sci U S A. 1995 Feb 28;92(5):1431–1435. doi: 10.1073/pnas.92.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscoso L. M., Merlie J. P., Sanes J. R. N-CAM, 43K-rapsyn, and S-laminin mRNAs are concentrated at synaptic sites in muscle fibers. Mol Cell Neurosci. 1995 Feb;6(1):80–89. doi: 10.1006/mcne.1995.1008. [DOI] [PubMed] [Google Scholar]
- Mudge A. W. Neural development: new ligands for Neu? Curr Biol. 1993 Jun 1;3(6):361–364. doi: 10.1016/0960-9822(93)90201-x. [DOI] [PubMed] [Google Scholar]
- Peles E., Yarden Y. Neu and its ligands: from an oncogene to neural factors. Bioessays. 1993 Dec;15(12):815–824. doi: 10.1002/bies.950151207. [DOI] [PubMed] [Google Scholar]
- Piette J., Huchet M., Houzelstein D., Changeux J. P. Compartmentalized expression of the alpha- and gamma-subunits of the acetylcholine receptor in recently fused myofibers. Dev Biol. 1993 May;157(1):205–213. doi: 10.1006/dbio.1993.1124. [DOI] [PubMed] [Google Scholar]
- Plowman G. D., Culouscou J. M., Whitney G. S., Green J. M., Carlton G. W., Foy L., Neubauer M. G., Shoyab M. Ligand-specific activation of HER4/p180erbB4, a fourth member of the epidermal growth factor receptor family. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):1746–1750. doi: 10.1073/pnas.90.5.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riese D. J., 2nd, van Raaij T. M., Plowman G. D., Andrews G. C., Stern D. F. The cellular response to neuregulins is governed by complex interactions of the erbB receptor family. Mol Cell Biol. 1995 Oct;15(10):5770–5776. doi: 10.1128/mcb.15.10.5770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon A. M., Hoppe P., Burden S. J. Spatial restriction of AChR gene expression to subsynaptic nuclei. Development. 1992 Mar;114(3):545–553. doi: 10.1242/dev.114.3.545. [DOI] [PubMed] [Google Scholar]
- Sliwkowski M. X., Schaefer G., Akita R. W., Lofgren J. A., Fitzpatrick V. D., Nuijens A., Fendly B. M., Cerione R. A., Vandlen R. L., Carraway K. L., 3rd Coexpression of erbB2 and erbB3 proteins reconstitutes a high affinity receptor for heregulin. J Biol Chem. 1994 May 20;269(20):14661–14665. [PubMed] [Google Scholar]
- Tang J., Jo S. A., Burden S. J. Separate pathways for synapse-specific and electrical activity-dependent gene expression in skeletal muscle. Development. 1994 Jul;120(7):1799–1804. doi: 10.1242/dev.120.7.1799. [DOI] [PubMed] [Google Scholar]
- Tzahar E., Levkowitz G., Karunagaran D., Yi L., Peles E., Lavi S., Chang D., Liu N., Yayon A., Wen D. ErbB-3 and ErbB-4 function as the respective low and high affinity receptors of all Neu differentiation factor/heregulin isoforms. J Biol Chem. 1994 Oct 7;269(40):25226–25233. [PubMed] [Google Scholar]
- Witzemann V., Barg B., Nishikawa Y., Sakmann B., Numa S. Differential regulation of muscle acetylcholine receptor gamma- and epsilon-subunit mRNAs. FEBS Lett. 1987 Oct 19;223(1):104–112. doi: 10.1016/0014-5793(87)80518-5. [DOI] [PubMed] [Google Scholar]
- Woodruff M. L., Theriot J., Burden S. J. 300-kD subsynaptic protein copurifies with acetylcholine receptor-rich membranes and is concentrated at neuromuscular synapses. J Cell Biol. 1987 Apr;104(4):939–946. doi: 10.1083/jcb.104.4.939. [DOI] [PMC free article] [PubMed] [Google Scholar]