Abstract
Aims
To examine patient and medication characteristics associated with retention and continued illicit opioid use in methadone (MET) versus buprenorphine/naloxone (BUP) treatment for opioid dependence.
Design/Settings/Participants
This secondary analysis included 1,267 opioid-dependent individuals participating in 9 opioid treatment programs between 2006 and 2009 and randomized to receive open-label BUP or MET for 24 weeks.
Measurements
The analyses included measures of patient characteristics at baseline (demographics; use of alcohol, cigarettes, and illicit drugs; self-rated mental and physical health), medication dose and urine drug screens during treatment, and treatment completion and days in treatment during the 24 week trial.
Findings
The treatment completion rate was 74% for MET vs. 46% for BUP (p<.01); the rate among MET participants increased to 80% when the maximum MET dose reached or exceeded 60mg/day. With BUP, the completion rate increased linearly with higher doses, reaching 60% with doses of 30–32mg/day. Of those remaining in treatment, positive opioid urine results were significantly lower (OR=0.63, 95%CI=0.52–0.76, p<.01) among BUP relative to MET participants during the first 9 weeks of treatment. Higher medication dose was related to lower opiate use, more so among BUP patients. A Cox proportional hazards model revealed factors associated with dropout: (1) BUP (vs. MET, HR=1.61, CI:1.20–2.15), (2) lower medication dose (<16mg for BUP, <60mg for MET; HR=3.09, CI:2.19–4.37), (3) the interaction of dose and treatment condition (those with higher BUP dose were 1.04 times more likely to drop out than those with lower MET dose, and (4) being younger, Hispanic, and using heroin or other substances during treatment.
Conclusions
Provision of methadone appears to be associated with better retention in treatment for opioid dependence than buprenorphine, as does use of provision of higher doses of both medications. Provision of buprenorphine is associated with lower continued use of illicit opioids.
The early years of the 21st century have witnessed a striking increase in rates of opioid misuse and addiction1,2 with concomitant increases in opioid overdose deaths.3 This situation calls for improved treatments for this potentially lethal disorder. Both methadone (MET) and buprenorphine (BUP) are effective treatments for opioid addiction. MET is the most widely used opioid agonist therapy in the world, and BUP is widely available in selected European countries, the United States (U.S.), Canada, and Australia. Several studies have reported lower treatment retention associated with BUP relative to MET.4,5 A recent Cochrane review6 of randomized clinical trials has indicated that retention with MET is better than BUP, although the two medications were equivalent in suppressing illicit opiate use. Many of the trials included in the Cochrane review used relatively low doses of BUP and slow, inflexible induction, with a maximum dose of 16mg per day. Nevertheless, even when patient preference was taken into consideration, a study with flexible dosing (a maximum BUP dose of 20mg) showed that those prescribed MET were more than twice as likely to be retained relative to those self-selecting BUP.7
Treatment retention is an important predictor of favorable treatment outcomes,8 so improving BUP treatment outcomes necessitates enhancing retention rates. Potentially negative aspects of BUP treatment reported by patients include the potential to precipitate withdrawal symptoms at induction,9 the unpleasant taste of the sublingual formulation,10 and slow dissolution of the sublingual tablet.11 Factors that facilitate retention have not been widely studied, but a recent meta-analysis12 based on 21 randomized clinical trials indicates that a higher BUP dose (16–32mg per day) predicted better retention in treatment compared with a lower dose (less than 16mg per day), and that positive urine drug screens for opiates predicted treatment dropout. Furthermore, retention in treatment predicted less illicit opiate use, and positive urine drug screens for cocaine predicted more illicit opiate use.
The present study takes advantage of a large randomized trial recently conducted in the U.S. in which opioid-dependent participants were randomized to MET vs. BUP for 24 weeks to compare liver health outcomes.13 This study compares treatment completion and retention rates for BUP and MET groups, and identifies participant and medication factors that influenced retention.
METHODS
Participants
As described elsewhere,13 the recently completed original study was a multisite, open-label, phase IV study to assess liver function in participants randomized to medication condition (BUP [given in the form of buprenorphine/naloxone], MET). A total of 1,269 eligible patients from 9 federally licensed opioid treatment programs across the U.S. were randomized (within site) and inducted on study medication (BUP = 740, MET = 529) from 2006 to 2009. The unequal sample sizes in the two conditions occurred because the study needed to achieve target sample sizes for each medication (300 each who completed 24 weeks of medication and provided the required liver tests), so the initial randomization scheme of 1:1 (BUP:MET) was changed to 2:1 in December 2007 (18 months after initiation) due to the higher dropout in the BUP condition. Because the present study is focused on effects of participant and medication characteristics on treatment retention, excluded were 2 women who became pregnant and were reassigned from BUP to MET, resulting in the use of data from 1,267 participants (BUP=738, MET=529) in the present analysis.
Procedure/Intervention
Participants were inducted on medication after being instructed to abstain from opioids for 12–24 hours to present in mild to moderate opioid withdrawal (Clinical Opioid Withdrawal Scale score ≥8) or as deemed appropriate by the study physician.13 Participants came to the clinic daily for observed medication administration except Sundays and holidays or when take-home medications were permitted by Federal/State regulations. Participants were titrated to an appropriate medication dose as determined by the local study physician, remained on study medication for 24 weeks, and were then tapered off medication over ≤8 weeks or referred for ongoing clinical treatment, with study completion by Week 32. Mean maximum daily doses throughout the trial were 22.1mg (range = 2–32mg) of BUP and 93.1mg (range = 5–397mg) of MET.
Weekly assessments included urine drug screens and adverse event assessments, and self-reported drug use data were collected every four weeks. Participants who missed 3 or more consecutive days of medication, returned to the clinic within 14 days of the last dose, and wished to continue medication, were re-inducted if deemed appropriate. Participants who missed 14 or more consecutive days of study medication were terminated from the study. Compensation was provided in accordance with local site policies, but typically included compensation for each research assessment. No differences were found between the two conditions in the number of serious adverse events.13
Outcomes
Treatment completion is defined as the participant continuing in the assigned medication group for 24 weeks without being withdrawn. Treatment retention is calculated as days in treatment since randomization until the last day of medication during the 24 weeks of treatment.
Measurements
Measurements were conducted at baseline for participant characteristics and during the trial for dose and urine drug screens.
Participant Characteristics
Baseline assessments included demographics (participants’ age, gender, race and ethnicity), number of cigarettes smoked per day, alcohol use, drug injection in the past 30 days, and Short Form 36-item Health Survey14 (SF-36, a self-report instrument to assess health status over a 4-week-period providing summary scores of physical and mental health components).
Urine Drug Screens (UDS)
Urine specimens were collected using a drug test cup with temperature controlled monitoring and test strips for opiates, methadone, oxycodone, cocaine metabolite, amphetamines, methamphetamines, cannabinoids and benzodiazepines. UDS were collected during baseline and weekly through Week 24.
Medication Dose
Date, medication, dose, and administration location data were captured in the Dose Log. Maximum doses in mg during the 24 weeks were categorized into 0–10, 12–14, 16–20, 22–28, 30–32 for BUP and 0–40, 41–60, 61–80, 81–120, and 121 or higher for MET. Weekly average dose was the dose averaged over a week, including dose=0 for no show. Doses on the last day of treatment were categorized as higher (>=16mg for BUP, or >=60mg for MET) versus lower in the Cox model.
Statistical Analyses
Group differences between BUP vs. MET were tested using chi-square (for categorical variables) or t-tests (for continuous measures). The unadjusted survival function was assessed using the Kaplan-Meier method, with a log rank test of group differences.15 Cox proportional hazard models16 were conducted to examine comparative survival rates between the two conditions as well as to identify predictors of retention including patient and medication characteristics. The generalized estimating equations (GEE) approach,17 a regression technique, was applied to assess the relationships between dose and opiate use (evidenced by positive urine results) over time. The GEE is particularly appropriate for analyzing longitudinal data because it takes time and time-invariant and time-varying covariates into account and allows adjustment for within-subject correlation. The logit link function with binary distribution and the unstructured correlation structure were applied in the GEE analyses. All statistical analyses were conducted with procedures FREQ for tables and chi-square tests, TTEST for t-tests, LIFETEST for the log rank test, PHREG for Cox models, and GENMOD for GEE approach of SAS version 9.2.18
RESULTS
Baseline characteristics of the total sample as well as for each group separately by treatment condition (BUP vs. MET) are provided in Table 1. With the exception of cigarette smoking and cocaine positive UDS, there were no significant differences between the two conditions in all of the variables examined. Basically, these participants were early middle-aged (M=37, SD=11), two-thirds male, with the highest percentage of white participants (71%, but also included 12% Hispanics and 9% African Americans). Almost 90% smoked cigarettes (more among participants on MET, p<.05), 27% used alcohol, and 69% injected drugs in the past 30 days. Many also tested positive for drugs other than opioids; 37% positive for cocaine (42% for MET vs. 34% for BUP, p<.01), 9% for amphetamine, and 24% for marijuana. Days of opioid use in the past 30 days did not differ between the two groups. Physical Component Summary scores on the SF-36 were comparable to the average among the general population of similar age and gender, but Mental Component Summary scores were at about 39, 2 standard deviations below the average for the general population.
Table 1.
Clinical Profiles at Baseline
| Buprenorphine/Naloxone | Methadone | Total | |
|---|---|---|---|
| (n=738) | (n=529) | (n=1,267) | |
| Age, % | |||
| 18–24 | 14.9 | 14.6 | 14.8 |
| 25–34 | 32.0 | 34.0 | 32.8 |
| 35–44 | 22.2 | 23.4 | 22.7 |
| 45–54 | 23.6 | 22.7 | 23.2 |
| 55+ | 7.3 | 5.3 | 6.5 |
| Age, Mean (SD) | 37.5 (11.2) | 37.3 (10.9) | 37.4 (11.1) |
| Female Gender, % | 32.0 | 32.1 | 32.0 |
| Race/Ethnicity, % | |||
| White | 69.4 | 74.1 | 71.4 |
| African American | 8.5 | 8.9 | 8.7 |
| Hispanic | 13.4 | 10.0 | 12.0 |
| Other | 8.7 | 7.0 | 8.0 |
| Clinic site location, % | |||
| West coast | 70.3 | 67.3 | 69.1 |
| East coast | 29.8 | 32.7 | 30.9 |
| # cigarettes smoked per day, %* | |||
| 0 | 12.2 | 9.5 | 11.1 |
| <10 | 28.6 | 26.8 | 27.9 |
| 11–20 | 44.9 | 46.1 | 45.4 |
| 21–30 | 11.9 | 12.1 | 12.0 |
| 31+ | 2.4 | 5.5 | 3.7 |
| Days using opioids in past 30 days | 26.8 (6.5) | 26.7 (6.6) | 26.8 (6.5) |
| Alcohol use, % | 26.3 | 27.5 | 26.8 |
| Cocaine positive UDS,1 %** | 34.0 | 42.0 | 37.3 |
| Amphetamine positive UDS, % | 8.7 | 9.5 | 9.0 |
| Cannabinoids positive UDS, % | 25.3 | 21.4 | 23.7 |
| Drug injection in past 30 days, % | 68.3 | 69.3 | 68.7 |
| SF-36 Physical Component Summary, Mean (SD) | 49.5 (9.0) | 49.2 (9.4) | 49.4 (9.2) |
| SF-36 Mental Component Summary, Mean (SD) | 39.4 (12.3) | 38.8 (12.9) | 39.1 (12.6) |
p<0.05;
p<0.01
UDS=urine drug screen
Treatment Completion and Retention
As shown in Table 2, significantly fewer BUP participants (46%) than MET participants (74%) completed treatment (p<.01) at 24 weeks. The mean number of days in treatment was 103.8 (66.9) for BUP and 141.3 (50.8) for MET. Significantly more BUP participants than MET participants (24.8% vs. 8.3%, p<.001) dropped out within the first 30 days of treatment; among those who stayed more than 30 days, BUP participants still showed a significantly lower completion rate than MET participants (61.3% vs. 80.3%, p<.001). Survival curves to 24 weeks for the two groups are displayed in Figure 1. A log-rank test comparing MET to BUP indicated significantly different survival functions (p<.001). More than 60% of the dropouts in both MET and BUP were due to having missed 14 or more days, but more BUP participants than MET participants dropped out because they no longer wished to participate in their assigned treatment (25.6% vs. 12.4%, p< .001).
Table 2.
Treatment Completion and Dropout Reasons (%)
| Buprenorphine/Naloxone | Methadone | Total | |
|---|---|---|---|
| (n=738) | (n=529) | (n=1,267) | |
| Dropout during the first 30 days of treatment, %** | 24.8 | 8.3 | 17.9 |
| Completed the 24-week trial, %** | 46.1 | 74.1 | 57.8 |
| Retention (days in treatment), Mean (SD)** | 103.8 (66.9) | 141.3 (50.8) | 119.4 (63.5) |
| Dropout reasons, %** | n=398 | n=137 | n=535 |
| Missed 14 or more days | 63.1 | 68.6 | 64.5 |
| No longer wish to participate | 25.6 | 12.4 | 22.2 |
| Administrative discharged | 3.5 | 4.4 | 3.7 |
| Not medically appropriate | 3.8 | 3.7 | 3.7 |
| Other reasons (incarceration, moved) | 4.0 | 11.0 | 5.8 |
| Participants who stayed in treatment more than 30 days | n=555 | n=529 | n=1,040 |
| Completed the 24-week trial, % ** | 61.3 | 80.8 | 70.4 |
| Retention (days in treatment), Mean (SD) ** | 133.3 (49.2) | 152.8 (34.8) | 142.4 (44.1) |
P<0.01
Figure 1.
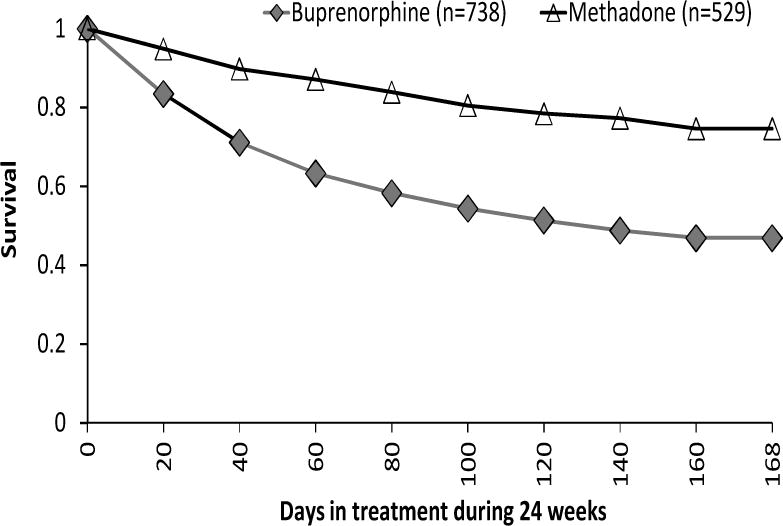
Survival Curves for Buprenorphine Versus Methadone
Maximum Dose of Medication and Retention
To explicate the dose and retention relationship and for ease of understanding, we classified the maximum dose by discrete categories and plotted the dose categories and retention rates in Figure 2. Doses of MET greater than 60mg demonstrated 80% or better retention with 120mg or higher showing a 91% completion rate. In contrast, BUP doses and retention rates showed a linear relationship, with increasing dose yielding improved retention, with the highest dose category of 30–32mg BUP resulting in a completion rate of about 60%.
Figure 2.
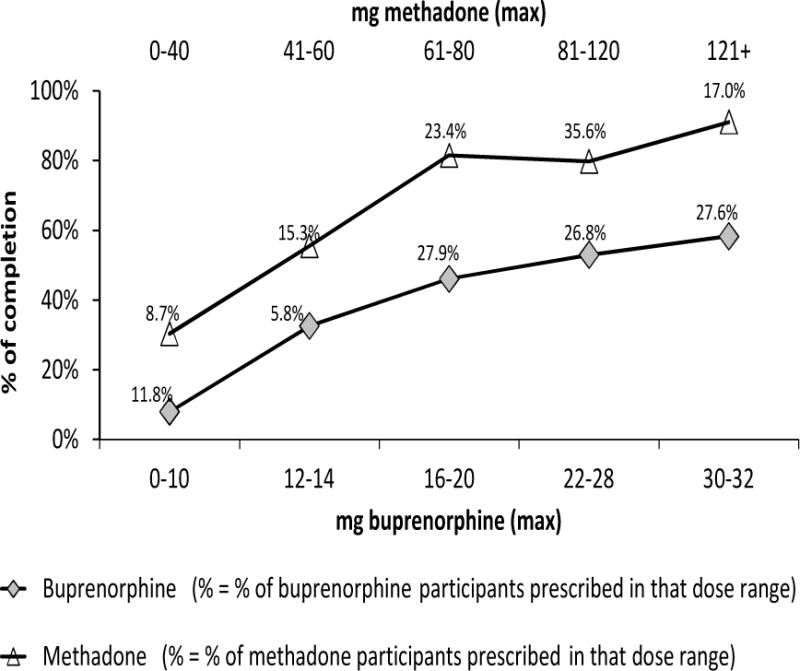
Comparing Retention at 24 Weeks by Maximum Dose of Medication Prescribed
Urine Results during the Trial
The underlying mechanism for increased retention with higher doses could be that higher doses reduce craving and opiate use, thereby retaining participants in treatment. To test this hypothesis, we further examined if a lower dose predicts opiate use (as indicated by a positive opiate urine test) over time during the trial. Figure 3 presents the trajectories of the weekly average dose and positive opiate test results over the 24 weeks of the trial for MET and BUP separately. GEE were applied, demonstrating a significant main effect of dose and a significant interaction effect of dose and treatment condition. Specifically, increased dose was negatively related to continued opiate use (OR=0.99, 95%CI= 0.98–0.99, p<.001), with BUP participants relative to MET participants showing a lower likelihood of positive opiate test results for every mg dose increase (OR=0.98, 95%CI=0.97–0.99, p<.001). Furthermore, opiate use was significantly lower among BUP than MET participants during the first 9 weeks of the treatment (OR=0.63, 95%CI=0.52–0.76, p<.01).
Figure 3.
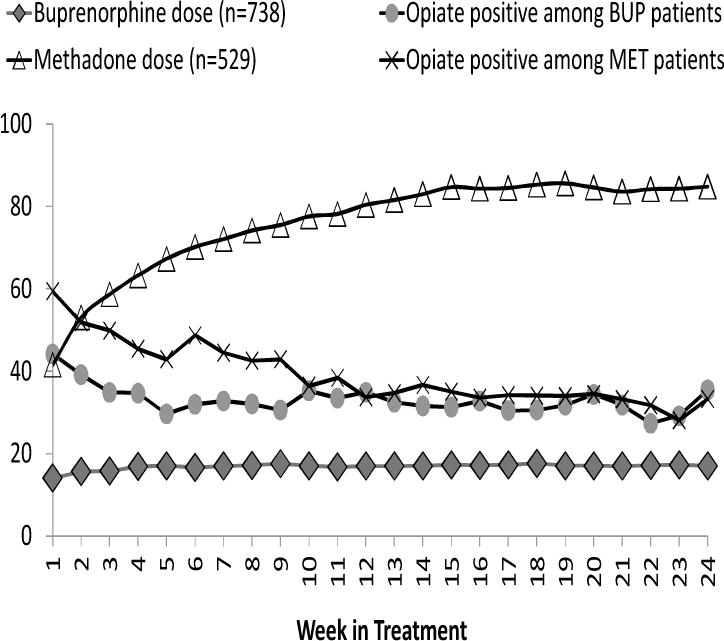
Averaged Dose and Positive Opiate Urine Test Across Weeks by Treatment Group (n=1,267)
Cox Model Predicting Retention
The unadjusted hazard ratio for retention on MET compared with BUP over the 24 weeks of the trial was 2.65 (95% CI= 2.18–3.22, p<.01). Adjusted hazard ratio was 1.61 (95% CI=1.20–2.15, p<.01) controlling for covariates at baseline in the Cox model (Table 3).
Table 3.
Cox Model Predicting Treatment Retention
| Buprenorphine/Naloxone | Methadone | Total | |
|---|---|---|---|
| (n=738) | (n=529) | (n=1,267) | |
|
|
|||
| Hazard Ratios (95% CI) |
Hazard Ratios (95% CI) |
Hazard Ratios (95% CI) |
|
| Experimental group, Buprenorphine (vs. Methadone) | – | – | 1.61 ** (1.20 – 2.15) |
| Age | 0.99 (0.98 – 1.00) |
0.98 (0.96 – 1.00) |
0.99 * (0.98 – 0.99) |
| Gender, Males (vs. Females) | 0.71 ** (0.57 – 0.88) |
2.08 ** (1.35 – 3.21) |
0.92 (0.76 – 1.12) |
| Race/Ethnicity | |||
| African American (vs. White) | 1.10 (0.73 – 1.67) |
0.72 (0.29 – 1.76) |
1.05 (0.73 – 1.53) |
| Hispanic (vs. White) | 1.26 (0.93 – 1.69) |
1.64 (0.96 – 2.80) |
1.40 * (1.08 – 1.81) |
| Other (vs. White) | 1.08 (0.75 – 1.54) |
1.89 (1.04 – 3.45) |
1.22 (0.90 – 1.66) |
| Site | |||
| West coast (vs. East coast) | 0.54 ** (0.43 – 0.68) |
0.59 * (0.38 – 0.90) |
0.57 ** (0.47 – 0.70) |
| Baseline characteristics | |||
| Alcohol use | 1.09 (0.86 – 1.37) |
0.88 (0.57 – 1.36) |
0.98 (0.80 – 1.20) |
| Number of cigarettes smoked per day | 1.10 (0.98 – 1.23) |
0.85 (0.70 – 1.04) |
1.03 (0.93 – 1.13) |
| SF-36 scores | |||
| Physical Component Summary | 1.00 (0.98 – 1.01) |
1.01 (0.98 – 1.03) |
1.00 (0.99 – 1.01) |
| Mental Component Summary | 1.00 (0.99 – 1.01) |
1.00 (0.98 – 1.01) |
1.00 (0.99 – 1.01) |
| Urine drug test across 24 weeks | |||
| Opioids, positive | 2.17 ** (1.51 – 3.13) |
2.07 * (1.05 – 4.07) |
2.09 ** (1.52 – 2.88) |
| Amphetamine, positive | 4.50 ** (3.32 – 6.10) |
6.85 ** (4.00 – 11.72) |
4.87 ** (3.75 – 6.34) |
| Cannabinoids, positive | 1.78 ** (1.32 – 2.40) |
3.43 ** (2.01 – 5.88) |
2.10 ** (1.62 – 2.71) |
| Cocaine, positive | 2.41 ** (1.78 – 3.27) |
2.65 ** (1.51 – 4.66) |
2.40 ** (1.84 – 3.14) |
| Days of heroin/opiates use in the past 30 days | 1.00 (0.98 – 1.01) |
1.00 (0.97 – 1.03) |
1.00 (0.98 – 1.01) |
| Dose on last day of treatment | |||
| Higher, dose>=16 for Bup or Dose>=60 for Met (vs. lower) | 0.63 ** (0.51 – 0.78) |
0.31 ** (0.21 – 0.44) |
0.32 ** (0.23 – 0.46) |
| Interactions of experimental group with last dose | – | – | 2.00 ** (1.33 – 2.99) |
p<0.05,
P<0.01
Results of the Cox model identified additional measures predicting retention. For the total sample, younger age, Hispanic (relative to white), and use of opioids, amphetamine, cannabinoids or cocaine were associated with dropout from the trial resulting in shorter retention. However, site (i.e., programs on West Coast; HR=0.57, 95% CI=0.47–0.70, p<.01) and higher medication dose (at least 16mg for BUP and 60mg for MET HR=0.32, 95% CI=0.23–0.46, p<.01) were associated with lower risk for dropout. In other words, participants receiving an lower medication dose were 3.09 times (CI=2.19–4.37) more likely than those receiving an higher dose to drop out of treatment. Furthermore, the interaction term of experimental condition and dose level (on the last day of treatment) was also significant. Taking methadone-with-lower-dose (HR=1) as the reference group, the HR of BUP was 1.61 (p< .01) indicating that BUP-with-lower-dose was 1.61 times more likely to drop out than the reference group. On the other hand, methadone-with-higher-dose was 0.32 less likely to drop out than the reference group, whereas the significant interaction of 2.00 (p<.01) indicated that BUP-with-higher-dose was more likely to drop out (although only about 1.04 times) than the reference group. Separate analyses for the two groups showed that site (i.e., programs in West Coast, relative to those in the East Coast) and use of opioids, amphetamine, cannabinoids, or cocaine during treatment were significant for both the BUP and MET groups. The influence of gender was in an opposite direction for the two groups. Males in the BUP group were less likely to drop out, and in contrast, males in the MET group were more likely to drop out.
DISCUSSION
This is the first large scale randomized trial that provides the opportunity to compare the treatment retention of participants on buprenorphine and methadone in community treatment programs in the U.S. The results demonstrate that those treated with BUP were more than 50% less likely to remain in treatment for 24 weeks than those receiving MET. This finding is consistent with other controlled trials or observational (nonrandomized) studies, even including studies that focused on special populations such as pregnant patients.19 Other findings of the study are also consistent with prior literature, including higher dose related to longer retention, use of other drugs (e.g., cocaine, amphetamine) associated with lower retention, and opiate use during treatment negatively related to dose for both groups. The unique contribution of the present study is that these findings were confirmed with this randomized trial conducted in community treatment programs in the U.S.
The study also revealed additional findings regarding BUP dose and treatment retention. First, approximately 25% of the BUP participants dropped out within first 30 days of treatment, suggesting a critical period calling for special efforts in retaining these participants. Second, of those who remained in treatment, positive opiate urine results were significantly lower among BUP relative to MET participants during the first 9 weeks of treatment. This finding confirms the advantage of BUP requiring a much shorter time for induction than MET. Third, there was a linear positive relationship between BUP dose and the treatment completion rate, suggesting the benefit of dosing greater than the common practice of a maximum dose of 16mg. However, even with the highest BUP dose level of 30–32mg allowed in this trial, the retention rate was less than the rate in the MET group (e.g., 60% vs 74%), and approximately 30% of the participants continued opioid use. Besides additional strategies focusing on retaining BUP participants beyond 30 days of treatment, these findings suggest that participants may yet fare better with BUP doses higher than the 32mg used in this study. Given the generally high safety profile of BUP and the lack of any significant side effects and other adverse effects at the high dose range observed in this study, we believe with proper monitoring safety will not be a clinical concern in such an effort.13
Finally, the study has identified additional participant characteristics predicting dropout, including being young, Hispanic, and concomitant use of heroin or other drugs such as cannabinoids, cocaine or amphetamine during treatment. Treatment programs may consider paying special attention to patients with these characteristics to prevent dropout. It should be noted that our finding on cannabinoids is in contrast to most prior studies that have found no effect of the use of cannabis on methadone treatment outcomes including treatment retention.20 A potential explanation for this discrepancy may be that strains of marijuana available more recently, when the current study was conducted, have been deliberately bred to have high concentrations of delta-9-tetrahydrocannabinol making them much more potent and potentially more destabilizing for patients on opioid maintenance therapy.
The study has several limitations. There were limited measures of participant motivation and program and community characteristics that are likely to influence treatment retention. Reasons for dropout were coarsely recorded; although the data did indicate that many more BUP participants simply discontinued their treatment. A qualitative study of this sample has suggested that BUP participants reported negative physical reactions or did not like BUP, being previously familiar with MET, and thus dropped out.21 The findings must also be taken in the context of an open-label, unblinded clinical trial.
Despite these limitations, the study revealed several important findings as discussed earlier. Given the linear trend of higher BUP dose related to increased retention, future studies should investigate if BUP doses greater than 32mg should be considered to increase retention, and perhaps to further reduce opioid use during treatment. One study which examined BUP doses of 24–56mg/d among 650 patients showed a retention rate over 92% at 30 months and, similar to the present investigation, decreasing rates of illicit substance use at the higher doses.22 In research or clinical practice, BUP is often prescribed at a dose of 16mg or lower, as BUP is an opioid partial agonist with a ‘ceiling effect’ for respiratory depression23 and in previous human laboratory studies, higher doses of BUP revealed a flattening out of the dose-effect curve, i.e. increasing doses do not result in greater changes in subjective measures or respiratory rate.24 A recent review article indicates that the risk of respiratory depression associated with BUP is lower than with other opioids including methadone.25 It appears that the current common practice of prescribing at a dose of 16mg per day and not to exceed 32mg maximum is driven by the ceiling effect observed and a few PET scan studies26,27 showing high mu receptor occupancy (79–95% at 16mg,26 84% at 32mg27) as well as concerns about diversion.28,29 However, these studies were based on small samples and do not take into account inter-individual variation and the other potential non-mu effects of BUP. History has taught that for about 20 years it was common to have MET dose ceilings of 40mg per day, but it is now well established that this is inadequate to maintain the necessary plasma concentrations to be effective; the effective range is between 60mg and 120mg or higher per day for most patients.30 The current study suggests the possibility of BUP at a dose of 32mg or higher having a potential impact on improving treatment retention and outcome. Thus, further investigations of the safety, efficacy, and clinical utilities of higher doses of BUP should be considered.
The high dropout rates at the early phase of BUP treatment suggest the need for early interventions to prevent dropout. It is possible that more intensive psychosocial support could help, although studies to date indicate little benefit to increased counseling for patients treated with buprenorphine.31,32,33 An alternative approach as propounded by Kakko et al. and demonstrated efficacious in a randomized trial is prompt, early transfer to MET of patients who do not quickly stabilize on BUP.34 A more widespread introduction of contingency management into opioid treatment programs could also help to optimize retention and outcomes for both BUP and MET.35 Finally, heroin-assisted treatment is used in several European countries with success, particularly for people with opioid dependence who continue intravenous heroin while on methadone maintenance or who are not enrolled in treatment.36
BUP retention may also be improved with take-home doses as permitted by regulation and as standard in office-based practice supervised by physicians, which obviates the need for daily attendance in clinic for dosing. As described earlier, the trial was conducted among community methadone maintenance programs and thus may not be optimal for delivering BUP treatment. The nine clinics were all federally licensed opioid treatment programs that have provided treatment with methadone for many years. All staff and most participants had previous experience with MET, and participants came to these programs for methadone treatment. Because BUP was delivered within these methadone maintenance programs, all programs delivered BUP following the same rules and regulations for methadone prescription (e.g., daily dose in clinic), precluding the flexibility and individualization possible in office-based treatment that may help to retain some patients.
The differential retention of males by medication condition has no definitive explanation and deserves future investigation. Since opioids suppress the hypothalamic-pituitary-gonadal axis leading to lower testosterone levels, it may be that BUP, as a partial agonist, does so less than MET, and thus males find BUP more tolerable in that regard.
In conclusion, the study findings suggest the need to (1) investigate the use of higher medication doses, particularly for BUP, (2) address continued use of opiate and other drugs such as cocaine, amphetamines, and cannabinoids, and (3) further study and identify factors/strategies influencing BUP retention, particularly during the first 30 days of treatment in order to help in efforts to improve engagement and retention in opioid treatment programs.
Acknowledgments
Funding has been provided by the National Institute on Drug Abuse through Grants U10 U10DA13045 and K05DA017648.
Footnotes
Declaration of Interest: Authors disclosing relevant financial interests, activities, relationships, and affiliations are:
Yih-Ing Hser: Received a small educational grant from Reckitt Benckiser Pharmaceuticals to support a Summer Institute on Promoting Recovery within the Changing Health Service System.
Andrew Saxon: Consultant to Reckitt Benckiser Pharmaceuticals. Advisory board member for Alkermes, Inc.
Walter Ling: Consultant to Reckitt Benckiser Pharmaceuticals.
All other authors report no financial or other possible conflicts of interest.
Trial registration: Starting Treatment with Agonist Replacement Therapies (START) on ClinicalTrials.gov (NCT00315341).
The corresponding author, Yih-Ing Hser, has full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Author contributions to the manuscript are as follows:
Study concept and design: Hser, Saxon, Thomas, Jacobs, Ling
Acquisition of data: Saxon, Thomas, Hasson, Hillhouse, Wiest, Cohen, Ling
Analysis and interpretation of data: Hser, Saxon, Huang, Teruya, McLaughlin, Cohen, Ling
Drafting of the manuscript: Hser, Saxon, Huang, Ling
Critical revision of the manuscript for important intellectual content: Hser, Saxon, Hasson, Thomas, Hillhouse, Jacobs, Teruya, McLaughlin, Wiest, Cohen, Ling
Statistical analysis: Huang, Hillhouse
Obtaining funding: Hser, Saxon, Hasson, Ling
Administrative, technical, or material support: Saxon, Hasson, Thomas, Hillhouse, Teruya
Study supervision: Hser, Saxon, Thomas, Jacobs, Ling
References
- 1.Substance Abuse and Mental Health Services Administration. Results from the 2010 National Survey on Drug Use and Health: Summary of National Findings. Rockville, MD: 2011. [Google Scholar]
- 2.Degenhardt L, Hall W. Extent of illicit drug use and dependence, and their contribution to the global burden of disease. Lancet. 2012 Jan 7;379(9810):55–70. doi: 10.1016/S0140-6736(11)61138-0. [DOI] [PubMed] [Google Scholar]
- 3.Warner M, Chen LH, Makuc DM, Anderson RN, Miniño AM. Drug poisoning deaths in the United States, 1980–2008. Hyattsville, MD: National Center for Health Statistics; 2011. [Google Scholar]
- 4.Connock M, Juarez-Garcia A, Jowett S, Frew E, Liu Z, Taylor RJ, Fry-Smith A, Day E, Lintzeris N, Roberts T, Burls A, Taylor RS. Methadone and buprenorphine for the management of opioid dependence: a systematic review and economic evaluation. Health Technol Assess. 2007;11(9):1–171. iii–iv. doi: 10.3310/hta11090. [DOI] [PubMed] [Google Scholar]
- 5.Bell J, Trinh L, Butler B, Randall D, Rubin G. Comparing retention in treatment and mortality in people after initial entry to methadone and buprenorphine treatment. Addiction. 2009;104(7):1193–200. doi: 10.1111/j.1360-0443.2009.02627.x. [DOI] [PubMed] [Google Scholar]
- 6.Mattick RP, Kimber J, Breen C, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2008;16(2):CD002207. doi: 10.1002/14651858.CD002207.pub3. [DOI] [PubMed] [Google Scholar]
- 7.Pinto H, Maskrey V, Swift L, Rumball D, Wagle A, Holland R. The SUMMIT trial: a field comparison of buprenorphine versus methadone maintenance treatment. J Subst Abuse Treat. 2010;39(4):340–352. doi: 10.1016/j.jsat.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 8.Gerstein DR, Harwood HJ, editors. Treating Drug Problems. Vol. 1. National Academy Press; Washington, DC: 1990. [PubMed] [Google Scholar]
- 9.Walsh SL, June HL, Schuh KJ, Preston KL, Bigelow GE, Stitzer ML. Effects of buprenorphine and methadone in methadone-maintained subjects. Psychopharmacology. 1995;119(3):268–276. doi: 10.1007/BF02246290. [DOI] [PubMed] [Google Scholar]
- 10.Awgu E, Magura S, Rosenblum A. Heroin-dependent inmates’ experiences with buprenorphine or methadone maintenance. J Psychoactive Drugs. 2010 Sep;42(3):339–346. doi: 10.1080/02791072.2010.10400696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Strain EC, Harrison JA, Bigelow GE. Induction of opioid-dependent individuals onto buprenorphine and buprenorphine/naloxone soluble-films. Clin Pharmacol Ther. 2011 Mar;89(3):443–449. doi: 10.1038/clpt.2010.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fareed A, Vayalapalli S, Casarella J, Drexler K. Effect of buprenorphine dose on treatment outcome. J Addict Dis. 2012;31(1):8–18. doi: 10.1080/10550887.2011.642758. [DOI] [PubMed] [Google Scholar]
- 13.Saxon AJ, Ling W, Hillhouse M, et al. Buprenorphine/Naloxone and methadone effects on laboratory indices of liver health: A randomized trial. Drug Alcohol Depend. 2013;128:71–76. doi: 10.1016/j.drugalcdep.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–483. [PubMed] [Google Scholar]
- 15.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J of the American Statistical Association. 1958;53:457–481. [Google Scholar]
- 16.Cox DR. Regression models and life tables (with discussion) J Roy Stat Soc. 1972;B34:187–220. [Google Scholar]
- 17.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–30. [PubMed] [Google Scholar]
- 18.SAS Institute, Inc. SAS/STAT User’s Guide, Version 9.2. Cary, NC: SAS Institute, Inc; 2009. [Google Scholar]
- 19.Jones HE, Kaltenback K, Heil SH, Stine SM, Coyle MG, Arria AM, O’Grady, Selby P, Martin PR, Fischer G. Neonatal abstinence syndrome after methadone or buprenorphine exposure. The New England Journal of Medicine. 2010;363(24):2320–31. doi: 10.1056/NEJMoa1005359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Epstein DH, Preston KL. Does cannabis use predict poor outcome for heroin-dependent patients on maintenance treatment? Past findings and more evidence against. Addiction. 2003 Mar;98(3):269–279. doi: 10.1046/j.1360-0443.2003.00310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Teruya C, et al. Patient perspectives on buprenorphine: A qualitative study of retention during the Starting Treatment with Agonist Replacement Therapies (START) study. (Under Review) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Di Petta Gilberto, Leonardi Claudio. Buprenorphine high-dose, broad spectrum, long-term treatment: A new clinical approach to opiate alkaloid dependency. Heroin Add & Rel Clin Probl. 2005;7(3):21–26. [Google Scholar]
- 23.Mégarbane B, Hreiche R, Pirnay S, Marie N, Baud FJ. Does high-dose buprenorphine cause respiratory depression?: possible mechanisms and therapeutic consequences. Toxicol Rev. 2006;25(2):79–85. doi: 10.2165/00139709-200625020-00002. [DOI] [PubMed] [Google Scholar]
- 24.Walsh SL, Preston KL, Stitzer ML, Cone EJ, Bigelow GE. Clinical pharmacology of buprenorphine: ceiling effects at high doses. Clinical Pharmacology & Therapeutics. 1994;55(5):569–580. doi: 10.1038/clpt.1994.71. [DOI] [PubMed] [Google Scholar]
- 25.Kress HG. Clinical update on the pharmacology, efficacy and safety of transdermal buprenorphine. Eur J Pain. 2009 Mar;13(3):219–30. doi: 10.1016/j.ejpain.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 26.Zubieta J, Greenwald MK, Lombardi U, et al. Buprenorphine-induced changes in mu-opioid receptor availability in male heroin-dependent volunteers: a preliminary study. Neuropsychopharmacology. 2000;23(3):326–334. doi: 10.1016/S0893-133X(00)00110-X. [DOI] [PubMed] [Google Scholar]
- 27.Greenwald MK, Johanson CE, Moody DE, et al. Effects of buprenorphine maintenance dose on mu-opioid receptor availability, plasma concentrations, and antagonist blockade in heroin-dependent volunteers. Neuropsychopharmacology. 2003 Nov;28(11):2000–2009. doi: 10.1038/sj.npp.1300251. [DOI] [PubMed] [Google Scholar]
- 28.Stimmel B. Buprenorphine misuse, abuse, and diversion: when will we ever learn? J Addict Dis. 2007;26(3):1–3. doi: 10.1300/J069v26n03_01. [DOI] [PubMed] [Google Scholar]
- 29.Johanson CE, Arfken CL, di Menza S, Schuster CR. Diversion and abuse of buprenorphine: findings from national surveys of treatment patients and physicians. Drug Alcohol Depend. 2012 Jan 1;120(1–3):190–195. doi: 10.1016/j.drugalcdep.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 30.Strain EC, Bigelow GE, Liebson IA, Stitzer ML. Moderate- vs high-dose methadone in the treatment of opioid dependence: a randomized trial. JAMA. 1999;281(11):1000–1005. doi: 10.1001/jama.281.11.1000. [DOI] [PubMed] [Google Scholar]
- 31.Fiellin DA, Pantalon MV, Chawarski MC, et al. Counseling plus buprenorphine-naloxone maintenance therapy for opioid dependence. N Engl J Med. 2006 Jul 27;355(4):365–374. doi: 10.1056/NEJMoa055255. [DOI] [PubMed] [Google Scholar]
- 32.Weiss RD, Potter JS, Fiellin DA, et al. Adjunctive counseling during brief and extended buprenorphine-naloxone treatment for prescription opioid dependence: a 2-phase randomized controlled trial. Arch Gen Psychiatry. 2011 Dec;68(12):1238–1246. doi: 10.1001/archgenpsychiatry.2011.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ling W, Hillhouse M, Ang A, Jenkins J, Fahey J. Comparison of behavioral treatment conditions in buprenorphine maintenance. Addiction. 2013 Jun 4; doi: 10.1111/add.12266. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kakko J, Gronbladh L, Svanborg KD, et al. A stepped care strategy using buprenorphine and methadone versus conventional methadone maintenance in heroin dependence: a randomized controlled trial. Am J Psychiatry. 2007 May;164(5):797–803. doi: 10.1176/ajp.2007.164.5.797. [DOI] [PubMed] [Google Scholar]
- 35.Peirce JM, Petry NM, Stitzer ML, Blaine J, Kellogg S, Satterfield F, et al. Effects of lower-cost incentives on stimulant abstinence in methadone maintenance treatment: a National Drug Abuse Treatment Clinical Trials Network study. Arch Gen Psychiatry. 2006;63(2):201–8. doi: 10.1001/archpsyc.63.2.201. [DOI] [PubMed] [Google Scholar]
- 36.Haasen C, Verthein U, Degkwitz P, Berger J, Krausz M, Naber D. Heroin-assisted treatment for opioid dependence: randomised controlled trial. Br J Psychiatry. 2007 Jul;191:55–62. doi: 10.1192/bjp.bp.106.026112. [DOI] [PubMed] [Google Scholar]


