Abstract
Objective
Survivors of critical illness are frequently left with long-lasting disability. The association between delirium and disability in critically ill patients has not been described. We hypothesized that the duration of delirium in the ICU would be associated with subsequent disability and worse physical health status following a critical illness.
Design
Prospective cohort study nested within a randomized controlled trial of a paired sedation and ventilator weaning strategy.
Setting
A single-center tertiary-care hospital
Patients
One hundred twenty-six survivors of a critical illness
Measurements
Confusion assessment method for the ICU (CAM-ICU), Katz activities of daily living (ADL), Functional Activities Questionnaire (FAQ, measuring instrumental activities of daily living), Medical Outcomes Study 36-item Short Form General Health Survey Physical Components Score (SF-36 PCS) and Awareness Questionnaire (AQ). Associations between delirium duration and outcomes were determined via proportional odds models with generalized estimating equations (GEE) (for ADL and FAQ scores) or via nonlinear mixed effects models (for SF-36 PCS and AQ scores).
Main Results
Excluding patients who died prior to follow-up but including those who withdrew or were lost to follow-up, we assessed 80/99 patients (81%) at 3-months and 63/87 (72%) at 12-months. After adjusting for covariates, delirium duration was associated with worse ADL scores (p=0.002) over the course of the 12-month study period but was not associated with worse IADL scores (p=0.15) or worse SF-36 PCS scores (p=0.58). Duration of delirium was also associated with lower AQ motor-sensory function scores (p=0.02).
Conclusion
In the setting of critical illness, longer delirium duration is independently associated with disability in ADLs and worse motor-sensory function in the following year. These data point to a need for further study into the determinants of functional outcomes in ICU survivors.
Keywords: Delirium, Intensive Care Unit, Activities of Daily Living
INTRODUCTION
The number of patients, particularly the elderly, admitted to the intensive care unit (ICU) is rapidly increasing.(1) The majority of these patients are functionally independent prior to their acute critical illness, but survivors are frequently unable to carry out basic activities of daily living (ADLs) that are essential to independent living (e.g., bathing, dressing, toileting, transferring, continence, and feeding) and instrumental activities of daily living (IADLs) that facilitate independent living (e.g., handing financial matters, assembling business affairs or papers, shopping alone for groceries, playing a game of skill or working on a hobby, making a cup of coffee, preparing a balanced meal, keeping track of current events, understanding a book or TV show, remembering appointments and traveling outside of one’s neighborhood).(2–4) Thus, new disability in these activities among the large and growing number of patients leaving the hospital after critical illness represents an important health care and societal problem in years to come that may disproportionately affect the elderly.
Knowledge about risk factors for disability after critical illness is an important but unmet need. In non-ICU hospitalizations, delirium is associated with disability in the months and years following the index illness.(5–9) Delirium in the ICU occurs in 60%-80% of mechanically ventilated patients and 20%-40% of non-ventilated patients.(10–15) The longer a patient is delirious in the ICU, the more likely he or she is to develop cognitive impairment or die.(15–17) The duration of ICU delirium has never been examined as a risk factor for long-term disability. Older patients are particularly susceptible to the development of delirium and therefore may be placed at a disproportional risk to suffer the long-term consequences of this syndrome. Given the high prevalence of delirium in the ICU and the association between delirium and newly acquired disabilities in other populations, we hypothesized that the duration of delirium in the ICU is associated with subsequent disability and poorer physical health status in the year following critical illness. To test this hypothesis, we conducted a prospective cohort study to determine whether the duration of delirium in the ICU is associated with functional outcomes up to one year later, regardless of age, in adult medical ICU patients.
MATERIALS AND METHODS
Study Design and Participants
We nested this single-center, prospective cohort study within the Awakening and Breathing Controlled (ABC) Trial (ClinicalTrials.gov NCT 00097630), a multicenter randomized trial that compared a paired sedation and ventilator weaning protocol with usual care.(18) Between October 2003 and March 2006, study personnel recruited patients at a large, private, tertiary care center, Saint Thomas Hospital in Nashville, TN. The medical intensive care unit (ICU) census was reviewed daily to identify patients ≥18 years old who were mechanically ventilated for more than 12 hours. Patients meeting these inclusion criteria were excluded from the parent study if they were mechanically ventilated for more than two weeks prior to screening, admitted following cardiac arrest, not committed to receiving aggressive therapy and/or moribund, unable to live independently at baseline due to profound neurologic deficits, or were enrolled in another trial that prohibited co-enrollment. Patients who survived until hospital discharge, did not have severe neurologic deficits (e.g., severe dementia or stroke), and had not undergone cardiac or neurologic surgery were included in this follow-up study.
Surrogate decision-makers provided written informed consent at the time of enrollment since patients were typically unable to consent while mechanically ventilated; the participants themselves consented prior to hospital discharge if they were competent to do so. The Vanderbilt Coordinating Center (Nashville, TN) supervised and conducted the trial, and the institutional review boards at Saint Thomas Hospital and Vanderbilt University (Nashville, TN) approved the study protocol.
Exposure
The primary exposure, chosen a priori, was duration of delirium in the ICU during the 28-day study period. We chose duration of ICU delirium as the exposure, rather than the dichotomous “ever/never” presence of delirium, because we expected that a patient who had short-lived delirium (e.g., 1 day of delirium) would more closely resemble the patient who had no delirium than a patient who was delirious for multiple days. Moreover, there is a growing body of literature suggesting that delirium duration (i.e., the “dose” of delirium), rather than simply its presence or absence, is associated with multiple long-term outcomes, including mortality and long-term cognitive impairment.(16, 17, 19) Finally, dichotomization of a continuous variable results in a significant loss of power, increases the risk of type I error, and may conceal non-linearity in the relation between the variable and the outcome.(20)
Each day, trained study personnel (either research nurses or physicians) assessed patients who were in the ICU and not comatose for delirium using the Confusion Assessment Method for the ICU (CAM-ICU), a brief, well-validated screening instrument for detection of delirium in the ICU.(10, 11) The Richmond Agitation-Sedation Scale (RASS) (21, 22) was used to assess level of consciousness and coma was defined as a RASS of −5 (no response to verbal or physical stimulation) or −4 (response to physical stimulation without response to verbal stimulation). Delirium duration was defined as the number of days a patient was CAM-ICU positive in the ICU during the 28-day study period.
Outcomes and Covariates
At 3 and 12 months following hospital discharge, a clinical neuropsychologist (JCJ)—who was blinded to and thus unaware of any details concerning the course of each patient’s critical illness (including duration of delirium)—assessed survivors using a battery of functional and physical health status measures. We assessed multiple aspects of physical functioning using four validated questionnaires. Specifically, we used the Katz ADL(23) and the Functional Activities Questionnaire (FAQ)(24) to assess activities of daily living (ADLs) and instrumental activities of daily living (IADL), respectively. To assess physical health status, we used the Medical Outcomes Study 36-item Short Form General Health Survey (SF-36) and calculated the physical component score (PCS) using the standard approach.(25) Finally, we used the Motor/Sensory Factors component of the Awareness Questionnaire(26) to specifically assess the patient’s perceptions of change in motor-sensory function following critical illness.
For each of the 6 ADLs assessed, we assigned patients reporting independence a score of 0, those reporting partial dependence a score of 1 and those reporting complete dependence a score of 2. Thus, a patient who was completely dependent in all ADLs would receive a score of 12, whereas a patient who was completely independent would receive a score of 0.
For IADL outcomes, patients were given 0 points if they reported no difficulty completing an IADL, 1 point if they reported difficulty doing the IADL but could do it without assistance, 2 points if they reported requiring assistance with the IADL, and 3 points if the patient reported complete dependency in the IADL. Therefore, a patient with complete dependence in all 10 IADLs would receive a score of 30 and a patient with complete IADL independence would have a score of 0.
The SF-36 PCS is comprised of the 21 questions from the SF-36 sub-domains of physical function, role physical, bodily pain and general health.(27) Each question is scored from 0–100 with lower scores indicating poor physical health status. Individual question scores are combined into sub-domain scores, which are used to derive the PCS score.
The Awareness Questionnaire (AQ) measures self-ratings of functional abilities.(26) The Motor/Sensory Factors component is comprised of four questions regarding arm and leg movement, eyesight, coordination, and hearing. Patients are asked to compare current function to pre-illness function. Scoring is done on a 5-point scale from 1–5, where 1 indicates ‘much worse’ function and 5 indicates ‘much better’ function (i.e., a higher score indicates better function). Thus, the possible scores range from 4 (much worse in all four areas) to 20 (much better in all four areas).
To adjust for potential confounding, we measured covariates that we selected a priori based on clinical and biologic plausibility. These covariates included age, severity of illness at the time of enrollment in the parent study, severe sepsis, duration of coma in the ICU during the 28-day study period, and baseline ADL and IADL scores. Severity of illness was calculated using the acute physiology score portion of the Acute Physiologic and Chronic Health Evaluation (APACHE) II score.(28) Severe sepsis at ICU admission was identified using the treating physicians’ diagnosis and confirmed using international consensus definitions.(29) Baseline (i.e., pre-critical illness) ADL scores were determined using surrogate responses to the Katz ADL,(30) and baseline IADL scores were determined using surrogate responses to the FAQ.(24) The surrogate who completed these questionnaires was a person who knew the patient well enough to answer detailed questions about the patient’s functional abilities during the time shortly before the onset of their critical illness. Both the Katz ADL and the FAQ have been shown to be valid and demonstrate good agreement when completed by the patient (as was done during the follow-up phase of the current study) or by their surrogate (as was done when assessment of baseline functioning was performed).(24, 31)
Statistical Analysis
Baseline demographics and clinical characteristics were examined using median and interquartile ranges for continuous variables and proportions for categorical variables. We analyzed patients from this trial population as a single cohort rather than according to intervention group assignment since previous analyses did not find differences in functional outcomes between patients randomized to the intervention protocol and those randomized to usual care.(32)
We used proportional odds models with generalized estimating equations (GEE) for ADL and IADL outcomes, evaluated as continuous outcome measures, to determine whether duration of delirium was independently associated with subsequent disability.(33) To assess the relationship between delirium duration and physical health status and between delirium duration and subsequent perception of motor-sensory function, we used nonlinear mixed effects models that contained SF-36 PCS scores and AQ scores as continuous outcome measures. We chose these statistical models based on the distribution of the outcomes (e.g., ADL and IADL outcomes were highly skewed and clustered around 0). Age, severe sepsis, APACHE II acute physiology score, and duration of coma were included in all regression models, regardless of statistical significance. In addition, we included baseline Katz ADL and FAQ scores as covariates in the respective Katz ADL and FAQ score models, to adjust for pre-ICU functional abilities. Nonlinearity of the associations between the primary exposure variable, delirium duration, as well as the covariates age and coma duration, was assessed by inclusion of restricted cubic splines in the regression models; these associations were assumed to be nonlinear unless the p value of the nonlinear term was greater than 0.20, in which case the nonlinear term was excluded from the model. We used R (version 2.8.1 patched) for all statistical analyses.
RESULTS
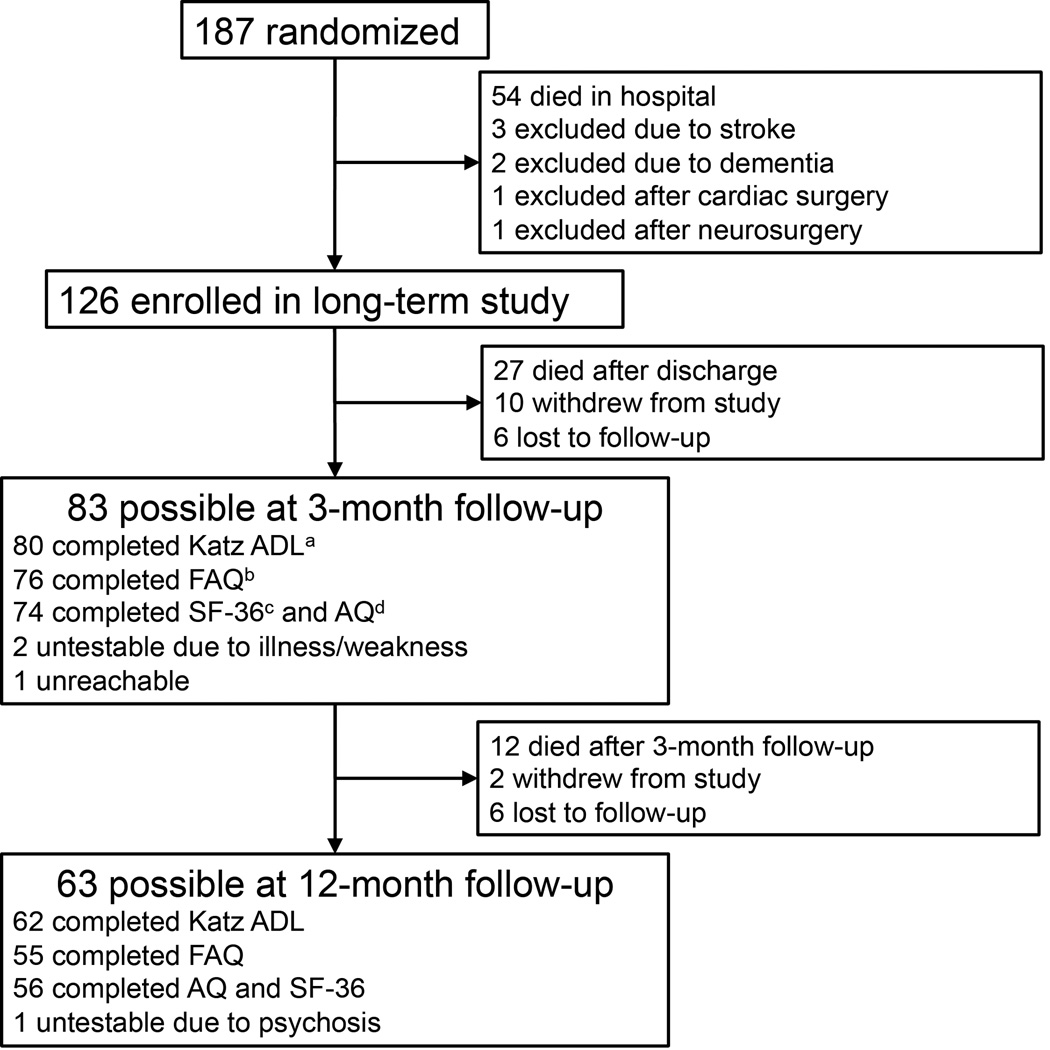
Of the 187 patients who were enrolled in the parent study at Saint Thomas Hospital, 54 died during the hospitalization, and seven were excluded from this long-term study (Figure 1). Thus, 126 patients survived and were eligible for inclusion in this long-term prospective cohort. Follow-up was achieved in 80 of the 99 (81%) patients alive at 3-month follow-up and in 62 of the 87 (71%) patients alive at 12-month follow-up (52 patients completed all four questionnaires at both time points).
Figure 1.
Enrollment and follow-up diagram. a Katz ADL: Activities of daily living, b FAQ: Functional Activities Questionnaire (measuring instrumental activities of daily living), c SF-36 Medical Outcomes Study 36-item Short Form General Health Survey Physical Components Score, d AQ: Awareness Questionnaire.
Baseline demographics and clinical characteristics of the study population are shown in Table 1. The majority of patients were 61 years or older, and more than a quarter were 71 years of age or older. Baseline disability in ADLs (defined as an ADL score ≥1) was present in 25% of patients and disability in IADLs (defined as an FAQ score ≥9) was present in 21% of patients. Patients had a high severity of illness at ICU admission and nearly half were admitted with sepsis and/or ARDS. Patients were mechanically ventilated for a median [interquartile range] of 5.0 [1.9–9.1] days.
Table 1.
Baseline Demographics and Clinical Characteristics a
| Characteristic | Cohort N=80b |
|---|---|
| Age, years | 61 (47–71) |
| Female, % (n/total) | 48 (38/80) |
| Years of education | 12 (10.2–12) |
| Total ADL score at enrollment | 0 (0–0.25) |
| Total FAQ (IADL) score at enrollment | 0 (0–7) |
| ADL disability c | 25% (20/80) |
| IADL disability d | 21% (16/76) |
| Acute Physiologic and Chronic Health Evaluation II | 29 (22–34) |
| Admission Diagnoses, % (n/total) | |
| Sepsis and/or ARDS | 50% (40/80) |
| MI/CHF | 19% (15/80) |
| Altered Mental Status | 15% (12/80) |
| COPD/Asthma | 9% (7/80) |
| Renal or Hepatic Failure | 4% (3/80) |
| HIV infection | 1% (1/80) |
| Upper airway obstruction | 1% (1/80) |
| Malignancy | 1% (1/80) |
| Delirium days in the intensive care unit | 2 (1–5) |
| Coma days in the intensive care unit | 2 (0–4) |
| Days of Mechanical Ventilation in intensive care unit | 5 (1.9–9.1) |
Values are expressed in median (interquartile range) or % (n/total).
N represents the total number of patients with at least one functional outcome assessed.
ADL disability at baseline was considered present if the Katz ADL score was ≥1, where higher scores indicate greater disability.
IADL disability at baseline was considered if scores on the FAQ assessment were ≥9, with higher scores indicating greater disability.(24)
Delirium was highly prevalent, with 84% of patients developing delirium in the ICU. Delirium persisted for at least 2 days in half the patients, and one quarter of patients were delirious for 5 days or more. Patients who died following discharge, withdrew following discharge or were lost to follow-up did not differ significantly from those patients who followed up with respect to delirium duration (2 [1–6] days vs. 2 [1–5] days, respectively).
At the 3- and 12-month follow-up assessments, the median [IQR] Katz ADL scores were 0 [0–1] and 0 [0–1], respectively. Disability in ADLs was present in one in three patients at both 3- and 12-month follow-ups (Table 2). Scores on the FAQ (IADL function) were 3 [0–6] at 3-month follow-up and 1 [0–5] at 12-month follow-up. Disability in IADLs was present in 17% of those assessed at 3-months and 5% of those assessed at 12-months. (Table 2). Physical health status was poor for nearly all patients throughout follow-up, with SF-36 PCS scores of 27 [19–35] at 3 months and 28 [21–37] at 12 months. Finally, Motor-Sensory Factors scores were 11 [9–12] and 11 [9–12] at 3- and 12-month follow-up, respectively, with over 60% of patients reporting worse motor-sensory function at both follow-up time points (Table 2).
Table 2.
Functional outcomes during follow-up
| Outcome, % (n/total a) | 3-months | 12-months |
|---|---|---|
| ADL disability b | 35% (28/80) | 32% (20/62) |
| IADL disability c | 17% (13/76) | 5% (3/55) |
| Impaired Physical Health Status d | 84% (62/74) | 80% (45/56) |
| Worse or Much Worse | ||
| Motor/Sensory Factors Score e | 62% (46/74) | 73% (41/56) |
Patients completing each outcome measure. The most common reason patients were unable to complete the full outcome assessment was fatigue (e.g., a patient may have completed the Katz ADL but have been too fatigued to complete other questionnaires). Results presented in proportions for descriptive purposes.
Proportion of patients with Katz ADL scores ≥1.
Proportion of patients with FAQ scores ≥9.
Proportion of patients with SF-36 PCS score ≤40.
Proportion of patients with Motor/Sensory Factors Score <12.
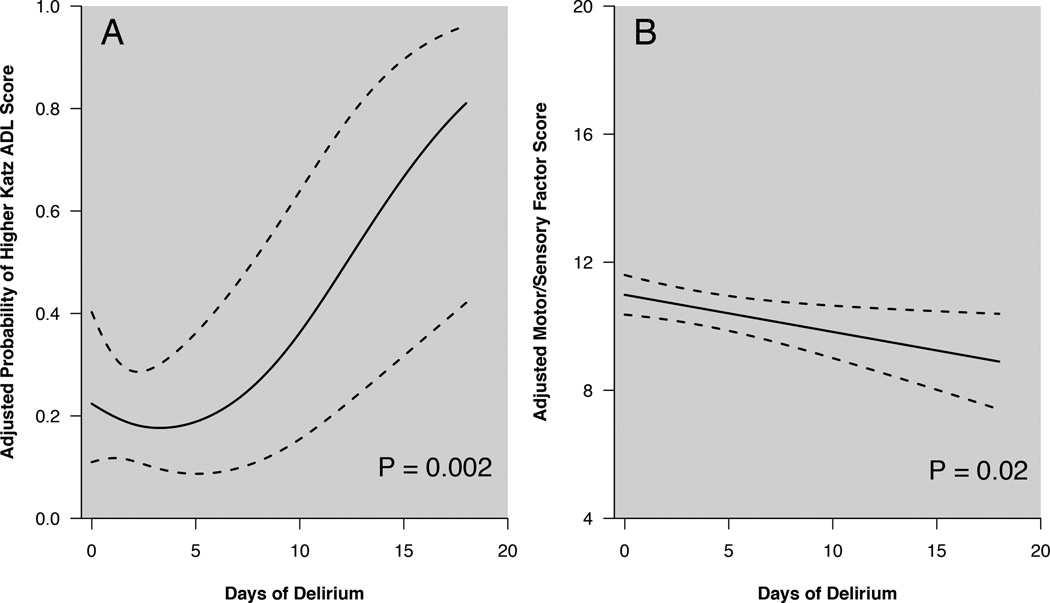
After adjusting for age, baseline ADL function, severity of illness at admission, sepsis at admission, and duration of coma, a longer duration of delirium in the ICU was associated with a higher ADL score over the course of the 12-month follow-up period (Table 3, p=0.002). Figure 2A models the non-linear association between duration of delirium in the ICU and probability of a higher Katz ADL score during long-term follow-up (i.e., patients with longer periods of delirium in the ICU were most likely to report worse ADL function).
Table 3.
Associations between duration of ICU delirium and outcomes
| Multivariable Regression Results |
|||
|---|---|---|---|
| Outcomea | Point Estimateb | 95% Confidence Interval | p-value |
| Katz ADL score | Non-linear c | 0.002 | |
| FAQ Score | 1.4 | 0.9 to 2.1 | 0.15 |
| SF-36 PCS Score | −0.7 | −3.2 to 1.8 | 0.58 |
| Motor/Sensory Factors Score |
−0.6 | −1.1 to −0.1 | 0.018 |
Delirium duration is dependent variable in each model. All outcomes were evaluated as continuous measures.
Point Estimate indicates the odds ratio for proportional odds models (Katz ADL and FAQ) or the β-coefficient for mixed effects models (Awareness Questionnaire and SF-36) for outcome after adjusting for covariates over the course of the 12-month follow-up period from the 25th percentile to the 75th percentile.
Because the association is non-linear, the magnitude of the association cannot be summarized using a single odds ratio, therefore the association is graphed in Figure 2A.
Figure 2.
Panel A. Association between duration of delirium in the ICU and probability of a higher Katz ADL score (indicating disability) over the course of the year following hospitalization for critical illness, after adjusting for age, baseline ADL function, severity of illness at admission, sepsis at admission, and duration of coma. As the number of days of ICU delirium increases, so does the probability of developing worse ADL function (p=0.002). Dashed lines indicate 95% confidence intervals.
Panel B. Association between duration of delirium in the ICU and score on the Motor/Sensory Factor of the Awareness Questionnaire over the course of the year following hospitalization for critical illness, after adjusting for age, severity of illness at admission, sepsis at admission, and duration of coma. As the number of days of ICU delirium increased, the Awareness Questionnaire Motor/Sensory Factors score decreased (p=0.02), indicating greater perceived impairment in limb movement, eyesight, coordination and hearing compared with pre-illness state. Possible scores on this measure range from 4–20, with lower scores indicating worse perceived functioning compared to pre-illness functioning. Dashed lines represent 95% confidence intervals.
Duration of ICU delirium was not associated with increased odds of a higher IADL score over the follow-up period (p=0.15, Table 3). Similarly, delirium duration was not associated with lower scores on the PCS component of the SF-36 (p=0.58, Table 3). After adjusting for covariates, duration of ICU delirium was associated with lower Awareness Questionnaire Motor/Sensory Factors scores over the course of the follow-up period (p=0.02, Table 3). Figure 2B illustrates the negative association between duration of delirium in the ICU and scores on the Motor/Sensory Factors component of the Awareness Questionnaire. Thus, the longer a patient was delirious the more likely he or she was to perceive his or her motor-sensory function as worse during follow-up compared to his or her pre-illness condition.
DISCUSSION
The main findings of this study are that the duration of delirium in the ICU was independently associated with disability in ADLs in the year following critical illness after adjusting for covariates, including baseline ADL function, and the longer a patient was delirious in the ICU, the more likely he or she was to report worse motor-sensory function during follow-up compared with his or her pre-illness state. Delirium duration was not independently associated with IADL disability or worse physical health status. Nevertheless, these results indicate that the long-term deleterious effects of delirium extend beyond cognition and mortality to functional outcomes as well. They highlight the need for effective delirium prevention and treatment strategies to reduce functional disabilities among survivors of critical illness.
While in non-critically ill and post-operative populations, delirium has been associated with ADL disability during both medium- (e.g., 3–6 months) and longer-term (e.g., 12 months) periods of follow-up,(6–9, 34, 35) to our knowledge, no previous study has shown an association between delirium duration and ADL disability in survivors of critical illness. Marcantonio and colleagues found that elderly patients with persistent delirium 1 month following hip fracture surgery had worse ADL function than those who had delirium that resolved before hospital discharge; both of these groups had worse ADL function than patients who were never delirious.(6) Similarly, Quinlan et al. found that functional decline was more prevalent in a population of elderly, non-cardiac surgery patients whose delirium persisted for 3 or more days compared with those who were delirious for 1–2 days or were never delirious.(9) Despite these findings in non-ICU populations, it cannot be assumed the adverse outcomes associated with delirium outside the ICU are applicable to those patients with delirium in the ICU. In this case, our results are indeed consistent with findings in other, non-critically ill populations and support our hypothesis that duration of ICU delirium is associated with post-ICU disability.
The mechanisms underlying the association between delirium duration and ADL disability are not yet known. One potential mechanism relates to the fact that most delirious ICU patients have hypoactive delirium (i.e., with reduced physical activity).(36, 37) As a result of reduced spontaneous physical activity, patients may be subject to disuse muscle atrophy, predisposing them to ICU-acquired weakness (ICU-AW).(38–41) Thus, developing weakness in the ICU may lead to functional disability in the months and years following critical illness. The extent to which delirium may contribute to the overall development of ICU-AW and subsequent long-term disability remains unknown.
Interestingly an intervention designed to prevent ICU-AW through the use of early physical and occupational therapy (PT/OT) in mechanically ventilated patients (e.g., beginning within 1–2 days of initiation of mechanical ventilation) was associated with a reduction in delirium duration compared with those who began PT/OT later (7 days after initiation of mechanical ventilation).(42) Furthermore, patients in the early PT/OT group were more likely to return to their baseline functional status (e.g. free of ADL & IADL disability). Thus, early physical and occupational rehabilitation is associated with improvements in both delirium duration and functional outcomes. The directionality of these effects remains unknown but these data do suggest a brain-body connection.
The brain-body connection may represent different manifestations of a common underlying mechanism. Inflammation, which is often present in critical illness syndromes such as severe sepsis and ARDS may link delirium and muscle wasting associated with critical illness.(43, 44) Additionally, the geriatric syndromes, delirium and physical frailty, have been hypothesized to be linked via inflammation, atherosclerosis, genetics and/or nutritional deficiencies.(45)
Two in three patients reported worse motor/sensory functioning during the follow-up period and worse motor/sensory functioning was associated with delirium duration. These results highlight a link between delirium and lesser grades of impaired physical functioning not rising to the level of disability. This finding indicates that the effects on physical functioning are far reaching.
Duration of delirium in the ICU has been previously associated with numerous other short-term adverse outcomes, including prolonged mechanical ventilation(19) and prolonged hospitalization,(46) as well as long-term adverse outcomes, such as persistent cognitive impairment(15) and increased mortality up to one year after critical illness.(16, 17) Our findings add to the growing body of evidence regarding the long-term outcomes associated with delirium duration and for clinicians, highlight the importance of monitoring and treating delirium in the critically ill.
As noted above, duration of ICU delirium was not associated with IADL disability during follow-up, nor was it associated with physical components of health status. Our study was likely underpowered to detect significant outcomes with respect to these outcomes. Alternatively, the mechanisms linking delirium and ADL function may not be relevant to IADL function, since some of the skills and abilities important to the former are different from those required for the latter. Additionally, IADL impairment could have been present early in the post-ICU course but resolved by the time of follow-up assessment as evidenced by the smaller proportion of patients reporting IADL disability at 12-month follow-up compared to those reporting disability at 3-month follow-up. A similar finding was reported by Rudolph and colleagues in a cohort of post-operative cardiac surgery patients where worse IADL function was present at 1-month follow-up, but the association was no longer present at 12-months.(5)
Our study has both strengths and weaknesses. Strengths include its prospective cohort design, daily delirium assessment with a validated delirium assessment tool administered by trained research personnel, blinding of the outcomes assessor as to delirium status in the ICU, high follow-up rate and the use of multiple, well-validated tools to assess outcomes. Weaknesses include its size, single-center nature, which may limit generalizability, use of self-reported measures of functioning rather than objective performance based measures of physical capabilities, and lack of pre-ICU administration of questionnaires necessitating reliance on proxy assessments of baseline functional status, the latter being limitations of nearly all ICU follow-up studies, since critical illness is unplanned and emergent. We were able, though, to determine the association between delirium and functional disability by estimating pre-critical illness ADL and IADL function via surrogate-completed Katz ADL and FAQ questionnaires; both provide good agreement between patients and surrogate ratings.(24, 31)
CONCLUSIONS
In summary, this study found that duration of delirium in the ICU was independently associated with newly acquired disability, as measured by ADL function and awareness of change in physical function, during the first year following critical illness. These results add to the growing body of literature regarding adverse outcomes associated with delirium and highlight that poor outcomes associated with delirium extend beyond delirium’s association with impaired cognition and death. Further study into the underlying mechanism(s) linking delirium and long-term disability as well as whether measures aimed at preventing and treating delirium may also prevent poor functional outcomes in survivors of critical illness is needed. These findings further the understanding of the long-term consequences of delirium and elevate the importance of monitoring for delirium for ICU clinicians.
ACKNOWLEDGEMENTS
Dr. Brummel is supported by the National Institutes of Health (T32HL087738). Dr. Jackson is supported by the National Institutes of Health (AG031322). Dr. Pandharipande is supported by the VA Clinical Science Research and Development Service (VA Career Development Award). Ms. Thompson is supported, in part, by the National Institutes of Health (AG027472). Dr. Shintani is supported, in part, by (AG027472) Dr. Ely is supported by the VA Clinical Science Research and Development Service (VA Merit Review Award) and the National Institutes of Health (AG027472 and AG035117). Dr. Gill is the recipient of a Midcareer Investigator Award in Patient-Oriented Research (K24AG021507) from the National Institute on Aging and is supported, in part, by the Yale Claude D. Pepper Older Americans Independence Center (P30AG21342). Drs. Bernard are Dittus are supported, in part, by the Vanderbilt Clinical-Translational Science Award (2UL1TR000445–06) from the NCRR/NIH. Dr. Dittus also is supported, in part by the National Institutes of Health (AG027472 and AG035117). Dr. Girard is supported by the National Institutes of Health (AG034257). Drs. Ely, Dittus and Girard are supported by the Veterans Affairs Tennessee Valley Geriatric Research, Education and Clinical Center (GRECC). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, And Blood Institute, the National Institute on Aging or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Prior Presentations:
Presented in abstract form at the American Thoracic Society International Meeting, May 2011, Denver, Colorado.
Author Contributions
Study Design: NEB, JCJ, PPP, JLT, AKS, RSD, GRB, EWE, TDG. Interpretation of data, critical revision of the article, and approval of the final version of the article: NEB, JCJ, PPP, JLT, AKS, RSD, TMG, GRB, EWE, TDG. Data analysis: JLT, AKS. Collection of participant data: TDG and JCJ.
Dr. Thompson has disclosed that she does not have any potential conflicts of interest.
REFERENCES
- 1.Bagshaw SM, Webb SA, Delaney A, et al. Very old patients admitted to intensive care in Australia and New Zealand: a multi-centre cohort analysis. Crit Care. 2009;13(2):R45. doi: 10.1186/cc7768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Herridge MS, Tansey CM, Matte A, et al. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011;364(14):1293–1304. doi: 10.1056/NEJMoa1011802. [DOI] [PubMed] [Google Scholar]
- 3.Iwashyna TJ, Ely EW, Smith DM, et al. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304(16):1787–1794. doi: 10.1001/jama.2010.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barnato AE, Albert SM, Angus DC, et al. Disability among elderly survivors of mechanical ventilation. Am J Respir Crit Care Med. 2011;183(8):1037–1042. doi: 10.1164/rccm.201002-0301OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rudolph JL, Inouye SK, Jones RN, et al. Delirium: An Independent Predictor of Functional Decline After Cardiac Surgery. J Am GeriatrSoc. 2010 doi: 10.1111/j.1532-5415.2010.02762.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marcantonio ER, Flacker JM, Michaels M, et al. Delirium is independently associated with poor functional recovery after hip fracture. J Am Geriatr Soc. 2000;48(6):618–624. doi: 10.1111/j.1532-5415.2000.tb04718.x. [DOI] [PubMed] [Google Scholar]
- 7.Inouye SK, Rushing JT, Foreman MD, et al. Does delirium contribute to poor hospital outcomes? A three-site epidemiologic study. J Gen Intern Med. 1998;13(4):234–242. doi: 10.1046/j.1525-1497.1998.00073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCusker J, Cole M, Dendukuri N, et al. Delirium in older medical inpatients and subsequent cognitive and functional status: a prospective study. CMAJ. 2001;165(5):575–583. [PMC free article] [PubMed] [Google Scholar]
- 9.Quinlan N, Rudolph JL. Postoperative delirium and functional decline after noncardiac surgery. J Am Geriatr Soc. 2011;59(Suppl 2):S301–S304. doi: 10.1111/j.1532-5415.2011.03679.x. [DOI] [PubMed] [Google Scholar]
- 10.Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU) JAMA. 2001;286(21):2703–2710. doi: 10.1001/jama.286.21.2703. [DOI] [PubMed] [Google Scholar]
- 11.Ely EW, Margolin R, Francis J, et al. Evaluation of delirium in critically ill patients: validation of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) Crit Care Med. 2001;29(7):1370–1379. doi: 10.1097/00003246-200107000-00012. [DOI] [PubMed] [Google Scholar]
- 12.Pandharipande P, Cotton BA, Shintani A, et al. Prevalence and risk factors for development of delirium in surgical and trauma intensive care unit patients. J Trauma. 2008;65(1):34–41. doi: 10.1097/TA.0b013e31814b2c4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McNicoll L, Pisani MA, Zhang Y, et al. Delirium in the intensive care unit: occurrence and clinical course in older patients. J Am Geriatr Soc. 2003;51(5):591–598. doi: 10.1034/j.1600-0579.2003.00201.x. [DOI] [PubMed] [Google Scholar]
- 14.Pandharipande PP, Pun BT, Herr DL, et al. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: the MENDS randomized controlled trial. JAMA. 2007;298(22):2644–2653. doi: 10.1001/jama.298.22.2644. [DOI] [PubMed] [Google Scholar]
- 15.Girard TD, Jackson JC, Pandharipande PP, et al. Delirium as a predictor of long-term cognitive impairment in survivors of critical illness. Crit Care Med. 2010;38(7):1513–1520. doi: 10.1097/CCM.0b013e3181e47be1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ely EW, Shintani A, Truman B, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291(14):1753–1762. doi: 10.1001/jama.291.14.1753. [DOI] [PubMed] [Google Scholar]
- 17.Pisani MA, Kong SY, Kasl SV, et al. Days of delirium are associated with 1-year mortality in an older intensive care unit population. Am J Respir Crit Care Med. 2009;180(11):1092–1097. doi: 10.1164/rccm.200904-0537OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Girard TD, Kress JP, Fuchs BD, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): a randomised controlled trial. Lancet. 2008;371(9607):126–134. doi: 10.1016/S0140-6736(08)60105-1. [DOI] [PubMed] [Google Scholar]
- 19.Shehabi Y, Riker RR, Bokesch PM, et al. Delirium duration and mortality in lightly sedated, mechanically ventilated intensive care patients. Crit Care Med. 2010;38(12):2311–2318. doi: 10.1097/CCM.0b013e3181f85759. [DOI] [PubMed] [Google Scholar]
- 20.Altman DG, Royston P. The cost of dichotomising continuous variables. BMJ. 2006;332(7549):1080. doi: 10.1136/bmj.332.7549.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ely EW, Truman B, Shintani A, et al. Monitoring sedation status over time in ICU patients: reliability and validity of the Richmond Agitation-Sedation Scale (RASS) JAMA. 2003;289(22):2983–2991. doi: 10.1001/jama.289.22.2983. [DOI] [PubMed] [Google Scholar]
- 22.Riker RR, Picard JT, Fraser GL. Prospective evaluation of the Sedation-Agitation Scale for adult critically ill patients. Crit Care Med. 1999;27(7):1325–1329. doi: 10.1097/00003246-199907000-00022. [DOI] [PubMed] [Google Scholar]
- 23.Katz S, Ford AB, Moskowitz RW, et al. Studies of Illness in the Aged. The Index of Adl: A Standardized Measure of Biological and Psychosocial Function. JAMA. 1963;185:914–919. doi: 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- 24.Pfeffer RI, Kurosaki TT, Harrah CH, Jr, et al. Measurement of functional activities in older adults in the community. J Gerontol. 1982;37(3):323–329. doi: 10.1093/geronj/37.3.323. [DOI] [PubMed] [Google Scholar]
- 25.Ware JE, Jr, Sherbourne CD. The MOS36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–483. [PubMed] [Google Scholar]
- 26.Sherer M, Bergloff P, Boake C, et al. The Awareness Questionnaire: factor structure and internal consistency. Brain Inj. 1998;12:63–68. doi: 10.1080/026990598122863. [DOI] [PubMed] [Google Scholar]
- 27.Ware JE. SF-36 Physical and Mental Health Summary Scales: a user's manual. Boston: Health Assessment Lab, New England Medical Center; 1994. [Google Scholar]
- 28.Knaus WA, Draper EA, Wagner DP, et al. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13(10):818–829. [PubMed] [Google Scholar]
- 29.Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31(4):1250–1256. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 30.Katz S, Akpom CA. A measure of primary sociobiological functions. Int J Health Serv. 1976;6(3):493–508. doi: 10.2190/UURL-2RYU-WRYD-EY3K. [DOI] [PubMed] [Google Scholar]
- 31.Santos-Eggimann B, Zobel F, Berod AC. Functional status of elderly home care users: do subjects, informal and professional caregivers agree? J Clin Epidemiol. 1999;52(3):181–186. doi: 10.1016/s0895-4356(98)00155-3. [DOI] [PubMed] [Google Scholar]
- 32.Jackson JC, Girard TD, Gordon SM, et al. Long-term cognitive and psychological outcomes in the awakening and breathing controlled trial. Am J Respir Crit Care Med. 2010;182(2):183–191. doi: 10.1164/rccm.200903-0442OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42(1):121–130. [PubMed] [Google Scholar]
- 34.Andrew MK, Freter SH, Rockwood K. Incomplete functional recovery after delirium in elderly people: a prospective cohort study. BMC Geriatr. 2005;5:5. doi: 10.1186/1471-2318-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murray AM, Levkoff SE, Wetle TT, et al. Acute delirium and functional decline in the hospitalized elderly patient. J Gerontol. 1993;48(5):M181–M186. doi: 10.1093/geronj/48.5.m181. [DOI] [PubMed] [Google Scholar]
- 36.Peterson JF, Pun BT, Dittus RS, et al. Delirium and its motoric subtypes: a study of 614 critically ill patients. J Am Geriatr Soc. 2006;54(3):479–484. doi: 10.1111/j.1532-5415.2005.00621.x. [DOI] [PubMed] [Google Scholar]
- 37.Pandharipande P, Cotton BA, Shintani A, et al. Motoric subtypes of delirium in mechanically ventilated surgical and trauma intensive care unit patients. Intensive Care Med. 2007;33(10):1726–1731. doi: 10.1007/s00134-007-0687-y. [DOI] [PubMed] [Google Scholar]
- 38.Griffiths RD, Hall JB. Intensive care unit-acquired weakness. Crit Care Med. 2010;38(3):779–787. doi: 10.1097/CCM.0b013e3181cc4b53. [DOI] [PubMed] [Google Scholar]
- 39.Ali NA, O'Brien JM, Jr, Hoffmann SP, et al. Acquired weakness, handgrip strength, and mortality in critically ill patients. Am J Respir Crit Care Med. 2008;178(3):261–268. doi: 10.1164/rccm.200712-1829OC. [DOI] [PubMed] [Google Scholar]
- 40.Stevens RD, Marshall SA, Cornblath DR, et al. A framework for diagnosing and classifying intensive care unit-acquired weakness. Crit Care Med. 2009;37(10 Suppl):S299–S308. doi: 10.1097/CCM.0b013e3181b6ef67. [DOI] [PubMed] [Google Scholar]
- 41.Latronico N, Bolton CF. Critical illness polyneuropathy and myopathy: a major cause of muscle weakness and paralysis. Lancet Neurol. 2011;10(10):931–941. doi: 10.1016/S1474-4422(11)70178-8. [DOI] [PubMed] [Google Scholar]
- 42.Schweickert WD, Pohlman MC, Pohlman AS, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet. 2009;373(9678):1874–1882. doi: 10.1016/S0140-6736(09)60658-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ebersoldt M, Sharshar T, Annane D. Sepsis-associated delirium. Intensive Care Med. 2007;33(6):941–950. doi: 10.1007/s00134-007-0622-2. [DOI] [PubMed] [Google Scholar]
- 44.Schefold JC, Bierbrauer J, Weber-Carstens S. Intensive care unit-acquired weakness (ICUAW) and muscle wasting in critically ill patients with severe sepsis and septic shock. J Cachexia Sarcopenia Muscle. 2011;1(2):147–157. doi: 10.1007/s13539-010-0010-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Quinlan N, Marcantonio ER, Inouye SK, et al. Vulnerability: the crossroads of frailty and delirium. J Am Geriatr Soc. 2011;59(Suppl 2):S262–S268. doi: 10.1111/j.1532-5415.2011.03674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ely EW, Gautam S, Margolin R, et al. The impact of delirium in the intensive care unit on hospital length of stay. Intensive Care Med. 2001;27(12):1892–1900. doi: 10.1007/s00134-001-1132-2. [DOI] [PMC free article] [PubMed] [Google Scholar]