Abstract
Low serotonin function is associated with alcoholism, leading to speculation that increasing serotonin function could decrease ethanol consumption. Mice with one or two deletions of the serotonin transporter (SERT) gene have increased extracellular serotonin. To examine the relationship between SERT genotype and motivation for alcohol, we compared ethanol self-administration in mice with zero (KO), one (HET), or two copies (WT) of the SERT gene. All three genotypes learned to self-administer ethanol. The SSRI, fluvoxamine, decreased responding for ethanol in the HET and WT, but not the KO mice. When tested under a progressive ratio schedule, KO mice had lower breakpoints than HET or WT. As work requirements were increased across sessions, behavioral economic analysis of ethanol self-administration indicated that the decreased breakpoint in KO as compared to HET or WT mice was a result of lower levels of unconstrained demand, rather than differences in elasticity, i.e., the proportional decreases in ethanol earned with increasing work requirements were similar across genotypes. The difference in unconstrained demand was unlikely to result from motor or general motivational factors, as both WT and KO mice responded at high levels for a 50% condensed milk solution. As elasticity is hypothesized to measure essential value, these results indicate that KO value ethanol similarly to WT or HET mice despite having lower break points for ethanol.
Keywords: Alcoholism, Behavioral Economics, Serotonin, Serotonin Transporter, Selective Serotonin Reuptake Inhibitor
1.0 INTRODUCTION
Alcoholism is associated with low extracellular serotonin (Sellers et al 1992). Therefore, increasing extracellular serotonin might decrease ethanol intake. Extracellular serotonin can be pharmacologically increased by blocking the serotonin transporter (SERT) using selective serotonin reuptake inhibitors (SSRIs; Blakely & Bauman 2000), and SSRIs are reported to decrease ethanol intake and ethanol maintained behavior (Gardell et al 1997; Ginsburg et al 2005; Lamb & Järbe, 2001; Murphy et al 1988). Extracellular serotonin can also be genetically determined. Mice with one copy of the SERT gene (HET) or lacking SERT (KO) have 5- and 9-fold higher extracellular serotonin levels (Mathews et al., 2004; Shen et al., 2004), thus, ethanol intake and ethanol-maintained behavior may also be decreased by deleting the gene encoding SERT., as While SERT KOs might be expected to consume and work less for ethanol, they should be relatively insensitive to response decreasing effects of SSRIs on ethanol-maintained behavior, as the substrate of SSRI action is missing. Conversely, HETs might be more sensitive to SSRI effects, as their transporter capacity is already reduced.
In the present experiments, we compared unconstrained ethanol consumption in SERT WT, HET and KO mice using both a post-prandial drinking procedure and consumption under a fixed ratio 1 schedule; compared alcohol's ability to maintain behavior in the three genotypes in two ways, by (1) using a within session progressive-ratio schedule, and (2) increasing ratio values across sessions; and compared the effects of fluvoxamine, an SSRI, on ethanol-maintained behavior in the three genotypes.
2.0 MATERIALS & METHODS
2.1 Animals
Twenty-four male mice were used: 8 of each SERT genotype. Mice bred on a congenic C57Bl/6J background were derived from an in house colony established from breeding pairs generously provided by Dr. Dennis Murphy (NIMH) in 1999 (see Bengel et al., 1998). Mice in this colony are backcrossed (WT × KO to yield HET) every 6th generation. Male SERT KO, HET or WT used in the present study came from the 23rd generation of HET × HET matings, following the third backcross. Littermates were raised and housed together in same-sex groups from weaning until the start of this experiment when they were singly housed. Mice were housed in a temperature- and humidity-controlled vivarium, and under a 12 h light/dark cycle (lights on 0600 h). Water was freely available except during experimental sessions (and as outlined below, during drinking induction). Food was limited to 2.5 g rodent chow each day. This food-restriction increased longevity, kept mice at similar weights and made development of ethanol self-administration simpler and its comparison to milk-maintained behavior possible. All procedures conducted on the mice were approved by the local institutional animal care and use committee and were in accordance with the NIH Guide for the Care and Use of Laboratory Animals.
2.2 Apparatus
These experiments were conducted in 8 mouse operant chambers (Med Associates, St. Albans, VT). Chambers had three head entry detectors with a yellow LED light at the rear. Two head entry detectors were located on either side of the front wall of the chamber and had dipper mechanisms. The one on the left was used in these experiments. Chambers were also equipped with a house-light. These chambers were housed inside large fan equipped sound-attenuating chambers. A white noise generator in the room provided masking noise.
2.3 Procedures
2.3.1 Overview
Experimental sessions were conducted each weekday. Initially, mice were trained to drink ethanol solutions using a post-prandial drinking procedure. Once drinking was established, mice were trained to respond for 16% (w/v) ethanol over several sessions. Once responding was maintained under a fixed ratio 20 (FR 20) schedule, mice were placed under progressive ratio schedule that began at FR 5 with the ratio value increasing by 5 following completion of each ratio. Mice were exposed to 16%, 8%, 32% and 16% ethanol under this schedule, though not all mice were exposed to all conditions. Following this, responding for ethanol was re-established using a sucrose fading procedure. Mice were then again exposed to a progressive ratio schedule for 12% ethanol that began at FR 10 with the ratio value increasing by 10 with each ratio completion. The effects of fluvoxamine on this responding were determined. Subsequently, mice were placed on an FR 1 schedule for 12% ethanol. Following ten sessions the FR value was doubled, and this doubling continued every 10th session until responding was substantially decreased. Once responding was substantially decreased, the FR was reset to 1 for 10 sessions. Finally, this procedure of increasing the FR across sessions was repeated using a 50% solution of condensed milk instead of 12% ethanol.
2.3.2 Post-Prandial Drinking
At mid-day mice were fed their daily food ration. Two hours prior, drinking water was removed and one hour before feeding another bottle containing an ethanol solution was placed in the cage and remained available for two hours (i.e., until one hour after food was presented). Over 30 sessions the ethanol concentration was gradually increased from 0% to 16% (w/v).
2.3.3 Progressive Ratio
Experimental sessions were nominally 90 minutes, but ended earlier if no response occurred within 15 minutes of the last response. Completion of the current FR resulted in a 10 second, 0.01 ml volume dipper presentation. When FR contingencies were in effect, the LED in the response hole was on and the house-light was off. When the dipper was up, the LED was off and the house-light was lit. When the session was completed, both were off. Completion of each FR not only resulted in dipper presentation, but also incremented the next FR value. In the first phase of these experiments the starting FR was 5 and this value increased by 5 with each ratio completion. When the session ended because of the programmed session time for 3–5 sessions in a row, the starting FR value was increased by 10. A condition ended when all of the following criteria were met: 1) at least 5 sessions in a row ended because of a pause in responding; and 2) there was no obvious upward or downward trend in responding. When behavior met these criteria (5 WT, 6 HET and 5 KO), testing began with another ethanol concentration [8% (2 WT, 1 HET and 4 KO) then 32% (2 WT, 1 HET and 4 KO) and finally 16% (2 WT and 4 KO) again] with a starting FR of 5. This procedure did not produce stable results and is not reported further. Therefore the procedure was modified to obtain more stable and reliable results. In this second set of progressive ratio experiments, the initial FR was 10 and the FR was increased by 10 with the completion of each FR. Mice were run on this condition until finishing ratio values did not differ by more than 20 over the last five sessions if these were <100 and by no more than 30 if these were ≥100. After behavior was stable, the effects of fluvoxamine were determined. All 24 mice were exposed to this progressive ratio procedure, but one HET died before completing this experiment.
2.3.4 Sucrose Fading
Following initial exposure to the progressive ratio procedure and 32% ethanol concentration, responding by most mice was at low levels. In order to reinvigorate responding, mice were exposed to a sucrose fading procedure (Samson 1986) under which they were initially exposed to ethanol either at 12% or lower concentrations and 12% sucrose under a small FR value. FR, and if needed ethanol concentrations, were increased across sessions, while sucrose concentration was decreased. This continued until stable responding was obtained under an FR 10 and a 12% ethanol concentration and a 0%sucrose concentration.
2.3.5 Fixed Ratio
The FR schedule used was identical in all respects to the progressive ratio schedule except that the FR value was constant throughout a session. In this final experiment FR values were doubled following every 10th session. Once responding was substantially decreased, the FR was returned to 1 for another 10 sessions, the experiment was repeated using 50% condensed milk rather than 12% ethanol. During the experiment with ethanol, one KO died after completing the first FR 1 manipulation, and one HET died during the FR 128 manipulation. Their data are not included in the results. Zeros are imputed for mice averaging less than 1 ethanol delivery on the last 5 sessions of an FR for ethanol deliveries at higher FR values (1 WT for FR 128; 1 HET for FR 32 and higher; and 2 KOs for FR 64 and higher and 3 KOs for FR 128). Fifty percent condensed milk deliveries across ratio values of 1 to 256 were examined in 4 KO and 6 WT mice that completed the ethanol study. All mice earned milk presentations at all FRs studied and thus, no data are imputed.
2.3.6 Drug Administration
Fluvoxamine was a gift of Solvay Pharmaceuticals (Weesp, The Netherlands). It was dissolved in normal saline, and injected i.p. 30 minutes pre-session in a volume of 1.0 ml/100 g. Fluvoxamine doses of 10 and 30 mg/kg were examined. The effects of vehicle, 10 and 30 mg/kg fluvoxamine were determined four times in each mouse responding under the progressive ratio schedule (in one mouse the effects of 30 mg/kg were only determined 3 times, in one mouse the effects of 30 mg/kg was determined 5 times, this occurred for two mice with 10 mg/kg and with one for vehicle).
Ethanol solutions were made by measuring the appropriate volume of room temperature 190 proof alcohol (Decon Labs; King of Prussia, PA) and then adding acidified filtered water to make the appropriate volume of solution. Rats had access to this water outside the experimental sessions. Solutions were then allowed to stand for at least an hour after which additional water was added to bring the solution up to the desired volume. Solutions were kept in a sealed flask at room temperature and were used within a week of preparation.
2.4 Data Analysis
The False Discovery Rate of all sets of comparisons was controlled using the procedure developed by Benjamini and Hochberg (1995). Breakpoints were compared between strains using analysis of variance (ANOVA) followed by the appropriate contrasts. For the effects of fluvoxamine on breakpoint, data were highly skewed as a result of outliers. We examined three ways of dealing with this. First, we used median values. Second, we eliminated data from mice with a last ratio completed < 10 following any vehicle injection, as the skewness and outliers appeared to result from greater sensitivity to receiving an injection. Lastly, we used median values eliminating data from any mouse without a median value of 1 or more ratios completed following vehicle. This approach produced orderly, interpretable data and resulted in eliminating data from 1 WT, 2 HETs, and 1 KO. As fluvoxamine was expected to decrease breakpoint compared to vehicle and these data were relatively non-normal, the effects of fluvoxamine were compared to vehicle using a one-tailed binomial test divided upon and below vehicle values.
For the FR size manipulation, responding at the beginning and end of the experiment under an FR1 and differences in responding at FR1 between genotypes were analyzed using repeated measures ANOVA followed by appropriate contrasts. Responding across ratio values was analyzed using repeated measures ANOVA. To more formally analyze these data across ratio values, we fit the elasticity function proposed by Hursh & Silberberg (2008) to the mean data for each genotype:
Q is the number of dipper presentations earned; P is the FR value, and Q0, α and k are estimated parameters. The equation was fitted using the nl function of STATA and initial estimates of Q0=amount earned at FR 1 (rounded up to the nearest whole integer), and k=4.5 are used. As k is a scaling parameter, and comparisons facilitated by having k constant across groups and k did not differ significantly between groups [WT: 3.77 (95% CL, 3.22–4.31); HET: 3.79 (1.24–6.34); KO: 3.66 (1.63–5.70)], we refit equations setting k to 3.74, the mean value of the three groups. We compared these values using confidence limits for the WT mice.
The effects of the FR manipulation on milk consumption were analyzed similarly to the effects of the FR manipulation on ethanol consumption. k was set to 6 for this analysis.
4 RESULTS
4.1 Post-Prandial Drinking
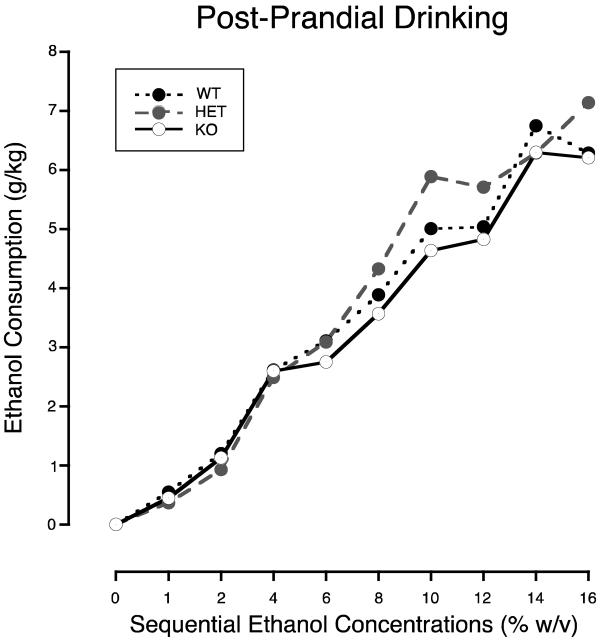
Figure 1 shows that as ethanol concentration increased across days, the amount of ethanol consumed in all three groups increased similarly. At 16% ethanol, WTs drank on average 6.3 (SD=1.2), HETs 7.1 (2.2), and the KOs 6.2 g/kg (1.5).
Figure 1.
Amount of ethanol consumed (g/kg) by WT (solid circles with small dashed line), HET (gray filled circles with dashed line) and SERT KO mice (open circles with solid line) across sequential phases of the post-prandial drinking procedure. Each ethanol concentration was presented for three sequential days. The mean of each individual's median consumption on those three days is plotted. Ethanol was available for one hour before and for one hour after the presentation of the mouse's daily food allotment (2.5 g).
4.2 Progressive Ratio Responding
4.2.1 Breakpoints
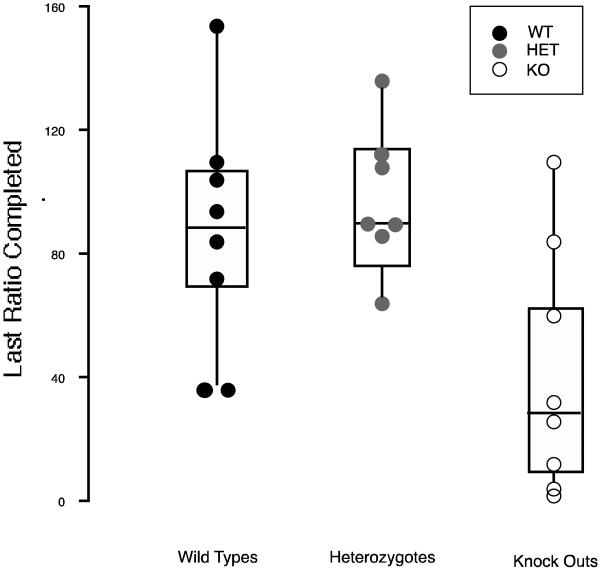
Figure 2 shows a box plot of finishing ratio values for each genotype. These were virtually identical for WTs and HETs, but were significantly less for KOs (ANOVA on genotype F(2,20)=5.53, P<0.05; contrasts between KO & HET (Z=3.10) or KO & WT (Z=2.55) were significant (both P<0.05) while the contrast between the WT & HET was not significant (Z=0.64)).
Figure 2.
Box plots of the last ratio completed by WT (solid circles), HET (gray filled circles) and SERT KO mice (open circles) when responding for 12% (w/v) ethanol under a progressive ratio schedule. Values plotted are means for each mouse for the five sessions in which the stability criteria were initially met. Boxes around points represent the mid-fifty percent values.
4.2.2 Effects of Fluvoxamine on Progressive Ratio Responding
The SSRI, fluvoxamine, as expected was ineffective in SERT KO mice (median number of ratios completed following vehicle, 10 & 30 mg/kg fluvoxamine: 2.0, 2.0, & 3.5). The lowest fluvoxamine dose tested did not decrease the number of ratios completed in either WTs or HETs (6.5 v 6.3 in WT for vehicle v 10 mg/kg and 9.5 v 5.0 in HET), though this effect began to approach significance in HET (P=0.188). The highest dose of fluvoxamine tested (30 mg/kg) significantly decreased the last ratio completed in HETs (median number of ratios completed=3.5; P<0.05) and in WTs (median number of ratios completed=2.0; P<0.05).
4.3 Ethanol Consumption Across Increasing Fixed-Ratio Values
Responding for ethanol under an FR1 was similar at the beginning and end of this experiment, but appeared to differ by genotype (mean (SD) 1st/last: WT: 67.8 (35.2)/83.4 (47.6); HET: 65.5 (32.6)/60.1 (30.6); KO: 39.3 (18.3)/36.9 (18.8)). This was confirmed by a repeated measures ANOVA that found a main effect of genotype (F(2, 17)=2.25, P<0.05), but not of order (F(1,17)=0.10, P>0.10) nor an interaction between genotype and order (F(2,17)=0.63, P>0.10). To explore the genotype effect, we averaged the first and second determination for each mouse and conducted an ANOVA on these data, conducting post-hoc contrasts between genotypes. This showed the difference between the KO and WT mice to be significant (F(1, 17)=7.14, P<0.05), but the differences between the KO and the HET (F(1, 17)=0.76, P>0.10) or the HET and the WT mice (F(1, 17)=2.87, P>0.10) to not be significant.
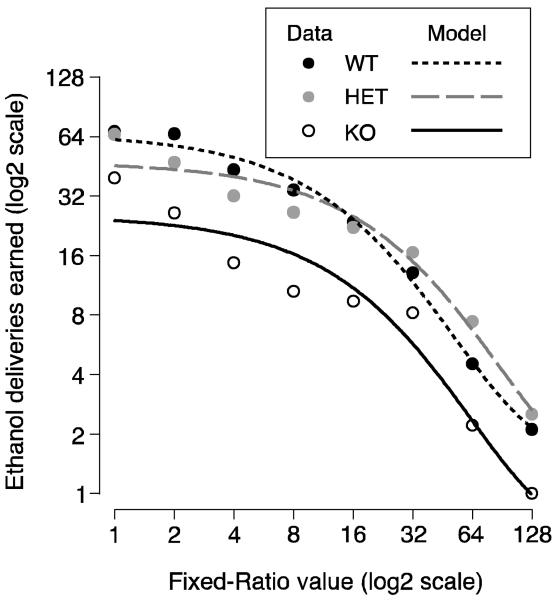
As can be seen in Figure 3, the WT mice continued to earn more ethanol than the KO mice as the FR requirement was increased, but to earn similar numbers of ethanol deliveries as the HET. This was confirmed by a repeated measures ANOVA that found a significant main effect for genotype (F(2, 17)=4.32, P<0.05), FR requirement (F(7, 119)=63.14, P<0.05, with Huynh-Feldt correction) and an interaction between genotype and FR requirement (F(14, 119)=3.47, P<0.05, with Huynh-Feldt correction). The proportional differences in consumption appeared to be relatively constant across FR values as can be seen in Figure 3 by the similar distance between points for KO mice compared to WT or HET mice (equal distances a log scale are equal proportional distances on an arithmetic scale).
Figure 3.
Mean number of ethanol dipper presentations earned at each fixed-ratio value during the last five sessions at this value are plotted against value of the fixed-ratio (both on a log2 scale). The mean data are represented by the circles (WT - solid; HET - gray; KO - open) and the fitted behavioral economic model log(Q)=log(Q0) + k(e−αP − 1) is represented by the lines (WT - short dash; HET – long dash; KO – solid).
The KO mice also tended to stop working for ethanol at lower FR values than the HET or WT mice. For instance, 5 of 7 KO mice averaged completing 1 or fewer ratios at an FR value of 64 or less compare to 1 of 8 WT mice (P=0.04, Fisher's Exact Test).
Behavioral Economic Analysis: The behavioral economic model of Hursh & Silberberg (2008) fit well (WT: R2=0.99; HET: R2=0.96; KO: R2=0.93). The measure of elasticity, α, was not significantly different between WTs and KOs [WT: 0.0196 (95% CL: 0.0153–0.238); HET: 0.0116 (0.0070–0.0161); KO: 0.0160 (.0058–0.0261)]. However, Q0, a measure of unconstrained demand, did differ significantly between WTs and KOs [WT: 66.4 (54.9–77.9); HET: 47.3 (33.0–61.6); KO: 25.6 (12.8–38.5)]. α was smaller in the HETs than the WTs, but α was similar in the HETS compared to the KOs. Q0 in HETs was intermediate between Q0 in WTs and KOs and differed significantly from Q0 in both the WTs and KOs.
4.4 Milk Consumption Across Increasing Fixed-Ratio Values
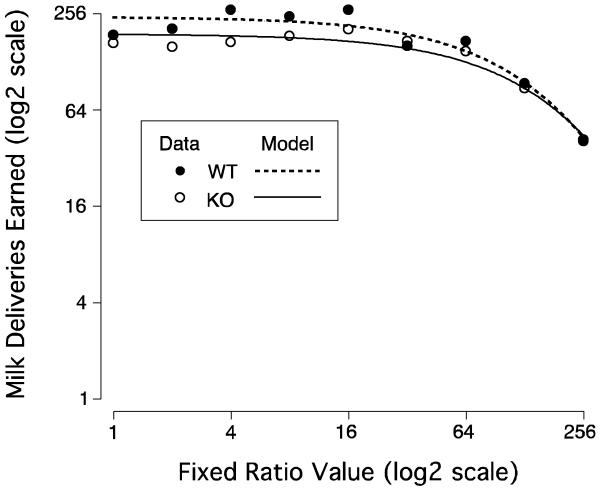
WTs earned an average (SD) of 187.9 (35.7) and KOs an average of 166.7 (28.0) milk presentations at the first FR 1 determination, and an average of 218.7 (33.6) and 165.6 (29.7) presentations respectively at the final FR 1 determination. Repeated measures ANOVA on these data indicated that there was no effect of determination (F(1,8)=1.19, P>0.10) nor was there a determination by genotype interaction (F(1,8)=1.7, P>0.10); however the difference in the number of presentations earned between the two genotypes was significant (F(1,8)=5.36, P<0.05). Additionally, while as can be seen in Figure 4, responding for milk was very similar between the WT and KO mice, repeated measures ANOVA confirms what was seen in the analysis of responding at FR 1 (genotype, F(1,8)=5.45, P<0.05; FR value, F(8,64)=29.27, P<0.05 with Huynh-Feldt correction; and genotype by FR value interaction F(8,64)=2.29, P<0.05 with Huynh-Feldt correction).
Figure 4.
Mean number of 50% condensed milk dipper presentations earned at each fixed-ratio value during the last five sessions at this value are plotted against the value of the fixed-ratio (both on a log2 scale). The mean data are represented by the circles (WT - solid; KO - open) and the fitted behavioral economic model log(Q)=log(Q0) + k(e−αP − 1) is represented by the lines (WT - short dash; KO – solid).
The milk discounting curves for WTs and KOs appeared very similar (Figure 4). However, more formal analysis reiterates the general trend of milk consumption at FR1 and the ANOVA results: in WTs unconstrained demand was higher (Q0 = 241.5 [199.6 – 283.4]) than in KOs (Q0 = 190.2 [165.0 – 215.5]). However, the elasticity was similar in the two genotypes (WT: α = 0.00132 [0.000093 – 0.00171]; KO: α = 0.00109 [0.00081 –0.00138]). The model fit these data well in both genotypes (WT: R2 = 0.93; KO: R2 = 0.94).
5.0 DISCUSSION
All three SERT genotypes worked for and consumed ethanol. Similarly, all three develop an ethanol conditioned place preference (Boyce-Rustay et al 2006). However, SERT KOs earned less ethanol than WTs or HETs. Also, KOs had lower breakpoints for ethanol than WTs or HETs. One possible conclusion from these results is that KOs value ethanol less. However, ethanol consumption in all three genotypes decreased at similar rates as work requirements increased, i.e., ethanol's elasticity was similar in all three genotypes. Elasticity has been argued to measure value (Hursh & Silberberg, 2008). Here, between genotype differences in breakpoint resulted from differences in unconstrained demand, rather than from differences in elasticity.
Under the post-prandial drinking procedure, all three genotypes drank substantial and similar amounts of ethanol. However, when available under an FR 1, KOs earned substantially less ethanol than either WTs or HETs. This continued across all FR values. Further, KOs drink less ethanol than WTs in a two-bottle choice procedure (Kelai et al 2003). The similarity among the three genotypes in ethanol consumption with post-prandial drinking and differences when responding for ethanol or drinking ethanol in a two-bottle choice procedure may reflect increased consumption of fluids generally with food consumption in the post-prandial drinking procedure, rather than consumption controlled by factors specific to the fluid consumed.
When the Fixed Ratio was increased within a session and when it was increased across sessions, ethanol maintained lower breakpoints in KOs than in HETs or WTs. Breakpoints in WTs and HETs were about twice those in KOs. This two-fold difference parallels a similar difference in unconstrained demand. Consumption and breakpoint are both increased by variables thought to increase value, e.g., food deprivation in animals responding for food (Aoyama 2000; Hodos, 1961) or ethanol dependence in animals responding for ethanol (Schulteis et al 1996; Walker & Koob, 2007). Thus, consumption and breakpoint have both been proposed to measure value. In the present experiment, consumption and breakpoint for ethanol were both lower in KOs than in WTs or HETs, suggesting ethanol's value was less for KOs than for either WTs or HETs.
On the other hand, ethanol consumption decreased similarly in all three genotypes with increases in work requirements: note the similar slopes of the elasticity functions in Figure 3. This is not a result of the particular function used, as slopes would be similar if one simply connected the individual data points for each genotype. Further, the amount of ethanol earned by the WTs or HETs was always about double that of KOs at any particular ratio. Hursh & Silberberg (2008) propose elasticity as a measure of value. In the present experiments, elasticity is not equivalent to breakpoint or consumption levels. Ethanol's elasticity was similar across genotypes, but consumption and breakpoint were lower in KOs.
Breakpoint is jointly determined by unconstrained demand and elasticity. In the present experiments, differences in breakpoint were the result of differences in unconstrained demand. This will not always be the case. For instance, the unconstrained demand for food is similar in dopamine D2 receptor KO mice and WT mice, but the elasticity of demand is greater in the KO mice (Soto et al 2011); and thus, the predicted breakpoint for food is lower in the KO mice. Therefore, breakpoint can be modulated by differences in either unconstrained demand or elasticity. The equating of value with elasticity has, however, led to an emphasis on elasticity in thinking about the causes of problem drinking. The present results suggest that unconstrained demand could also play an important causal role in the development of problem drinking. For instance, the KOs' lower unconstrained demand (with similar elasticity) results in them earning less ethanol at all ratio values. Further, the amount of drinking at low cost, but not the elasticity of drinking, was associated with alcohol related problems in college students (McKillop et al 2009). Similarly, in heavy drinking community volunteers, the intensity of drinking, but not its elasticity, is associated with alcohol use disorders (McKillop et al 2010). Thus, the amount of unconstrained drinking may be an equal or greater determinant of problem drinking as elasticity.
Therefore, understanding the determinants of unconstrained demand may be key to understanding the causes of problem drinking. A potentially important determinant of unconstrained demand and problem drinking is ethanol sensitivity. Sons of alcoholics are at increased risk for problem drinking. These men are less sensitive to alcohol induced sway than those at less risk (Schukit 1988), and this decreased sensitivity appears to mediate the development of problem drinking (Schuckit & Smith 1996). However, decreased ethanol sensitivity does not predict ethanol being valued. For instance, ethanol does not reinforce behavior in the less sensitive Short Sleep mice, but does reinforce behavior in the more sensitive Long Sleep mice (Elmer et al 1990). This suggests that decreased ethanol sensitivity does not necessarily increase ethanol value. Rather, decreased ethanol sensitivity may increase the risk of problem drinking by increasing the amount that can be drunk in those whose behavior is reinforced by ethanol, i.e., by increasing unconstrained demand. There are a number of mechanisms by which decreased ethanol sensitivity might increase unconstrained demand. For instance, ethanol's consumption may be limited by its aversive actions; and thus, a decreased sensitivity to its aversive actions may result in greater ethanol consumption (Cunningham 1994). Thus, one could hypothesize the HETs and WTs drank more than the KOs because they were less sensitive to ethanol's aversive effects; and thus, HETs and WTs could drink more before encountering these aversive effects. Another possibility is that the HETs and WTs are less sensitive to ethanol's response impairing effects than KOs. If this is true, then KOs might reach the point where they are unable to respond after fewer ethanol deliveries than either WTs or HETs; and thus, be unable to drink more. Therefore, those more sensitive to some of ethanol effects may be at less risk for problem drinking, because they encounter these consumption limiting effects after consuming less; and this lower consumption puts them at lower risk for problem drinking, rather than because they value alcohol less.
KOs may be more sensitive than WTs to some effects of ethanol, but not others. For example, when given a high-dose of ethanol, KOs slept longer than WTs or HETs (Boyce-Rustay et al 2006; Daws et al 2006). Also, KOs are more sensitive to ethanol induced motor incoordination (Boyce-Rustay et al 2006), though it should be noted that KOs generally perform less well on this measure. Regardless, published data show that KOs are more sensitive to certain ethanol effects, and perhaps this greater sensitivity accounts for their lower unconstrained demand. However, KO and WT mice are similarly sensitive to other ethanol effects. For instance, ethanol-induced hypothermia has been postulated as an aversive effect that might limit ethanol consumption (Cunningham 1994), however ethanol-induced hypothermia is similar across the genotypes (Boyce-Rustay et al 2006).
Presumably, the lower ethanol consumption of the KOs is a result of either higher extracellular serotonin levels or adaptations to these. To the extent that we were able to test it, pharmacologically increasing extracellular serotonin levels by administering fluvoxamine decreased breakpoints for ethanol in HETs and WTs. This decrease was not seen in KOs, which lack SERT and therefore, could not have their serotonin levels increased further by fluvoxamine. Similarly, another SSRI, fluoxetine, decreased ethanol intake in WT, but not SERT KO mice (Kelai et al 2003). These observations parallel reports that in some circumstances SSRIs can decrease problem drinking (Sellers et al 1992).
Some limitations of our work need to be considered. First, the differences among genotypes in unconstrained ethanol consumption could reflect differences in maximal behavioral output. However, our results using milk make this an unlikely explanation. WTs and KOs both worked more for milk than ethanol. Second, differences among genotypes in unconstrained ethanol consumption could reflect differences in fluid consumption. The similar level of milk consumption and the similar (very low) levels of water consumption in the post-prandial drinking procedure before food was available (data not shown) make among genotype differences in baseline fluid consumption an unlikely explanation. Thirdly, the differences in unconstrained demand for ethanol could reflect general motivational differences. While WTs tend to have higher breakpoints than KOs for food (Sanders et al 2007; Trigo et al 2007) or water (Trigo et al 2007), these differences disappear with prolonged training. Further, the similar and much higher unconstrained demand and elasticity for milk in WTs and KOs make this explanation unlikely to account for the between genotype differences in unconstrained ethanol demand. Fourth, our findings are clearly limited in the sense that these findings may only apply to adult male mice. Adolescent mice consume more alcohol than adult mice (Tambour et al 2008), therefore between genotype differences we report here for adults, may be diminished in adolescent mice. Similarly, female mice consume more ethanol than male mice (Middaugh et al 1999; Tambour et al 2008), and there are reports suggesting that the difference in unconstrained demand between genotypes is less in females than in males (compare Kelai et al 2003 using males to Boyce-Rustay et al 2006 using females). Interestingly, the relationship between low serotonin function and risk for problem drinking results mostly from studies in adult males (e.g., Rausch et al 1991).
In conclusion, several findings are clear. The breakpoint for ethanol was lower in KOs than in either WTs or HETs. This likely resulted from the lower unconstrained ethanol demand by KOs than WTs or HETs, rather than differences in elasticity. This difference in unconstrained demand and breakpoint was not a result of a general inability or willingness of KOs to respond at higher levels, for when milk was substituted for ethanol, KOs responded at high levels similar to those of WTs. The observed differences with ethanol may, however, be a result of KOs being more sensitive to some ethanol effects than WTs or HETs (Boyce-Rustay et al 2006; Daws et al 2006). Interestingly, these results parallel human studies showing that alcohol-related problems correlate with higher levels of unconstrained demand, but not with elasticity (McKillop et al 2009; McKillop et al 2010), and that susceptibility to developing alcoholism is associated both with a decreased sensitivity to some ethanol effects (Schuckit 1988) and lower serotonin function (Sellers 1992).
Acknowledgements
We thank Gerrado Martinez for his assistance in conducting these experiments. This work was supported by grants from the National Institutes on Health (RJL: AA012337; LCD: MH064489).
References
- Aoyama K. Effects of hunger state on within-session response decreases under CRF schedule. Learning Motivation. 2000;31:1–20. [Google Scholar]
- Bengel D, Murphy DL, Andrews AM, Wichems CH, Feltner D, Heils A, Mossner R, Westphal H, Lesch KP. Alter brain serotonin homeostasis and locomotor insensitivity to 3,4-methylenedioxymethamphetamine (`ecstasy') in serotonin transporter-deficient mice. Mol Pharmacol. 1998;53:649–655. doi: 10.1124/mol.53.4.649. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Statist. Soc. B. 1995;57(1):289–300. [Google Scholar]
- Blakely RD, Bauman AL. Biogenic amine transporters: regulation in flux. Cur Op Neurobiol. 2000;10:328–336. doi: 10.1016/s0959-4388(00)00088-x. [DOI] [PubMed] [Google Scholar]
- Boyce-Rustay JM, Wiedholz LM, Millstein RA, Caroll J, Murphy DL, Daws LC, Holmes A. Ethanol-related behaviors in serotonin transporter knockout mice. Alc Clin Exp Res. 2006;30:1957–1965. doi: 10.1111/j.1530-0277.2006.00241.x. [DOI] [PubMed] [Google Scholar]
- Cunningham CL. Modulation of ethanol reinforcement by conditioned hypothermia. Psychopharm. 1994;115:79–85. doi: 10.1007/BF02244755. [DOI] [PubMed] [Google Scholar]
- Daws LC, Montañez S, Munn JL, Owens WA, Baganz NL, Boyce-Rustay JM, Millstein RA, Wiedholz LM, Murphy DL, Holmes A. Ethanol inhibits clearance of serotonin by a serotonin transporter-independent mechanism. J Neurosci. 2006;26:6431–6438. doi: 10.1523/JNEUROSCI.4050-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmer GI, Meisch RA, Goldberg SR, George FR. Ethanol self-administration in Long Sleep and Short Sleep mice indicates reinforcement is not inversely related to neurosensitivity. J. Pharmacol. Exp. Ther. 1990;254(3):1054–1062. [PubMed] [Google Scholar]
- Gardell LR, Whalen CA, Chattophadyay S, Cavallaro CA, Hubbell CL, Reid LD. Combination of naltrexone and fluoxetine on rats' propensity to take alcoholic beverage. Alc Clin Exp Res. 1997;21:1435–1439. [PubMed] [Google Scholar]
- Ginsburg B, Koek W, Javors MA, Lamb RJ. Effects of fluvoxamine on a multiple schedule of ethanol- and food- maintained behavior in two rat strains. Psychopharm. 2005;180:249–257. doi: 10.1007/s00213-005-2156-z. [DOI] [PubMed] [Google Scholar]
- Hodos W. Progressive ratio as a measure of reward strength. Sci. 1961;134:943–944. doi: 10.1126/science.134.3483.943. [DOI] [PubMed] [Google Scholar]
- Hursh SR, Silberberg A. Economic demand and essential value. Psyc Rev. 2008;115:186–198. doi: 10.1037/0033-295X.115.1.186. [DOI] [PubMed] [Google Scholar]
- Kelaï S, Aïssi F, Lesch KP, Cohen-Salmon C, Hamon M, Lanfumey L. Alcohol intake after serotonin transporter inactivation in mice. Alc. Alcoholism. 2003;38:386–389. doi: 10.1093/alcalc/agg095. [DOI] [PubMed] [Google Scholar]
- Lamb RJ, Järbe TUC. Effects of Fluvoxamine on Ethanol-Reinforced Behavior in the Rat. J Pharmacol Exp Ther. 2001;297:1001–1009. [PubMed] [Google Scholar]
- Mathews TA, Fedele DE, Coppelli FM, Avila AM, Murphy DL, Andrews AM. Gene dose-dependent alterations in extraneuronal serotonin but not dopamine in mice with reduced serotonin transporter expression. J Neurosci Meth. 2004;140:169–181. doi: 10.1016/j.jneumeth.2004.05.017. [DOI] [PubMed] [Google Scholar]
- McKillop J, Murphy JG, Tidey JW, Kahler CW, Ray LA, Bickel WR. Latent structure of facets of alcohol reinforcement from a behavioral economic demand curve. Psychopharm. 2009;203:33–40. doi: 10.1007/s00213-008-1367-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKillop J, Miranda R, Monti PM, Ray L, Murphy JG, Rohsenow DJ, McGeary JE, Swift RM. Alcohol demand, delayed reward discounting, and craving in relation to drinking and alcohol use disorders. J Ab Psyc. 2010;119:106–114. doi: 10.1037/a0017513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisch RA. Relative persistence of behavior: a fundamental measure of relative reinforcing effects. Exp Clin Psychopharm. 2000;8:333–349. doi: 10.1037//1064-1297.8.3.333. [DOI] [PubMed] [Google Scholar]
- Middaugh LD, Kelley BM, Bandy A-L, McGroarty KK. Ethanol consumption by C57BL/6 mice: influence of gender and procedural variables. Alc. 1999;17:175–183. doi: 10.1016/s0741-8329(98)00055-x. [DOI] [PubMed] [Google Scholar]
- Murphy JM, Waller MB, Gatto GJ, McBride WJ, Lumeng L, Li T-K. Effects of fluoxetine on the intragastric self-administration of ethanol in the alcohol preferring P line of rats. Alc. 1988;5:283–286. doi: 10.1016/0741-8329(88)90066-3. [DOI] [PubMed] [Google Scholar]
- Nevin JA, Grace RC. Behavioral momentum and the law of effect. Behav Brain Sci. 2000;23:73–90. doi: 10.1017/s0140525x00002405. [DOI] [PubMed] [Google Scholar]
- Rausch JL, Monteiro MG, Schuckit MA. Platelet serotonin uptake in men with family histories of alcoholism. Neuropsychopharm. 1991;4(2):83–86. [PubMed] [Google Scholar]
- Samson HH. Initiation of ethanol reinforcement using a sucrose-substitution procedure in food- and water-sated rats. Alc Clin Exp Res. 1986;10:436–442. doi: 10.1111/j.1530-0277.1986.tb05120.x. [DOI] [PubMed] [Google Scholar]
- Sanders AC, Hussain AJ, Hen R, Zhuang X. Chronic blockade or constitutive deletion of the serotonin transporter reduces operant responding for food reward. Neuropsychopharm. 2007;32:2321–2329. doi: 10.1038/sj.npp.1301368. [DOI] [PubMed] [Google Scholar]
- Sellers EM, Higgins GA, Sobell MB. 5-HT and alcohol abuse. Trends Pharmacol Sci. 1992;13:69–75. doi: 10.1016/0165-6147(92)90026-3. [DOI] [PubMed] [Google Scholar]
- Schukit MA. Reactions to alcohol in sons of alcoholics and controls. Alc Clin Exp Res. 1988;12:465–470. doi: 10.1111/j.1530-0277.1988.tb00228.x. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL. An 8-Year follow-up of 450 sons of alcoholic and control subjects. Arch Gen Psych. 1996;53:202–210. doi: 10.1001/archpsyc.1996.01830030020005. [DOI] [PubMed] [Google Scholar]
- Schulteis G, Hyytiä P, Heinrichs SC, Koob GF. Effects of chronic ethanol exposure on oral self-administration of ethanol or saccharin by Wistar rats. Alc Clin Exp Res. 1996;20:164–171. doi: 10.1111/j.1530-0277.1996.tb01060.x. [DOI] [PubMed] [Google Scholar]
- Shen H-W, Hagino Y, Kobayashi H, Shinohara-Tanaka K, Ikeda K, Yamamoto H, Yamamoto T, Lesch K-P, Murphy DL, Hall FS, Uhl GR, Sora I. Regional differences in extracellular dopamine and serotonin assessed by in vivo microdialysis in mice lacking dopamine and/or serotonin transporters. Neuropsychopharm. 2004;29:1790–1799. doi: 10.1038/sj.npp.1300476. [DOI] [PubMed] [Google Scholar]
- Tambour S, Brown LL, Crabbe JC. Gender and age at drinking onset affect voluntary alcohol consumption, but neither the alcohol deprivation effect nor the response to stress in mice. Alc Clin Exp Res. 2008;32:2100–2106. doi: 10.1111/j.1530-0277.2008.00798.x. [DOI] [PubMed] [Google Scholar]
- Trigo JM, Renoir T, Lanfumey L, Hamon M, Lesch K-P, Robledo P, Maldonado R. 3,4-methylenedioxymethamphetamine self-administration is abolished in serotonin transporter knockout-mice. Biol Psych. 2007;62:669–679. doi: 10.1016/j.biopsych.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Walker BM, Koob GF. The gamma-aminobutyric acid-B receptor antagonist baclofen attenuates responding for ethanol in ethanol-dependent rats. Alc Clin Exp Res. 2007;31:11–18. doi: 10.1111/j.1530-0277.2006.00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]