Abstract
Androgen Receptor (AR), a steroid hormone receptor, is critical for prostate cancer growth. However, activation of AR by androgens can also lead to growth suppression and differentiation. Transcriptional cofactors play an important role in this switch between proliferative and anti-proliferative AR target gene programs. TBLR1, a core component of the nuclear receptor corepressor (NCoR) complex, shows both co-repressor and co-activator activities on nuclear receptors, but little is known about its effects on AR and prostate cancer. We characterized TBLR1 as a coactivator of AR in prostate cancer cells and the activation is both phosphorylation and 19S proteosome dependent. We showed that TBLR1 physically interacts with AR and directly occupies the androgen response elements of affected AR target genes in an androgen-dependent manner. TBLR1 is primarily localized in the nucleus in benign prostate cells and nuclear expression is significantly reduced in prostate cancer cells in culture. Similarly, in human tumor samples, the expression of TBLR1 in the nucleus is significantly reduced in the malignant glands compared to the surrounding benign prostatic glands (p<0.005). Stable ectopic expression of nuclear TBLR1 leads to androgen-dependent growth suppression of prostate cancer cells in vitro and in vivo by selective activation of androgen regulated genes associated with differentiation (e.g. KRT18) and growth suppression (e.g. NKX3.1), but not cell proliferation of the prostate. Understanding the molecular switches involved in the transition from AR dependent growth promotion to AR dependent growth suppression will lead to more successful prostate cancer treatments.
Keywords: prostate cancer, androgen receptor, tumor suppressor, corepressor, coactivator
INTRODUCTION
It has long been known that androgens are critical in the growth and progression of prostate cancer (Dehm and Tindall 2006). Androgens signal through the Androgen Receptor (AR), a member of the steroid receptor family of transcription factors that plays an important role in the regulation of genes controlling growth suppression/differentiation and growth of prostate cells. Androgens activate AR, facilitating its translocation to the nucleus, binding at androgen response elements, and interacting with cofactors (coactivators or corepressors) leading to the activation or repression of AR target genes. Although androgen activation leads to increased proliferation in the prostate, activation by androgen can also lead to differentiation and growth suppression (Whitacre, et al. 2002; Yuan, et al. 1993). The specific mechanism responsible for this switch is not well understood, although transcriptional cofactors have been shown to be important in the activation and repression of AR target genes (Gelmann 2002; Heinlein and Chang 2002; Janne, et al. 2000). The presence or absence of these cofactors may control the balance between proliferation and differentiation (Ligr, et al. 2010; Peng, et al. 2008a). ARA70α and p44/Mep50/WDR77 are both AR coactivators that act as androgen dependent tumor suppressors.
Transducin beta like related protein 1 (TBLR1), a transcriptional cofactor, was initially identified as a core component of the nuclear receptor corepressor (NCoR) complex and also coimmunoprecipitates with silencing mediator of retinoic acid and thyroid receptor (SMRT) (Tomita, et al. 2004). The NCoR complex also contains closely related protein TBL1X, and GPS2, IR10, and HDAC3 (Yoon et al. 2003). The NCoR complex is responsible for repression of many different transcription factors, including AR and other nuclear hormone receptors (NHR) (Burke and Baniahmad 2000).
As an intrinsic component of the NCoR complex, TBLR1 has been shown to have essential functions as a corepressor. TBLR1 is necessary for targeting of the NCoR complex to histones H2B and H4 and preferentially binds to acetylated histones, allowing for repression via deacetylation by HDAC3 (Yoon, et al. 2005). TBLR1 also has been shown to be necessary for activation of liganded NHRs. Removal of TBLR1 by single cell microinjection of TBLR1 antibodies leads to repression of nuclear receptor transcription by β-gal reporter assay including Thyroid receptor (TR), Estrogen receptor (ER), Retinoic acid receptor (RAR), and Peroxisome proliferator-activated receptor gamma (PPARγ). This activation is mediated through NCoR and SMRT, as removal of these proteins by antibody injection or siRNA rescues the repressive effect. TBLR1, an F-box like domain containing protein, has been shown to recruit ubiquitin ligases to the NHR promoter and target NCoR for ubiquitination and dismissal, allowing for the binding of coactivators (Perissi, et al. 2004). Additionally, TBLR1 has been shown to be phosphorylated in 293T cells by PKCδ and the ability to activate NHR transcription by TBLR1 is dependent on this phosphorylation (Perissi, et al. 2008). Recent evidence has also shown that TBLR1 is sumoylated and this sumoylation is required for activation of Wnt target genes through beta-catenin (Choi, et al. 2011).
In this study, we investigate the role of TBLR1 in the modulation of AR mediated transcription and prostate cancer growth. We show that TBLR1 is a coactivator of AR and acts as a tumor suppressor in prostate cancer, consistent with its decreased nuclear expression in human prostate cancer compared to benign glands in clinical samples by immunohistochemistry. TBLR1 selectively activates AR target genes important for growth suppression and differentiation but not pro-proliferative AR target genes.
MATERIALS AND METHODS
Cell Lines and Culture
LNCaP, PC3, and 293T cells were procured from ATCC (Manassas, VA, USA) with documentation of authentication by STR analysis. RC165, an immortalized benign prostate cell, was from Dr. J. Rhim lab (Uniformed Services University of the Health Sciences) (Gu, et al. 2005). LNCaP-AI, an androgen-independent derivative of LNCaP, was from Dr. A. Ferrari lab (New York University Cancer Institute) (Gao, et al. 1999). PC3-AR was from Dr. Z. Wang lab (MD Anderson Cancer Center) (Peng, et al. 2008b). RC-165, RC-170, LNCaP-AI, and PC3-AR were all previously used in publications from our lab (Li, et al. 2009; Ligr, et al. 2012; Peng et al. 2008b). Passages from 5 to 20 of each cell line were used for experiments. LNCaP, LNCaP-AI, PC3, RC-165, RC-170 and 293T cells were maintained in regular medium [RPMI medium 1640 (High glucose DMEM for 293T) plus 10% FBS], androgen-free medium (phenol-free RPMI medium with 10% charcoal-treated FBS), or androgen media (androgen-free medium with 10 nM R1881). Cell lines were maintained at 37°C with 5% CO2.
Luciferase assay
Luciferase assays were performed (Peng et al. 2008a) with Lipofectamine (Invitrogen) with 4XARE reporter, 10 ng of pCDNA-AR, 100 ng of reporter, 1 ng of pR-LUC internal control plasmids, and varying amounts of pCDNA TBLR1 plasmid or mutants. Cells were treated with androgen media for 24 hours prior to reading. For the appropriate experiments, cells were also treated with 1 μM Rottlerin or 1 μM MG132 for 24 hours prior to reading. Dual-Luciferase Reporter Assay System (E1910, Promega) was used according to manufacturer’s instructions.
Co-immunoprecipitation
Immunoprecipitation was performed using either anti-AR, anti-TBLR1 or M2FLAG agarose antibodies to precipitate complexes. Complexes were incubated overnight at 4°C. Anti-AR/TBLR1 complexes were then incubated with protein G beads for 4 hours at 4°C. The beads/agarose were washed three times with lysis buffer and eluted with 2X Laemmi loading buffer at 100°C for 5 minutes.
Whole cell lysates, cell fractionation, and immunoblot analysis
Whole-cell extracts were prepared from LNCaP, RC165, and PC3 cells containing NLSTBLR1, pBabe, or native in RIPA buffer (50mM Tris-HCl ph 8.0, 150 mM NaCl, 1% NP-40, 1mM EDTA, 0.1% SDS). For cell fractionation, cells were lysed with cytoplasmic buffer (10mM HEPES ph 7.6, 50mM NaCl, 0.5M sucrose, 1mM DTT, 5mM MgCl2, 0.1% Triton X-100). After centrifugation, the supernatant was the cytoplasmic fraction and the nuclear pellet was washed 3 times with cytoplasmic buffer followed by lysis with RIPA buffer. Extracts were subjected to electrophoresis on SDS/PAGE and then transferred to nitrocellulose membranes for immunoblot analysis as described (Li et al. 2009) with antibodies for TBLR1 and ERK2 (Santa Cruz antibodies), beta-actin and Anti-FLAG M2 (Sigma), H2B and GAPDH (Cell Signaling), KRT18, NKX3.1, p21, p27, HUS1, and JAG1 (Abcam).
Immunofluorescence microscopy
Cells were fixed in 4% PFA for 20 minutes and permeabilized in 1:1 Methanol:Acetone for 20 minutes. Cells were incubated in 1:100 TBLR1 antibody in 10% sheep serum overnight, washed with PBS, and incubated in anti-mouse IgG-Cy2-conjugated antibodies (Molecular probes 1:500 in 10% sheep serum) for 1 hour, washed with PBS and counterstained with DAPI or Hoescht for 2 minutes as previously described (Li et al. 2009).
TMA Construction and IHC
The study protocol was approved by the New York University institution review board. An IHC study with antibody against TBLR1 was used to characterize the expression pattern of TBLR1 in prostate cancer. Whole tissue sections from thirty cases of prostatic adenocarcinoma were studied. TBLR1 nuclear and cytoplasmic expression was compared between malignant glands and the surrounding benign prostatic glands on the same slide. The intensity of nuclear and cytoplasmic TBLR1 expression were scored semi-quantitatively; 0 as negative, 1 as weak, 2 as moderate and 3 as strong expression. The score represents the overall level of TBLR1 immunoreactivity including staining intensity and the percentage of positive cells. The nuclear staining and cytoplasmic staining were scored separately. TBLR1 nuclear and cytoplasmic expression pattern was studied in 86 samples of prostatic adenocarcinoma on a tissue microarray (TMA). Each sample is represented by four 0.6 mm cores on the TMA. The samples on the TMA were stratified by various clinicopathological factors including age at diagnosis, preoperative PSA, grade and stage.
Construction of Retroviral pBabe Vectors Expressing NLSTBLR1 to construct stable cell lines
We created a TBLR1 fusion protein in which the strong NLS PKKKRKV was fused to the N terminus of TBLR1 to create NLSTBLR1. A retroviral-based mammalian expression vector, pBabe, was used to stably express NLSTBLR1 proteins in LNCaP cells as described previously (Peng et al. 2008a). Briefly, Phoenix A amphotropic packaging cells (American Type Culture Collection) were transfected with NLSTBLR1, and pBabe retroviral constructs to produce virus. The virus-containing supernatant was collected by centrifugation and filtered before retroviral infection. Stable cell lines were selected in 1μg/mL puromycin.
Cell Proliferation Assays
For cell proliferation assay by WST-1 (Roche), cells (1 × 104) were plated into 24-well plates and measured at A450 every other day. For cell proliferation assays, cells (4 x 104) were plated into 6-well plates and counted with a hemocytometer every other day as previously described (Peng et al. 2008a).
Flow cytometry and apoptosis assay
The cells were grown in 6 well plates and were dissociated by trypsin, resuspended in HBSS, and fixed in ice cold 70% ethanol. Cells were incubated in propidium iodide/RNAse solution (1 mg propidium iodide, 10 mg EDTA, 250 μL Igepal, 1 ml of 10mg/ml of RNAse dissolved in 50 ml of PBS) at 37 °C for 2 hours. The cell cycle analysis was performed on a FACSC flow cytometer (BD Biosciences) and analyzed using Weasel software (The Walter and Eliza Hall Institute of Medical Research, Melbourne, Australia). For measurement of apoptosis, Promega Apo-ONE homogeneous caspase 3/7 assay was used as per manufacturer. Cells were incubated in 10nM R1881 media for 24 hours prior to addition of reagent for 18 hour incubation.
RNA isolation and qRT-PCR
Total RNA was extracted from the cell lines using RNAqueous-4PCR kit (AM1914, Ambion). RetroScript kit (AM1710, Ambion) was used for cDNA synthesis with isolated RNA as template, according to manufacturer’s instructions. RNA was isolated from LNCaP NLSTBLR1 and LNCaP pBabe cells after 48 hours of hormone free incubation, followed by 24 hours of 10nM R1881 stimulation. Primers for qPCR were kindly donated by Dr. Susan Logan as reported previously (Nwachukwu, et al. 2009). PCR was performed using the BioRad CFX96 machine and Biorad iQ SYBR Green supermix.
Gene knockdown by siRNA
siRNAs for AR, TBLR1, and control siRNA were purchased from Ambion and transfected with HiperFect Transfection Reagents (Qiagen) 48 h before immunoblot analysis of whole-cell extract, RNA extraction, or cell kinetic studies.
Anchorage-independent growth
LNCaP NLSTBLR1 and pBabe control were seeded into 0.35% agarose media, containing 10% charcoal stripped FBS with 10nM R1881 or ethanol vehicle at 4 x 104 cells per 60mm plate. Fresh 0.35% agarose media was added every 3 days and colonies were allowed to grow for three weeks as previously described (Cai, et al. 2008). Average number of colonies of three duplicate plates is displayed. Pictures were taken of representative colonies in each condition.
Chromatin immunoprecipitation
LNCaP cells were grown in androgen-free media for 72 hours prior to 4 hours of stimulation in 10 nM R1881 or ethanol vehicle. Cells were cross-linked for 10 minutes in 1% formaldehyde solution, followed by quenching with 0.125M glycine. Cells were washed in PBS and lysed in 0.5% NP-40, 0.25% Triton X-100, 50mM HEPES pH 7.5, 10% glycerol, 140mM NaCl, 1mM EDTA. Nuclei were resuspended in 50mM Tris-HCl pH 8, 10mM EDTA, 1% SDS and sonicated 4 times for 10 seconds each. 25 μg of chromatin was used for each IP reaction. 2 μg of TBLR1, AR, or rabbit IgG antibody was used and incubated overnight at 4 °C. Samples were incubated with Protein G beads for 4 hours at 4 °C, and washed 5 times in wash buffer. Immunoprecipitated complexes containing DNA were eluted in 1% SDS Tris buffer and reverse crosslinked at 65 °C overnight. Quantitative PCR analysis was performed. The primer sets used were: NKX3.1 ARE I: F (GATGGGTGGGAGGAGATGA) R (TGTCTTGGACAAGCGGAG) NKX3.1 ARE II: F (GGTTCTGCTGTTACGTTTG), R (CTTGCTTGCTCAGTGGAC), HUS1 ARE: F (CTGCTGCTTCTCCTGCTTTT), R (CCACAGAGACCAGGGTGAGT), GAPDH 3′UTR: F (ATGGTTGCCACTGGGGATCT) R (TGCCAAAGCCTAGGGGAAGA).
Nude Mouse Xenografts
Male nude mice (5 weeks old) were purchased from NCI and maintained in accordance with the Institutional Animal Care and Use Committee-approved protocol. Ten million cells were used to perform subcutaneous injection into the flank region of the mice with Matrigel ECM (reconstituted basement membrane) to support tumor growth. Each experimental group contained 10 mice. Tumors were measured with calipers two times per week as previously described (Ligr et al. 2010). At the end of the experiment, tumors were removed and weighed.
Site-directed mutagenesis
Point mutations in pCDNA TBLR1 and pBabe NLSTBLR1 were made using the Stratagene Quikchange XL (200521) mutagenesis kit as previously described (Cai et al. 2008). Mutation 1 is S123 to A123. Mutation 2 is S199, T203, S204 to A199, A203, A204.
Statistical Analysis
Statistical analyses of the above results were performed by Student’s t test. * denotes p < 0.05, ** denotes p < 0.01, and *** denotes p < 0.001. Differences are considered statistically significant if p < 0.05.
RESULTS
TBLR1 functions as AR coactivator in prostate cancer cells
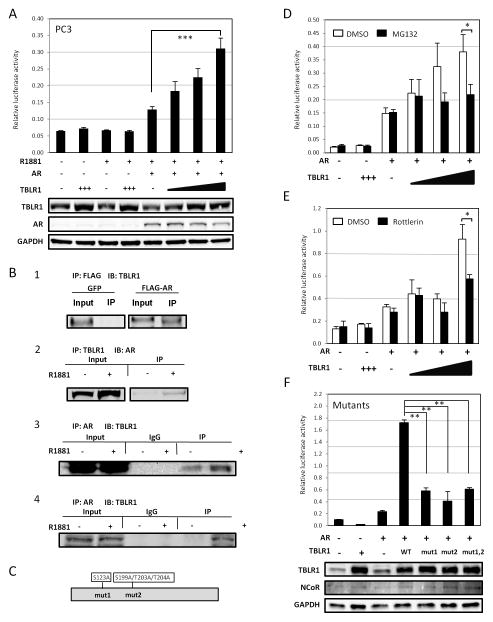
Although initially identified as a core component of the NCoR corepressor complex and necessary for repression of Thyroid Receptor, TBLR1 has also been found to be important in the activation of several transcription factors, including but not limited to, RAR, PPARgamma, and NFkappaB (Perissi et al. 2004). We examined the function of TBLR1 on AR mediated transcription using a dual luciferase assay, with a luciferase reporter containing four androgen response elements (4XARE) and pRL renilla internal control reporter in PC3 (AR negative) prostate cancer cells in the presence of synthetic androgen, R1881. In the absence of androgen or AR, increased TBLR1 had no effect on reporter activity. However, after transfection with AR, TBLR1 lead to up to 2.5 fold increase in AR activation in a dose-dependent manner (Figure 1A).
Figure 1. TBLR1 is a coactivator of AR in prostate cells.
A. TBLR1 acts as a coactivator for AR in PC3 prostate cancer cells in a dose-dependent manner in the presence of 10nM R1881 by dual luciferase assay with a 4X androgen response element (ARE) luciferase reporter plasmid. B. (Panel 1) Coimmunoprecipitation of AR and TBLR1. TBLR1 physically interacts with AR in 293T cells transfected with pCDNA-AR plasmid. (Panel 2) Coimmunoprecipitation with TBLR1 antibody shows AR interaction with TBLR1 in an androgen-dependent manner in LNCaP cells overexpressing NLSTBLR1, and (Panel 3) in reciprocal by coimmunoprecipitation with AR antibody. (Panel 4) Coimmunoprecipitation with AR antibody confirms endogenous interaction between AR and TBLR1 in LNCaP-AI cells. C. Diagram of previously identified phosphorylation sites important for coactivator activity in TBLR1. D. Treatment with the proteosome inhibitor MG132 reduces the activation ability of TBLR1 on AR mediated transcription in the presence of 10nM R1881. E. Treatment with the PKC inhibitor Rottlerin also reduces the ability of TBLR1 to activate AR in the presence of 10nM R1881. F. Mutant TBLR1 constructs, mut1 with S123A, mut2 with S199A/T203A/T204A, or double mutants show reduced activation ability on AR mediated transcription in the presence of 10nM R1881. All experiments were performed in triplicate.
We next determined if there was a physical interaction between AR and TBLR1. First, we overexpressed a FLAG tagged AR plasmid or a GFP control plasmid in 293T cells. Using FLAG antibody to immunoprecipitate, and immunoblotting for TBLR1 we identified an interaction between AR and TBLR1 (Figure 1B, panel 1). Next, to confirm this interaction in prostate cancer cells we used AR-positive LNCaP cells stably overexpressing NLSTBLR1 (TBLR1 with a nuclear localization sequence tag). We immunoprecipitated using TBLR1 antibody and immunoblotted for AR. The cells were grown in androgen-free media for 48 hours prior to stimulation with 10nM R1881 or ethanol vehicle for 6 hours. We observed interaction between the two proteins in prostate cancer cells in the presence of R1881 (Figure 1B, panel 2). Additionally, we performed the experiment in reciprocal manner, using AR antibody to immunoprecipitate and TBLR1 antibody to probe to confirm the physical interaction (Figure 1B, panel 3). Finally, to test this interaction in an endogenous system without any overexpression, we used the LNCaP-AI cell line. LNCaP-AI cells are an androgen-independent derivative of LNCaP cells that still express AR and are sensitive to androgens. Immunoprecipitating with AR antibody and immunoblotting for TBLR1, we were also able to observe the interaction between TBLR1 and AR in an androgen-dependent manner (Figure 1B, panel 4).
Post-translational modifications important for TBLR1 activity on AR mediated transcription
Several posttranslational modifications affect TBLR1 activity (Perissi et al. 2004; Perissi et al. 2008). The ability of TBLR1 to activate RAR is both 19S proteosome dependent and PKCδ phosphorylation dependent. Phosphorylation sites of TBLR1 are marked on the diagram in figure 1C. Upon phosphorylation at S123 and/or S199/T203/T204, TBLR1 targets NCoR for ubiquitination and dismissal in the activation of RAR (Perissi et al. 2008). We tested if this was also true in the case of TBLR1 acting as a coactivator of AR in prostate. In the presence of a specific 19S proteosome inhibitor, MG132, a specific PKC inhibitor, Rottlerin, or mutant TBLR1 constructs, S123A, S199A/T203A/T204A, or the double mutant, TBLR1 activation of AR was diminished up to three fold (Figure 1D,E,F). We found that mutation of site 1, site 2, or both had the same diminished transcriptional effect, suggesting that both sites are involved in transcriptional activation. It is important to note that the phospho-mutant TBLR1 plasmids did retain partial activation ability. For Figure 1F, we also performed a parallel western blot to check overall NCoR levels and confirm TBLR1 overexpression after transfection with wildtype TBLR1 and the mutant constructs. However, we found no variations in total NCoR levels among each condition.
Sumoylation is another posttranslational modification reported to affect TBLR1 activity (15). However, the mutation of the critical site for sumoylation K497 did not affect its AR mediated transcriptional coactivator activities in our luciferase assay (Supplemental Figure 1A).
TBLR1 expression and subcellular localization in prostate cancer
Altered cofactor expression and localization have been shown to correlate with prostate cancer (Ligr et al. 2010; Peng et al. 2008a). Here, we examined the expression levels and intracellular localization of TBLR1 in benign and malignant prostate cell lines and human tissue.
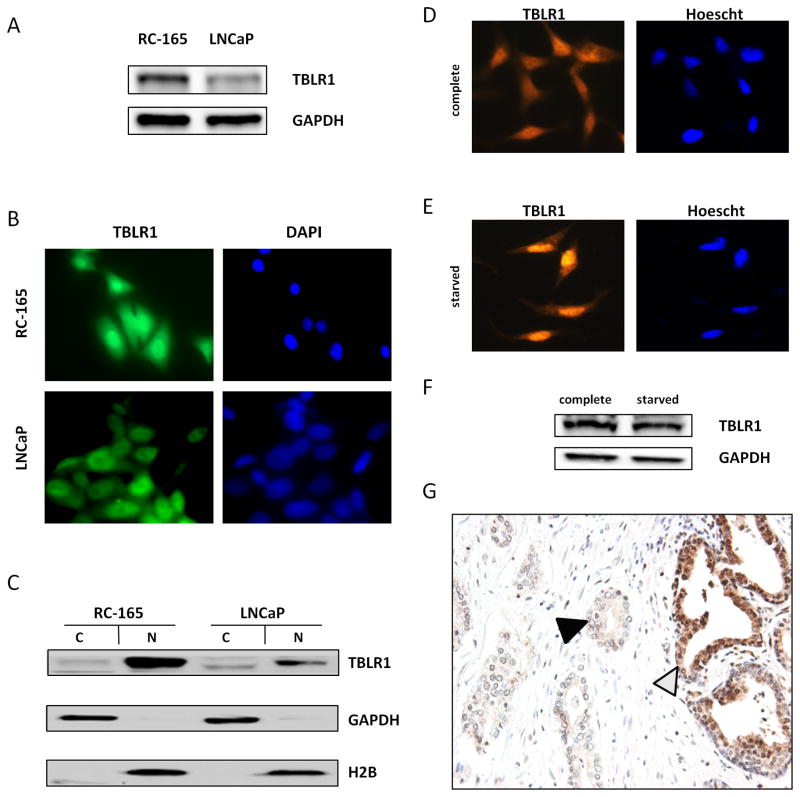
The level of expression in the benign prostate cell line, RC-165 (Gu et al. 2005), and the malignant prostate cell line, LNCaP were compared. TBLR1 protein expression in LNCaP was lower than RC-165 (Figure 2A). By immunoflouresence, we showed TBLR1 was primarily localized in the nucleus of RC-165 but distributed in both the cytoplasm and nucleus in LNCaP cells (Figure 2B). We confirmed these results by western blot with cellular fractionation (Figure 2C). To further confirm variable levels of nuclear TBLR1 in benign versus malignant prostate cancer cells, we also tested protein levels in two additional androgen-sensitive cell lines, benign RC-170 cells and malignant LNCaP-AI cells relative to LNCaP cells with cell fractionation followed by western blot (Supplemental Figure 2A). Consistently, benign RC-170 cells showed high nuclear TBLR1 and malignant LNCaP-AI showed low nuclear TBLR1 levels.
Figure 2. Expression of TBLR1 in prostate cancer.
A. TBLR1 expression in immortalized benign prostate RC-165 is high compared to malignant LNCaP cells by western blot. GAPDH is used as a loading control. B. Cytolocalization of TBLR1 by immunoflourescence shows primarily nuclear in RC-165 and both nuclear and cytoplasmic in LNCaP. C. Confirmation of cytolocalization of TBLR1 in RC-165 and LNCaP cells by cell fractionation followed by western blot. D. In complete media, TBLR1 is primarily located in the cytoplasm of LNCaP-AI cells, but induced growth arrest upon 16 hour serum starvation leads to E. TBLR1 migration to the nucleus. F. Total levels of TBLR1 do not change after serum starvation induced growth arrest. G. In human prostate cancer tissue, TBLR1 expression is lower in malignant glands (black triangle) compared to neighboring benign glands (white triangle) by immunohistochemistry. Picture is a representative sample from the 19 of 30 tissue samples showing reduced nuclear TBLR1 expression in malignant glands.
It has previously been shown that TBLR1 translocates to the nucleus upon serum starvation in NIH 3T3 cells (Zhang, et al. 2006). These results, combined with the fact we observed higher nuclear TBLR1 expression in benign RC-165 vs. malignant LNCaP cells suggested nuclear TBLR1 conveyed a growth arrest signal. To test if this phenomenon was true in the prostate, LNCaP-AI cells were treated with serum starvation for 16 hours in 0.2% FBS. Serum starved LNCaP-AI cells showed increased nuclear and reduced cytoplasmic staining compared to cells grown in normal 10% FBS growth media (Figure 2D, E). Serum starvation induced growth arrest did not lead to changes in overall TBLR1 expression (Figure 2F).
We next looked at TBLR1 expression in human prostate cancer tissue samples. TBLR1 nuclear and cytoplasmic expression were measured by immunohistochemistry (IHC) using whole tissue sections from thirty cases of prostatic adenocarcinoma. TBLR1 nuclear and cytoplasmic expression was compared between malignant glands and the surrounding benign prostatic glands on the same slide.
Comparing malignant glands to the surrounding benign prostatic glands on the same tissue section, the expression of TBLR1 in nucleus is significantly lower in the malignant glands (p < 0.005) (Figure 2G). Among the thirty cases of prostatic adenocarcinoma we studied, the levels of TBLR1 nuclear expression in malignant glands decreased in 19 cases, stayed the same in 10 cases and increased in 1 case (Table 1). Additionally, TBLR1 nuclear and cytoplasmic expression pattern was studied in 86 samples of prostatic adenocarcinoma on a tissue microarray (TMA). When stratified with clinicopathological factors, the levels of TBLR1 nuclear expression have a positive correlation with the Gleason scores (R2= 0.9621, p<0.01) (Supplemental Figure 2C). We also tested 14 additional cases with high grade prostatic intraepithelial neoplasia (HGPIN), a precursor lesion of prostate cancer, adjacent to benign prostate glands on the same section. The data showed decreased TBLR1 expression in 10 of 14 cases (71%) of HGPIN (Supplemental Table 1 and Supplemental Figure 2B). No statistically significant difference between the level of TBLR1 cytoplasmic expression in malignant prostatic glands and that of the benign prostatic glands was observed.
Table 1. Expression of nuclear TBLR1 in prostate cancer.
30 cases of prostate cancer were stained for TBLR1 by immunohistochemistry in which both malignant and benign glands could be observed on the same slide. Benign and malignant cells were scored based on overall level of TBLR1 immunoreactivity including staining intensity and the percentage of positive cells in each sample. Data in the table represents number of samples that were decreased, unchanged, or increased in the malignant glands vs. benign glands.
| TBLR1 nuclear staining (malignant vs. adjacent benign gland) | |
|---|---|
| Decreased | 19 |
| Unchanged | 10 |
| Increased | 1 |
| Total | 30 |
Nuclear TBLR1 inhibits prostate cancer growth in vitro
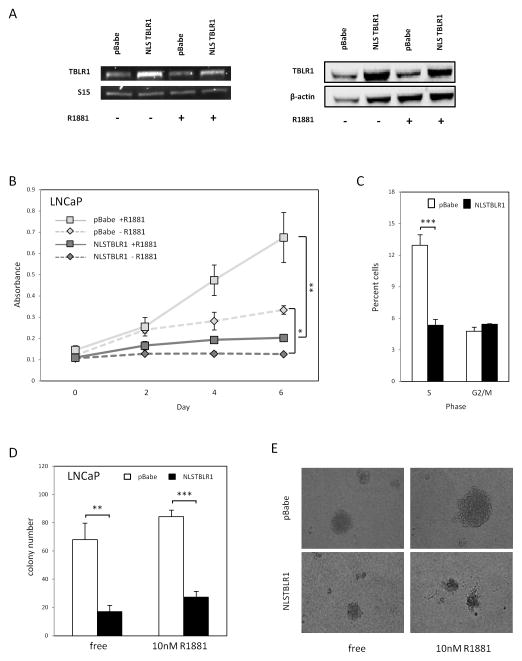
To study the functional effects of nuclear TBLR1 in prostate cells, we aimed to overexpress nuclear TBLR1 in LNCaP cells and measure its effects. In order to achieve nuclear localization of exogenenous TBLR1 expression, we created an NLSTBLR1 (containing a nuclear localization sequence PKKKRKV) fusion construct. GFP-NLSTBLR1 was confirmed to localize to the nucleus upon transfection into 293T cells (Supplemental Figure 3A). In comparison, GFP-TBLR1 (wild type) construct expression was exclusively observed in the cytoplasm (Supplemental Figure 3B). Control cells were made from pBabe vector alone. Overexpression of NLSTBLR1 is shown both at the RNA and protein level (Figure 3A). We also confirmed that overexpression of nuclear TBLR1 had no effect on AR protein levels in these cells (Supplemental Figure 3C). Nuclear TBLR1 dramatically reduced proliferation of LNCaP cells in 10nM R1881 media and also showed reduced proliferation in hormone-free media (Figure 3B), although the degree of inhibition is more substantial in androgen containing media. Cell cycle analysis by flow cytometry revealed that overexpression of nuclear TBLR1 in androgen containing media led to a two-fold decrease of S phase cells suggesting a G0/G1 arrest mechanism as the cause of reduced proliferation (Figure 3C). Similar findings were observed in hormone free media as well (data not shown). We found no evidence of increased apoptosis in LNCaP NLSTBLR1 compared to LNCaP pBabe in 10nM R1881 media, using a caspase 3/7 activity assay (Supplemental Figure 3D). We found similar proliferative effects of nuclear TBLR1 at both 1 nM and 10 nM of R1881 (Supplemental Figure 4A–D). Additionally, we performed TBLR1 silencing with siRNA in to test effects on proliferation and cell cycle. Interestingly, loss of TBLR1 expression in LNCaP cell lines led to dramatic decrease of growth and G0/G1 cell cycle arrest (Supplemental Figure 5B,C). TBLR1 knockdown in LNCaP-AI cells also led to decreased growth and G0/G1 cell cycle arrest (Supplemental Figure 5D,E).
Figure 3. Nuclear TBLR1 on in vitro prostate cancer cell growth.
A. Expression of TBLR1 by RT-PCR and western blot of LNCaP-NLSTBLR1 stable line compared to LNCaP pBabe control. B. WST-1 proliferation assay of LNCaP-NLSTBLR1 compared to LNCaP-pBabe control shows reduced growth in both hormone free and 10nM R1881 media. C. Cell cycle analysis with PI staining by flow cytometry comparing LNCaP pBabe to LNCaP NLSTBLR1 in 10nM R1881 media. D. Anchorage-independent growth assay displaying average number of colonies observed per plate in LNCaP pBabe and LNCaP NLSTBLR1 in hormone free and 10nM R1881 media. E. Representative pictures of colonies in anchorage-independent growth assay.
To test nuclear TBLR1 effect on cellular transformation, we performed an anchorage-independent growth assay in soft agar, comparing LNCaP cells expressing NLSTBLR1 or control vector. After 14 days, significantly fewer colonies were observed in cells overexpressing NLSTBLR1. In hormone free media, colony numbers of cells expressing NLSTBLR1 were reduced 75%, and in androgen media they were reduced 67% (Figure 3D). In addition, the average size of colonies was reduced as shown in representative pictures of colonies (Figure 3E). Thus, increased nuclear TBLR1 not only led to reduced growth of LNCaP cells but also inhibited their anchorage-independent growth, an in vitro assay of transformation.
Additionally, we created LNCaP cell lines expressing NLSTBLR1 phospho-mutants as described above, however, the growth inhibitory function of nuclear TBLR1 was not affected by either mutation (Supplemental Figure 3E).
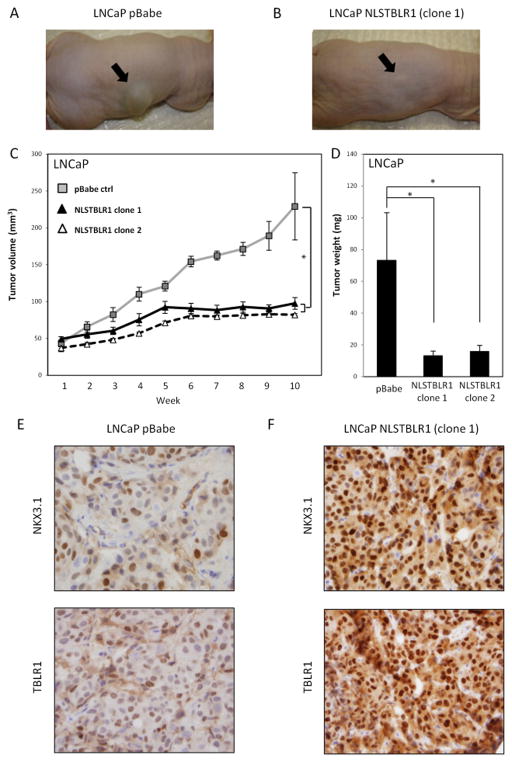
Nuclear TBLR1 suppresses prostate tumor growth in vivo
To determine if the inhibitory effect of nuclear TBLR1 on growth in culture could be replicated in vivo, we examined tumor growth in subcutaneous nude mouse xenografts with LNCaP-pBabe and LNCaP-NLSTBLR1 cells (Figure 4A,B). In 10 mice of each group, 1 × 107 cells were injected subcutaneously in the right flank and measured twice per week. Tumors produced by LNCaP pBabe cells showed steady growth over the 10 week time course of the experiment. However, tumors produced by LNCaP-NLSTBLR1 cells showed minimal growth over the same time period (Figure 4C). Two separate single clones of LNCaP NLSTBLR1 were used to confirm the inhibitory effect of nuclear TBLR1 on in vivo growth. After the 10 week time course tumors were harvested and weighed. Tumor weight of LNCaP pBabe tumors were significantly higher than LNCaP NLSTBLR1 tumors (Figure 4D). IHC staining of xenograft tumors confirmed increased TBLR1 expression in the NLSTBLR1 tumors compared to pBabe control by IHC (Figure 4E, F). There is a strong increase in the tumor suppressor NKX3.1, an AR target gene, reactivity in NLSTBLR1 tumors compared to pBabe control (Figure 4E,F). There was no difference in cleaved Caspase 3 levels between NLSTBLR1 and control tumors (Supplemental Figure 7A,B), consistent with our in vitro data (Supplemental Figure 3D).
Figure 4. Nuclear TBLR1 inhibits in vivo prostate cancer growth.
A. Representative pictures of subcutaneous tumor xenografts from LNCaP pBabe and B. LNCaP NLSTBLR1 clone 1 groups. C. Growth suppression by nuclear TBLR1 on subcutaneous tumor xenografts, Tumor volume over 10 weeks showing LNCaP-NLSTBLR1 cells have greatly reduced in vivo tumor growth. Grey squares, control LNCaP-pBabe cells; black triangles, LNCaP-NLSTBLR1-clone 1 cells; white triangles, LNCaP-NLSTBLR1-clone 2 cells. D. Average tumor weights from harvested tumors after 10 weeks; Error bars represent SE. E. IHC on LNCaP pBabe tumor for NKX3.1 and TBLR1. F. IHC on LNCaP NLSTBLR1 clone 1 tumor for NKX3.1 and TBLR1.
AR-dependence of nuclear TBLR1 function on prostate cancer growth suppression
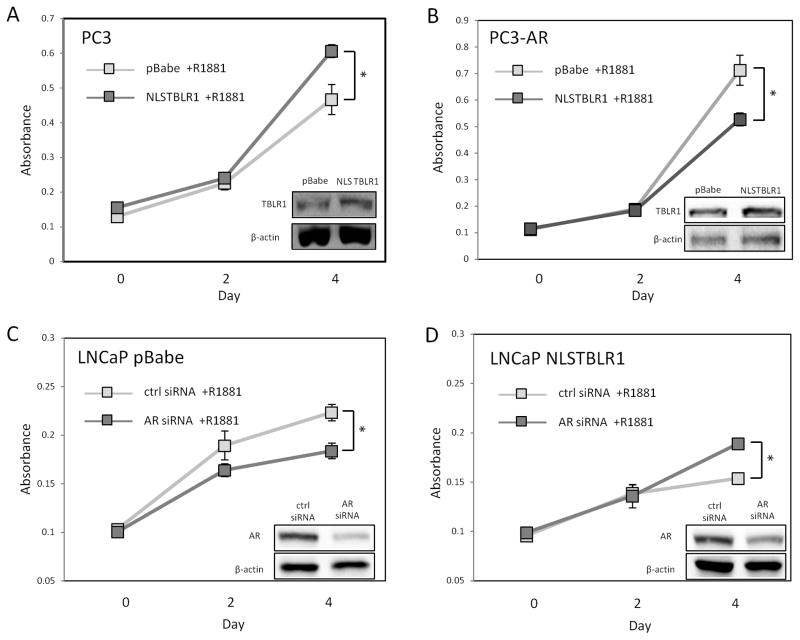
To test if the effect of nuclear TBLR1 on prostate cancer cell growth is mediated through AR, we created cell lines expressing NLSTBLR1 in PC3 cells, an AR-negative prostate cancer cell line, and PC3-AR, a derivative of PC3 expressing wild-type AR. Increased nuclear TBLR1 slightly increased growth in AR-negative PC3 cells in 10nM R1881 media, (Figure 5A) but PC3-AR cells expressing NLSTBLR1 showed reduced growth in 10nM R1881 media (Figure 5B). In hormone-free media, PC3-AR NLSTBRL1 cells showed a slightly increased growth rate compared to PC3-AR control cells (Supplemental Figure 6A) similar to what we observed in PC3-NLSTBLR1 cells in androgen media (Figure 5A). Although AR transfected PC-3 cells lines primarily show a negative growth response to androgen (Garcia-Arenas, et al. 1995; Yuan et al. 1993), more recently it has been observed that the level of AR expression can modulate the androgen response in these cells (Altuwaijri, et al. 2007; Yu, et al. 2009). The PC3-AR cell line used in our experiments showed a slight increase in growth rate upon stimulation with 10 nM R1881 compared to hormone free media (Supplemental Figure 6B).
Figure 5. AR-dependence of nuclear TBLR1 growth inhibition.
A. Proliferation assay of PC3-NLSTBLR1 compared to PC3-pBabe control shows increased growth in 10nM R1881 media. B. Proliferation assay of PC3-AR-NLSTBLR1 compared to PC3-AR-pBabe control shows decreased growth in 10nM R1881 media. C. Proliferation assay of LNCaP pBabe cells treated with AR siRNA or control siRNA in 10nM R1881 media shows decreased growth after AR knockdown. D. Proliferation assay of LNCaP NLSTBLR1 cells treated with AR siRNA or control siRNA in 10nM R1881 media shows increased growth after AR knockdown. The growth experiments were performed in triplicate in two independent experiments.
Additionally, we examined the effects of loss of AR using AR knockdown with siRNA in LNCaP pBabe control cells (Figure 5C) and LNCaP NLSTBLR1 containing cells (Figure 5D), followed by cell proliferation assays. AR knockdown on LNCaP pBabe control cells reduced growth while AR knockdown in LNCaP NLSTBLR1 stimulated growth, partially reversing the negative growth effects of overexpressed nuclear TBLR1. Together, these data shows that there is at least partial AR-dependence for nuclear TBLR1 suppression of prostate cancer growth.
Selective regulation of AR target genes by nuclear TBLR1
Because TBLR1 acts as a coactivator for AR in prostate cancer cells in a luciferase reporter assay and alterations in nuclear TBLR1 expression led to growth arrest, we sought to determine what specific AR-responsive genes were regulated by TBLR1. Quantitative RT-PCR was used to measure the effect of increased nuclear TBLR1 on the transcription of a set of androgen-regulated genes. AR target genes are involved in a broad spectrum of variable cellular processes including cell cycle progression, tumor suppression, differentiation, and apoptosis. We selected a limited number of genes from different groups as examples to measure nuclear TBLR1 activity on AR mediated transcriptional activation. This panel contained an assortment of exemplary androgen-regulated genes in various categories including Cyclin A2 and CDC6 (promoting cell cycle activation), HUS1 (involved in cell cycle arrest), NKX3.1 (prostate specific tumor suppressor), and KRT18 (luminal epithelial differentiation).
We compared gene expression between LNCaP NLSTBLR1 and LNCaP pBabe control cells after stimulation with androgen. The panel of genes we tested was divided into two groups. Genes that showed greater than 1.7 fold activation by NLSTBLR1 in set 1 (Figure 6A, B), and genes that showed little to no activation in set 2 (Supplemental Figure 8A). Subset 1 were genes primarily associated with growth suppression/differentiation, including NKX3.1, PMEPA1, HUS1, and KRT18. These genes were confirmed to be androgen-regulated in our assay by comparing hormone free to androgen media in LNCaP control cells (Supplemental Figure 8B). Additionally, BMPR1B, a gene associated with reduced expression in prostate cancer (Kim, et al. 2000) also showed activation. JAG1, the ligand for Notch1 receptor and suggested as both oncogenic and tumor suppressor roles in varying tumor types, was highly upregulated by increased TBLR1. Subset 2, that showed little to no activation from overexpression of NLSTBLR1, were genes primarily associated with cell proliferation, including CCNA2 and CDC6 (Supplemental Figure 8A).
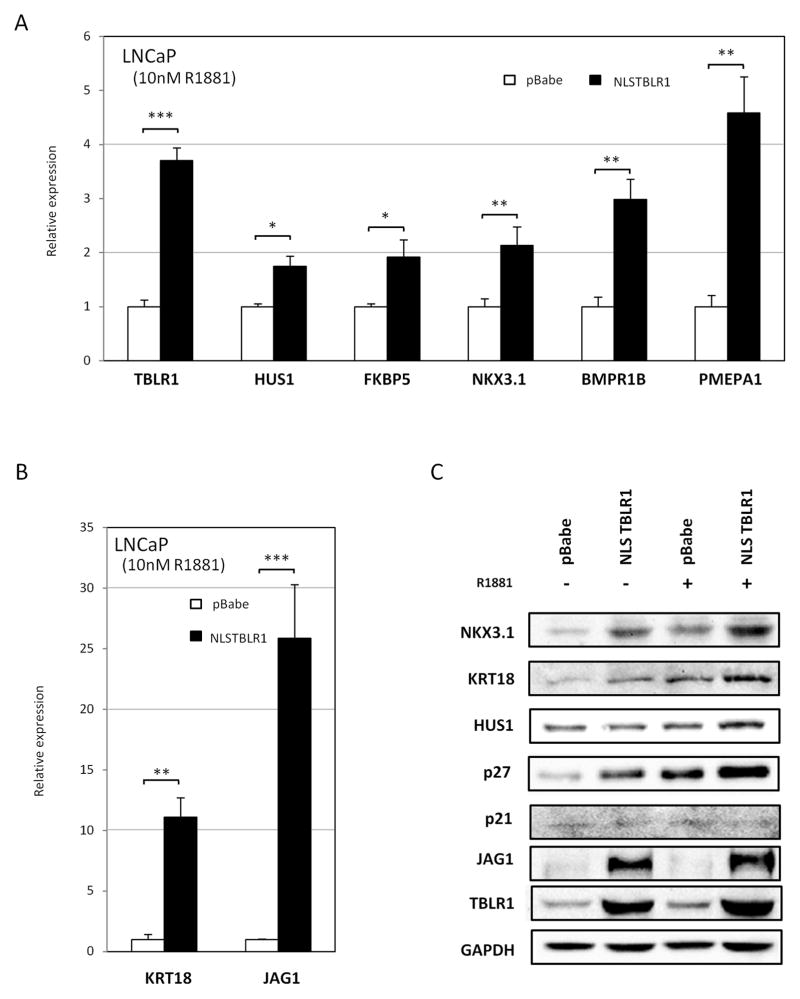
Figure 6. Nuclear TBLR1 upregulates growth suppressive AR target genes.
A, B Comparison of expression of androgen-regulated genes between LNCaP pBabe and LNCaP NLSTBLR1 in 10nM R1881 media by qRT-PCR. Panel is divided between genes that were increased less than 10 fold (A) and genes increased more than 10 fold (B) in LNCaP NLSTBLR1 cells compared to LNCaP pBabe cells in the presence of 10nM R1881. C. Comparison of protein expression of androgen-regulated genes between LNCaP pBabe and LNCaP NLSTBLR1 in hormone free media or 10nM R1881 media by western blot.
Changes in expression of NKX3.1, KRT18, HUS1, and JAG1 after nuclear TBLR1 overexpression were also confirmed at the protein level by western blot. Additionally, we tested expression of the androgen-regulated cell cycle regulators p27 and p21. Overexpression of nuclear TBLR1 showed no effect on p21, but did show a strong increase in p27 expression, suggesting a role in p27 in growth arrest initiated by nuclear TBLR1 (Figure 6C). Additionally, we tested the effect of increased nuclear TBLR1 on p27 levels in two additional androgen-sensitive cell lines, RC-165 and LNCaP-AI. Overexpression of nuclear TBLR1 also led to increased p27 protein levels by western blot analysis in these cells (Supplemental Figure 8C).
To further confirm these results of TBLR1 acting as a coactivator of AR, we also tested the effect of TBLR1 knockdown across this panel of genes. Loss of TBLR1 led to a reduction of expression across the panel of genes (Supplemental Figure 9A). Loss of NKX3.1, KRT18, and HUS1 expression at the protein level was confirmed (Supplemental Figure 9B). No change in p27 levels was observed upon TBLR1 knockdown.
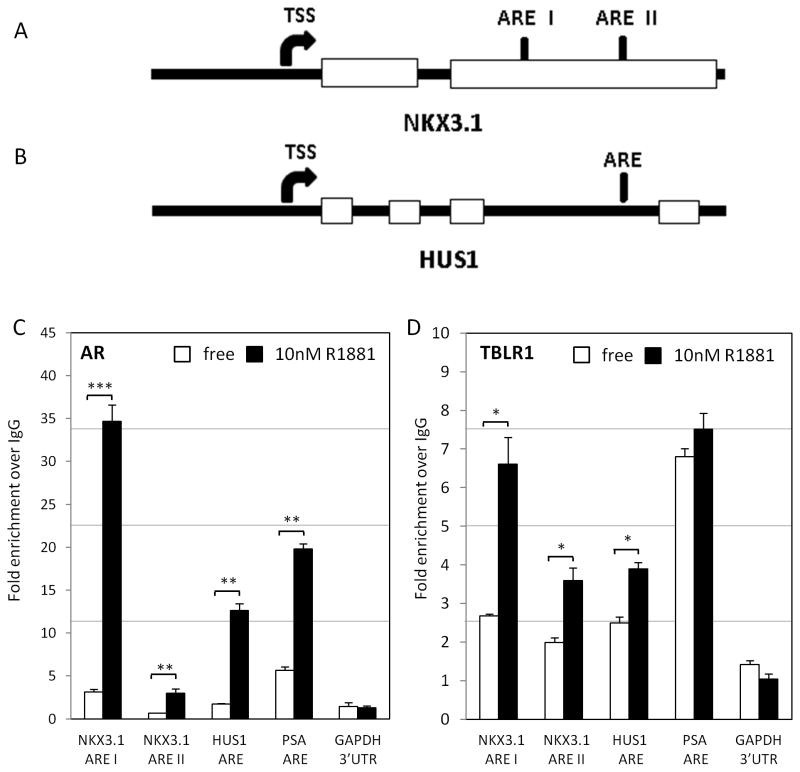
We next used chromatin immunoprecipitation (ChIP) to test the direct regulation of the affected AR target genes by TBLR1. NKX3.1 has been confirmed as a direct AR target gene with the primary androgen response elements (ARE) identified within the 3′ UTR of the gene (Thomas, et al. 2010). There is also an ARE for the HUS1 gene between exons 3 and 4 (see diagrams Figure 7A, B). In addition, we used the well characterized AR target gene prostate-specific antigen (PSA) as a positive control. By ChIP, using antibody specific to AR, we first confirmed these four ARE with increased occupation of AR by androgen stimulation (Figure 7C). Using a polyclonal antibody specific to TBLR1, we showed TBLR1 occupancy at both the primary and secondary ARE of the NKX3.1 gene, HUS1 ARE, and PSA ARE but not at the GAPDH 3′ UTR negative control. Upon androgen stimulation, TBLR1 occupancy at both ARE of NKX3.1 increased over 2 fold. We also showed TBLR1 occupancy on the ARE of the HUS1 gene with an increase of approximately 1.4 fold upon androgen stimulation. Androgen stimulation did not show increased TBLR1 at the PSA promoter, corresponding with its lack of activation observed in LNCaP cells by increased nuclear TBLR1 (Figure 7D).
Figure 7. TBLR1 occupancy on growth suppressive AR target genes.
A. Diagram of androgen response element(s) (ARE) on NKX3.1 gene and B. HUS1 gene. C. Chromatin immunoprecipitation with AR antibody in LNCaP cells in hormone free or 10nM R1881 media at NKX3.1 ARE I, ARE II, HUS1 ARE, PSA ARE, and GAPDH 3′UTR negative control to confirm ARE at each of the AR target genes. D. Chromatin immunoprecipitation with TBLR1 antibody in LNCaP cells in hormone free or 10nM R1881 media at NKX3.1 ARE I, ARE II, HUS1 ARE, PSA ARE and GAPDH 3′UTR negative control. All experiments were performed in triplicate.
Since we identified that increased nuclear TBLR1 led to increased activation of NKX3.1 and HUS1 genes in the presence of androgen, we next tested if increased nuclear TBLR1 modified occupancy of the corepressor NCoR at their regulatory elements after androgen stimulation. We compared LNCaP NLSTBLR1 cells and LNCaP pBabe control cells by ChIP analysis using a polyclonal NCoR antibody. There was significantly reduced NCoR occupancy at the NKX3.1 AREI and the HUS1 ARE in LNCaP NLSTBLR1 cells compared to LNCaP pBabe control cells after androgen stimulation (Supplemental Figure 10A,B).
DISCUSSION
AR signaling is critical for prostate cancer growth and progression. Transcriptional cofactors, including both co-activators and co-repressors, are important in regulating AR transcriptional activity. Tumor development and progression are affected by changes in expression, localization, and activation of various cofactors. In this paper we examined the role of the nuclear receptor cofactor, TBLR1, as a regulator of AR transcriptional activity and its expression and function in prostate cancer.
TBLR1 is ubiquitously expressed and has been shown to serve as a co-repressor for a number of transcription factors of the Nuclear Hormone Receptor (NHR) family, serving as a docking function for NCoR and SMRT. These interact with histone deacetylase (HDAC3) to form an active deacetylase and render chromatin less accessible. Little is known about the interactions of TBLR1 with AR and ensuing function in prostate cancer. We showed TBLR1 acts as a coactivator of AR in prostate cancer cells by both luciferase assay and AR target gene expression. TBLR1 interacts with AR in an androgen dependent fashion and directly co-occupies androgen response elements with AR.
Overwhelming evidence in the literature indicates the importance of AR transcriptional activation to drive prostate cancer cell proliferation. Recently, a number of AR coactivators have been described to have tumor suppressor function, including ARA70α and nuclear p44/MEP50 (Ligr et al. 2010; Peng et al. 2008a). Understanding the balance between proliferation and growth suppression, and the role specific cofactors play in regulating this switch is critical in understanding prostate biology and prostate cancer treatment methods. The benign prostate cancer cell line, RC-165, has high nuclear TBLR1 expression compared to the cancer cell line LNCaP. As observed in our cell line model, human tumor samples show significant reduction of TBLR1 in the nucleus of malignant glands in comparison to neighboring benign glands. Additionally, serum starvation induced growth arrest leads to translocation of TBLR1 from the cytoplasm to the nucleus in cancer cells. This data together strongly suggests a role of nuclear TBLR1 as a tumor suppressor in prostate cancer. Interestingly, although overall level of expression of TBLR1 is reduced in cancer vs. benign cells, we did observe a correlation of increased nuclear TBLR1 with increased Gleason score. This could be representative of a feedback mechanism that higher grade cancers cells are producing more TBLR1 in an unsuccessful attempt to control cancer cell proliferation.
Indeed, overexpression of nuclear TBLR1 in AR positive prostate cancer cells leads to growth suppression in vitro in cell proliferation and anchorage-independent assays, as well as tumor growth inhibition in nude mice xenografts in vivo. It appears that there is an androgen-independent component of tumor suppression by TBLR1 also, since the growth of LNCaP cells is reduced even in growth medium free of androgen (Figure 3B). Importantly, there is a higher degree of androgen-dependent element of growth inhibition as nuclear TBLR1 promotes cell growth in PC3 cells and this process is reversed to growth inhibition in PC3-AR cells (Figure 5A, B). To confirm an androgen-dependent mechanism of nuclear TBLR1 mediated growth inhibition, AR knockdown in LNCaP-NLSTBLR1 cells was also able to partially rescue growth suppressive effects (Figure 5C,D).
AR can regulate select sets of AR target genes (Agoulnik and Weigel 2009; Wang, et al. 2011) under various conditions. A number of AR target genes have been identified to regulate cell growth including cell cycle genes, apoptosis, tumor suppressors and differentiation genes. In this study, we have shown that TBLR1 is an AR coactivator that acts as a tumor suppressor in prostate by selectively activating growth suppression and differentiation androgen-regulated genes. Overexpressing nuclear TBLR1 in the prostate cancer model cell line LNCaP leads to increased expression of important prostate pro-differentiation (i.e. KRT18) and tumor suppressor genes (i.e. NKX3.1 and HUS1). Overexpression of nuclear TBLR1 in LNCaP cells also leads to increased expression of the cell cycle inhibitor p27, an androgen regulated gene which may be responsible for the G0/G1 cell cycle arrest observed in our proliferation studies. Increased expression of NKX3.1 and p27 has been previously reported to work synergistically to decrease prostate cancer cell proliferation (Wang, et al. 2009). Our data indicate that TBLR1 interacts with AR in an androgen-dependent fashion, and that TBLR1 directly occupies androgen response elements of AR target genes NKX3.1 and HUS1 in an androgen-dependent manner. Together, this data shows nuclear TBLR1 as a direct regulator of growth suppressive AR target genes.
Interestingly, upon knockdown of TBLR1 in LNCaP or LNCaP-AI cells by siRNA we also observed a reduction of proliferative ability, showing that either overexpression or knockdown of TBLR1 leads to G0/G1 growth arrest. Although this data at first seems contradictory, it appears that the growth arrest observed in each of these instances could be through different mechanisms. Overexpression of TBLR1 showed increased p27 levels whereas knockdown of TBLR1 did not affect p27 levels (Figure 6C and Supplemental Figure 9B). Additionally, we showed that overexpression of nuclear TBLR1 leads to selective activation of growth suppression/differentiation AR target genes but not pro-proliferative genes (Figure 6A), however, knockdown of TBLR1 leads to a more global downregulation of both sets of genes (Supplemental Figure 9A). This shows that although increased nuclear TBLR1 does selectively activate a subset of AR target genes, a baseline level may be required for most AR target genes and cell proliferation. It is possible the loss of expression of proliferation genes (ie. CCNA2, CDC6) upon TBLR1 knockdown overrides the effect of decreased tumor suppressor target genes (ie. NKX3.1) on overall growth rate. Additionally, since TBLR1 functions in the context of a protein complex with HDAC and NCoR proteins, loss of expression of the TBLR1 component may disrupt the complex and interfere with transcription and cell growth..
Previous studies showed the mechanism of TBLR1 activation of nuclear receptors could be due to phosphorylation of TBLR1 by PKCδ at two specific sites that targets NCoR for ubiquitination and degradation by the 19S proteosome (Perissi et al. 2004). In prostatic tissues we showed by luciferase assay that activation of AR by TBLR1 also is indeed dependent on both 19S proteosome activity and phosphorylation (Figure 1C–E). Indirect evidence has suggested that TBLR1 serves as an F-box protein with E3 ligase activity on BCL-3 (Keutgens, et al. 2010). It has been suggested that since it contains an F-box like motif, it acts as an E3 ligase and promotes the degradation of NCoR, leading to increased chromatin accessibility. The F-box in TBLR1 is unusual in that it lacks a tryptophan near the NH2-end of the motif that is required for Skp1 binding (Kipreos and Pagano 2000). Without this interaction E3 ligase activity does not develop. However, no interaction of TBLR1 with SKP1 could be demonstrated by our lab (G Daniels, P Lee, unpublished data), suggesting that the role of TBLR1 in the proteosome pathway may be indirect. When we tested LNCaP NLSTBLR1 phospho-mutants in proliferation assays, however, there was no difference in growth observed between NLSTBLR1 and all three phospho-mutants, revealing phosphorylation at these sites alone is not critical for growth suppression by nuclear TBLR1 (Supplemental Figure 3E), supported by our data showing phospho-mutants still retaining some coactivation ability in our luciferase assay (Figure 1F). Phosphorylation of TBLR1 may affect other functions of TBLR1 other than growth inhibition. A recent report also showed that TBLR1 is sumolyated at K497 and sumolyation is required for TBLR1 activation of Wnt target genes through interaction with beta-catenin (15). However, using a sumolyation mutant K497R TBLR1 plasmid in our luciferase assay we observed equal AR activation compared to wild type TBLR1 (Supplemental Figure 1A). Sumolyation at K497 does not appear to be important for AR transcriptional activation in our limited luciferase system.
In conclusion, nuclear TBLR1 acts as a tumor suppressor in prostate cancer and AR coactivator directly and selectively activates androgen-regulated genes important for growth suppression/differentiation. Better understanding of AR regulation in the prostate and the molecular switches that occur to transition from growth suppression to pro-proliferation target genes will lead to more successful prostate cancer treatments.
Supplementary Material
Acknowledgments
FUNDING
This study is supported by NIH (1U01CA149556-01), DOD PCRP (PC080010 and PC11624) and VA Merit (1I01BX001505-01) grants to PL, NYU CTSI TL1 (1UL1RR029893) and NYU Molecular Oncology and Immunology Training grant (T32 CA009161) postdoctoral fellowships to GD, DOD postdoctoral fellowship (PC081578) to YRL and (PC094557) to XYW.
This material is based upon work supported in part by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development (Biomedical Laboratory Research and Development). We wish to thank the Department of Defense supported Prostate Cancer Biorepository Network (PCBN).
Footnotes
CONFLICT OF INTEREST
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Agoulnik IU, Weigel NL. Coactivator selective regulation of androgen receptor activity. Steroids. 2009;74:669–674. doi: 10.1016/j.steroids.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altuwaijri S, Wu CC, Niu YJ, Mizokami A, Chang HC, Chang C. Expression of human AR cDNA driven by its own promoter results in mild promotion, but not suppression, of growth in human prostate cancer PC-3 cells. Asian J Androl. 2007;9:181–188. doi: 10.1111/j.1745-7262.2007.00258.x. [DOI] [PubMed] [Google Scholar]
- Burke LJ, Baniahmad A. Co-repressors 2000. FASEB J. 2000;14:1876–1888. doi: 10.1096/fj.99-0943rev. [DOI] [PubMed] [Google Scholar]
- Cai CQ, Peng Y, Buckley MT, Wei J, Chen F, Liebes L, Gerald WL, Pincus MR, Osman I, Lee P. Epidermal growth factor receptor activation in prostate cancer by three novel missense mutations. Oncogene. 2008;27:3201–3210. doi: 10.1038/sj.onc.1210983. [DOI] [PubMed] [Google Scholar]
- Choi HK, Choi KC, Yoo JY, Song M, Ko SJ, Kim CH, Ahn JH, Chun KH, Yook JI, Yoon HG. Reversible SUMOylation of TBL1-TBLR1 regulates beta-catenin-mediated Wnt signaling. Mol Cell. 2011;43:203–216. doi: 10.1016/j.molcel.2011.05.027. [DOI] [PubMed] [Google Scholar]
- Dehm SM, Tindall DJ. Molecular regulation of androgen action in prostate cancer. J Cell Biochem. 2006;99:333–344. doi: 10.1002/jcb.20794. [DOI] [PubMed] [Google Scholar]
- Gao M, Ossowski L, Ferrari AC. Activation of Rb and decline in androgen receptor protein precede retinoic acid-induced apoptosis in androgen-dependent LNCaP cells and their androgen-independent derivative. J Cell Physiol. 1999;179:336–346. doi: 10.1002/(SICI)1097-4652(199906)179:3<336::AID-JCP11>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Garcia-Arenas R, Lin FF, Lin D, Jin LP, Shih CC, Chang C, Lin MF. The expression of prostatic acid phosphatase is transcriptionally regulated in human prostate carcinoma cells. Mol Cell Endocrinol. 1995;111:29–37. doi: 10.1016/0303-7207(95)03544-h. [DOI] [PubMed] [Google Scholar]
- Gelmann EP. Molecular biology of the androgen receptor. J Clin Oncol. 2002;20:3001–3015. doi: 10.1200/JCO.2002.10.018. [DOI] [PubMed] [Google Scholar]
- Gu Y, Kim KH, Ko D, Srivastava S, Moul JW, McLeod DG, Rhim JS. Androgen and androgen receptor antagonist responsive primary African-American benign prostate epithelial cell line. Anticancer Res. 2005;25:1–8. [PubMed] [Google Scholar]
- Heinlein CA, Chang C. Androgen receptor (AR) coregulators: an overview. Endocr Rev. 2002;23:175–200. doi: 10.1210/edrv.23.2.0460. [DOI] [PubMed] [Google Scholar]
- Janne OA, Moilanen AM, Poukka H, Rouleau N, Karvonen U, Kotaja N, Hakli M, Palvimo JJ. Androgen-receptor-interacting nuclear proteins. Biochem Soc Trans. 2000;28:401–405. [PubMed] [Google Scholar]
- Keutgens A, Shostak K, Close P, Zhang X, Hennuy B, Aussems M, Chapelle JP, Viatour P, Gothot A, Fillet M, et al. The repressing function of the oncoprotein BCL-3 requires CtBP, while its polyubiquitination and degradation involve the E3 ligase TBLR1. Molecular and cellular biology. 2010;30:4006–4021. doi: 10.1128/MCB.01600-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim IY, Lee DH, Ahn HJ, Tokunaga H, Song W, Devereaux LM, Jin D, Sampath TK, Morton RA. Expression of bone morphogenetic protein receptors type-IA, -IB and -II correlates with tumor grade in human prostate cancer tissues. Cancer research. 2000;60:2840–2844. [PubMed] [Google Scholar]
- Kipreos ET, Pagano M. The F-box protein family. Genome Biol. 2000;1:REVIEWS3002. doi: 10.1186/gb-2000-1-5-reviews3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Wang L, Zhang M, Melamed J, Liu X, Reiter R, Wei J, Peng Y, Zou X, Pellicer A, et al. LEF1 in androgen-independent prostate cancer: regulation of androgen receptor expression, prostate cancer growth, and invasion. Cancer Res. 2009;69:3332–3338. doi: 10.1158/0008-5472.CAN-08-3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligr M, Li Y, Logan SK, Taneja S, Melamed J, Lepor H, Garabedian MJ, Lee P. Mifepristone inhibits GRbeta coupled prostate cancer cell proliferation. J Urol. 2012;188:981–988. doi: 10.1016/j.juro.2012.04.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligr M, Li Y, Zou X, Daniels G, Melamed J, Peng Y, Wang W, Wang J, Ostrer H, Pagano M, et al. Tumor suppressor function of androgen receptor coactivator ARA70a in prostate cancer. Am J Pathol. 2010;176:1891–1900. doi: 10.2353/ajpath.2010.090293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nwachukwu JC, Mita P, Ruoff R, Ha S, Wang Q, Huang SJ, Taneja SS, Brown M, Gerald WL, Garabedian MJ, et al. Genome-wide impact of androgen receptor trapped clone-27 loss on androgen-regulated transcription in prostate cancer cells. Cancer Res. 2009;69:3140–3147. doi: 10.1158/0008-5472.CAN-08-3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Y, Chen F, Melamed J, Chiriboga L, Wei J, Kong X, McLeod M, Li Y, Li CX, Feng A, et al. Distinct nuclear and cytoplasmic functions of androgen receptor cofactor p44 and association with androgen-independent prostate cancer. Proc Natl Acad Sci U S A. 2008a;105:5236–5241. doi: 10.1073/pnas.0712262105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Y, Li CX, Chen F, Wang Z, Ligr M, Melamed J, Wei J, Gerald W, Pagano M, Garabedian MJ, et al. Stimulation of prostate cancer cellular proliferation and invasion by the androgen receptor co-activator ARA70b. Am J Pathol. 2008b;172:225–235. doi: 10.2353/ajpath.2008.070065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perissi V, Aggarwal A, Glass CK, Rose DW, Rosenfeld MG. A corepressor/coactivator exchange complex required for transcriptional activation by nuclear receptors and other regulated transcription factors. Cell. 2004;116:511–526. doi: 10.1016/s0092-8674(04)00133-3. [DOI] [PubMed] [Google Scholar]
- Perissi V, Scafoglio C, Zhang J, Ohgi KA, Rose DW, Glass CK, Rosenfeld MG. TBL1 and TBLR1 phosphorylation on regulated gene promoters overcomes dual CtBP and NCoR/SMRT transcriptional repression checkpoints. Mol Cell. 2008;29:755–766. doi: 10.1016/j.molcel.2008.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas MA, Preece DM, Bentel JM. Androgen regulation of the prostatic tumour suppressor NKX3.1 is mediated by its 3′ untranslated region. The Biochemical journal. 2010;425:575–583. doi: 10.1042/BJ20091109. [DOI] [PubMed] [Google Scholar]
- Tomita A, Buchholz DR, Shi YB. Recruitment of N-CoR/SMRT-TBLR1 corepressor complex by unliganded thyroid hormone receptor for gene repression during frog development. Mol Cell Biol. 2004;24:3337–3346. doi: 10.1128/MCB.24.8.3337-3346.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Garcia-Bassets I, Benner C, Li W, Su X, Zhou Y, Qiu J, Liu W, Kaikkonen MU, Ohgi KA, et al. Reprogramming transcription by distinct classes of enhancers functionally defined by eRNA. Nature. 2011;474:390–394. doi: 10.1038/nature10006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Ma Q, Luo J, Liu B, Tan F, Zhang Z, Chen Z. Nkx3.1 and p27(KIP1) cooperate in proliferation inhibition and apoptosis induction in human androgen-independent prostate cancer cells. Cancer investigation. 2009;27:369–375. doi: 10.1080/07357900802232749. [DOI] [PubMed] [Google Scholar]
- Whitacre DC, Chauhan S, Davis T, Gordon D, Cress AE, Miesfeld RL. Androgen induction of in vitro prostate cell differentiation. Cell Growth Differ. 2002;13:1–11. [PubMed] [Google Scholar]
- Yoon HG, Chan DW, Huang ZQ, Li J, Fondell JD, Qin J, Wong J. Purification and functional characterization of the human N-CoR complex: the roles of HDAC3, TBL1 and TBLR1. EMBO J. 2003;22:1336–1346. doi: 10.1093/emboj/cdg120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon HG, Choi Y, Cole PA, Wong J. Reading and function of a histone code involved in targeting corepressor complexes for repression. Mol Cell Biol. 2005;25:324–335. doi: 10.1128/MCB.25.1.324-335.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu SQ, Lai KP, Xia SJ, Chang HC, Chang C, Yeh S. The diverse and contrasting effects of using human prostate cancer cell lines to study androgen receptor roles in prostate cancer. Asian journal of andrology. 2009;11:39–48. doi: 10.1038/aja.2008.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan S, Trachtenberg J, Mills GB, Brown TJ, Xu F, Keating A. Androgen-induced inhibition of cell proliferation in an androgen-insensitive prostate cancer cell line (PC-3) transfected with a human androgen receptor complementary DNA. Cancer Res. 1993;53:1304–1311. [PubMed] [Google Scholar]
- Zhang XM, Chang Q, Zeng L, Gu J, Brown S, Basch RS. TBLR1 regulates the expression of nuclear hormone receptor co-repressors. BMC Cell Biol. 2006;7:31. doi: 10.1186/1471-2121-7-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.