Abstract
A complication of diabetes is the inability of wounds to heal in diabetic patients. Diabetic wounds are refractory to healing due to the involvement of activated matrix metalloproteinases (MMPs), which remodel the tissue resulting in apoptosis. There are no readily available methods that identify active unregulated MMPs. With the use of a novel inhibitor-tethered resin that binds exclusively to the active forms of MMPs, coupled with proteomics, we quantified MMP-8 and MMP-9 in a mouse model of diabetic wounds. Topical treatment with a selective MMP-9 inhibitor led to acceleration of wound healing, re-epithelialization, and significantly attenuated apoptosis. In contrast, selective pharmacological inhibition of MMP-8 delayed wound healing, decreased re-epithelialization, and exhibited high apoptosis. The MMP-9 activity makes the wounds refractory to healing, whereas that of MMP-8 is beneficial. The treatment of diabetic wounds with a selective MMP-9 inhibitor holds great promise in providing heretofore-unavailable opportunities for intervention of this disease.
Keywords: Diabetic wound healing, matrix metalloproteinases, selective inhibition
Diabetes mellitus is a complex metabolic disease that affects >340 million individuals worldwide.1 Diabetic patients have impaired ability to metabolize glucose, and the ensuing hyperglycemia results in many complications, which include damage to the vasculature and the inability to heal wounds. The vascular damage in diabetes results in ischemia as a contributing factor to the persistence of wounds,2 causing inflammation and triggering production of reactive oxygen species, which prevent wound closure by damaging the extracellular matrix (ECM).2, 3 The complications in wound healing in diabetics results in 66,000 lower-limb amputations in the United States every year.1 Matrix metalloproteinases (MMPs), a family of 26 zinc-dependent endopeptidases, normally restructure the ECM in an effort to repair the wound, but the ischemic condition in diabetic wounds presents an obstacle. As will be outlined in this report, expression of MMPs in diabetic wounds is altered, and contributes to the refractory nature of the wounds to heal.
MMPs are expressed as inactive zymogens (proMMPs), requiring proteolytic removal of the pro domain for their activation, which is mediated by other proteinases, including MMPs. MMPs are further regulated by complexation with tissue inhibitors of matrix metalloproteinases (TIMPs), which block access to the active site. Furthermore, MMPs are expressed at low levels in healthy tissues, but their expression increases during many diseases that involve remodeling of the ECM. This is known to be the case for chronic wounds,4, 5 except the methods that have been employed do not differentiate among proMMPs and TIMP-complexed MMPs (both inactive as enzymes) and activated MMPs.6 While MMPs play both beneficial and detrimental roles,7 most research has focused on the detrimental roles of MMPs with limited studies conducted to ascertain the beneficial actions of MMPs. Without identifying the active unregulated MMPs, we actually do not know which MMP is relevant for disease and which MMP may play a beneficial repair role.
Various techniques are available to profile MMPs,8 however, these tools generally do not reveal whether the elevated levels of MMPs that are being monitored are due to zymogenic forms, the active MMPs, or TIMP-complexed MMPs. Quantification of mRNA levels by northern blot analysis and RT-PCR are limited in that these methods measure mRNA levels and not the amount and activity of the protein. Immunohistochemistry and western blot require specific antibodies, which usually cannot distinguish between the zymogen, the active or TIMP-complexed MMPs. Zymography detects both zymogen and active MMPs, however inactive TIMP-complexed MMPs appear as active MMP bands due to dissociation of the non-covalent TIMP-MMP complex under the denaturing conditions of zymography. In-situ zymography is limited by the availability of fluorescent proteinase substrates and has limitations for quantitative determinations. Activity-based enzyme profiling of MMPs requires a library of selective MMP-directed probes.9–13 A TAPI-2 affinity resin has been reported to identify active MMPs,14–16 however the starting materials are very expensive. With the exception of the TAPI-2 resin, the other methods do not identify and quantify the active form of MMPs. Thus, the existing methods have deficiencies in shedding definitive light on the roles of MMPs in various diseases.
In addressing what active MMPs might play roles in disease, we have devised a resin that has been covalently tethered to a broad-spectrum MMP inhibitor (compound 1, Figure 1a), based on the structure of batimastat.17 The first feature, the large breadth of inhibitory property by the tethered inhibitor, is of central importance, enabling binding by all active MMPs. Second, by immobilizing the inhibitor to the solid support (Sepharose 6B resin), one should not abrogate its activity. The design paradigms that addressed these criteria have been described previously,17 but the judicious linkage of the resin via a 12-atom linker segment was incorporated in a portion of the inhibitor that points to the milieu from the MMP active site. The resin binds only to active MMPs, to the exclusion of MMP zymogens and TIMP-inhibited MMPs. After incubation with the resin, the resin-bound proteins are subjected to trypsin-digest on the resin. The resultant peptide mixtures are desalted and analyzed by nanoultraperformance liquid chromatography (UPLC) coupled to a tandem mass spectrometer interfaced with a protein database search engine. We detect and quantify the bound active MMPs in wound tissue by our mass spectrometry protocol with a limit of quantification of 6 fmol (10−15 mol, equivalent to 0.4 pg; see Supporting Information). We used an excisional wound-healing model in diabetic mice18 and covered the wounds with occlusive dressing, which effectively splints the wounds open.18 Splinted wounds impede wound contraction and allow wounds to heal by re-epithelialization and granulation tissue formation,19 replicating the mechanisms of human wound healing. Using our resin method, we identified active MMP-8 and MMP-9 in wound tissues of diabetic mice. We also analyzed the tissues by zymography, which is a commonly used method in detection of MMPs. Zymography showed the presence of proMMP-2, active MMP-2, and proMMP-9 (Supplementary Figure 1a), whereas active MMP-9 was conspicuously not detectable by this method. While MMP-2 and MMP-9 have been proposed to exist in diabetic wounds,20, 21 we did not find active MMP-2 in diabetic wounds with our resin. Since the resin binds only to uninhibited active MMP(s), the active MMP-2 band observed by gelatin zymography (Supplementary Figure 1a) has to be TIMP-inhibited, resulting in an inactive form of the enzyme. In contrast, active MMP-9 is not detectable by gelatin zymography (Supplementary Figure 1a), however it is the major active MMP determined by the resin method (Figure 1b). This is due to the lack of sufficient sensitivity by gelatin zymography, for which we determined a lower limit of detection of 10 pg (Supplementary Figure 1b). In contrast, the lower limit of quantification using the resin is 0.4 pg. While zymography is useful, its limitation is its lower limit of detection.
Figure 1.
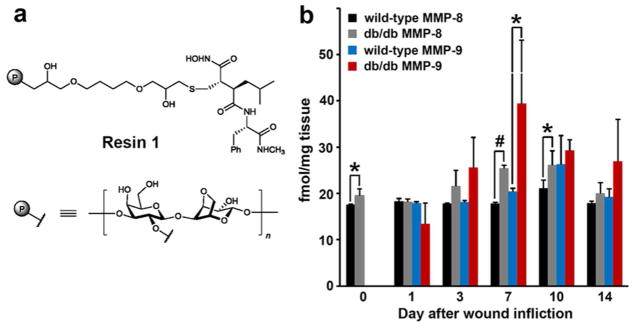
Identification and quantification of active MMPs in the course of diabetic wound healing. (a) Broad-spectrum MMP inhibitor-tethered resin (compound 1). (b) Levels of active MMP-8 and MMP-9 in wound tissues quantified by the inhibitor-tethered resin coupled with nano UPLC with MRM detection. The skin plugs collected on day 0 using a biopsy punch were analyzed and served as negative controls. Levels of MMP-9 on day 0 were below the limit of quantification (<6 fmol mg−1 tissue). Data represent mean ± SD, n = 3; * p < 0.05, # p < 0.01.
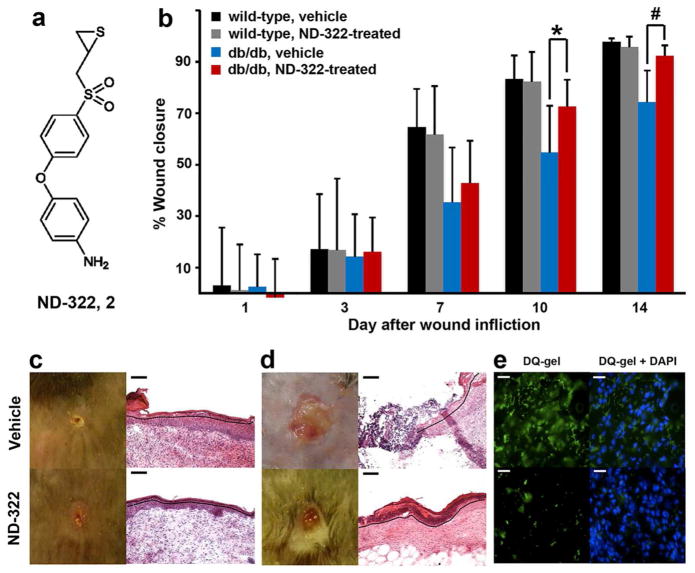
Methods for quantification of active MMP-8 and MMP-9 were developed using LC-multiple-reaction monitoring (MRM), a highly specific quantitative mass spectrometry method that measures the signal arising when a defined precursor peptide selected in the first quadrupole gives rise to a selected fragment ion in the third quadrupole. We note that active MMP-8 and MMP-9 are present in the wounds of both wild-type and diabetic mice, except that MMP-9 is elevated at statistically significant levels only in diabetic wounds (Figure 1b). Gutiérrrez-Fernández et al. have reported that MMP-8 is involved in healing of normal skin wounds.22 The questions that become pertinent are whether MMP-9 is the culprit in refractory diabetic wound healing and if active MMP-8 in both wild-type and diabetic mice is a reflection of the effort by the tissue in healing? If active MMP-9 plays a detrimental effect on diabetic wound healing, can we intervene pharmacologically? Our laboratories have worked over the years on a class of selective thiirane MMP inhibitors, which inhibit this enzyme by a unique mechanism involving a reaction catalyzed by the target MMP.23 This inhibitor class shows selectivity in targeting MMPs because of the unique mechanism of action.23 We assessed the effect of inhibitor 2 (also known as ND-322, Figure 2a), which exhibits selectivity in inhibition toward MMP-2, MMP-9, and MMP-14,24 on wound healing as a function of time as a percentage of the initial wound area (Figure 2b). Therefore, ND-322 would inhibit selectively active MMP-9 found upregulated in diabetic wounds, while sparing MMP-8. If the activity of MMP-9 were to be an impediment to healing of diabetic wounds, then treatment with ND-322 should accelerate wound healing. This is indeed what was observed, as described below.
Figure 2.
ND-322 accelerates wound healing by re-epithelialization and abrogates MMP-9 activity in diabetic db/db wounds. (a) Chemical structure of inhibitor 2 (ND-322); Ki values:24 10% inhibition at 250 μM for MMP-1, 24 ± 15 nM for MMP-2 (slow-binding), 23.4 ± 1.6 μM for MMP-3 (competitive), 16% inhibition at 250 μM for MMP-7, 2.6 ± 0.4 μM for MMP-8 (noncompetitive), 870 ± 111 nM for MMP-9 (slow-binding), 210 ± 20 nM for MMP-14 (slow-binding), 9% inhibition at 30 μM for MMP-19, 30% inhibition at 20 μM for ADAM9, 21% inhibition at 20 μM for ADAM10, 15.7 ± 2.0 μM for ADAM17 (competitive). (b) Wound healing in db/db and wild-type mice. Mean ± SD; n = 35, 28, 21, 14, and 7 on days 1, 3, 7, 10, and 14, respectively; *p < 0.05, # p < 0.01 indicate statistically significant differences in wound healing between ND-322- and vehicle-treated db/db mice. Representative wound images (left, all to the same scale) and H&E staining (right for day 14) for (c) wild-type and (d) db/db mice. Re-epithelialization is indicated by black dotted line. Scale bars in panels c and d are 100 Um. (e) In-situ zymography with MMP fluorogenic substrate DQ-gel (green in left panels) merged with nuclear DNA staining by DAPI (blue in right panels); scale bars, 25 Um. The use of ND-322 diminished the MMP-9 activity in vivo, evidenced by diminution of fluorescence (lower left).
Differences in wound healing in diabetic and wild-type mice treated with vehicle were statistically significant on days 7, 10, and 14 (day 7: 35 ± 21% vs. 65 ± 15%, p < 0.01; day 10: 53 ± 19% vs. 83 ± 9%, p < 0.01; day 14: 74 ± 12% vs. 98 ± 1%, p < 0.01). Wounds in wild-type mice were essentially healed on day 14 (Figure 2c, top left), while those in diabetic mice lagged behind significantly (Figure 2d, top left). Hematoxylin-eosin (H&E) staining revealed that wild-type mice showed complete re-epithelialization on day 14 (Figure 2c, top right), whereas diabetic mice treated with vehicle had partial re-epithelialization (Figure 2d, top right).
Topical treatment of wounds with ND-322 in wild-type mice did not accelerate healing, compared to vehicle-treatment (day 7: 62 ± 19% vs. 65 ± 15%, n = 21; day 10: 82 ± 11% vs. 83 ± 9%, n = 14; day 14: 96 ± 4% vs. 98 ± 1%, n = 7; p > 0.25, Figure 2b). Since active MMP-9 is not upregulated in wounds of wild-type mice, treatment with an MMP-9 inhibitor does not appear to have any beneficial effect on wound healing in wild-type animals.
In contrast, topical treatment of wounds with ND-322 in diabetic mice accelerated wound healing (Figure 2b). On days 1, 3, and 7, wound healing in ND-322-treated and vehicle-treated diabetic mice was not statistically significant (p > 0.2, n = 35, 28, and 21 on days 1, 3, and 7, respectively). On days 10 and 14, wound healing was significantly greater in ND-322-treated diabetic mice than in vehicle-treated diabetic mice (day 10: 70 ± 16% vs. 53 ± 19%, p < 0.05, n = 14; day 14: 92 ± 4% vs. 74 ± 12%, p < 0.01, n = 7). Remarkably, the extent of wound healing of diabetic mice treated with ND-322 on day 14 was comparable to that of wild-type mice (92 ± 4% vs. 96 ± 4%, p > 0.14, n = 7). Not only was wound healing in ND-322-treated diabetic mice more rapid, it also entailed complete re-epithelialization (Figure 2d, bottom right); as is true for wild-type vehicle-treated wounds (Figure 2c, top right) and wild-type ND-322-treated wounds (Figure 2c, bottom right).
In-situ zymography detects MMP activity localized within tissues by fluorescence. This method showed considerable gelatinase activity (due to MMP-9) in wound tissues of diabetic mice treated with vehicle (Figure 2e, top left), which was significantly decreased on treatment with ND-322 (Figure 2e, bottom left). Nuclei, as visualized with DAPI in vehicle-treated mice, were comparable to those in ND-322-treated animals (Figure 2e, top right and bottom right).
If active MMP-8 is involved in repairing diabetic wounds, can we assess its beneficial role by selective inhibition of its activity? Compound 3 (Figure 3a) has been reported as a non-zinc chelating selective MMP-8 inhibitor.25 We synthesized racemic compound 3 and documented its selectivity toward inhibition of MMP-8 by enzymology. Compound 3 inhibits MMP-8 with a Ki value of 25 ± 2 nM, while it inhibits MMP-9 poorly with a Ki value of 76 ± 8 μM. We evaluated this inhibitor in the excisional diabetic wound model (Figure 3b). Wound healing was delayed in MMP-8 inhibitor-treated wounds (Figures 3b and 3c, 33 ± 14% vs. 46 ± 19% on day 10, p = 0.23, n = 6; 58 ± 9% vs. 73 ± 10% on day 14, p = 0.02, n = 6). Re-epithelialization in MMP-8 inhibitor-treated wounds (Figure 3c, bottom left) was decreased relative to vehicle controls (Figure 3c, top left). In-situ zymography showed that treatment with the MMP-8 inhibitor significantly decreased collagenase activity (due to MMP-8, Figure 3d, bottom left) compared to vehicle-treated wounds (Figure 3d, top left), while the number of nuclei were comparable (Figure 3d, right).
Figure 3.
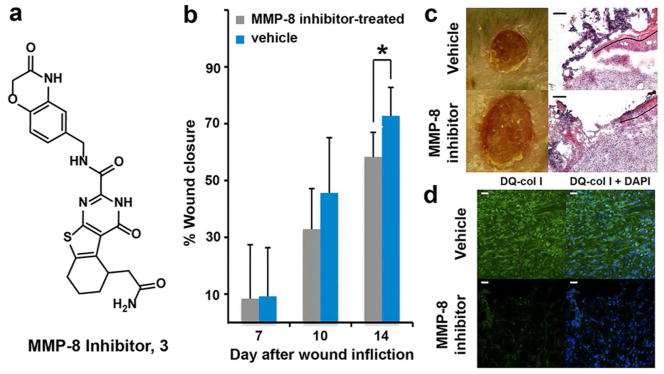
MMP-8 inhibition delays healing of db/db wounds. (a) Chemical structure of MMP-8 inhibitor 3. (b) Wound healing in db/db mice. Mean ± SD; n = 12, 6, and 6 on days 7, 10, and 14, respectively; *p < 0.05. (c) Representative wound images (left, all to the same scale) and H&E staining (right for day 14) for db/db mice. Re-epithelialization is indicated by the dotted black line; scale bars 100 Um. (d) In-situ zymography with MMP fluorogenic substrate DQ-col I (green in left panels) merged with nuclear DNA staining by DAPI (blue); scale bars, 25 Um.
MMP-9 activity is an instigator of apoptosis.26 Does treatment with ND-322 decrease apoptosis in diabetic wounds? Analyses of wound tissues by the terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) to detect DNA fragmentation resulting from apoptosis revealed that diabetic mice treated with vehicle showed numerous apoptotic cells (Figure 4, left). Apoptosis was significantly decreased in diabetic mice treated with ND-322 (Figure 4, center), while mice treated with the MMP-8 inhibitor (Figure 4, right) had similar number of apoptotic cells as vehicle-treated controls (Figure 4, left).
Figure 4.
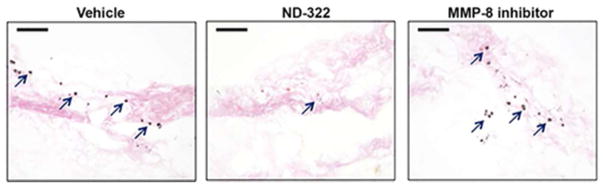
Representative TUNEL images of vehicle-, ND-322-, and MMP-8 inhibitor-treated wounds. Dark spots indicate apoptotic cells; arrows point to representative TUNEL-positive cells; scale bars, 100 Um.
We used a uniquely versatile inhibitor-tethered resin for identification of active MMP-8 and MMP-9 in both diabetic and non-diabetic wounds, and showed that the levels of the latter were elevated at statistically significant levels only in diabetic wounds. Working with the hypothesis that MMP-9 is detrimental to healing of diabetic wounds, but that MMP-8 likely plays a beneficial effect, we inhibited MMP-9 selectively by the use of ND-322. The diabetic wounds healed more rapidly in a process that involved re-epithelialization of the wounds, as is the case for the non-diabetic wounds in wild-type mice. In addition, apoptosis was significantly attenuated. We also used a selective MMP-8 inhibitor and showed that healing of the diabetic wounds was delayed, accompanied by decreased re-epithelialization, and undiminished apoptosis. The present study reveals a beneficial effect of selective inhibition of MMP-9 in healing of diabetic wounds. Whereas the use of the selective inhibitor ND-322 does not show any effect on non-diabetic wounds—neither detrimental nor beneficial—it is intriguing that the use of the broad-spectrum MMP inhibitor ilomastat (also known as GM-6001) in non-diabetic wounds in rats,27 pigs,28 and humans29 delayed wound closure and diminished epithelialization. These findings reveal that broad inhibition of the “good” and the “bad” MMPs simultaneously is detrimental to the wound-healing process. Clinical management of diabetic wounds presently involves merely debridement of the wound and attempts at keeping it clean and free of infection. The selective MMP-9 inhibition strategy that we have disclosed here is a first potential pharmacological intervention in treatment of diabetic wounds, which holds great promise in addressing an unmet medical need.
METHODS
Syntheses and Formulation of Inhibitors 2 and 3
Inhibitor 2 (ND-322) was synthesized as reported previously24 and dissolved in saline (5.0 mg mL−1). Inhibitor 3 was synthesized by the literature method;27 its spectroscopic data were identical to that reported in the literature.25 and was formulated in 80% propylene glycol/20% DMSO at a concentration of 5.0 mg mL−1. The dosing solutions and the vehicle solutions (saline or 80% propylene glycol/20% DMSO) were sterilized by filtration (Acrodisc syringe filter, Pall Life Sciences, 0.2 Um, 13 mm diameter, PTFE membrane).
Synthesis of MMP Inhibitor-tethered Resin
Resin 1 was synthesized in our laboratories in 12 synthetic steps as previously reported.17
MMP-Expression Profiling
Wound tissues (10 mg) were homogenized in 100 μL of cold lysis buffer (25 mM Tris-HCl pH 7.5, 100 mM NaCl, 1% v/v Nonidet P-40 and protease inhibitors, with the exception of metalloproteinase inhibitors), analyzed for protein concentration by the BCA protein assay, and mixed with 100 μL of resin 1 at 4 °C for 18 h. After centrifugation (15000 g, 1 min), the supernatant was removed, the resin beads were washed with CB buffer and water, and the resin bound proteins were subjected to trypsin-digest. The procedure for on-resin reduction, alkylation, and tryptic digestion was adapted from the Pierce In-Solution Tryptic Digestion Kit (Thermo Scientific). Briefly, proteins were covered with 100 mM dithiothreitol in HPLC grade water, incubated at 65 °C for 20 min, then cooled to room temperature. Samples were then alkylated (3 UL of 100 mM iodoacetamide in HPLC-grade water), followed by incubation in the dark at room temperature for 20 min. Samples were then enzymatically digested overnight at 37 °C with trypsin (2 μL of 0.1 Ug UL−1 in 50 mM ammonium bicarbonate).
Supplementary Material
Acknowledgments
We thank S. Chapman for the preparation of wound-tissue sections and M. Ikejiri for synthesis of the internal standard 4 (Supporting Information). This work was supported in part by a pilot core grant (to M.C.) from the Indiana-Clinical and Translational Science Institute, supported by grant RR025761 from the National Institutes of Health. M.G. is a Ruth L. Kirschstein National Research Service Award Fellow of the Chemistry-Biochemistry-Biology Interface Program at the University of Notre Dame, supported by training grant GM075762 from the National Institutes of Health.
Footnotes
The authors declare no competing financial interest.
Animals, excisional diabetic wound model, zymography, histological evaluation, in-situ zymography, apoptosis detection, quantification of MMPs in wound tissues, blood collection and sample analysis, liquid chromatography/mass spectrometry, and statistical analyses. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Centers for Disease Control and Prevention. 2011 National Diabetes Fact Sheet 2011 [Google Scholar]
- 2.Mustoe T. Understanding chronic wounds: a unifying hypothesis on their pathogenesis and implications for therapy. Am J Surg. 2004;187:65S–70S. doi: 10.1016/S0002-9610(03)00306-4. [DOI] [PubMed] [Google Scholar]
- 3.Alleva R, Nasole E, Di Donato F, Borghi B, Neuzil J, Tomasetti M. alpha-Lipoic acid supplementation inhibits oxidative damage, accelerating chronic wound healing in patients undergoing hyperbaric oxygen therapy. Biochem Biophys Res Commun. 2005;333:404–410. doi: 10.1016/j.bbrc.2005.05.119. [DOI] [PubMed] [Google Scholar]
- 4.Dalton SJ, Mitchell DC, Whiting CV, Tarlton JF. Abnormal extracellular matrix metabolism in chronically ischemic skin: a mechanism for dermal failure in leg ulcers. J Invest Dermatol. 2005;125:373–379. doi: 10.1111/j.0022-202X.2005.23789.x. [DOI] [PubMed] [Google Scholar]
- 5.Rayment EA, Upton Z, Shooter GK. Increased matrix metalloproteinase-9 (MMP-9) activity observed in chronic wound fluid is related to the clinical severity of the ulcer. Br J Dermatol. 2008;158:951–961. doi: 10.1111/j.1365-2133.2008.08462.x. [DOI] [PubMed] [Google Scholar]
- 6.Toth M, Fridman R. Assessment of Gelatinases (MMP-2 and MMP-9 by Gelatin Zymography. Methods Mol Med. 2001;57:163–174. doi: 10.1385/1-59259-136-1:163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Agrawal SM, Lau L, Yong VW. MMPs in the central nervous system: where the good guys go bad. Semin Cell Dev Biol. 2008;19:42–51. doi: 10.1016/j.semcdb.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 8.Fisher JF, Mobashery S. Mechanism-based profiling of MMPs. In: Clark IM, editor. Matrix Metalloproteinase Protocols. Humana Press; 2010. pp. 471–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barglow KT, Cravatt BF. Activity-based protein profiling for the functional annotation of enzymes. Nat Methods. 2007;4:822–827. doi: 10.1038/nmeth1092. [DOI] [PubMed] [Google Scholar]
- 10.Evans MJ, Cravatt BF. Mechanism-based profiling of enzyme families. Chem Rev. 2006;106:3279–3301. doi: 10.1021/cr050288g. [DOI] [PubMed] [Google Scholar]
- 11.Saghatelian A, Jessani N, Joseph A, Humphrey M, Cravatt BF. Activity-based probes for the proteomic profiling of metalloproteases. Proc Natl Acad Sci U S A. 2004;101:10000–10005. doi: 10.1073/pnas.0402784101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sieber SA, Cravatt BF. Analytical platforms for activity-based protein profiling--exploiting the versatility of chemistry for functional proteomics. Chem Commun (Camb) 2006:2311–2319. doi: 10.1039/b600653c. [DOI] [PubMed] [Google Scholar]
- 13.Sieber SA, Niessen S, Hoover HS, Cravatt BF. Proteomic profiling of metalloprotease activities with cocktails of active-site probes. Nat Chem Biol. 2006;2:274–281. doi: 10.1038/nchembio781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Freije JR, Bischoff R. Activity-based enrichment of matrix metalloproteinases using reversible inhibitors as affinity ligands. J Chromatogr A. 2003;1009:155–169. doi: 10.1016/s0021-9673(03)00920-8. [DOI] [PubMed] [Google Scholar]
- 15.Freije JR, Klein T, Ooms JA, Franke JP, Bischoff R. Activity-based matrix metalloprotease enrichment using automated, inhibitor affinity extractions. J Proteome Res. 2006;5:1186–1194. doi: 10.1021/pr050483b. [DOI] [PubMed] [Google Scholar]
- 16.Freije R, Klein T, Ooms B, Kauffman HF, Bischoff R. An integrated high-performance liquid chromatography-mass spectrometry system for the activity-dependent analysis of matrix metalloproteases. J Chromatogr A. 2008;1189:417–425. doi: 10.1016/j.chroma.2007.10.059. [DOI] [PubMed] [Google Scholar]
- 17.Hesek D, Toth M, Krchnak V, Fridman R, Mobashery S. Synthesis of an inhibitor-tethered resin for detection of active matrix metalloproteinases involved in disease. J Org Chem. 2006;71:5848–5854. doi: 10.1021/jo060058h. [DOI] [PubMed] [Google Scholar]
- 18.Sullivan SR, Underwood RA, Gibran NS, Sigle RO, Usui ML, Carter WG, Olerud JE. Validation of a model for the study of multiple wounds in the diabetic mouse (db/db) Plast Reconstr Surg. 2004;113:953–960. doi: 10.1097/01.prs.0000105044.03230.f4. [DOI] [PubMed] [Google Scholar]
- 19.Galiano RD, Michaels Jt, Dobryansky M, Levine JP, Gurtner GC. Quantitative and reproducible murine model of excisional wound healing. Wound Repair Regen. 2004;12:485–492. doi: 10.1111/j.1067-1927.2004.12404.x. [DOI] [PubMed] [Google Scholar]
- 20.Lobmann R, Ambrosch A, Schultz G, Waldmann K, Schiweck S, Lehnert H. Expression of matrix-metalloproteinases and their inhibitors in the wounds of diabetic and non-diabetic patients. Diabetologia. 2002;45:1011–1016. doi: 10.1007/s00125-002-0868-8. [DOI] [PubMed] [Google Scholar]
- 21.Wall SJ, Bevan D, Thomas DW, Harding KG, Edwards DR, Murphy G. Differential expression of matrix metalloproteinases during impaired wound healing of the diabetes mouse. J Invest Dermatol. 2002;119:91–98. doi: 10.1046/j.1523-1747.2002.01779.x. [DOI] [PubMed] [Google Scholar]
- 22.Gutierrez-Fernandez A, Inada M, Balbin M, Fueyo A, Pitiot AS, Astudillo A, Hirose K, Hirata M, Shapiro SD, Noel A, Werb Z, Krane SM, Lopez-Otin C, Puente XS. Increased inflammation delays wound healing in mice deficient in collagenase-2 (MMP-8) FASEB J. 2007;21:2580–2591. doi: 10.1096/fj.06-7860com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Forbes C, Shi Q, Fisher JF, Lee M, Hesek D, Llarrull LI, Toth M, Gossing M, Fridman R, Mobashery S. Active site ring-opening of a thiirane moiety and picomolar inhibition of gelatinases. Chem Biol Drug Des. 2009;74:527–534. doi: 10.1111/j.1747-0285.2009.00881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gooyit M, Lee M, Schroeder VA, Ikejiri M, Suckow MA, Mobashery S, Chang M. Selective water-soluble gelatinase inhibitor prodrugs. J Med Chem. 2011;54:6676–6690. doi: 10.1021/jm200566e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pochetti G, Montanari R, Gege C, Chevrier C, Taveras AG, Mazza F. Extra binding region induced by non-zinc chelating inhibitors into the S1′ subsite of matrix metalloproteinase 8 (MMP-8) J Med Chem. 2009;52:1040–1049. doi: 10.1021/jm801166j. [DOI] [PubMed] [Google Scholar]
- 26.Gu Z, Kaul M, Yan B, Kridel SJ, Cui J, Strongin A, Smith JW, Liddington RC, Lipton SA. S-nitrosylation of matrix metalloproteinases: signaling pathway to neuronal cell death. Science. 2002;297:1186–1190. doi: 10.1126/science.1073634. [DOI] [PubMed] [Google Scholar]
- 27.Mirastschijski U, Haaksma CJ, Tomasek JJ, Agren MS. Matrix metalloproteinase inhibitor GM 6001 attenuates keratinocyte migration, contraction and myofibroblast formation in skin wounds. Exp Cell Res. 2004;299:465–475. doi: 10.1016/j.yexcr.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 28.Agren MS. Matrix metalloproteinases (MMPs) are required for re-epithelialization of cutaneous wounds. Arch Dermatol Res. 1999;291:583–590. doi: 10.1007/s004030050459. [DOI] [PubMed] [Google Scholar]
- 29.Agren MS, Mirastschijski U, Karlsmark T, Saarialho-Kere UK. Topical synthetic inhibitor of matrix metalloproteinases delays epidermal regeneration of human wounds. Exp Dermatol. 2001;10:337–348. doi: 10.1034/j.1600-0625.2001.100506.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.