Abstract
Objectives:
Recent studies have shown that brief periods of mechanical ventilation (MV) in animals and humans can lead to ventilator induced diaphragmatic dysfunction (VIDD), which includes muscle atrophy, reduced force development and impaired mitochondrial function. Animal work has shown that short periods of increased diaphragm activity during MV support can attenuate VIDD, but corresponding human data are lacking. The purpose of this study was to examine the effect of intermittent diaphragm contractions during cardiothoracic surgery, including controlled MV, on mitochondrial respiration in the human diaphragm.
Method:
In five patients (age 65.6 ± 6.3 yrs) undergoing cardiothoracic surgery, one phrenic nerve was stimulated hourly (30 pulses per minute, 1.5 msec duration, 17.0 ± 4.4 mA) during the surgery. Subjects received 3.4 ± 0.6 stimulation bouts during surgery. Thirty minutes following the last stimulation bout, samples of diaphragm muscle were obtained from the antero-lateral costal regions of the stimulated and inactive hemidiaphragms. Mitochondrial respiration was measured in permeabilized muscle fibers with high-resolution respirometry.
Results:
State III mitochondrial respiration rates (pmol O2/sec/mg wet weight) were 15.05 ± 3.92 and 11.42 ± 2.66 for the stimulated and unstimulated samples respectively, p < 0.05. State IV mitochondrial respiration rates were 3.59 ± 1.25 and 2.11 ± 0.97 in the stimulated samples and controls samples, respectively, p < 0.05.
Conclusion:
These are the first data examining the effect of intermittent contractions on mitochondrial respiration rates in the human diaphragm following surgery/MV. Our results indicate that very brief periods (duty cycle ~1.7%) of activity can improve mitochondrial function in the human diaphragm following surgery/MV.
Keywords: ventilator induced diaphragm dysfunction, mechanical ventilation, diaphragm stimulation, phrenic nerve, mitochondrial respiration, thoracic surgery
Inspiratory pressure developed by diaphragmatic contractions is a major determinant of the ability to wean patients from mechanical ventilation (MV) (1). Growing evidence from animal models (2) documents that MV use can induce ventilator-induced diaphragmatic dysfunction (VIDD). Humans have been reported to also be susceptible to VIDD by many (3-7), but not all researchers (8). Mitochondrial dysfunction is also a component of VIDD, as demonstrated by increased reactive oxygen species (ROS) emission and diminished respiration (9, 10).
Diaphragm activity prior to and during MV has been shown to attenuate the contractile component of VIDD in murine models of MV. Smuder et al recently reported in a rat model that endurance training prior to a 12 hour bout of controlled MV offered protection against MV-induced diaphragm mitochondrial oxidative damage and uncoupling of oxidative phosphorylation (10). Gayan-Ramirez et al. found that allowing rats to breathe spontaneously for 5 minutes every 6 hours reduced diaphragm contractile losses by ~45% over 24 hours of controlled MV (11). Sassoon compared animals receiving controlled MV versus assist control ventilation, which requires some diaphragm activity. Animals receiving assist control MV support maintained diaphragm strength to a greater extent than animals receiving controlled MV (12).
Humans have been found to be susceptible to developing VIDD following exposure to MV support (3-6). Long-term intermittent diaphragm activity has been shown to offer some protection from VIDD in humans. In a tetraplegic patient, 30 min of pacing one hemidiaphragm each day attenuated atrophy of the paced hemidiaphragm over eight months of controlled MV compared to a non-paced hemidiaphragm (13). There is wide spread appreciation of VIDD in animal models and human patients, but no data examining the effects of diaphragm contractile activity on mitochondrial respiration following surgery/MV in humans exists.
The purpose of this brief report was to examine the effect of intermittent diaphragm twitches during cardiothoracic surgery/MV on diaphragm mitochondrial respiration in humans. We hypothesized that intermittent hemidiaphragm stimulation during surgery/MV would result in higher mitochondrial respiration rates in the stimulated hemidiaphragm compared to the unstimulated hemidiaphragm in the same subject.
Methods
Subjects
Five patients undergoing elective cardiothoracic surgery at Shands Hospital at the University of Florida were recruited. Demographic and operative data are given in the Table. Postoperative weaning difficulties are rare in patients undergoing elective cardiothoracic surgery and for ethical considerations, we chose to study patients that we believed were at low risk for postoperative complications and weaning difficulties. The University of Florida IRB approved this study, and all subjects consented to participation.
Table.
Patient demographics and surgery description
| Pt # | Age | Gender | Height (cm) |
Mass (kg) |
MV start to biopsy (hrs) |
Surgical procedure |
|---|---|---|---|---|---|---|
| 1 | 58 | M | 183 | 78.2 | 5.3 | Aortic valve replacement, aortic repair |
| 2 | 61 | F | 165 | 73.2 | 4.2 | Aortic valve replacement, aortic repair,
Florida Sleeve, ligation of patent ductus arteriosus |
| 3 | 69 | M | 173 | 76.4 | 5.0 | Aortic valve replacement, coronary artery
bypass graft |
| 4 | 74 | F | 166 | 90.9 | 5.6 | Replacement of the ascending aorta, aortic
repair |
| 5 | 66 | F | 163 | 65.5 | 4.5 | Aortic valve replacement, mitral valve
replacement, coronary artery bypass graft |
| Mean | 65.6 ± 6.3 | 170.0 ±8.2 | 76.8 ± 9.2 | 4.92 ± 0.54 |
Exclusion criteria
prior surgery to the heart, diaphragm, pleura or phrenic nerves resulting in anatomical changes that would complicate obtaining muscle samples or interfere with phrenic stimulation
neuromuscular or inflammatory muscle diseases,
obstructive lung disease (FEV1.0 < 60% of predicted),
other lung disease (bronchiectasis, lung cancer, pulmonary hypertension, tuberculosis or pulmonary fibrosis etc.),
NYHA Class IV heart failure,
implanted cardiac pacemaker or defibrillators,
use of immunosuppressants, corticosteroids or aminoglycoside antibiotics within 60 days of surgery and
serum creatinine > 1.6 mg/dl.
Anesthetic management
Medications used during induction included Etomidate or Propofol, plus Midazolam and Fentanyl, as well as Pancuronium and Vecuronium, which were not reversed at the end of the case. Patients underwent tracheal intubation, arterial line, central venous line and pulmonary artery catheter placement, intra-operative evaluation by transesophageal echocardiography, and were maintained under general anesthesia with Isoflurane or Sevoflurane. Vasoactive agents, such as Epinephrine or Nitroglycerin, were started if needed at the end of cardiopulmonary bypass. Supplemental Digital Content 1 provides a complete listing of medications used for each patient.
Diaphragm stimulation
All patients underwent midline sternotomies. The right and left phrenic nerves were alternately selected between patients for stimulation with an external cardiac pacer (Medtronic 5388) with temporary cardiac pacing wire electrodes*. The pacing wires were sutured adjacent (~ 5 mm) to either side of the phrenic nerve on the stimulated side in the upper thoracic space. Once appropriate locations for the stimulating electrodes were identified, the wires remained in the same location for the entire duration of the experiment. Phrenic stimulation was initiated at 5 mA and increased by 3-5 mA until hemidiaphragm twitches were observed. The stimulus intensity was then increased to three times the threshold value up to the stimulator’s maximal setting of 25 mA. Stimulation was conducted for one minute (30 pulses per minute, 1.5 msec duration) as soon as the phrenic nerve and diaphragm were exposed and hourly thereafter. Adequacy of the stimulation was determined by visually observing hemidiaphragm contractions entrained with stimulation (see video in online supplement 2). Full thickness diaphragm samples (20- 50 mg) were obtained 30 minutes following the last stimulation bout.
Determination of mitochondrial function using high-resolution respirometry of permeabilized muscle fibers Permeabilized diaphragm muscle samples were prepared for respirometry as described Kuznetsov, A.V., et al (14) and analyzed by a blinded investigator. Briefly, small portions (~10-15 mg wet weight) of freshly collected muscle were dissected and placed in ice-cold Buffer X, containing 60 mM K-MES, 35 mM KCl, 7.23 mM K2EGTA, 2.77 mM CaK2EGTA, 20 mM imidazole, 0.5 mM DTT, 20 mM taurine, 5.7 mM ATP, 15 mM PCr, and 6.56 mM MgCl2·6H2O (pH 7.1). Skeletal muscle strips were separated into thin muscle-fiber bundles, permeabilized in Buffer X containing 50 μg/mL saponin, and incubated on a rotator for 30 min at 4°C. The permeabilized fiber bundles were then placed in ice-cold Buffer Z containing 110 mM K-MES, 35 mM KCl, 1 mM EGTA, 5 mM K2HPO4, 3 mM MgCl2·6H2O, 0.005 mM glutamate, 0.002 mM malate, and 0.05% BSA (pH 7.1). Permeabilized muscle fibers remained in Buffer Z on a rotator at 4°C for 45 min prior to being incubated for 10 min in Buffer Z containing 10mM sodium pyrophosphate. Buffer Z containing 20 mM creatinine was added to the high-resolution Oxygraph 2k (OROBOROS INSTRUMENTS, Innsbruck, Austria), equilibrated to the required experimental temperature (37 °C) and oxygenated with atmospheric oxygen to ~500 μM O2. The permeabilized muscle fiber bundles were blotted dry, weighed and added to the chamber. Respiratory function of mitochondria and flux ratios were determined using the following titration protocol (final concentrations and assessment in parenthesis). After assessment of resting respiration, the following reagents were added: pyruvate/malate (5mM/2mM; State IV); ADP (1-5 mM; State III, complex I supported respiration); cytochrome c (10 μM; integrity of the outer mitochondrial membrane); succinate (10 mM; State III, complex II supported respiration); oligomycin (2 μg mL−1; inhibition of ATP synthase); stepwise titration with the uncoupler FCCP (0.5-5 μM; ETC capacity and limitation of OXPHOS relative to ETC by the phosphorylation system).
The respiratory control ratio (RCR) was determined by calculating the ratio of State III to State IV. The RCR is a general measure of mitochondrial function and higher values denote better function. State IV respiration is a model of steady-state, basal activity, while State III respiration is measured following the addition of ADP and mimics mitochondrial activity during exercise with high oxygen consumption and ATP turnover (15).
Data Analysis
T tests for matched pairs were used to compare distributions and statistical significance was set at p < .05. Data are shown mean ± SD.
Results and Discussion
All subjects tolerated the stimulation and biopsy procedures without complication, and were successfully extubated on the first attempt within 30 hours of leaving the operating room. None of the patients exhibited elevated hemidiaphragms on post-operative chest X rays.
The first stimulation period was administered as soon as the phrenic nerve and diaphragm could be safely visualized (1.6 ± 0.3 hours following the start of MV). Subjects received 3.4 ± 0.6 stimulation bouts and the mean time from the start of MV support to obtaining the muscle samples was 4.92 ± 0.54 hours. The mean stimulation amplitude was 17.0 ± 4.4 mA. Four subjects received the neuromuscular blockers Vecuronium or Pancuronium (8 mg ± 0.8) during anesthetic induction. Two subjects received small doses of Vecuronium or Pancuronium during the early stages of surgery. Vigorous hemidiaphragm twitches were observed during all stimulation bouts. Supplemental Digital Content 2, contains a brief movie of a representative stimulation bout showing a left hemidiaphragm contracting in response to stimulation. The neuromuscular blocking drugs doses administered were low and given primarily during the intubation/anesthetic induction phase of the procedure approximately 1.5 hours before the first stimulation bout. The diaphragm is more resistant to non-depolarizing neuromuscular blocking agents than limb muscle (16). The average esophageal temperature at the time of the stimulation periods and tissue harvest was 31.3 ± 2.6°C. Muscle samples were obtained 29 ± 3 minutes following the last stimulation period.
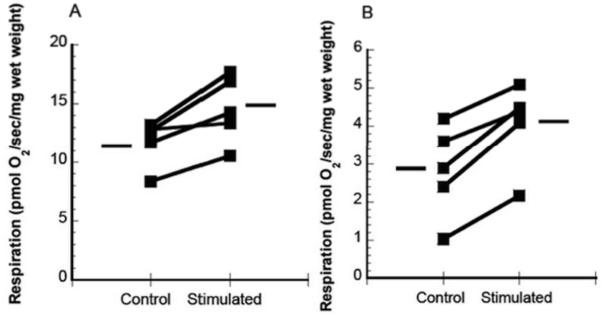
State III mitochondrial respiration rates (pmol O2/sec/mg wet weight) were 15.05 ± 3.92 and 11.42 ± 2.66 in the stimulated and unstimulated samples respectively, p < 0.05, Fig. State IV respiration rates were 3.59 ± 1.25 and 2.11 ± 0.97 (pmol O2/sec/mg wet weight) in the stimulated samples and controls samples, respectively, p < 0.05, Fig. The RCR for the stimulated samples was 3.75 ± .86 and 4.87 ± 2.08 for the unstimulated samples, p = 0.08. Supplemental Digital Content 3 contains a table with substrate-uncoupler-inhibitor respiration data. Post hoc power calculations (N = 5, two-tailed, p = .05) revealed statistical power of .83 and .93 for States III and IV, respectively.
The human diaphragm is a highly active muscle, with a duty cycle of approximately 30%. This high level of chronic activity may explain why the diaphragm is highly susceptible to VIDD following short periods of MV-induced inactivity. Mitochondrial dysfunction, including increased reactive oxygen species emissions and impaired respiration has been documented in animals following brief (~6-12 hours) periods of MV support (9, 10). Several laboratories have shown that the human diaphragm is susceptible to VIDD (4-7, 17, 18), and recent work has reported altered mitochondrial gene expression and impaired mitochondrial respiration, accompanied by elevated oxidative stress in the diaphragm tissue of mechanically-ventilated humans during surgery (19). The fact that 30 contractions per minute for 1 minute of every hour (~1.7% duty cycle) increased mitochondrial respiration suggests that brief periods of activity during MV support may offer some protection against mitochondrial dysfunction. Additional studies are needed to identify the cellular and/or molecular basis of the improved mitochondrial respiration we observed. The cardiothoracic surgery/MV scenario we studied is not just a model of diaphragm inactivity as is possible with experimental animal models. Prolonged surgeries have well known systemic inflammatory effects (20), however major surgery is a frequent precursor of weaning difficulty, and thus our methodology is clinically relevant.
In summary, we found that periodic hemidiaphragm stimulation during cardiothoracic surgery/MV induced physiologically important changes in State III and IV mitochondrial respiration in the active hemidiaphragms. Further studies examining the cellular basis of our results and the effects of stimulation and exercise training on the human diaphragm as a possible modality to prevent or treat VIDD are warranted.
Supplementary Material
Medications used during intubation and anesthesia induction and redoses during surgery for each subject.
The video was taken looking into the thoracic cavity from the head of the operating table. A green arrow will appear in the video pointing to the left hemidiaphragm contracting and separating from the inferior surface of the heart once every two seconds in response to left phrenic nerve stimulation.
author name: Daniel Martin
videographer: Daniel Martin
participant: study subject
length: 15 seconds
size: 4 MB
Respiration data for substrate-uncoupler-inhibitor protocols.
Individual data points for State III (A) and State IV (B) mitochondrial respiration in permeabilized fiber bundles in the stimulated and control hemidiaphragms. Simulated vs control, p < 0.05 for both States. The horizontal lines indicate the means for each condition.
Acknowledgments
This study was supported by the University of Florida Clinical Translational Science Institute and Pilot grant (UL1 RR029890), the Claude D. Pepper Older Americans Independence Center (OAIC) Metabolism and Translational Science Core (CL) and K12-HD055929 (BKS). The OAIC is supported by a grant from the National Institutes of Health/National Institute on Aging (1P30AG028740).
We are grateful Nancy Staples, RN for assisting in the recruitment of the subjects and Carlos Campos for video editing. We would also like to thank the subjects for their participation.
Dr. Beaver, Dr. Martin, Dr. Joseph, Dr. Smith, Dr. Hess, Dr. Berg, Dr. Leeuwenburgh’s institution received grant support from the National Institutes of Health (NIH). Dr. Deoghare, Dr. Beaver, and Dr. Martin received grant support from NIH. Dr. Beaver received grants from the State of Florida, Atricure, and Terumo. Dr. Christiaan is employed by the University of Florida. Dr. Martin consults for Terumo. Dr. Beaver consults for Atricure, Mardil, and Abbott. Dr. Martin was issued a U.S. patent to alter clinical mechanical ventilators to allow patients to undergo inspiratory muscle strength training while receiving mechanical ventilation support (#8307827).
Footnotes
This work was performed at the university of Florida, Gainesville, FL.
AE Medical Corp. Farmingdale, NJ. Myowire temporary cardiac pacing wire, Cat # 025-200
Contributor Information
A. Daniel Martin, Department of Physical Therapy, University of Florida.
Anna M. Joseph, Department of Aging and Geriatric Research, University of Florida.
Thomas M. Beaver, Department of Surgery, Division of Cardiothoracic Surgery, University of Florida.
Barbra K. Smith, Department of Physical Therapy, University of Florida.
Tomas D. Martin, Department of Surgery, Division of Cardiothoracic Surgery, University of Florida.
Kent Berg, Department of Anesthesiology, University of Florida.
Philip J. Hess, Department of Surgery, Division of Cardiothoracic Surgery, University of Florida.
Harsha V. Deoghare, Department of Physical Therapy (current Address: Department of Physical Therapy California State University, Fresno, CA 93740.
Christiaan Leeuwenburgh, Department of Aging and Geriatric Research, University of Florida.
References
- 1.Karakurt Z, Fanfulla F, Ceriana P, et al. Physiologic determinants of prolonged mechanical ventilation in patients after major surgery. J Crit Care. 2012;27:221. doi: 10.1016/j.jcrc.2011.08.009. e229-216. [DOI] [PubMed] [Google Scholar]
- 2.Vassilakopoulos T. Ventilator-induced diaphragm dysfunction: the clinical relevance of animal models. Intensive Care Med. 2008;34:7–16. doi: 10.1007/s00134-007-0866-x. [DOI] [PubMed] [Google Scholar]
- 3.Hermans G, Agten A, Testelmans D, et al. Increased duration of mechanical ventilation is associated with decreased diaphragmatic force: a prospective observational study. Crit Care. 20101;14:R127. doi: 10.1186/cc9094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hussain SN, Mofarrahi M, Sigala I, et al. Mechanical ventilation-induced diaphragm disuse in humans triggers autophagy. Am J Respir Crit Care Med. 2010;182:1377–1386. doi: 10.1164/rccm.201002-0234OC. [DOI] [PubMed] [Google Scholar]
- 5.Levine S, Nguyen T, Taylor N, et al. Rapid disuse atrophy of diaphragm fibers in mechanically ventilated humans. N Engl J Med. 2008;358:1327–1335. doi: 10.1056/NEJMoa070447. [DOI] [PubMed] [Google Scholar]
- 6.Knisely AS, Leal SM, Singer DB. Abnormalities of diaphragmatic muscle in neonates with ventilated lungs. J Pediatr. 1988;113:1074–1077. doi: 10.1016/s0022-3476(88)80585-7. [DOI] [PubMed] [Google Scholar]
- 7.Huang TT, Deoghare HV, Smith BK, et al. Gene expression changes in the human diaphragm after cardiothoracic surgery. J Thorac Cardiovasc Surg. 2011;142:1214–1222. doi: 10.1016/j.jtcvs.2011.02.025. 1222 e1211-1220. [DOI] [PubMed] [Google Scholar]
- 8.Hooijman PE, van Hees HW, Paul MA, et al. Preservation Of Diaphragm Muscle Fiber Contractility In Mechanically Ventilated Humans. Am J Respir Crit Care Med. 2012;185:A6871. [Google Scholar]
- 9.Kavazis AN, Talbert EE, Smuder AJ, et al. Mechanical ventilation induces diaphragmatic mitochondrial dysfunction and increased oxidant production. Free Radic Biol Med. 2009;46:842–850. doi: 10.1016/j.freeradbiomed.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smuder AJ, Min K, Hudson MB, et al. Endurance exercise attenuates ventilator-induced diaphragm dysfunction. J Appl Physiol. 2011:501–510. doi: 10.1152/japplphysiol.01086.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gayan-Ramirez G, Testelmans D, Maes K, et al. Intermittent spontaneous breathing protects the rat diaphragm from mechanical ventilation effects. Crit Care Med. 2005;33:2804–2809. doi: 10.1097/01.ccm.0000191250.32988.a3. [DOI] [PubMed] [Google Scholar]
- 12.Sassoon CS, Zhu E, Caiozzo VJ. Assist-control mechanical ventilation attenuates ventilator-induced diaphragmatic dysfunction. Am J Respir Crit Care Med. 2004;170:626–632. doi: 10.1164/rccm.200401-042OC. [DOI] [PubMed] [Google Scholar]
- 13.Ayas NT, McCool FD, Gore R, et al. Prevention of human diaphragm atrophy with short periods of electrical stimulation. Am J Respir Crit Care Med. 1999;159:2018–2020. doi: 10.1164/ajrccm.159.6.9806147. [DOI] [PubMed] [Google Scholar]
- 14.Kuznetsov AV, Veksler V, Gellerich FN, et al. Analysis of mitochondrial function in situ in permeabilized muscle fibers, tissues and cells. Nature protocols. 2008;3:965–976. doi: 10.1038/nprot.2008.61. [DOI] [PubMed] [Google Scholar]
- 15.Di Meo S, Venditti P. Mitochondria in exercise-induced oxidative stress. Biol Signals Recept. 2001;10:125–140. doi: 10.1159/000046880. [DOI] [PubMed] [Google Scholar]
- 16.Moerer O, Baller C, Hinz J, et al. Neuromuscular effects of rapacuronium on the diaphragm and skeletal muscles in anaesthetized patients using cervical magnetic stimulation for stimulating the phrenic nerves. Eur J Anaesthesiol. 2002;19:883–887. doi: 10.1017/s0265021502001412. [DOI] [PubMed] [Google Scholar]
- 17.Jaber S, Petrof BJ, Jung B, et al. Rapidly progressive diaphragmatic weakness and injury during mechanical ventilation in humans. Am J Respir Crit Care Med. 2011;183:364–371. doi: 10.1164/rccm.201004-0670OC. [DOI] [PubMed] [Google Scholar]
- 18.Welvaart WN, Paul MA, Stienen GJ, et al. Selective diaphragm muscle weakness after contractile inactivity during thoracic surgery. Ann Surg. 2011;254:1044–1049. doi: 10.1097/SLA.0b013e318232e75b. [DOI] [PubMed] [Google Scholar]
- 19.Tang H, Lee M, Budak MT, et al. Intrinsic apoptosis in mechanically ventilated human diaphragm: linkage to a novel Fos/FoxO1/Stat3-Bim axis. FASEB J. 2011;25:2921–2936. doi: 10.1096/fj.11-183798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Becher RD, Hoth JJ, Miller PR, et al. Systemic inflammation worsens outcomes in emergency surgical patients. The journal of trauma and acute care surgery. 2012;72:1140–1149. doi: 10.1097/TA.0b013e3182516a97. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Medications used during intubation and anesthesia induction and redoses during surgery for each subject.
The video was taken looking into the thoracic cavity from the head of the operating table. A green arrow will appear in the video pointing to the left hemidiaphragm contracting and separating from the inferior surface of the heart once every two seconds in response to left phrenic nerve stimulation.
author name: Daniel Martin
videographer: Daniel Martin
participant: study subject
length: 15 seconds
size: 4 MB
Respiration data for substrate-uncoupler-inhibitor protocols.