Abstract
The aim of the study was to investigate the effect of different support surfaces on feedforward and feedback components of postural control. Nine healthy subjects were exposed to external perturbations applied to their shoulders while standing on a rigid platform, foam, and wobble board with eyes open or closed.
Electrical activity of nine trunk and leg muscles and displacements of the center of pressure were recorded and analyzed during the time frames typical of feedforward and feedback postural adjustments. Feedforward control of posture was characterized by earlier activation of anterior muscles when the subjects stood on foam compared to a wobble board or a firm surface. In addition, the magnitude of feedforward muscle activity was the largest when the foam was used. During the feedback control, anterior muscles were activated prior to posterior muscles irrespective of the nature of surface. Moreover, the largest muscle activity was seen when the supporting surface was foam. Maximum CoP displacement occurred when subjects were standing on a rigid surface.
Altering support surface affects both feedforward and feedback components of postural control. This information should be taken into consideration in planning rehabilitation interventions geared towards improvement of balance.
Keywords: Postural control, support surface, EMG
Introduction
Control of upright posture requires a unique integration of inputs from the three major sensory systems of the body: visual, vestibular, and somatosensory (Manchester et al., 1989, Nashner and Berthoz, 1978). It is believed that important afferent information that is necessary to maintain posture, comes from the two types of specialized mechanoreceptors located on the sole of the feet (Magnusson et al., 1990). Slowly adapting mechanoreceptors provide spatial information about the pressure distribution between the feet and the ground whereas rapidly adapting mechanoreceptors provide information about the amplitude and changes in the pressure distribution (Kavounoudias et al., 1999). Also, it is important to note that such mechanoreceptors not only supply information about surface contact pressure (Vallbo and Johansson, 1984), but they also help sense small continuous changes of posture.
Individuals with diabetic neuropathy, elderly individuals with peripheral neuropathy, or individuals with traumatic injury that involves one of the nerves of the lower extremity, commonly have diminished ability to utilize somatosensory information (Greene DA, 1990, van Deursen and Simoneau, 1999). Balance assessment techniques frequently involve standing on a rigid surface or foam, a more compliant supporting surface positioned on the top of the force platform (Allum et al., 2002, NeuroCom, 2010). It is also reported that wobble boards could be used in balance retraining (Burton, 1986).
Both foam and wobble board distort the normal proprioceptive inputs from the lower extremity. Standing on a compliant surface such as foam induces body instability in both the sagittal and frontal planes and also alters inputs to both joint receptors and cutaneous mechanoreceptors in the sole. However, the stimulation of stretched muscle is not affected while standing on foam (Chiang and Wu, 1997). Past studies have shown that standing on foam results in a significant challenge to postural control (Patel et al., 2011) (Blackburn et al., 2003, Jeka et al., 2004, Vrancken et al., 2005). Moreover, standing on foam is considered to be an even more complex balance task than pitch controlled ankle-sway referencing (Allum, Zamani, 2002).
Standing on wobble board induces body instability in one plane. Furthermore, due to the differences in the physical properties of the surface in contact with the sole (firm or soft), somatosensory inputs from the foot are different between standing on a wobble board versus standing on foam (Roll et al., 2002). Standing on a wobble board stimulates activity of lower limb musculatures as well as lumbar erector spinae (Burton, 1986).
Maintenance of vertical posture is regulated by feedforward and feedback components of postural control. Feedforward control involves activation of leg and trunk muscles prior to an expected body perturbation also known as anticipatory postural adjustments (APA) (Belen’kii et al., 1967, Massion, 1992). Feedback control is initiated by the sensory feedback signals after the perturbation onset and is known as compensatory postural adjustments (CPA) (Alexandrov et al., 2005, Horak et al., 1996, Park et al., 2004). There are differences in the function between the two: APAs serve to minimize the displacement of the body’s Center of Mass (CoM) prior to a perturbation (Aruin and Latash, 1995, Bouisset and Zattara, 1987) while CPAs serve as a mechanism of restoration of the position of CoM after a perturbation has already occurred (Macpherson et al., 1989, Maki et al., 1996).
Changes in the stability of the supporting surface and associated changes in the available somatosensory information could affect both of the components of postural control. Thus, APAs are reduced when body posture is unstable (Nouillot et al., 1992) or very stable (Nardone and Schieppati, 1988). In addition, APAs were also reduced when subjects performed backward bending while standing on a narrow support (Pedotti et al., 1989). Moreover, earlier and smaller APAs were observed in experiments involving fast arm movements while standing on a wobble board as compared to standing on a stationary surface (Gantchev and Dimitrova, 1996). Instability of the supporting surface also affects the feedback component of postural control. Thus, standing on a narrow beam (Gatev et al., 1999, Horak and Nashner, 1986) or on one leg (Tropp and Odenrick, 1988) results in subjects utilizing primarily the hip strategy when recovering from a perturbation induced by a moving support. Moreover, an increase in the amplitude of compensatory EMG activity of the leg and trunk muscles was observed while subjects wore unstable foot wear (Sousa et al., 2010), while standing on foam (Fransson et al., 2007) or while standing on a wobble board (Burton, 1986).
While the effect of standing on foam or a wobble board was investigated individually, to the best of our knowledge there are no studies that evaluate the effect of both of these supports in the control of vertical posture in the presence of an external perturbation.
Thus, the current experiment was designed to study the role of different support surfaces upon APAs and CPAs. The subjects were exposed to similar perturbations induced at the shoulder level while standing either on stable or unstable surfaces (foam, wobble board). We hypothesized that: a) APAs will be reduced in conditions with diminished stability induced by foam (that causes instability in both sagittal and frontal planes) or wobble board (that induces instability in sagittal plane), and b) CPAs will be different between the two unstable conditions with greater EMG activity in the foam condition, which is the most unstable.
Materials and methods
Participants
Nine healthy participants (4 males and 5 females) with no history of lower extremity injury, chronic ankle instability or clinically diagnosed balance disorders within last 6 month participated in the study. Mean age, height and weight of the participants were 23±0.5 years, 1.7±0.02 m and 67±5.8 kg respectively. The right side was the dominant side for all subjects. The protocol was approved by the University’s Institutional Review Board prior to participant recruitment, and all participants provided written informed consent before taking part in the experimental procedures.
Procedure
The subjects were instructed to stand on the different surfaces and maintain standing balance while being subjected to external perturbations at the shoulder level induced by an aluminum pendulum attached to the ceiling. An additional load (mass = 5% of subject’s body weight) was fixed to the pendulum at its lower end. The width of the padded hitting surface of the pendulum was adjusted to match the subject’s shoulder width. The pendulum was positioned at an initial angle of 30 degrees to the vertical (distance of 0.6 m from the body) and released by an experimenter. Perturbations consisted of unidirectional forces applied by the pendulum on the shoulders of the subjects. The subjects were instructed to look straight towards a target attached to the pendulum at eye level and maintain their balance after the perturbation (Mohapatra et al., 2011, 2012). The supporting surface was either stationary (RIGID) as the subjects stood on the force platform or unstable. Instability was induced by a piece of foam, 12.7cm thickness (FOAM) or a wooden wobble board, 7.6 cm in height, (WOBBLE) positioned on the top of the force platform. The subjects stood barefoot on these surfaces while keeping eyes open or closed. Thus the eyes open conditions were REO (Rigid-Eyes Open), FEO (Foam-Eyes Open) and WEO (Wobble- Eyes Open), and the eyes closed conditions were REC (Rigid-Eyes Closed), FEC Foam-Eyes Closed) and WEC (Wobble- Closed). Accordingly, when their eyes were open, the subjects were able to see the upcoming pendulum and generate an anticipatory postural adjustment, while in the conditions with their eyes closed only compensatory adjustments were generated (Santos et al., 2010a). The experimenter made sure that the feet position in relation to the center of the force platform was the same across all the conditions.
The subjects wore wireless headphones playing music throughout all of the conditions to mask any kind of auditory information. For safety, the participants remained in a harness with two straps attached to the ceiling and wore protective glasses during the experiment. The subjects performed two to three practice trials in each experimental condition prior to the start of data collection. Five trials, each of 5s in duration, were collected in each experimental condition and the order of the conditions was randomized across subjects.
Instrumentation and Data processing
Ground reaction forces and moments were recorded using a force platform (Model OR-5, AMTI, USA). An accelerometer (Model 208CO3, PCB Piezotronics Inc., USA) was attached to the subject’s proximal clavicle to record the moment of pendulum impact (defined as T0). Electrical activity of muscles (EMGs) was recorded unilaterally (right side) from the following muscles: tibialis anterior (TA, at one-third on the line between the tip of the fibula and the tip of the medial malleolus), lateral gastrocnemius (GL, at one third line from lateral side of the popliteus cavity to the lateral side of the Achilles tendon insertion), rectus femoris (RF, at 50% on the line from the anterior superior iliac spine (ASIS) to the superior part of the patella), vastus lateralis (VL, lower 25% between ASIS and Gerdy prominence), vastus medialis (VM, lower 25% between ASIS and knee joint space), biceps femoris (BF, half way between the ischial tuberosity and the lateral epicondyle of the tibia), semitendinosus (ST, 5cm above the posterior knee joint medially), rectus abdominis (RA, 3 cm lateral to the umbilicus), and erector spinae lumborum (ESL, 3 cm lateral to the first lumbar vertebra) by disposable surface electrodes (Red Dot 3M). These specific leg and trunk muscles were selected because of their involvement in control of vertical posture while dealing with symmetrical perturbations induced in the sagittal plane and because these muscles were previously used to study anticipatory and compensatory control of posture (Aruin and Latash, 1995, Latash et al., 1995, Santos, Kanekar, 2010a). The placement of electrodes for recording EMG activity was based on recommendations reported in the literature (Basmajian, 1980). The electrodes were positioned in pairs with the center-to-center distance of 25 mm; the ground electrode was positioned on the bony anterior border of tibia. The skin was prepared by cleaning with alcohol swabs. The EMG signals were collected, filtered, and amplified (10–500 Hz, gain 2000) with a EMG system (Myopac, RUN Technologies, USA). The forces, moments of forces, EMG, and accelerometer signals were digitized with a 16-bit resolution at 1,000 Hz by means of customized LabVIEW 8.6.1 software (National Instruments, Austin TX, USA). Further processing included obtaining the muscle latencies, calculation and normalization of the integrals of EMG (IEMGNORM) and obtaining CoP displacements (Mohapatra, Krishnan, 2011). The analysis was conducted using customized MATLAB program (Math Works, Natick, MA, USA). Four epochs were selected (each 150 ms in duration) in relation to T0: (1) from −250 to −100 ms (anticipatory, APA1), (2) from −100 to +50 ms (anticipatory, APA2), (3) from +50 to 200 ms (compensatory, CPA1) and (4) from 200 to 350 ms (compensatory, CPA2) (Santos, Kanekar, 2010a, Santos et al., 2010b).
Muscle latency for a specific muscle was defined as the instant lasting for at least 50 ms when its EMG amplitude was greater (activation) or smaller (inhibition) than the mean ± 2 SD of the baseline. In addition, an exploratory data analysis was performed using Principal Component Analysis (PCA). Thus, PCA was applied to the correlation matrices of muscles latencies data of all the subjects. The PCs were further subjected to Oblimin with Kaiser Normalization rotation with factor extraction. CoP displacements in the anterior–posterior direction were calculated. The CoP signals were corrected by its respective baseline, and the CoP data windows were shifted 50 ms forward to account for the electro-mechanical delay (Cavanagh and Komi, 1979, Howatson et al., 2009). Peak magnitude of the CoP and the magnitude of CoP at the moment of perturbation (T0) were calculated.
Statistical analysis
Two separate multiple repeated-measures ANOVAs were performed for the IEMGNORMs. First analysis was focused on the feedforward postural control, which included two within subject factors: conditions (REO, FEO and WEO) and epochs (APA1 and APA2). Second analysis was focused on the feedback postural control that included two within subject factors: conditions (REC, FEC and WEC) and epochs (CPA1 and CPA2). The averaged data from the series of 5 trials for each of the condition was used in the analysis. A post hoc analysis (pairwise t-test) with Bonferroni correction was performed to compare between conditions and epochs. Other variables such as latency of trunk and leg muscles, magnitude of CoP at the moment of perturbation (T0) and peak magnitude of the CoP are also reported. For all the tests, statistical significance was set at p < 0.05. Statistical analysis was performed in SPSS 17 for Windows 7 (SPSS Inc., Chicago, USA). Reliability analysis assessing internal consistency was performed using Cronbach’s alpha. Additional post hoc power analysis was performed using SPSS program for each studied variable. The results of power analysis suggested that enrolling nine subjects would provide 88% power for the EMG integrals, and 86% and 92% for the CoP displacements and EMG latency respectively.
Results
Reliability analysis
The calculated Cronbach’s alpha values were as follows: for CoP (at T0 = 0.905, at Peak= 0.938), for EMG Latencies (TA = 0.831, GL =0.723, RF =0.902, VL =0.878, VM =0.915, BF =0.825, ST =0.814, RA =0.764 and ESL =0.849,) and for EMG Integrals (TA =0.979, GL=0.957, RF =0.965, VL=0.972, VM =0.965, BF=0.899, ST =0.895, RA=0.979 and ESL=0.929). Since the analysis shows that all the values are in fact > 0.7, the reliability is met.
Feedforward control
EMG patterns
Anticipatory postural adjustments were seen in all conditions with full vision: the majority of muscles showed activity prior to the perturbation (T0) (Fig 1). The first muscle to show activity in FOAM or WOBBLE conditions was RF (115±26 ms before T0 for FOAM and 62±37 ms before T0 for WOBBLE). Overall all the anterior muscles showed earlier activity in FOAM than WOBBLE. GL was the only muscle to show activity after perturbation in all surface conditions even when eyes were open.
Fig. 1.
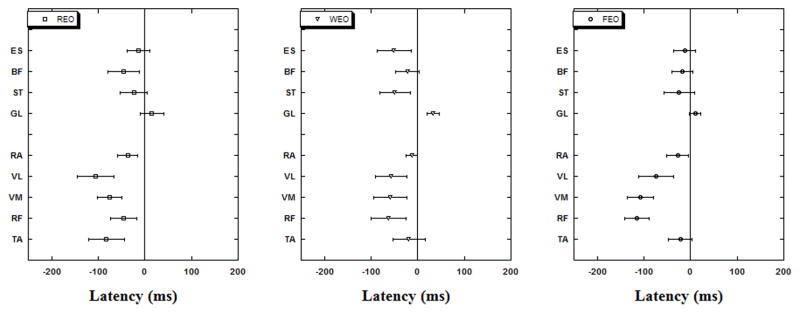
Muscle latencies (anterior vs. posterior groups) are shown for the three experimental conditions while subjects stood on RIGID (REO), FOAM (FEO) or WOBBLE (WEO) surface with eyes open.
Principal Component Analysis
PCA validity was confirmed by visual inspections of the scree plots. On an average, two principal components (PCs) (Table 1) accounted for the 75% total variance in the muscle activation space in the REO, 72 % in FEO, and 76 % of the total variance in the WEO conditions. The first PC in the REO showed high loading values (>0.6) for RF, VL, VM, BF and ST. In FEO however, the loading patterns for the first PC were significantly higher for RF, VL, VM and ST. Furthermore, when the subjects stood on the wobble board (WEO), the muscles which showed highest loading in the PC1 were TA, RF, VL, VM and ESL. The second PC in the REO showed high loading values for TA, GL and RA. In FEO however, the loading patterns for the second PC were significantly higher for GL, BF and ESL. Furthermore, when the subjects stood on the wobble board (WEO), the muscles which showed highest loading in the PC2 were GL, BF and ST.
Table 1.
Results of the Principal Component Analysis for the APA component in EO condition.
| Muscles | Components (REO) s2=0.75 |
Components (WEO) s2=0.76 |
Components (FEO) s2=0.72 |
|||
|---|---|---|---|---|---|---|
| 1 | 2 | 1 | 2 | 1 | 2 | |
| TA | .019 | .933 | .679 | −.180 | .520 | −.555 |
| GL | .492 | .619 | −.261 | −.889 | .078 | .765 |
| RF | .845 | −.035 | .928 | .177 | .827 | .124 |
| VL | .886 | .137 | .950 | .316 | .726 | −.063 |
| VM | .807 | −.105 | .989 | .120 | .967 | .099 |
| BF | .894 | −.158 | .279 | .910 | .002 | −.955 |
| ST | .836 | .016 | −.353 | .799 | .773 | −.506 |
| RA | −.337 | .870 | .341 | −.434 | .592 | .310 |
| ESL | .544 | .543 | .694 | −.426 | −.157 | −.927 |
Numbers in bold indicate significant loading components (>|0.6|). s2 indicates sample variance.
It is important to note that the PC1 revealed a co-contraction of the thigh muscles and PC2 component depicted a co-contraction for both the trunk and leg muscles in the FEO and REO conditions (Table 1). A co-contraction is defined as a pattern with significant loading coefficients on the same PC with the same sign (positive or negative) for two muscles with opposing actions at a particular joint (ankle, knee or hip) (Krishnan et al., 2012).
EMG integrals
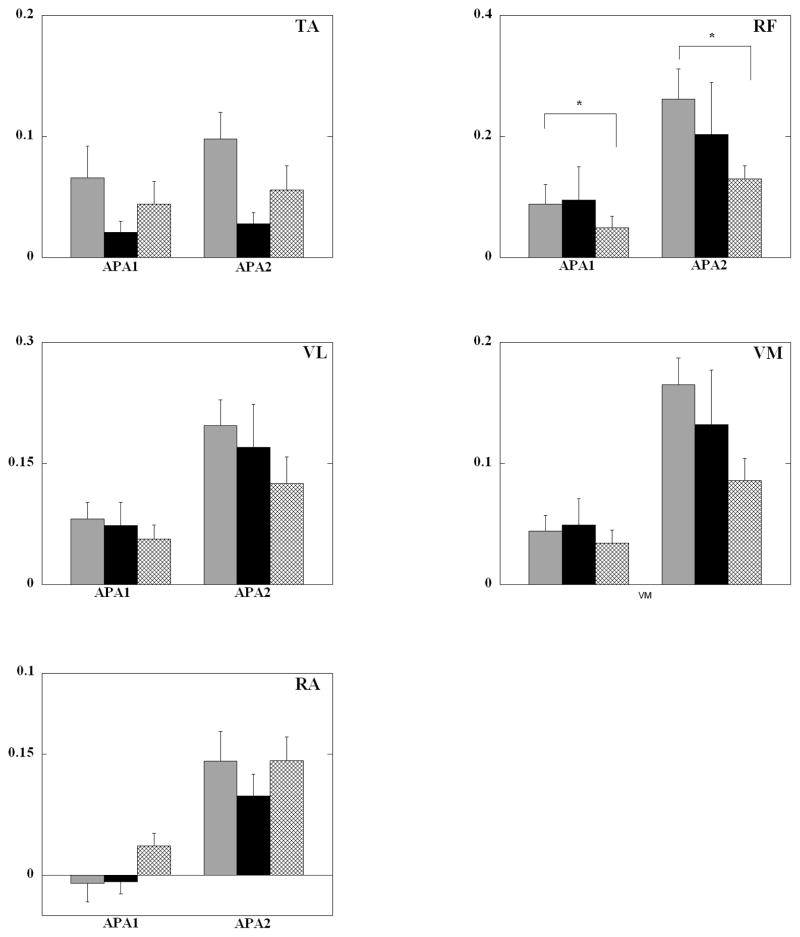
Anticipatory integrals of EMG (iEMGs) are shown in Fig 2. The only muscle to show a significant main effect of the surfaces was TA (F2, 16=4.95, p=0.021) (Table 2). In general, six out of the nine muscles TA, GL, RF, VL, VM and ESL showed the highest anticipatory activity especially in the APA2 epoch in the FOAM condition. Furthermore five of these six muscles (GL, RF, VL, VM and ESL) showed the least muscular activity especially in the APA2 epoch for the WOBBLE condition.
Fig. 2.
Mean normalized EMG integrals (in arbitrary units) of TA (tibialis anterior), RF (rectus femoris), VL (vastus lateralis), VM (vastus medialis) and RA (rectus abdominis) for the three surface support conditions for all subjects for the APA epochs (eyes open). Each column represents the IEMGNORMs for 150 ms of the APA1 and APA2 epochs with its standard error bars. * signifies statistical significant differences (p<0.05) between FEO and WEO.
Table 2.
(F and p values of conditions, epochs and their interactions during APA phase in EO Condition).
| Muscles | Conditions | Epochs | Conditions * Epochs | |||
|---|---|---|---|---|---|---|
| [F2,16] | p | [F1,8] | p | [F2,16] | p | |
| TA | 4.948* | 0.021 | 4.983 | 0.056 | 1.420 | 0.271 |
| GL | 1.399 | 0.275 | 0.115 | 0.743 | 0.039 | 0.962 |
| RF | 1.660 | 0.221 | 23.602* | 0.001 | 3.154 | 0.070 |
| VL | 1.747 | 0.206 | 14.943* | 0.005 | 2.736 | 0.095 |
| VM | 1.701 | 0.214 | 22.108* | 0.002 | 5.113* | 0.019 |
| BF | 0.903 | 0.420 | 0.222 | 0.065 | 1.923 | 0.178 |
| ST | 2.112 | 0.153 | 1.717 | 0.226 | 1.934 | 0.177 |
| RA | 3.272 | 0.064 | 24.108* | 0.001 | 0.892 | 0.429 |
| ESL | 0.403 | 0.675 | 2.032 | 0.192 | 1.528 | 0.247 |
indicates p<0.05
Four muscles (RF, VL, VM and RA) showed a significant main effect of the two APA epochs (Table 2). When the APA1 and APA2 epochs were compared across conditions, iEMGs were larger during the APA2 epoch as compared to the APA1 in all the muscles. The difference was statistically significant in TA (p=0.05), RF (p=0.001), VL (p=0.005), VM (p=0.002) and RA (p=0.001) muscles.
COP Displacements
In EO conditions the subjects demonstrated almost equal CoP T0 displacements (0.014±0.002 m) in the backward direction in all of the different surface conditions. The peak displacements of CoP in experiments with eyes open (EO) were 0.028±0.003 m in FOAM followed by WOBBLE (0.030±0.006 m) and 0.033±0.004 m in RIGID conditions.
Feedback control
EMG patterns
There was no anticipatory activity in any muscle in conditions with eyes closed (EC), instead all the muscles became active only after the perturbation onset (T0) (Fig 3). A group of muscles (TA, VL, BF and ST) showed the earliest activity in the WOBBLE condition followed by FOAM and RIGID conditions. Another group of muscles (GL and VM) showed a different pattern with the earliest activation seen in the FOAM condition followed by WOBBLE and RIGID conditions. It is interesting to note that irrespective of the supporting surface the pattern of activation of muscles was similar based on their location on the body (anterior or posterior). Thus, the anterior muscles were the first to show activity in response to a perturbation followed by the posterior muscles.
Fig. 3.
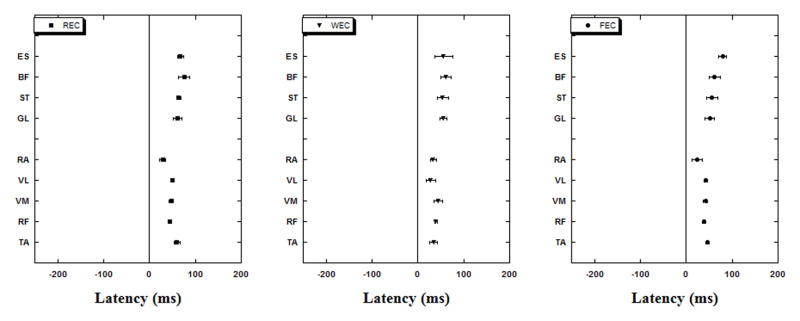
Muscle latencies (anterior vs. posterior groups) with their standard error bars for the three eyes closed experimental conditions while subjects stood on RIGID (REC), FOAM (FEC) or WOBBLE (WEC) surface. Note that anterior muscles are first to activate/inhibit but only after T0.
Principal Component Analysis
On an average, two principal components (PCs) accounted for the 74% total variance in the muscle activation space in the REC, 73 % in FEC, and 70 % of the total variance in the WEC conditions (Table 3). The first PC in the REC showed high loading values for TA, RF, VL, VM and ST. In FEC however, the loading patterns for the first PC were higher (>0.6) for TA, RF, VL, VM and ESL. Furthermore, when the subjects stood on the wobble board (WEC) the muscles which showed highest loading in the PC1 were GL, RF, BF, ST and RA. The second PC in the REC showed high loading values for GL, BF and ESL. In FEC however, the loading patterns for the second PC were significantly higher for BF and ST. Furthermore, when the subjects stood on the wobble board (WEC) the muscles which showed highest loading in the PC2 were TA, VL and VM.
Table 3.
Results of the Principal Component Analysis for the CPA component in EC condition.
| Muscles | Components (REC) s2=0.74 |
Components (WEC) s2=0.70 |
Components (FEC) s2=0.73 |
|||
|---|---|---|---|---|---|---|
| 1 | 2 | 1 | 2 | 1 | 2 | |
| TA | .985 | .083 | .280 | .963 | .933 | .071 |
| GL | .085 | −.873 | .854 | −.188 | .549 | −.129 |
| RF | .686 | −.536 | .913 | .074 | .910 | .098 |
| VL | .626 | −.564 | .041 | .914 | .883 | .117 |
| VM | .942 | −.154 | .531 | −.716 | .960 | −.257 |
| BF | −.364 | −.939 | .771 | .147 | .099 | .946 |
| ST | .653 | −.476 | .679 | −.447 | −.022 | .973 |
| RA | .263 | .107 | .670 | .291 | .498 | .100 |
| ESL | −.167 | .616 | .300 | −.259 | .760 | .161 |
Numbers in bold indicate significant loading components (>|0.6|). s2 indicates sample variance.
Significant loading coefficients for the PC1 and PC2 seen in the thigh muscles with opposing actions reveal a co-contraction in the WEC condition. Similarly, significant loading coefficients for PC1 depict a co-contraction in the leg muscles in the FEC conditions.
EMG integrals
Anticipatory integral of EMG (iEMGs) of TA, GL and RF, calculated for all the conditions when the subject stood with eyes closed are shown in Fig. 4. There was no statistically significant difference between different support surface conditions.
Fig. 4.
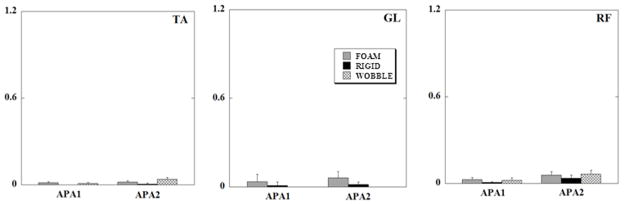
Mean normalized EMG integrals (in arbitrary units) of TA (tibialis anterior), GL (lateral gastrocnemius), and RF (rectus femoris) during the three surface support conditions for all subjects for the APA epochs (eyes closed). Each column represents the IEMGNORMs for 150 ms of the APA1 and APA2 epochs with its standard error bars.
Compensatory integrals of EMG (iEMGs) of TA, GL and RF, calculated for all the conditions are shown in Fig 5. There was a significant main effect of the support surface in TA, GL, RF, VL and VM (Table 4). Thus, compensatory iEMGs were significantly larger in TA (p<0.0001) and GL (p<0.0001) for FOAM when compared with the condition when the subject stood on the force-platform (RIGID). Additionally, compensatory iEMGs were significantly larger in TA (p=0.031), RF (p=0.022), VL (p=0.05) and VM (p=0.05) while standing on FOAM when compared to WOBBLE. The comparison of the epochs revealed that CPA1 was significantly larger than CPA2 for all the muscles (p<0.01).
Fig. 5.
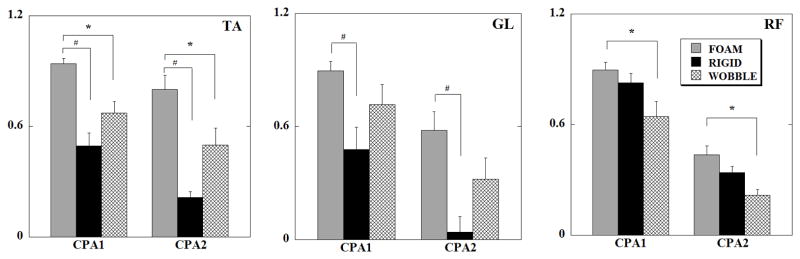
Mean normalized EMG integrals (in arbitrary units) of TA (tibialis anterior), GL (lateral gastrocnemius) and RF (rectus femoris), during the three surface support conditions for all subjects for the CPA epochs (eyes closed). Each column represents the IEMGNORMs for 150 ms of the CPA1 and CPA2 epochs with its standard error bars. * signifies statistical significant differences (p<0.05) between FEC and WEC and # denotes statistically significant differences (p<0.05) between FEC and REC.
Table 4.
(F and p values of conditions, epochs and their interactions during CPA phase in EC Condition)
| Muscles | Conditions | Epochs | Conditions * Epochs | |||
|---|---|---|---|---|---|---|
| [F2,16] | p | [F1,8] | p | [F2,16] | p | |
| TA | 19.614# | <0.0001 | 13.456# | 0.006 | 2.269 | 0.136 |
| GL | 10.123# | 0.001 | 26.901# | 0.001 | 0.437 | 0.653 |
| RF | 8.934# | 0.002 | 131.454# | <0.0001 | 0.281 | 0.759 |
| VL | 6.938# | 0.007 | 56.686# | <0.0001 | 1.889 | 0.184 |
| VM | 5.748* | 0.013 | 46.141# | <0.0001 | 2.257 | 0.137 |
| BF | 2.887 | 0.085 | 23.040# | 0.001 | 2.714 | 0.097 |
| ST | 2.380 | 0.124 | 12.261# | 0.008 | 2.295 | 0.133 |
| RA | 1.088 | 0.361 | 134.46# | <0.0001 | 1.092 | 0.359 |
| ESL | 1.841 | 0.191 | 61.840# | <0.0001 | 1.003 | 0.389 |
indicates p<0.05 and
indicates p<0.01
COP Displacements
When subjects stood with eyes closed, there was negligible anticipatory CoP displacement (0.003±0.001m for RIGID, 0.007±0.002m for WOBBLE and 0.006±0.001m for FOAM); the maximum CoP displacement after the perturbation was 0.055±0.003 m, 0.053±0.004 m and 0.052±0.004 m for RIGID, WOBBLE, and FOAM surface respectively.
Discussion
The ability of an individual to maintain posture is influenced by the quality of the sensory information about the characteristics of the body perturbation and support surface. In the current experiment the subjects stood on different support surfaces allowing us to examine the relationships between the feedforward and feedback components of postural control (used to maintain upright stance) and somatosensory information. Since the magnitudes of external perturbations were kept constant throughout all experimental conditions, the study outcome presents information on the sole effect of different support surfaces in control of vertical stance. Particularly, the results provide evidence on the role of body instability and deficient proprioceptive and somatosensory inputs on the generation of APAs and CPAs. Specially, the study revealed that both APAs and CPAs were larger in conditions with increased body instability. In addition, standing on foam (that created instability in both the sagittal and frontal planes as well as reduced somatosensory inputs), was associated with greater APAs as well as CPAs compared to the wobble board condition (that induces body instability in the sagittal plane with no major reduction in the somatosensory inputs). Hence our first hypothesis that there would be a reduction in APAs with increase in body instability was not supported but the second hypothesis was supported.
Role of support instability on feedforward postural control
Standing on foam (that is a compliant surface) induces instability in both the sagittal and frontal planes and also distorts somatosensory inputs from the sole of the feet. In the current study, standing on foam was associated with an overall increase of the activity of the postural muscles during the anticipatory epochs. The observed increase of the anticipatory activation of muscles contrasts previous literature reporting decreased APAs in experiments involving unstable posture (Aruin, Forrest, 1998). A possible explanation to this finding relates to the differences in the induced instability. Standing on boards with long and narrow support beams induces body instability while standing on foam induces body instability but also diminishes somatosensory input from the surface. It is known that the insufficient afferent information from the environment could be a reason for co-contraction of muscles during maintenance of vertical posture (Kennedy et al., 2012). Anticipatory co-contraction of muscles was reported in individuals with neurological disorders (Aruin and Almeida, 1997, Garland et al., 1997) and the elderly (Bleuse et al., 2006) who deliberately use muscle co-contraction trading efficacy for safety. A co-contraction of leg and trunk muscles was also described in healthy individuals positioned on an unstable surface (Behm and Colado, 2012, Lee et al., 2006). A PC analysis utilized in the current study also revealed the existence of co-contraction in a number of muscles. Thus, similar (positive) signs of the PC1 loadings seen in the FEO conditions for the anterior (RF, VL and VM) and posterior (ST) knee muscles confirm the existence of co-contraction of the thigh muscles. However, in the WEO condition PCA loadings in both the PC components reveal that co-contractions at both the ankle and knee joints were not present. At the same time, when the subjects stood on the rigid (REO) support the PC1 loadings revealed co-contraction at the knee joint and the PC2 loadings uncovered co-contraction at the ankle joint.
The observed muscle co-contraction could be considered as an indication of the increased stiffness of the joints that the CNS implemented to stabilize the COP displacement when dealing with the instability. This suggestion is in line with the outcome of a previous study that has reported the implementation of the stiffness strategy in conditions with the increased postural threat (Carpenter et al., 2001). Indeed, the use of co-contraction of muscles was reported while dealing with challenging postural conditions (Asaka et al., 2008, Krishnamoorthy et al., 2004) and pre-programmed reactions in young subjects (Robert and Latash, 2008) and in the elderly (Wolfson, 1997).
The outcome of the present study demonstrates that the level of co-contraction varies depending on the stability of the supporting surfaces. These results taken together with the literature suggest that increasing muscle co-contraction is a general strategy that the CNS uses to provide additional stability in conditions of increased muscle weakness and a slowing of sensory motor processing as it happens in case of advanced age or neurological disease or when standing on an unstable surface i.e. foam. This suggestion is in line with prior literature indicating that co-contraction can be used to augment trunk stiffness thereby increase body stability (Lee, Rogers, 2006). It is important to note that co-contraction was observed between the front and back muscles on the right side of the body. We believe that a similar co-contraction was present on the left side of the body. A co-contraction of analogous muscles on both the sides of the body could also be a part of a strategy focused on increasing the body stability in conditions of standing on foam (that is associated with the combination of the increased instability in both, the sagittal and frontal planes and the distorted somatosensory inputs from the sole of the feet). On the other hand, increased co-contraction of muscles could contribute to fatigue and consequentially impair postural control. These assumptions however, could not be supported with the experimental data since the EMGs were recorded unilaterally. As such, future studies involving EMG recording from the muscles on both sides of the body are needed to provide information needed to either support or reject the proposed suggestions.
Standing on the wobble board creates instability in the sagittal plan. At the same time there is no difference in the physical properties of the surface in contact with the feet compared to the standing on the force platform (in the RIGID condition). This resulted in the decreased anticipatory activation of muscles in the WOBBLE condition as compared to RIGID condition. Thus, this result is in agreement with the previous literature (Aruin, Forrest, 1998, Pedotti, Crenna, 1989) describing a decrease in anticipatory activation of muscles in experiments with body instability. It was also reported that when the support surface is unstable in the sagittal plane, the anticipatory EMG activity in BF and SOL (soleus) muscles is seen earlier compared to the standing on a stable surface (Gantchev and Dimitrova, 1996). In the current study such a finding was not observed for BF. The differences in the study outcomes could be due to the dissimilarity between the self-initiated perturbations (Gantchev and Dimitrova, 1996) and the external perturbation used in the current study. Moreover, the differences in the perturbation magnitudes could be another reason as it was reported that body perturbations induced by a support surface translation were more destabilizing than those applied to the body at the waist level (Mansfield and Maki, 2009).
It is interesting to note that although the anticipatory activity varied between different surface conditions, almost similar anticipatory CoP displacements were seen in the experimental conditions of standing on different supporting surfaces. We can speculate that the support-related changes in the EMG activity were not realized in significant changes in the body position as the CNS modulated anticipatory activity of the trunk and leg muscles to maintain the CoP relatively motionless. This could be a result of the implementation of two strategies. First, smaller APAs were generated to avoid additional destabilization of the body equilibrium in the condition of instability as it was in WOBBLE conditions. A possibility this to happen was described in the previous literature. For example, in a study involving self-initiated releases of a load from extended arms while standing on an unstable board, a clear attenuation of the CoP displacements was reported in conditions with induced body instability (Aruin, Forrest, 1998). Second, there was a simultaneous anticipatory activation in the ventral and dorsal muscles; such a co-contraction might lead to a cancellation of the effect of muscle activation on the CoP displacement and as a result increased body stability. Indeed, similar anticipatory co-contraction of muscles was described in the literature as an attempt to deal with the increased fear of falling (Adkin et al., 2000, Okada et al., 2001).
Role of support instability on feedback postural control
No anticipatory postural adjustments were generated when standing with eyes closed as the subjects did not know the moment of the pendulum release. As such, only compensatory postural adjustments were used to restore body position after the perturbation.
Alterations in somatosensation from the legs or trunk have been shown to modulate postural responses to unexpected surface translations. Thus, it was demonstrated that individuals with peripheral neuropathy exhibit delays of the muscle activation while being perturbed (Inglis et al., 1994). While in the current study muscles were activated 11% and 19% later in the FOAM and WOBBLE conditions than in RIGID conditions, respectively, these delays were not statistically significant. It is important to mention that the subjects in the current study were healthy young volunteers while the participants in the former study had impaired proprioception.
The observed differences in the compensatory integrals of EMG between the experimental conditions with standing on foam and wobble board (Fig. 4) provide support for the second hypothesis. Indeed, all the studied muscles except RF showed highest activity during the compensatory phase of postural control while standing on foam (that induces instability in the sagittal and frontal planes) compared to standing on wobble board (that induces instability in the sagittal plane only) or a rigid surface. Four muscles (TA, GL, BF and ESL) showed smallest CPA magnitudes for the most stable condition (RIGID).
In the current study, regardless of the nature of instability of the supporting surface, all anterior muscles were activated first after the perturbation followed by the activation of posterior muscles. An activation of muscles around the hip was reported in subjects standing on a narrow beam (Gatev, Thomas, 1999, Horak and Nashner, 1986) or on one leg (Tropp and Odenrick, 1988) while recovering from a perturbation induced by a moving support. The differences in the patterns of activation of muscles in the current study and the former ones could be explained by a different type of perturbation and its point of application. While past studies mainly used a support surface translation movements, our study utilized perturbations delivered to the shoulders.
There are certain limitations in this study that we would like to mention. While unilateral EMG recording was shown to be informative while studying postural control of standing on a rigid surface and being exposed to symmetrical perturbations (Girolami et al., 2010, Shiratori and Aruin, 2007), bilateral EMGs should be recorded to fully investigate postural control while standing on a compliant surface such as foam. However, due to a limitation in the number of channels we were unable to record bilateral EMGs. Future studies need to be based on either using the EMG systems with larger number of channels or reducing the number of studied muscles so EMGs from the left and right side muscles are recorded. Second, we used 12.7cm thickness foam and a wooden wobble board which was positioned in such a way that it induced instability only in the sagittal plane. Future studies are needed to investigate the role of different thickness of foam as well as the effect of the wobble board inducing instability in the frontal plane.
Conclusion
The outcome of the study revealed that the altering support surface affects both feedforward and feedback components of postural control. This information should be taken into consideration in planning rehabilitation interventions geared towards improvement of balance.
Acknowledgments
We would like to thank Vennila Krishnan for help during data collection. This study was supported in part by NIH grant HD-064838.
Biographies

Sambit Mohapatra is a postdoctoral fellow at the Neuroscience Research Center in MedStar National Rehabilitation Hospital and Georgetown University, Washington D.C. He completed his Bachelors degree with honors in Physical Therapy from University of Delhi, India in 2007. He then graduated with a Masters in Kinesiology and a PhD in Rehabilitation Sciences from University of Illinois at Chicago, in 2009 and 2012 respectively. His research has focused on understanding the role of sensory systems in control of posture in health and disease. His current research interests are to investigate and implement novel rehabilitation strategies by exploring behavioral and physiological correlates in individuals with neurological impairments.

Komal Kukkar obtained a bachelor degree in Physical Therapy with a focus on biomechanics from Baba Farid University of Health Sciences in India. Currently he is pursuing a Master degree in Biomechanics and Motor Control at the University of Illinois at Chicago. His current research interests include biomechanics of standing and contribution of various sensory systems in postural stability.

Alexander S. Aruin received his PhD in Medical Cybernetics from the Moscow Institute of Artificial Organs and Transplantation in 1978 and a D.Sc. (PhD) in Biomechanics from the Latvian Institute of Traumatic Injuries and Orthopedics in 1990. He was affiliated with the Moscow Institute of Electronic Engineering as Full Professor and Director of the Laboratory of Ergonomics and Biomechanics. After moving to the United States in 1992, he became a faculty at Rush-Presbyterian St. Luke’s Medical Center in Chicago and later a faculty member at Pennsylvania State University. He is currently a Professor of Physical Therapy, Bioengineering, and the Director of the Knecht Movement Science Laboratory at the University of Illinois at Chicago, a Professor of Physical Medicine & Rehabilitation at Rush University, Chicago, and a Senior Clinical Researcher at Marianjoy Rehabilitation Hospital in Wheaton, Illinois. He has coauthored more than 130 referred papers and two monographs in the fields of biomechanics, kinesiology, motor control and neuromuscular disorders. His primary research interests have been focused on motor disorders and rehabilitation, biomechanics, and motor control. Dr. Aruin develops new technologies for training healthy people and for providing physical therapy and rehabilitation to injured and disabled individuals. He is a recipient of a number of federally supported grants and is a member of the American Society for Biomechanics, Society for Neuroscience, and International Society of Motor Control.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adkin AL, Frank JS, Carpenter MG, Peysar GW. Postural control is scaled to level of postural threat. Gait Posture. 2000;12:87–93. doi: 10.1016/s0966-6362(00)00057-6. [DOI] [PubMed] [Google Scholar]
- Alexandrov AV, Frolov AA, Horak FB, Carlson-Kuhta P, Park S. Feedback equilibrium control during human standing. Biological cybernetics. 2005;93:309–22. doi: 10.1007/s00422-005-0004-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allum JH, Zamani F, Adkin AL, Ernst A. Differences between trunk sway characteristics on a foam support surface and on the Equitest ankle-sway-referenced support surface. Gait Posture. 2002;16:264–70. doi: 10.1016/s0966-6362(02)00011-5. [DOI] [PubMed] [Google Scholar]
- Aruin A, Almeida G. A coactivation strategy in anticipatory postural adjustment in persons with Down syndrome. Motor Control. 1997;2:178–91. [Google Scholar]
- Aruin AS, Forrest WR, Latash ML. Anticipatory postural adjustments in conditions of postural instability. Electroencephalogr Clin Neurophysiol. 1998;109:350–9. doi: 10.1016/s0924-980x(98)00029-0. [DOI] [PubMed] [Google Scholar]
- Aruin AS, Latash ML. The role of motor action in anticipatory postural adjustments studied with self-induced and externally triggered perturbations. Experimental brain research. Experimentelle Hirnforschung. 1995;106:291–300. doi: 10.1007/BF00241125. [DOI] [PubMed] [Google Scholar]
- Asaka T, Wang Y, Fukushima J, Latash ML. Learning effects on muscle modes and multi-mode postural synergies. Exp Brain Res. 2008;184:323–38. doi: 10.1007/s00221-007-1101-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basmajian JV. Electromyography--dynamic gross anatomy: a review. Am J Anat. 1980;159:245–60. doi: 10.1002/aja.1001590302. [DOI] [PubMed] [Google Scholar]
- Behm D, Colado JC. The effectiveness of resistance training using unstable surfaces and devices for rehabilitation. International journal of sports physical therapy. 2012;7:226–41. [PMC free article] [PubMed] [Google Scholar]
- Belen’kii VE, Gurfinkel VS, Pal’tsev EI. Control elements of voluntary movements. Biofizika. 1967;12:135–41. [PubMed] [Google Scholar]
- Blackburn JT, Riemann BL, Myers JB, Lephart SM. Kinematic analysis of the hip and trunk during bilateral stance on firm, foam, and multiaxial support surfaces. Clinical biomechanics (Bristol, Avon) 2003;18:655–61. doi: 10.1016/s0268-0033(03)00091-3. [DOI] [PubMed] [Google Scholar]
- Bleuse S, Cassim F, Blatt JL, Labyt E, Derambure P, Guieu JD, et al. Effect of age on anticipatory postural adjustments in unilateral arm movement. Gait Posture. 2006;24:203–10. doi: 10.1016/j.gaitpost.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Bouisset S, Zattara M. Biomechanical study of the programming of anticipatory postural adjustments associated with voluntary movement. J Biomechanics. 1987;20:735–42. doi: 10.1016/0021-9290(87)90052-2. [DOI] [PubMed] [Google Scholar]
- Burton AK. Trunk muscle activity induced by three sizes of wobble (balance) boards. J Orthop Sports Phys Ther. 1986;8:27–9. doi: 10.2519/jospt.1986.8.1.27. [DOI] [PubMed] [Google Scholar]
- Carpenter MG, Frank JS, Silcher CP, Peysar GW. The influence of postural threat on the control of upright stance. Exp Brain Res. 2001;138:210–8. doi: 10.1007/s002210100681. [DOI] [PubMed] [Google Scholar]
- Cavanagh PR, Komi PV. Electromechanical delay in human skeletal muscle under concentric and eccentric contractions. Eur J Appl Physiol Occup Physiol. 1979;42:159–63. doi: 10.1007/BF00431022. [DOI] [PubMed] [Google Scholar]
- Chiang J-H, Wu G. The influence of foam surfaces on biomechanical variables contributing to postural control. Gait & Posture. 1997:239–45. [Google Scholar]
- Fransson PA, Gomez S, Patel M, Johansson L. Changes in multi-segmented body movements and EMG activity while standing on firm and foam support surfaces. Eur J Appl Physiol. 2007;101:81–9. doi: 10.1007/s00421-007-0476-x. [DOI] [PubMed] [Google Scholar]
- Gantchev GN, Dimitrova DM. Anticipatory postural adjustments associated with arm movements during balancing on unstable support surface. Int J Psychophysiol. 1996;22:117–22. doi: 10.1016/0167-8760(96)00016-5. [DOI] [PubMed] [Google Scholar]
- Garland SJ, Stevenson TJ, Ivanova T. Postural responses to unilateral arm perturbation in young, elderly, and hemiplegic subjects. Archives of physical medicine and rehabilitation. 1997;78:1072–7. doi: 10.1016/s0003-9993(97)90130-1. [DOI] [PubMed] [Google Scholar]
- Gatev P, Thomas S, Kepple T, Hallett M. Feedforward ankle strategy of balance during quiet stance in adults. J Physiol. 1999;514 (Pt 3):915–28. doi: 10.1111/j.1469-7793.1999.915ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girolami GL, Shiratori T, Aruin AS. Anticipatory postural adjustments in children with typical motor development. Experimental brain research. Experimentelle Hirnforschung. 2010;205:153–65. doi: 10.1007/s00221-010-2347-7. [DOI] [PubMed] [Google Scholar]
- Greene DASA, Albers JW, Pfeifer MA. Diabetic neuropathy. 4. New York: Elsevier; 1990. [DOI] [PubMed] [Google Scholar]
- Horak FB, MacPherson JM, Peterson BW. Postural orientation and equilibrium. New York: Published for the American Physiological Society by Oxford University Press; 1996. [Google Scholar]
- Horak FB, Nashner LM. Central programming of postural movements: adaptation to altered support-surface configurations. J Neurophysiol. 1986;55:1369–81. doi: 10.1152/jn.1986.55.6.1369. [DOI] [PubMed] [Google Scholar]
- Howatson G, Glaister M, Brouner J, van Someren KA. The reliability of electromechanical delay and torque during isometric and concentric isokinetic contractions. J Electromyogr Kinesiol. 2009;19:975–9. doi: 10.1016/j.jelekin.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Inglis JT, Horak FB, Shupert CL, Jones-Rycewicz C. The importance of somatosensory information in triggering and scaling automatic postural responses in humans. Experimental brain research. Experimentelle Hirnforschung. 1994;101:159–64. doi: 10.1007/BF00243226. [DOI] [PubMed] [Google Scholar]
- Jeka J, Kiemel T, Creath R, Horak F, Peterka R. Controlling human upright posture: velocity information is more accurate than position or acceleration. J Neurophysiol. 2004;92:2368–79. doi: 10.1152/jn.00983.2003. [DOI] [PubMed] [Google Scholar]
- Kavounoudias A, Gilhodes JC, Roll R, Roll JP. From balance regulation to body orientation: two goals for muscle proprioceptive information processing? Experimental brain research. Experimentelle Hirnforschung. 1999;124:80–8. doi: 10.1007/s002210050602. [DOI] [PubMed] [Google Scholar]
- Kennedy A, Guevel A, Sveistrup H. Impact of ankle muscle fatigue and recovery on the anticipatory postural adjustments to externally initiated perturbations in dynamic postural control. Experimental brain research. Experimentelle Hirnforschung. 2012;223:553–62. doi: 10.1007/s00221-012-3282-6. [DOI] [PubMed] [Google Scholar]
- Krishnamoorthy V, Latash ML, Scholz JP, Zatsiorsky VM. Muscle modes during shifts of the center of pressure by standing persons: effect of instability and additional support. Exp Brain Res. 2004;157:18–31. doi: 10.1007/s00221-003-1812-y. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Latash ML, Aruin AS. Early and late components of feed-forward postural adjustments to predictable perturbations. Clin Neurophysiol. 2012;123:1016–26. doi: 10.1016/j.clinph.2011.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latash ML, Aruin AS, Neyman I, Nicholas JJ. Anticipatory postural adjustments during self inflicted and predictable perturbations in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1995;58:326–34. doi: 10.1136/jnnp.58.3.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee PJ, Rogers EL, Granata KP. Active trunk stiffness increases with co-contraction. J Electromyogr Kinesiol. 2006;16:51–7. doi: 10.1016/j.jelekin.2005.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macpherson JM, Horak FB, Dunbar DC, Dow RS. Stance dependence of automatic postural adjustments in humans. Exp Brain Res. 1989;78:557–66. doi: 10.1007/BF00230243. [DOI] [PubMed] [Google Scholar]
- Magnusson M, Enbom H, Johansson R, Pyykko I. Significance of pressor input from the human feet in anterior-posterior postural control. The effect of hypothermia on vibration-induced body-sway. Acta Otolaryngol. 1990;110:182–8. doi: 10.3109/00016489009122535. [DOI] [PubMed] [Google Scholar]
- Maki BE, McIlroy WE, Perry SD. Influence of lateral destabilization on compensatory stepping responses. J Biomech. 1996;29:343–53. doi: 10.1016/0021-9290(95)00053-4. [DOI] [PubMed] [Google Scholar]
- Manchester D, Woollacott M, Zederbauer-Hylton N, Marin O. Visual, vestibular and somatosensory contributions to balance control in the older adult. J Gerontol. 1989;44:M118–27. doi: 10.1093/geronj/44.4.m118. [DOI] [PubMed] [Google Scholar]
- Mansfield A, Maki BE. Are age-related impairments in change-in-support balance reactions dependent on the method of balance perturbation? J Biomech. 2009;42:1023–31. doi: 10.1016/j.jbiomech.2009.02.007. [DOI] [PubMed] [Google Scholar]
- Massion J. Movement, posture and equilibrium: interaction and coordination. Prog Neurobiol. 1992;38:35–56. doi: 10.1016/0301-0082(92)90034-c. [DOI] [PubMed] [Google Scholar]
- Mohapatra S, Krishnan V, Aruin AS. The effect of decreased visual acuity on control of posture. Clin Neurophysiol. 2011;123:173–82. doi: 10.1016/j.clinph.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohapatra S, Krishnan V, Aruin AS. Postural control in response to an external perturbation: effect of altered proprioceptive information. Experimental brain research. Experimentelle Hirnforschung. 2012;217:197–208. doi: 10.1007/s00221-011-2986-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardone A, Schieppati M. Postural adjustments associated with voluntary contraction of leg muscles in standing man. Experimental brain research. Experimentelle Hirnforschung. 1988;69:469–80. doi: 10.1007/BF00247301. [DOI] [PubMed] [Google Scholar]
- Nashner L, Berthoz A. Visual contribution to rapid motor responses during postural control. Brain Res. 1978;150:403–7. doi: 10.1016/0006-8993(78)90291-3. [DOI] [PubMed] [Google Scholar]
- NeuroCom. NeuroCom Users Manual for Smart Balance Master. Clackamas, OR: 2010. [Google Scholar]
- Nouillot P, Bouisset S, Do MC. Do fast voluntary movements necessitate anticipatory postural adjustments even if equilibrium is unstable? Neurosci Lett. 1992;147:1–4. doi: 10.1016/0304-3940(92)90760-5. [DOI] [PubMed] [Google Scholar]
- Okada S, Hirakawa K, Takada Y, Kinoshita H. Relationship between fear of falling and balancing ability during abrupt deceleration in aged women having similar habitual physical activities. Eur J Appl Physiol. 2001;85:501–6. doi: 10.1007/s004210100437. [DOI] [PubMed] [Google Scholar]
- Park S, Horak FB, Kuo AD. Postural feedback responses scale with biomechanical constraints in human standing. Experimental brain research. Experimentelle Hirnforschung. 2004;154:417–27. doi: 10.1007/s00221-003-1674-3. [DOI] [PubMed] [Google Scholar]
- Patel M, Fransson PA, Johansson R, Magnusson M. Foam posturography: standing on foam is not equivalent to standing with decreased rapidly adapting mechanoreceptive sensation. Experimental brain research. Experimentelle Hirnforschung. 2011;208:519–27. doi: 10.1007/s00221-010-2498-6. [DOI] [PubMed] [Google Scholar]
- Pedotti A, Crenna P, Deat A, Frigo C, Massion J. Postural synergies in axial movements: short and long-term adaptation. Experimental brain research. Experimentelle Hirnforschung. 1989;74:3–10. doi: 10.1007/BF00248275. [DOI] [PubMed] [Google Scholar]
- Robert T, Latash ML. Time evolution of the organization of multi-muscle postural responses to sudden changes in the external force applied at the trunk level. Neurosci Lett. 2008;438:238–41. doi: 10.1016/j.neulet.2008.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roll R, Kavounoudias A, Roll JP. Cutaneous afferents from human plantar sole contribute to body posture awareness. Neuroreport. 2002;13:1957–61. doi: 10.1097/00001756-200210280-00025. [DOI] [PubMed] [Google Scholar]
- Santos MJ, Kanekar N, Aruin AS. The role of anticipatory postural adjustments in compensatory control of posture: 1. Electromyographic analysis. J Electromyogr Kinesiol. 2010a;20:388–97. doi: 10.1016/j.jelekin.2009.06.006. [DOI] [PubMed] [Google Scholar]
- Santos MJ, Kanekar N, Aruin AS. The role of anticipatory postural adjustments in compensatory control of posture: 2. Biomechanical analysis. J Electromyogr Kinesiol. 2010b;20:398–405. doi: 10.1016/j.jelekin.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiratori T, Aruin A. Modulation of anticipatory postural adjustments associated with unloading perturbation: effect of characteristics of a motor action. Experimental brain research. Experimentelle Hirnforschung. 2007;178:206–15. doi: 10.1007/s00221-006-0725-y. [DOI] [PubMed] [Google Scholar]
- Sousa ASP, Macedo R, Santos R, Tavares JMRS. Influence of an unstable shoe on compensatory postural adjustments: An experimental evaluation. TMSi - Sixth International Conference on Technology and Medical Sciences; 2010. [Google Scholar]
- Tropp H, Odenrick P. Postural control in single-limb stance. J Orthop Res. 1988;6:833–9. doi: 10.1002/jor.1100060607. [DOI] [PubMed] [Google Scholar]
- Vallbo AB, Johansson RS. Properties of cutaneous mechanoreceptors in the human hand related to touch sensation. Hum Neurobiol. 1984;3:3–14. [PubMed] [Google Scholar]
- van Deursen RW, Simoneau GG. Foot and ankle sensory neuropathy, proprioception, and postural stability. J Orthop Sports Phys Ther. 1999;29:718–26. doi: 10.2519/jospt.1999.29.12.718. [DOI] [PubMed] [Google Scholar]
- Vrancken AM, Allum JH, Peller M, Visser JE, Esselink RA, Speelman JD, et al. Effect of bilateral subthalamic nucleus stimulation on balance and finger control in Parkinson’s disease. J Neurol. 2005;252:1487–94. doi: 10.1007/s00415-005-0896-7. [DOI] [PubMed] [Google Scholar]
- Wolfson L. Balance decrements in older persons: Effects of age and disease. 1997. [Google Scholar]