Abstract
Objective
Substantial variability exists in the timing of limitations in life support for critically ill patients. Our objective was to investigate how the timing of limitations in life support varies with changes in organ failure status and time since acute lung injury (ALI) onset.
Design, Setting, and Patients
This evaluation was performed as part of a prospective cohort study evaluating 490 consecutive ALI patients recruited from 11 intensive care units (ICUs) at three teaching hospitals in Baltimore, MD.
Interventions
None.
Measurements
The primary exposure was proportion of days without improvement in Sequential Organ Failure Assessment (SOFA) score, evaluated as a daily time-varying exposure. The outcome of interest was a documented limitation in life support defined as any of the following: (1) No CPR, (2) Do not re-intubate, (3) No vasopressors, (4) No hemodialysis, (5) Do not escalate care or (6) Other limitation (e.g., “comfort care only”).
Main Results
For medical ICU (MICU) patients without improvement in daily SOFA score, the rate of limitation in life support tripled in the first three days after ALI onset, increased again after Day 5, and peaked at Day 19. Compared to MICU patients, surgical ICU (SICU) patients had a rate of limitations that was significantly lower during the first five days after ALI onset. In all patients, more days without improvement in SOFA scores was associated with limitations in life support, independent of the absolute magnitude of the SOFA score.
Conclusions
Persistent organ failure is associated with an increase in the rate of limitations in life support independent of the absolute magnitude of SOFA score, and this association strengthens during the first weeks of treatment. During the first five days after ALI onset limitations were significantly more common in MICUs than SICUs.
Keywords: Intensive Care, Resuscitation Orders, Terminal Care, Withholding Treatment, Acute Lung Injury, Prospective Studies, Respiration, Artificial, Survival Rate, Time Factors, Intensive Care units, Life Support Care
Introduction
One in five Americans dies in an intensive care unit (ICU).[1] Between 50% and 90% of these deaths occur after limiting the use of life sustaining therapies.[2–5] Prior studies evaluating end-of-life decisions in the ICU have focused primarily on baseline characteristics of patients and physicians.[6, 7] While age, pre-existing comorbidities, and quality-of-life likely have a strong influence on ICU admission and the decision to initiate life support, over time, these factors may be over-shadowed by patients’ trajectory of critical illness while receiving treatment in the ICU. Decisions about the appropriate use of life support are especially relevant in patients with acute lung injury (ALI) because they experience a high severity of illness, frequent multiple organ dysfunction, and survivors often experience new physical, cognitive, and psychological comorbidities with associated decreases in quality of life.[8–13] Although previous cohorts of critically ill patients likely included some patients with ALI, the incidence and timing of decisions to limit life support specifically for ALI patients has not been thoroughly explored. Our objectives were to evaluate the timing of new limitations in life support among patients with ALI, and to determine whether the persistence of organ dysfunction is associated with new limitations during the course of an ICU stay.
Materials and Methods
Study Cohort
As part of a prospective cohort study, mechanically ventilated patients who met the American-European Consensus criteria for ALI [14] were consecutively enrolled from 13 ICUs at four hospitals in Baltimore, Maryland between October 2004 and October 2007.[15] Patients in neurologic specialty ICUs were excluded to avoid enrolling patients with head trauma or primary neurologic disease. Key exclusion criteria were: 1) pre-existing illness with a life expectancy of <6 months; 2) pre-existing cognitive impairment or communication/language barriers; 3) no fixed address; 4) transfer to a study site ICU with pre-existing ALI of >24 hours duration; 5) >5 days of mechanical ventilation before ALI; and 6) a pre-existing limitation in life support at the time of study eligibility (except for a sole order for “no cardiopulmonary resuscitation” (CPR) in the event of a cardiac arrest). For this analysis we excluded research participants who had a sole “no CPR” limitation at ALI onset (n=24), so that all participants had no limitations in life support at ALI onset. In addition, participants recruited from two ICUs at the Veterans Affairs hospital study site (n=6) were excluded because medical records were inaccessible for independent verification of limitations of life support at the time of this analysis. Consequently, a total of 490 patients from 11 ICUs were available for this analysis. The institutional review boards of Johns Hopkins University and all participating study sites approved this research.
Primary Outcome
Patient records were evaluated on a daily basis while in the ICU for limitations in the use of life support. A limitation in life support was defined as any of the following: (1) No CPR, (2) Do not re-intubate, (3) No vasopressors, (4) No hemodialysis, (5) Do not escalate care or (6) Other limitation (e.g., “comfort care only”). These limitations were considered in this analysis when initiated either with or without a concomitant decision to withdraw life support.
Primary Exposure
The primary exposure for this analysis was the proportion of days since ALI onset during which a patient’s SOFA score remained the same or increased, corresponding to no improvement or worsening of organ function. The SOFA score was developed as an objective quantitative description of the degree of organ dysfunction in critically ill patients over time.[16]. Total score (range 0 to 24) is calculated as the sum of a 4-point scale used to measure organ dysfunction for each of the following six body systems: respiratory, circulatory, renal, hematologic, hepatic, and central nervous system, with greater dysfunction given a higher score. SOFA scores were missing for only 9 of 8,673 (0.10%) days of observation. These missing scores were imputed by multiple imputation and included in analysis. The proportion of ICU days since ALI onset without improvement in SOFA score was calculated as the number of days that a patient failed to improve divided by the number of days since ALI onset. The proportion of improved days was first measured on Day 1 by comparing a patient’s Day 1 SOFA score with that on the day of ALI onset (Day 0). We also considered additional measures of SOFA score for inclusion in the analysis, including whether the SOFA score had improved in the previous 24-hours, change in SOFA score from the day of ALI onset, and change in SOFA score over the past 3 or 7 days. However, the addition of these measures to the existing model did not substantially improve fit, as evaluated using likelihood ratio tests, and thus were excluded from the final model.
Covariates
The following baseline covariates hypothesized to be potentially associated with the use of life support were included in the analysis: patient age, sex, race (white vs non-white), days of hospitalization prior to ALI onset, indicator of hospital study site (1, 2, or 3), ICU type (medical vs surgical), Charlson comorbidity index[17], cancer history, Acute Physiology and Chronic Health Evaluation II (APACHE II) severity of illness score at ICU admission [18], and the Functional Comorbidity Index [19] measure of baseline physical functional status. In addition, the individual daily scores for each of the 6 body systems which comprise the SOFA score were entered into the model as time-varying covariates; thus, allowing the estimation of limitation rates for prototypical patients with the combination of organ system failures typical in this ALI cohort.
Statistical Analyses
All patients were analyzed from ALI onset until the day that a limitation in life support first occurred. For illustrative purposes, results are displayed to day 21 at which point the majority of outcomes had occurred. Cardiac arrest and discharge from the ICU were treated as censoring events. For this analysis, flexible parametric survival regression models were used, with restricted cubic splines of the Weibull distribution (with knots at 3, 7, and 21 days) used for the baseline hazard function.[20–22] The shape of the baseline hazard was determined by maximizing Akaike’s Information Criterion (AIC) and favoring simple models in the case of near ties of AIC values. All models were adjusted for covariates as previously described.
Each covariate was evaluated for a time-dependent effect by fitting interaction terms with time and comparing models with and without time-dependent effects using likelihood ratio tests. Only the estimated effect of the primary exposure (proportion of days without improvement in SOFA score) and a single covariate (ICU type) on the rate of limitations varied significantly with time. Thus, relative hazards obtained from the regression models for these variables were modeled as functions of time. Analysis was conducted using STATA 11.0 (StataCorp, College Station, TX).
Results
Among the 490 patients in our analysis, median age was 52 years (interquartile range 42 – 62), 43% were female, 80% were treated in medical ICUs, and 56% survived to ICU discharge (Table 1). Half of all patients were hospitalized for <2 days prior to ALI onset. Patients in SICUs were more likely to be male and white than MICU patients, and their median APACHE II score was lower at ICU admission (Table 1). During the first five days after ALI onset, 18% of MICU patients and 3% of SICU patients had new limitations in life support. A total of 192 patients (39%) had limitations prior to ICU discharge or cardiac arrest in the ICU. The median time from ALI onset to any limitation in life support was 7 days (interquartile range 3 – 16).
Table 1.
Patient characteristics and outcomes for acute lung injury cohort
| Patient Characteristics | All patients (N = 490) | MICU (N = 394) | SICU (N = 96) |
|---|---|---|---|
| Age; median (IQR) | 52 (42 – 62) | 51 (41 – 61) | 54 (44 – 65) |
| Female | 43% | 45% | 38% |
| Non-white | 41% | 45% | 24% |
| Charlson Comorbidity Index; median (IQR) | 2 (1 – 4) | 2 (1 – 4) | 2 (0 – 3) |
| Cancer History | 18% | 18% | 22% |
| Functional Comorbidity Index; median (IQR) | 1 (1 – 3) | 1 (1 – 3) | 2 (1 – 3) |
| Study site hospital indicator | |||
| 1 | 38% | 44% | 14% |
| 2 | 31% | 35% | 14% |
| 3 | 31% | 21% | 73% |
| ICU Admission Sourcea | |||
| Emergency department | 42% | 45% | 29% |
| Hospital floor | 39% | 42% | 28% |
| Another ICU | 13% | 12% | 16% |
| Operating room | 4% | 0% | 23% |
| Other | 1% | 1% | 4% |
| APACHE II at ICU admission; median (IQR) | 26 (20 – 33) | 27 (21 – 34) | 21 (17 – 27) |
| Total SOFA score at ALI onset; median (IQR) | 9 (7 – 12) | 10 (7 – 13) | 8 (6 – 11) |
| Days in Hospital Prior to ALI Onset; median (IQR) | 2 (1 – 6) | 2 (1 – 6) | 3 (1 – 8) |
| Days in ICU Prior to ALI Onset; median (IQR) | 1 (0 – 2) | 0 (0 – 2) | 1 (0 – 3) |
|
| |||
| Patient Outcomes | |||
|
| |||
| First eventa | |||
| Discharged alive from ICU | 48% | 45% | 61% |
| Limitation in use of life supportb | 39% | 42% | 28% |
| Cardiopulmonary resuscitationc | 13% | 13% | 10% |
| Survived to ICU discharge | 56% | 52% | 73% |
Abbreviations: IQR, Interquartile Range; ICU, intensive care unit; APACHE II, Acute Physiology and Chronic Health Evaluation II; SOFA, sequential organ failure assessment; ALI, acute lung injury; CNS, central nervous system
Proportions do not add to 100% due to rounding
Of these patients, 14%, 11%, and 26% survived to ICU discharge for All patients, MICU patients and SICU patients, respectively.
Of these patients 24%, 21%, and 40% survived to ICU discharge for All patients, MICU patients and SICU patients, respectively.
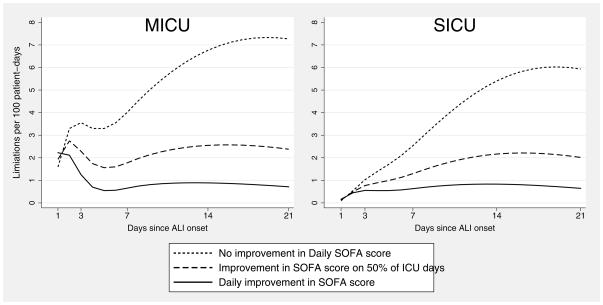
To illustrate how the timing of limitations in life support changed, the survival model was used to estimate the number of limitations in life support each day among 100 prototypical patients with median values for all continuous covariates and mode values for all binary covariates in a MICU and in a SICU (Figure 1). Notably, over the first three days in a MICU, the point estimate for the rate of first limitation was more than three-fold greater for patients with no improvement in daily SOFA score versus patients with daily improvement, and then decreased thereafter, reaching a nadir at Day 5. However, in SICU patients, this pattern was not observed, with the rate of limitations continuously increasing over time. Among both MICU and SICU patients with no improvement in daily SOFA scores, the rate of limitations peaked at Day 19.
Figure 1. Rate of Limitations in Life Support Over 21 Days After Acute Lung Injury Onset.
Estimates for the rate of limitations in life support over 21 days among prototypical patients in this acute lung injury cohort, assuming median values for continuous covariates and mode values for binary covariates.
Abbreviations: MICU, Medical Intensive Care Unit; SICU, Surgical Intensive Care Unit; SOFA, Sequential Organ Failure Assessment score; ALI, acute lung injury
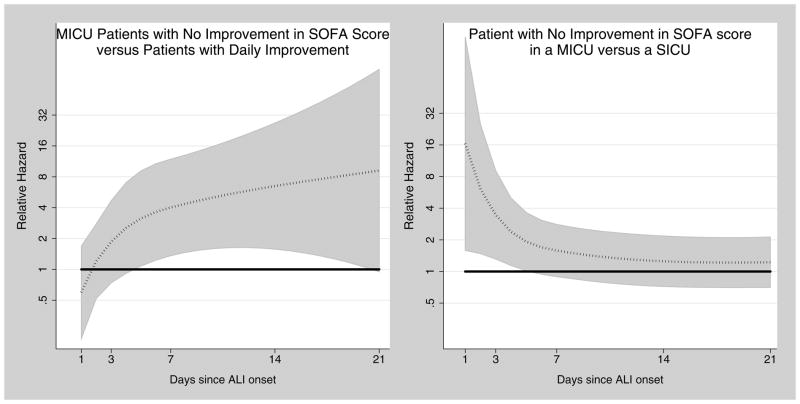
The time-varying, adjusted relative hazards for limitations in life support are reported in Table 2 and Figure 2. The relative hazard of a limitation was always numerically greater for patients with fewer days of improvement in SOFA score and for patients in MICUs. However, as depicted in Figure 2, only some of these differences reached statistical significance. More specifically, the relative hazard of a limitation for MICU patients without improvement in SOFA score compared to patients with daily improvement ranged from 1.85 to 9.20, and achieved statistical significance between days 5 and 20. Consequently, two ALI patients with the same baseline characteristics who have both survived in the ICU for one week and have the same SOFA scores in every organ system may not have the same immediate risk of a limitation in life support. Limiting the use of life support is more likely for the patient who has had fewer days of improvement in overall organ dysfunction during the past week. The relative hazard of a limitation for MICU patients without improvement in SOFA score relative to SICU patients without improvement ranged from 1.22 to 3.46 and was statistically significant on the first five days following ALI onset, after which time the rates of first limitations for MICU and SICU patients were similar.
Table 2.
Time-Dependent Adjusted Relative Hazards* for Limitation in Life Support after Acute Lung Injury Onset
| Proportion of ICU days with SOFA score improvement from prior day | Day 3 | Day 7 | Day 14 | Day 21 | |
|---|---|---|---|---|---|
| RH (95% CI) | RH (95% CI) | RH (95% CI) | RH (95% CI) | ||
|
| |||||
| MICU | 100% | Reference | Reference | Reference | Reference |
| 75% | 1.18 (0.93 – 1.52) | 1.44 (1.08 – 1.94) | 1.62 (1.10 – 2.42) | 1.78 (0.90 – 3.56) | |
| 50% | 1.39 (0.86 – 2.24) | 2.06 (1.16 – 3.64) | 2.60 (1.22 – 5.56) | 3.13 (0.87 – 11.21) | |
| 25% | 1.61 (0.80 – 3.26) | 2.89 (1.26 – 6.64) | 4.13 (1.38 – 12.36) | 5.39 (0.89 – 32.59) | |
| 0% | 1.85 (0.74 – 4.66) | 4.02 (1.36 – 11.89) | 6.49 (1.57 – 26.77) | 9.20 (0.95 – 89.39) | |
|
| |||||
| SICU | 100% | Reference | Reference | Reference | Reference |
| 75% | 1.39 (0.93 – 2.09) | 1.70 (1.04 – 2.80) | 1.72 (1.03 – 2.87) | 1.85 (0.81 – 4.25) | |
| 50% | 1.83 (0.90 – 3.68) | 2.71 (1.16 – 6.35) | 2.87 (1.12 – 7.37) | 3.33 (0.75 – 14.87) | |
| 25% | 2.31 (0.89 – 5.96) | 4.14 (1.32 – 13.00) | 4.72 (1.27 – 17.56) | 5.87 (0.75 – 46.08) | |
| 0% | 2.84 (0.88 – 9.15) | 6.13 (1.50 – 25.00) | 7.62 (1.46 – 39.85) | 10.18 (0.79 – 131.72) | |
|
| |||||
| ICU Type† | Surgical | Reference | Reference | Reference | Reference |
| Medical | 3.46 (1.31 – 9.11) | 1.59 (0.89 – 2.80) | 1.25 (0.72 – 2.18) | 1.22 (0.70 – 2.13) | |
Abbreviations: ICU, intensive care unit; SOFA, sequential organ failure assessment; MICU, medical intensive care unit; SICU, surgical intensive care unit; 95% CI, 95% confidence interval; ALI, acute lung injury
The relative hazard describes the relative risk of having a first limitation in life support on a specific day in the ICU. For example, a patient with a relative hazard of 1.66 on day 7 has a 66% increased risk of having a limitation on day 7 compared to the reference patient. Model is adjusted for age, race, sex, hospital study site indicator, days of hospitalization prior to ALI onset, Acute Physiology and Chronic Health Evaluation II score at ICU admission, Charlson Comorbidity Index, Cancer history, Functional Comorbidity Index, and time-varying SOFA score organ system components.
Calculated for patients with no improvement in SOFA score (i.e. proportion of ICU days with SOFA score improvement from prior day = 0%)
Figure 2. Time-Dependent Relative Hazards of Limitations in Life Support Over 21 Days After Acute Lung Injury Onset.
Time dependent relative hazards (dotted line) and 95% confidence intervals (shaded areas) for limitations in life support over 21 days of follow-up for prototypical patients in this acute lung injury cohort. Horizontal solid line at a relative hazard of 1.0 indicates no difference in the rate of limitations in life support for compared groups.
Abbreviations: ICU, intensive care unit; MICU, medical intensive care unit; SICU, surgical intensive care unit; SOFA, sequential organ failure assessment; ALI, acute lung injury
Discussion
Our analyses of this multi-site, prospective, cohort study of ALI patients had three major findings. First, the timing of limitations in life support for ALI patients treated in MICUs demonstrated an early peak around Day 3 and a second peak 2 – 3 weeks later for patients without consistent improvement in organ function. Second, patients in SICUs are initially much less likely to have limitations in life support than MICU patients, but this difference dissipated by Day 14. Finally, patients who failed to show consistent improvement in organ function had higher limitation rates than patients who displayed improvement, even when the absolute magnitude of organ dysfunction on the day of evaluation was the same.
The small number of MICU patients with a first limitation more than two weeks after ALI onset makes our estimates at those times imprecise. However, there are several clinical observations that may support the observed pattern of an early peak around Day 3 and a second peak later on. For instance, there may be early meetings with patient surrogates that include discussions about limiting the use of life support or about time-limited trials of life support therapies.[23] The pattern also may represent two distinct groups of patients: those for whom meetings about prognosis and goals of care occur during the first three days following ALI onset, and those for whom such meetings do not occur until the patient fails to show consistent improvement in organ function. Family meetings may not occur for some subgroups of patients because identifying appropriate patient surrogates and scheduling meetings can be time-consuming and difficult [24, 25]. It is also possible that some intensivists simply choose not to present the option of limiting life support unless patients show several days of steady deterioration.[26]
The observed difference in the timing of limitations between medical and surgical ICUs may be a result of differences in the patient populations which were not captured by the covariates in our models. The observed preference for life support interventions in the SICU is also consistent with previous research describing an informal “covenant of care” between surgeons and their patients.[27] In a survey of 2,100 surgeons practicing in the U.S., the majority reported refusing to operate on patients with preferences for limiting life support and only half considered it acceptable to honor a patient or surrogate request to withdraw life support on postoperative Day 7.[28, 29] In our study cohort, limiting life support postoperatively in the SICU also may have been viewed as undesirable if physicians viewed ALI as an avoidable complication.[30] However, for patients who remained critically ill for at least a week, the rate of limitations in life support in a SICU vs MICU was not significantly different. Further study is required to determine whether this indicates that informal agreements about the postoperative use of life support are of limited duration.
This study has a number of potential limitations. Not all potentially relevant factors associated with limitations in life support were evaluated in this study (e.g. proxy perception of patient’s pre-ICU quality of life, differences in physician specialty and training, and physician attitudes to limiting life support) which may have resulted in residual confounding in evaluating the association between persistent organ dysfunction and limitations in life support and in the comparison of medical versus surgical patients. Our cohort was limited to acute lung injury patients at three teaching hospitals in a single city, limiting generalizability of findings especially given known regional variability ICU care at the end-of-life [31, 32]. Our outcome of interest included both documented decisions not to initiate additional forms of life support as well as decisions to withdraw existing life support. We expect that isolated decisions not to initiate a treatment were less likely to be documented compared to decisions to withdrawal life support yielding some potential measurement error. However, we believe that the broader definition used in this study better reflects the range of decisions made in an ICU setting about the use of life-sustaining interventions. Finally, this study cannot describe how the shared decision-making process influenced the timing of limitations in life support. Without data on when meetings about the use of life support occurred, or on the goals of care expressed by patient surrogates, we cannot attribute the observed outcomes to differences in the behaviors of physicians or families.
In conclusion, the timing of limitations in life support for patients with ALI is complex and dynamic over the first weeks of treatment. Patients in MICUs versus SICUs had significantly more limitations during the first five days after ALI onset. In both MICUs and SICUs, a lack of consistent improvement in organ function is associated with new limitations in life support independent of the absolute magnitude of organ failure.
Acknowledgments
We thank all of the patients who participated in the study, as well as Mr. Robert LeGros, BA for help with data collection, Mr. Victor Dinglas, MPH for assistance with data management, and Pranita Tamma MD, MHS, for her editorial assistance.
This research was supported by the National Institutes of Health (Acute Lung Injury Specialized Centers of Clinically Oriented Research grant No. P050 HL 73994). AET is supported by the Johns Hopkins University Sommer Scholars Program and a postdoctoral training grant from the National Institute on Aging, T32AG000247.
Dr. Turnbull received grant support from the Johns Hopkins University Summer Scholars Program (scholarship for graduate students in public health). Dr. Shanholtz and his institution received grant support from NIH. Dr. Turnbull’s institution received grant support from NIH, National Institute on Aging (T32AG000247 - postdoctoral training grant). Dr. Needham’s institution received grant support from NIH.
Dr. Shanholtz received support for article research from NIH. Dr. Needham received support for article research from NIH (Acute Lung Injury Specialized Centers of Clinically Oriented Research grant - No. P050 HL 73994).
Footnotes
The remaining authors have disclosed that they do not have any potential conflicts of interest.
All authors contributed to the conception and design of the study. AET and BML analyzed the data. AET drafted the article. All authors contributed to the interpretation of analyses, critically revised the article for important intellectual content and gave final approval of the manuscript version to be published. DMN and AET are responsible for the overall content as guarantors.
References
- 1.Angus DC, Barnato AE, Linde-Zwirble WT, Weissfeld LA, Watson RS, Rickert T, Rubenfeld GD. Use of intensive care at the end of life in the United States: an epidemiologic study. Crit Care Med. 2004;32:638–643. doi: 10.1097/01.ccm.0000114816.62331.08. [DOI] [PubMed] [Google Scholar]
- 2.Prendergast TJ, Luce JM. Increasing incidence of withholding and withdrawal of life support from the critically ill. Am J Respir Crit Care Med. 1997;155:15–20. doi: 10.1164/ajrccm.155.1.9001282. [DOI] [PubMed] [Google Scholar]
- 3.Prendergast TJ, Claessens MT, Luce JM. A national survey of end-of-life care for critically ill patients. Am J Respir Crit Care Med. 1998;158:1163–1167. doi: 10.1164/ajrccm.158.4.9801108. [DOI] [PubMed] [Google Scholar]
- 4.Hall RI, Rocker GM. End-of-life care in the ICU: treatments provided when life support was or was not withdrawn. Chest. 2000;118:1424–1430. doi: 10.1378/chest.118.5.1424. [DOI] [PubMed] [Google Scholar]
- 5.Prendergast TJ, Puntillo KA. Withdrawal of life support: intensive caring at the end of life. Jama J Am Med Assoc. 2002;288:2732–2740. doi: 10.1001/jama.288.21.2732. [DOI] [PubMed] [Google Scholar]
- 6.Cook DJ, Guyatt GH, Jaeschke R, Reeve J, Spanier A, King D, Molloy DW, Willan A, Streiner DL. Determinants in Canadian health care workers of the decision to withdraw life support from the critically ill. Canadian Critical Care Trials Group. Jama J Am Med Assoc. 1995;273:703–708. [PubMed] [Google Scholar]
- 7.Frost DW, Cook DJ, Heyland DK, Fowler RA. Patient and healthcare professional factors influencing end-of-life decision-making during critical illness: a systematic review. Crit Care Med. 2011;39:1174–1189. doi: 10.1097/CCM.0b013e31820eacf2. [DOI] [PubMed] [Google Scholar]
- 8.Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353:1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 9.Rubenfeld GD, Herridge MS. Epidemiology and outcomes of acute lung injury. Chest. 2007;131:554–562. doi: 10.1378/chest.06-1976. [DOI] [PubMed] [Google Scholar]
- 10.Cox CE, Docherty SL, Brandon DH, Whaley C, Attix DK, Clay AS, Dore DV, Hough CL, White DB, Tulsky JA. Surviving critical illness: acute respiratory distress syndrome as experienced by patients and their caregivers. Crit Care Med. 2009;37:2702–2708. doi: 10.1097/CCM.0b013e3181b6f64a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dowdy DW, Eid MP, Dennison CR, Mendez-Tellez PA, Herridge MS, Guallar E, Pronovost PJ, Needham DM. Quality of life after acute respiratory distress syndrome: a meta-analysis. Intensive Care Med. 2006;32:1115–1124. doi: 10.1007/s00134-006-0217-3. [DOI] [PubMed] [Google Scholar]
- 12.Herridge MS, Tansey CM, Matté A, Tomlinson G, Diaz-Granados N, Cooper A, Guest CB, Mazer CD, Mehta S, Stewart TE, Kudlow P, Cook D, Slutsky AS, Cheung AM. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011;364:1293–1304. doi: 10.1056/NEJMoa1011802. [DOI] [PubMed] [Google Scholar]
- 13.Bienvenu OJ, Colantuoni E, Mendez-Tellez PA, Dinglas VD, Shanholtz C, Husain N, Dennison CR, Herridge MS, Pronovost PJ, Needham DM. Depressive symptoms and impaired physical function after acute lung injury: a 2-year longitudinal study. Am J Respir Crit Care Med. 2012;185:517–524. doi: 10.1164/rccm.201103-0503OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, Legall JR, Morris A, Spragg R. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149:818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 15.Needham DM, Dennison CR, Dowdy DW, Mendez-Tellez PA, Ciesla N, Desai SV, Sevransky J, Shanholtz C, Scharfstein D, Herridge MS, Pronovost PJ. Study protocol: The Improving Care of Acute Lung Injury Patients (ICAP) study. Crit Care. 2006;10:R9. doi: 10.1186/cc3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, Reinhart CK, Suter PM, Thijs LG. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22:707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 17.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 18.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–829. [PubMed] [Google Scholar]
- 19.Groll DL, To T, Bombardier C, Wright JG. The development of a comorbidity index with physical function as the outcome. J Clin Epidemiol. 2005;58:595–602. doi: 10.1016/j.jclinepi.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 20.Royston P, Parmar MKB. Flexible parametric proportional-hazards and proportional-odds models for censored survival data, with application to prognostic modelling and estimation of treatment effects. Stat Med. 2002;21:2175–2197. doi: 10.1002/sim.1203. [DOI] [PubMed] [Google Scholar]
- 21.Lambert P, Royston P. Further development of flexible parametric models for survival analysis. Stata J. 2009;9:265–290. [Google Scholar]
- 22.Hinchliffe SR, Lambert PC. Flexible parametric modelling of cause-specific hazards to estimate cumulative incidence functions. Bmc Med Res Methodol. 2013;13:13. doi: 10.1186/1471-2288-13-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quill TE, Holloway R. Time-limited trials near the end of life. Jama J Am Med Assoc. 2011;306:1483–1484. doi: 10.1001/jama.2011.1413. [DOI] [PubMed] [Google Scholar]
- 24.White DB, Curtis JR, Wolf LE, Prendergast TJ, Taichman DB, Kuniyoshi G, Acerra F, Lo B, Luce JM. Life support for patients without a surrogate decision maker: who decides? Ann Intern Med. 2007;147:34–40. doi: 10.7326/0003-4819-147-1-200707030-00006. [DOI] [PubMed] [Google Scholar]
- 25.Bandy RJ, Helft PR, Bandy RW, Torke AM. Medical decision-making during the guardianship process for incapacitated, hospitalized adults: a descriptive cohort study. J Gen Intern Med. 2010;25:1003–1008. doi: 10.1007/s11606-010-1351-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Charlson ME, Sax FL, MacKenzie CR, Fields SD, Braham RL, Douglas RG., Jr Resuscitation: how do we decide? A prospective study of physicians’ preferences and the clinical course of hospitalized patients. Jama J Am Med Assoc. 1986;255:1316–1322. doi: 10.1001/jama.255.10.1316. [DOI] [PubMed] [Google Scholar]
- 27.Cassell J, Buchman TG, Streat S, Stewart RM. Surgeons, intensivists, and the covenant of care: administrative models and values affecting care at the end of life--Updated. Crit Care Med. 2003;31:1551–1557. discussion 1557–1559. [PubMed] [Google Scholar]
- 28.Redmann AJ, Brasel KJ, Alexander CG, Schwarze ML. Use of advance directives for high-risk operations: a national survey of surgeons. Ann Surg. 2012;255:418–423. doi: 10.1097/SLA.0b013e31823b6782. [DOI] [PubMed] [Google Scholar]
- 29.Schwarze ML, Redmann AJ, Alexander GC, Brasel KJ. Surgeons Expect Patients to Buy-In to Postoperative Life Support Preoperatively: Results of a National Survey. Crit Care Med. 2012 doi: 10.1097/CCM.0b013e31826a4650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schwarze ML, Redmann AJ, Brasel KJ, Alexander GC. The role of surgeon error in withdrawal of postoperative life support. Ann Surg. 2012;256:10–15. doi: 10.1097/SLA.0b013e3182580de5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barnato AE, Herndon MB, Anthony DL, Gallagher PM, Skinner JS, Bynum JPW, Fisher ES. Are regional variations in end-of-life care intensity explained by patient preferences?: A Study of the US Medicare Population. Med Care. 2007;45:386–393. doi: 10.1097/01.mlr.0000255248.79308.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nicholas LH, Langa KM, Iwashyna TJ, Weir DR. Regional variation in the association between advance directives and end-of-life Medicare expenditures. Jama J Am Med Assoc. 2011;306:1447–1453. doi: 10.1001/jama.2011.1410. [DOI] [PMC free article] [PubMed] [Google Scholar]