Abstract
Objective
Inhaled anticoagulation regimens are increasingly being employed to manage smoke inhalation-associated acute lung injury (SI-ALI). We systematically reviewed published and unpublished pre-clinical and clinical trial data to elucidate the effects of these regimens on lung injury severity, airway obstruction, ventilation, oxygenation, pulmonary infections, bleeding complications, and survival.
Data Sources
PubMed, Scopus, EMBASE, and Web of Science were searched to identify relevant published studies. Relevant unpublished studies were identified by searching the Australian and New Zealand Clinical Trials Registry, World Health Organization International Clinical Trials Registry Platform, Cochrane Library, ClinicalTrials.gov, MINDCULL.com, Current Controlled Trials, and Google.
Study Selection
Inclusion criteria were any pre-clinical or clinical study in which (1) animals or subjects experienced smoke inhalation exposure, (2) were treated with nebulized or aerosolized anticoagulation regimens including heparin, heparinoids, antithrombins, or fibrinolytics (e.g., tissue plasminogen activator), (3) a control and/or sham group was described for pre-clinical studies, (4) and a concurrent or historical control group described for clinical studies. Exclusion criteria were (1) the absence of a group treated with a nebulized or aerosolized anticoagulation regimen, (2) the absence of a control or sham group, and (3) case reports.
Data Extraction
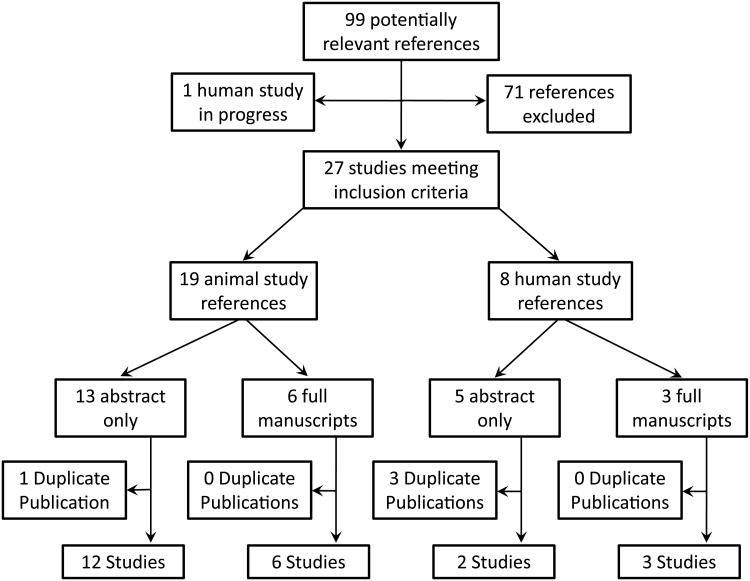
99 potentially relevant references were identified. 27 references met inclusion criteria including 19 pre-clinical references reporting 18 studies, and 8 clinical references reporting 5 clinical studies.
Data Synthesis
A systematic review of the literature is provided. Both clinical and methodological diversity precluded combining these studies in a meta-analysis.
Conclusions
The high mortality associated with SI-ALI results from airway damage, mucosal dysfunction, neutrophil infiltration, airway coagulopathy with cast formation, ventilation-perfusion mismatching with shunt and barotrauma. Inhaled anticoagulation regimens in both pre-clinical and clinical studies improve survival and decrease morbidity without altering systemic markers of clotting and anticoagulation. In some preclinical and clinical studies, inhaled anticoagulants were associated with a favorable effect on survival. This approach appears sufficiently promising to merit a well-designed prospective study to validate its use in patients with severe SI-ALI requiring mechanical ventilation.
Keywords: Smoke Inhalation Injury, Acute Lung Injury, Anticoagulants, Heparin, Antithrombins, Fibrinolytic Agents
Introduction
In the United States, more than 450,000 burn injuries occur annually (1). Smoke inhalation-associated acute lung injury (SI-ALI) constitutes a major cause of morbidity and mortality in victims of fire tragedies, affecting nearly 1.5-19.5% of burn patients worldwide (1.5% Israel; 8% China; 19.5% USA) (2-4). The incidence of SI-ALI is correlated with a greater percent of burn surface area (BSA) (3), however significant SI-ALI may exist in the absence of cutaneous burns. When combined with cutaneous burns, inhalation injury increases fluid requirements for resuscitation, the incidence of pulmonary complications, and the mortality rate (3, 5-8). In a retrospective analysis of 573 burn patients admitted over a 4 year period to a regional burn center, patients with inhalation injury had a > 70% incidence of respiratory failure (hypoxemia, multiple pulmonary infections, or prolonged ventilator support), and a 20% incidence of acute respiratory distress syndrome (9). The reported mortality associated with SI-ALI ranges from 11% to 80% (3, 4, 8-11). The variability in mortality rates from different regions of the world and time periods likely reflects the heterogeneity of the patient populations due to age, comorbidities, severity of the primary and secondary injuries, and variations in therapy.
Several mechanisms have been identified in animal models and in human pathologic studies that contribute to the development of SI-ALI. Smoke inhalation promotes airway damage and inflammation. Immediately following smoke inhalation, bronchial blood flow increases up to 20-fold (12). Increased microvascular permeability and airway edema results in plasma exudation into the airway resulting in intra-airway coagulation and fibrin deposition. Cellular debris, mucin, and fibrin deposition admix to form a fibrinocellular exudate or pseudomembranes that may develop into obstructive airway casts that promote significant mismatch in ventilation and perfusion (V/Q) (2, 13-23). The V/Q mismatch is further worsened by increased perfusion to under-ventilated lung regions due to impaired hypoxic vasoconstriction driven in part by increased nitric oxide production (24, 25). Moreover, partially obstructed airways may result in air trapping and alveolar hyperinflation via a ball-valve mechanism that may promote distal airway and alveolar damage. These mechanisms may lead to impaired oxygenation and ventilation. While on mechanical ventilation this may result in elevated peak and plateau pressures, auto-peep, and patient-ventilator dysynchrony. This may promote regional barotrauma in some lung segments while resulting in repetitive alveolar collapse and expansion injury. Additionally, casts may also occlude endotracheal and tracheostomy tubes necessitating emergent airway revision (26).
SI-ALI is an important yet understudied clinical problem. The use of inhaled anticoagulation regimens is increasingly being employed to manage SI-ALI with the goal of improving outcomes by decreasing airways fibrin deposition and obstruction (27). However, the data detailing the efficacy of this approach is limited and to our knowledge has not been compiled into a comprehensive review. We conducted this review to summarize the complex body of literature on the topic, assess the consistency of results across trials, clarify the strengths and weakness of available studies, and to document the need for further prospective clinical investigation. We systematically review published and unpublished pre-clinical and clinical study data to elucidate the effects of such regimens on lung injury severity, airway obstruction, ventilation, oxygenation, pulmonary infections, bleeding complications and survival.
Methods
A systematic search was performed to capture published and unpublished pre-clinical and clinical studies of nebulized anticoagulation regimens as treatment for SI-ALI. PubMed, Scopus, EMBASE, and Web of Science were searched to identify relevant published studies. The search strategies were adapted to accommodate the unique searching features of each database, including database-specific MESH and EMTREE controlled vocabulary terms. Searches were not limited by date, language or publication status. See Appendix 1 (supplemental online material) for detailed search strategies. The cited and citing references of selected studies were also searched for additional relevant material.
To minimize publication bias, relevant unpublished studies were identified by searching the Australian and New Zealand Clinical Trials Registry, World Health Organization International Clinical Trials Registry Platform, Cochrane Library, ClinicalTrials.gov, MINDCULL.com, Current Controlled Trials, and Google.
Inclusion criteria were any pre-clinical or clinical study in which (1) animals or subjects experienced smoke inhalation exposure, (2) were treated with nebulized or aerosolized anticoagulation regimens including heparin, heparinoids, antithrombins, or fibrinolytics (e.g., tissue plasminogen activator), (3) a control and/or sham group was described for pre-clinical studies, (4) and a concurrent or historical control group described for clinical studies. Exclusion criteria were (1) the absence of a group treated with a nebulized or aerosolized anticoagulation regimen, (2) the absence of a control or sham group, and (3) case reports.
Results
The search identified 99 potentially relevant studies. 27 references met inclusion criteria (Figure 1) including 19 pre-clinical references reporting 18 studies, and 8 clinical references reporting 5 clinical studies. 1 prospective clinical trial has been planned but results are not available (28). The presence of both clinical diversity and methodological diversity precluded combining the study reports in a meta-analysis.
Figure 1.
Schematic summary of literature review of nebulized anticoagulation regimens for the treatment of smoke inhalation associated acute lung injury.
Pre-Clinical Studies
The bulk of the pre-clinical studies were conducted using an ovine model of SI-ALI. In this model, the sheep are surgically prepared for chronic study via placement of arterial, left atrial, pulmonary artery, and lung lymphatic catheters. On post-operative day 5-7, smoke inhalation exposure is accomplished via either cooled cotton (<40°C) (13, 29-45) or pinewood (46) smoke administered via tracheostomy or endotracheal tube. In most models, the animals undergo cutaneous thermal burn (40% BSA) at the time of smoke exposure. The animals are mechanically ventilated and volume resuscitated with intravenous crystalloid infusion in accordance with the Parkland formula (47). Antibiotic therapy is not administered. The most commonly investigated nebulized anticoagulation regimens include: unfractionated heparin (5,000 or 10,000 IU), antithrombin (AT) (290 units), tissue plasminogen activator (TPA) (1mg or 2mg) either alone or in combination with anticoagulants. Depending on the study and treatment regimen, nebulized therapies are initiated at 1-4 hours post-injury and continued every 4 hours for, commonly, 24-48 hours. Endpoints are reported from 48-96 hours post-injury and usually include mortality, weaning from mechanical ventilation, and tracheostomy decannulation. Commonly reported markers of ventilation and oxygenation include peak and plateau airway pressures, PaO2/FiO2, and arterial-alveolar (a-A) oxygen gradient. Markers of pulmonary congestion and obstruction include the lung wet-to-dry ratio (W/D), lung lymph flow, pulmonary shunt fraction (Qs/Qt), and histological obstruction scores. Other end-points include the results of blood coagulation and clotting cascade variables and fluid balance. Appendix 2 (supplemental online material) summarizes the results of 18 pre-clinical studies assessing inhaled anticoagulation regimens as treatment for SI-ALI.
Characteristic findings of SI-ALI as described by findings from control groups include the following: increased mortality and impaired ventilation with elevations in peak and plateau airway pressures, decline in PaO2/FiO2 with concomitant rise in a-A gradient, and increased evidence of airway obstruction. Increasing pulmonary congestion is often manifested as an increase in lung lymph flow, QsQt, and W/D. With few exceptions, nebulized anticoagulation regimens significantly attenuated these findings (Table 1).
Table 1.
Summary of the pathophysiological effects of nebulized anticoagulation regimens in animal models of smoke inhalation associated acute lung injury.
| Author (Year) | Inhaled Anticoagulation Regimen | PaO2/FiO2 Ratio | a-A Gradient | Qs/Qt | W/D Ratio | Lymph Flow | Airway Pressures | Histological Obstruction | Survival |
|---|---|---|---|---|---|---|---|---|---|
| Characteristic Findings in SI-ALI | 1, 5 | ↓ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↓ |
| Desai (1986) | 1, 5 | ↓ | ↑ | ||||||
| Brown (1988)** | 4 | ↑ | |||||||
| Cindric (1996) | 1 | ↓ | |||||||
| Katahira (2001) | 1 | ↑ | ↓ | ||||||
| Murakami (2002) | 1 | ↑ | ↓ | ↓ | ↓ | ||||
| Taski (2002) | 1 | ↓ | No change | ↓ | |||||
| Enkhbaatar (2004) | 1, 3, 6 | ↑ | ↓ | ↓ | ↓ | ↓ | |||
| Enkhbaatar (2005) | 2, 6 | ↓ | ↓ | ↓ | |||||
| Nakano (2006) | 7 | ↑ | ↓ | ||||||
| Enkhbaatar (2007) | 1, 3, 6 | ↑ | ↓ | ↓ | ↓ | ↓ | |||
| Enkhbaatar (2008) | 7 | ↑ | ↑ | ↓ | ↓ | ↓ | ↓ | ||
| Rehberg (2009) | 8 | ↓ | ↓ | ↓ | |||||
| Asmussen (2010) | 8 | ↑ | ↑ | ||||||
| Rehberg (2010) | 8 | ↑ | ↓ | ↓ | |||||
| Asmussen (2011) | 8 | ↑ | ↑ | ||||||
| Rehberg (2011) | 8 | ↑ | ↓ | ↓ |
Alveolar to arterial (a-A) gradient; Shunt fraction (Qs/Qt), Wet-to-dry (W/D) ratio Inhaled (INH) anticoagulation regimens:
Heparin INH
Tissue plasminogen activator (tPA) INH
Antithrombin (AT) INH
GM 1892 INH
Heparin INH + DMSO INH
Heparin INH + AT INH
Heparin INH + AT IV
Heparin INH + tPA INH + AT IV
For studies presented, arrows indicate change from control or untreated groups, not overall change from baseline.
Due to high mortality in control and DMSO alone groups, further comparisons were only made between Heparin and DMSO + Heparin groups.
Clinical Studies
Eight references accounting for five human clinical studies were identified (Table 2). Additionally, a prospective human study is planned but not yet recruiting patients (ClinicalTrials.gov #NCT014548690) (28). All human studies conducted to date have been retrospective studies with historical controls. Four of the five studies were conducted around a change in institutional protocol (2, 27, 48, 49). Three studies assessed a low-dose heparin protocol (5,000 IU) (2, 49, 50) of which one treated according to physician discretion(50), and two studies assessed a high-dose heparin regimen (10,000 IU) (27, 48). The impact of the nebulized heparin protocols on common primary endpoints is summarized in Table 3. Both studies assessing a standardized high-dose heparin dosing regimen (10,000 IU nebulized every 4 hours) reported statistically significant mortality improvement (27, 48). Conversely, the one study that did not report a mortality benefit used a low-dose regimen, and unlike the aforementioned studies, suffered from significant selection bias in that patients were not allocated into treatment groups but instead were treated according to physician discretion(50). Each of three studies assessing lung injury score reported a statistically significant improvement (2, 27, 48). Only one standardized regimen (low-dose) has assessed the incidence of pneumonia and this study reported a significant decrease in the incidence of pneumonia (2). These results were not reproduced by the study without a standardized dosing regimen (50). Although no change in the duration of mechanical ventilation has been reported with nebulized heparin regimens, one study did report a decrease in the incidence of un-planned re-intubation (2). Similar to animal studies, nebulized heparin regimens of 5,000 IU or 10,000 IU every four hours in humans have not been shown to alter serum markers of anticoagulation (49).
Table 2.
Summary of human clinical investigations of inhaled anticoagulation for smoke inhalation associated acute lung injury.
| Author (Year); Reference | N | Protocol | Intervention | Results |
|---|---|---|---|---|
| Desai (1998); (6, 68) | 90 | Retrospective analysis with historical control | Standard therapy + Heparin 5000 units INH alternating with 3ml NAC INH every 2 hours for first 7 days following injury. | ↓ Mortality, ↓re-intubation rate, ↓ incidence pneumonia and atelectasis. No significant change in duration of intubation. |
| Rivero (2007);(52)* | 16 | Retrospective analysis with historical control | The nebulized heparin 10,000 IU + N-acetylcysteine 3ml of 20% group consisted of 9 patients with smoke inhalation acute lung injury diagnosed with bronchoscopy and necessitating mechanical ventilation and APACHE-III score > 35 (mean 46.66 vs. 44.86 (control), p=0.38). Additionally, daily Lung Injury Score were calculated for seven days. Lung Injury Score was determined by averaging the scores of chest X-ray, PaO2/FiO2, PEEP, and respiratory system compliance. | In the first two ICU days mean lung injury scores were significantly lower in the treatment group (0.76±0.53 vs. 1.23±0.88 (p=0.08). Mean lung injury score during the first week were 0.91±0.14 vs. 1.79±0.41, (p<0.01) for NH-NA and Non-NH-NA patients respectively. Mortality was 11% (1/9) in the treatment group and 43% (3/7) patients in the control group during the first ICU week. |
| Holt (2008);(54) | 150 | Retrospective analysis with historical control | No patient allocation. Patients treated according to attending discretion. Treated patients received inhaled heparin 5000 U/1 ml, NAC 3ml 20% solution, and albuterol 2.5mg of 0.083% solution (3ml) every 4 hours for the first 7 days following admission or until extubation. | No significant difference in mortality, duration of mechanical ventilation, length-of-stay, or pneumonia |
| Miller (2009);(51, 63) | 30 | Retrospective analysis with historical control | Standard therapy + Heparin 10,000 units INH every 4 hours alternating with NAC 3ml of 20% + Albuterol 0.5ml every 4 hours. | ↓ Mortality, ↓ lung injury score |
| Yip (2011);(53) | 63 | Retrospective analysis with historical control | 1. Treatment group: Mechanically ventilated adult patients with bronchoscopically confirmed SI-ALI admitted in a burn ICU (2006-2009). Treated with heparin 5000 IU INH +, NAC 600mg/3ml of 20% aerosolized solution INH + salbutamol 5mg INH, each every 4 hours. (n=52) 2. Control: mechanically ventilated SI-ALI patients (2001-2005) before protocol initiation. | No significant difference in trend of APTT, PT and platelet count over 7 days. No clinically significant increase in bleeding risk in treatment group. |
Published as abstract.
INH - Inhaled; NAC - N-acetylcystine; SI-ALI - smoke inhalation associate acute lung injury; PT – prothrombin time; APTT – activated partial thromboplastin time
Table 3.
Summary of the pathophysiological and clinical effects of nebulized heparin regimens in human clinical studies of smoke inhalation associated acute lung injury.
| Author (Date) | Lung Injury Score | Pneumonia Incidence | Mechanical Ventilation Duration | Unplanned Re-intubation | Hospital Length of Stay | Bleeding Risk | Mortality |
|---|---|---|---|---|---|---|---|
| Desai (1998) | ↓ | ↓ | No Change | ↓ | |||
| Rivero (2007) | ↓ | ↓ | |||||
| Holt (2008)* | No Change | No Change | No Change | ||||
| Miller (2009) | ↓ | ↓ | |||||
| Yip (2011) | No Change |
No randomization or allocation into treatment groups. Patients treated at attending physician discretion with a dosing regimen half the strength of the Rivero et al., and Miller et al. studies.
Discussion
Much of what is known of the early effects of smoke exposure on the respiratory tract has been described and validated in numerous ovine and lapine models of smoke inhalation (51-54). Although difficult to study in humans, the time course of these physiological effects is comparable to those found in burned patients with smoke inhalation injury (51).
Thermal inhalation injury only rarely affects the intrathoracic airways (55). Unless the patient is exposed to steam, which has much greater heat carrying capacity than dry air, the heat dissipating properties of the upper airway restricts direct thermal injury to the supraglottic structures (56, 57). Intrathoracic and lower airway injury is most often a chemical-associated injury related to substances that have lower water solubility and particles less than 5 microns in diameter (58).
Inhaled toxicants can be in the form of gases, vapors, particulate matter and aerosols (57). Smoke from combustible material contains a variety of toxic substances that may cause direct cell injury and initiate inflammatory responses that propagate airway damage (55, 57, 58). Additionally, inhaled particulate matter (i.e. soot) may have adsorbed toxic molecules on the surfaces and sustain inflammatory responses when adherent to the airway mucosa (57).
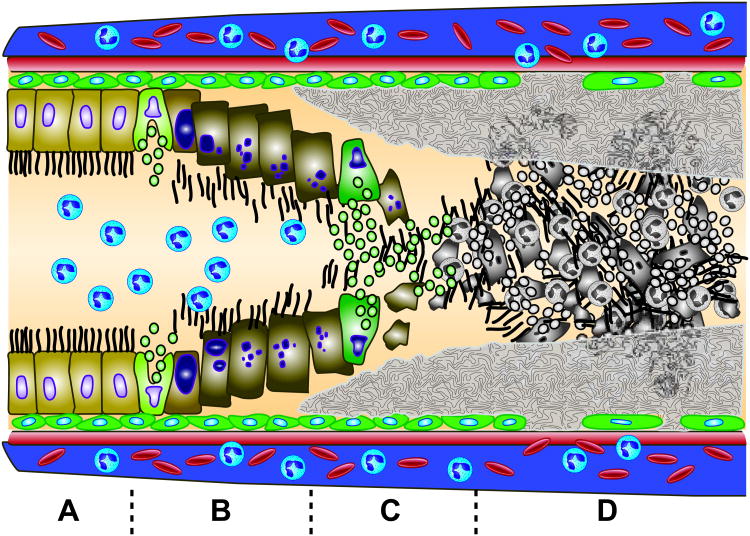
The initial injury from smoke inhalation is limited to the trachea and bronchi, and is characterized by mucosal hyperemia, increased microvascular permeability, exfoliation of the epithelial lining, mucous secretion, and an acute inflammatory cell influx (Figure 2A-C) (53). Necrosis and sloughing of tracheal epithelium has been reported as early as 15 minutes post-exposure (51). Bronchi obstruction peaks as early as 24 hours, whereas bronchiolar obstruction progressively increases to reach a maximum by 72 hours (Figure 2D) (51, 53). This may be in part due to obstructive material that migrates distally from larger airways to smaller bronchioles and disruption of mucociliary clearance (53). As highlighted in Figure 2, fundamental to the airway obstruction is the role of increased microvascular permeability and airway edema that results in plasma exudation into the airway producing intra-airway coagulation and fibrin deposits. These deposits lead to with the resultant fibrinocellular pseudomembranes composed of fibrin deposition, cellular debris and mucin. A dose-dependent relationship between degree of smoke exposure and severity of injury has been described (55).
Figure 2. Depiction of airway changes in the setting of SI-ALI.
Trachea and bronchi injury is characterized by mucosal hyperemia, increased microvascular permeability, exfoliation of the epithelial lining, mucous secretion, and an acute inflammatory cell influx (Figure 2A - C) (57). As early as 1 hour post-exposure, affected respiratory tract epithelium may display clumping, swelling, and loss of cilia, blebbing, and surface erosion (Figure 2B) (55). Within hours, sloughing of the respiratory mucosa progresses, a fibrinocellular pseudomembrane begins to form, and neutrophils begin to influx into the major airways (55). By 6 hours post-injury, the injured bronchi and bronchiole epithelium remains largely intact with focal areas of necrosis and sloughing (Figure 2A, B) (56). Surface lining cells appear enlarged with cytoplasmic vacuolization, and neutrophils have begun to marginate and focally concentrate at points of epithelial necrosis (56). In addition, basal cells are normal in appearance, and the subepithelial connective tissue may appear slightly edematous with contained neutrophil infiltrates (56). By 24 hours, the ciliated and secretory lining cells are largely destroyed (Figure 2C) (56). Cellular debris is admixed with fibroid material, mucus, and neutrophils creating a pseudomembraneous fibrinocellular network that is adherent to both the cell-denuded basal lamina and to the intact basal cells (Figure 2C, D) (56). By 72 hours, injured epithelial areas are largely resurfaced by a stratified reparative epithelium with interposed areas of fibrinocellular exudate (Figure 2D) (56). This epithelium is 3-5 cells thick, with flattened, non-ciliated cells along the surface and these cells are likely derived from proliferating and migrating basal cells (56). Subepithelial edema and inflammatory cell infiltrates begin to diminish (56). Complete repair of the respiratory tract epithelium with return of normal cilia populations may take up to 2 to 4 weeks depending on the severity of smoke exposure.
As intra-airway coagulation with fibrin deposition and fibrinocellular pseudomembranes begin to form within hours of smoke exposure, inhaled anticoagulation regimens may be most-effective when initiated early under the premise that impairing fibrin clot deposition may decrease airway cast formation, and improve oxygenation and ventilation while decreasing the risk of barotrauma. Pre-clinical models (Supplemental Table) most-commonly initiated therapy at 1-2 hours (range 0.5-4 hours) post-injury and continued every 4 hours for usually 48 hours (range 24-96 hours). Human studies did not reliably report time from smoke exposure to treatment initiation. Based on pre-clinical studies, it may be reasonable to initiate a regimen of inhaled heparin within 4 hours post-exposure (or as early as possible), and to continue it every 4 hours for 48 hours. In animal models, by 72 hours injured epithelial areas are largely resurfaced by a stratified reparative epithelium with only interposed areas of fibrinocellular exudate (Figure 2D) (52). Additional factors that contribute to airway cast formation are mucus secretion and the presence of a large burden of cellular debris. The addition of the mucolytic N-acetylcysteine to nebulized heparin regimens intervenes at an additional level to prevent or minimize formation of obstructive airway casts (2, 27, 48, 59). Future studies in SI-ALI may use combination therapy with inhaled heparin, an inhaled fibrinolytic (e.g., tissue plasminogen activator) and mucolytic therapy (e.g., N-acetylcysteine). Further, recombinant human deoxyribonuclease I (dornase alfa) cleaves extracellular DNA in mucous and when inhaled reduces the adhesiveness and viscoelasticity of airway mucous in patients with cystic fibrosis (60). This treatment might augment the breakdown of cellular debris and further impede the formation of fibrinocellular pseudomembranes. However, to date no studies using this strategy in the setting of SI-ALI have been reported.
Conclusion
The high mortality associated with SI-ALI results from airway epithelial cell injury and inflammation, mucosal dysfunction, airway coagulopathy with cast formation resulting in ventilation-perfusion mismatch with shunt and barotrauma. Inhaled anticoagulation regimens in both pre-clinical and clinical studies improve decrease morbidity without altering systemic markers of clotting and anticoagulation. In some preclinical and clinical studies, inhaled anticoagulants we re associated with a favorable effect on survival. This approach appears sufficiently promising to merit a well-designed prospective study to validate its use in patients with severe SI-ALI requiring mechanical ventilation.
Supplementary Material
Acknowledgments
We thank Judith Welsh for her assistance and expertise with the literature search and search strategies. Additionally, we thank Kelly Byrne for her assistance in developing the artwork.
Footnotes
Reprints: No reprints will be ordered.
Disclosure: This work was supported by the Intramural Research Program of the Clinical Center, National Institutes of Health and the Veterans Administration Hospital. Dr. Suffredini received support for article research from the National Institutes of Health. Dr. Elamin received support for travel from SuperDimension. Dr. Miller disclosed that he does not have any potential conflicts of interest.
Author Contributions: Drs. Miller, Elamin, and Suffredini contributed to all stages of manuscript planning, research, preparation, and revision.
References
- 1.Association AB. Burn Incidence and Treatment in the United States 2011 Fact Sheet. American Burn Association National Burn Repository (2011 report) 2011 cited Available from: http://www.ameriburn.org/resources_factsheet.php.
- 2.Desai MH, Mlcak R, Richardson J, et al. Reduction in mortality in pediatric patients with inhalation injury with aerosolized heparin/N-acetylcystine [correction of acetylcystine] therapy. J Burn Care Rehabil. 1998;19(3):210–212. doi: 10.1097/00004630-199805000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Luo G, Peng Y, Yuan Z, et al. Inhalation injury in southwest China--the evolution of care. Burns. 2010;36(4):506–510. doi: 10.1016/j.burns.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 4.Thompson PB, Herndon DN, Traber DL, et al. Effect on mortality of inhalation injury. The Journal of trauma. 1986;26(2):163–165. doi: 10.1097/00005373-198602000-00012. [DOI] [PubMed] [Google Scholar]
- 5.Dai NT, Chen TM, Cheng TY, et al. The comparison of early fluid therapy in extensive flame burns between inhalation and noninhalation injuries. Burns. 1998;24(7):671–675. doi: 10.1016/s0305-4179(98)00092-8. [DOI] [PubMed] [Google Scholar]
- 6.Suzuki M, Aikawa N, Kobayashi K, et al. Prognostic implications of inhalation injury in burn patients in Tokyo. Burns. 2005;31(3):331–336. doi: 10.1016/j.burns.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 7.Tredget EE, Shankowsky HA, Taerum TV, et al. The role of inhalation injury in burn trauma. A Canadian experience Ann Surg. 1990;212(6):720–727. doi: 10.1097/00000658-199012000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shirani KZ, Pruitt BA, Jr, Mason AD., Jr The influence of inhalation injury and pneumonia on burn mortality. Ann Surg. 1987;205(1):82–87. doi: 10.1097/00000658-198701000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hollingsed TC, Saffle JR, Barton RG, et al. Etiology and consequences of respiratory failure in thermally injured patients. Am J Surg. 1993;166(6):592–596. doi: 10.1016/s0002-9610(05)80662-2. discussion 596-597. [DOI] [PubMed] [Google Scholar]
- 10.Mlcak RP. Aerosolized heparin/N acetylcystine treatment of smoke inhalation injury in 596 burned children: A 15 year review, 1990-2005. In: Saliba MJ, editor. 6th International Heparin in Burns Symposium; Shanghai, China. 2005. [Google Scholar]
- 11.Blinn DL, Slater H, Goldfarb IW. Inhalation injury with burns: a lethal combination. J Emerg Med. 1988;6(6):471–473. doi: 10.1016/0736-4679(88)90402-7. [DOI] [PubMed] [Google Scholar]
- 12.Stothert JC, Jr, Ashley KD, Kramer GC, et al. Intrapulmonary distribution of bronchial blood flow after moderate smoke inhalation. J Appl Physiol. 1990;69(5):1734–1739. doi: 10.1152/jappl.1990.69.5.1734. [DOI] [PubMed] [Google Scholar]
- 13.Enkhbaatar P, Murakami K, Westphal M, et al. Combined antithrombin and heparin nebulization improves pulmonary function in sheep with burn and smoke inhalation: 96. Critical Care Medicine. 2004;32(12):A24. [Google Scholar]
- 14.Jacob S, Kraft R, Zhu Y, et al. Acute secretory cell toxicity and epithelial exfoliation after smoke inhalation injury in sheep: an electron and light microscopic study. Toxicol Mech Methods. 2010;20(8):504–509. doi: 10.3109/15376516.2010.511302. [DOI] [PubMed] [Google Scholar]
- 15.Choi WI, Syrkina O, Kwon KY, et al. JNK activation is responsible for mucus overproduction in smoke inhalation injury. Respir Res. 2010;11:172. doi: 10.1186/1465-9921-11-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cox RA, Burke AS, Traber DL, et al. Production of pro-inflammatory polypeptides by airway mucous glands and its potential significance. Pulm Pharmacol Ther. 2007;20(2):172–177. doi: 10.1016/j.pupt.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 17.Morita N, Enkhbaatar P, Maybauer DM, et al. Impact of bronchial circulation on bronchial exudates following combined burn and smoke inhalation injury in sheep. Burns. 2011;37(3):465–473. doi: 10.1016/j.burns.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hill IR. Reactions to particles in smoke. Toxicology. 1996;115(1-3):119–122. doi: 10.1016/s0300-483x(96)03499-3. [DOI] [PubMed] [Google Scholar]
- 19.Murakami K, Traber DL. Pathophysiological basis of smoke inhalation injury. News Physiol Sci. 2003;18:125–129. doi: 10.1152/nips.01427.2002. [DOI] [PubMed] [Google Scholar]
- 20.Cox RA, Mlcak RP, Chinkes DL, et al. Upper airway mucus deposition in lung tissue of burn trauma victims. Shock. 2008;29(3):356–361. doi: 10.1097/shk.0b013e31814541dd. [DOI] [PubMed] [Google Scholar]
- 21.Fitzpatrick JC, Cioffi WG, Jr, Cheu HW, et al. Predicting ventilation failure in children with inhalation injury. J Pediatr Surg. 1994;29(8):1122–1126. doi: 10.1016/0022-3468(94)90292-5. [DOI] [PubMed] [Google Scholar]
- 22.Saliba MJ., Jr Heparin in the treatment of burns: a review. Burns. 2001;27(4):349–358. doi: 10.1016/s0305-4179(00)00130-3. [DOI] [PubMed] [Google Scholar]
- 23.Hamahata A, Enkhbaatar P, Sakurai H, et al. Effect of ablated bronchial blood flow on survival rate and pulmonary function after burn and smoke inhalation in sheep. Burns. 2009;35(6):802–810. doi: 10.1016/j.burns.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Traber DL, Hawkins HK, Enkhbaatar P, et al. The role of the bronchial circulation in the acute lung injury resulting from burn and smoke inhalation. Pulm Pharmacol Ther. 2007;20(2):163–166. doi: 10.1016/j.pupt.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 25.Westphal M, Cox RA, Traber LD, et al. Combined burn and smoke inhalation injury impairs ovine hypoxic pulmonary vasoconstriction. Crit Care Med. 2006;34(5):1428–1436. doi: 10.1097/01.CCM.0000215828.00289.B9. [DOI] [PubMed] [Google Scholar]
- 26.Cancio LC. Airway management and smoke inhalation injury in the burn patient. Clin Plast Surg. 2009;36(4):555–567. doi: 10.1016/j.cps.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 27.Miller AC, Rivero A, Ziad S, et al. Influence of nebulized unfractionated heparin and N-acetylcysteine in acute lung injury after smoke inhalation injury. Journal of burn care & research : official publication of the American Burn Association. 2009;30(2):249–256. doi: 10.1097/BCR.0b013e318198a268. [DOI] [PubMed] [Google Scholar]
- 28.Salehi SH. The effect of heparin on inhalation injury : This study is not yet open for participant recruitment. [clinical trial] 2011 [cited This is a prospective randomized clinical trial about the efficacy of heparin nebulization on lung injury score in inhalation burn injury in Mothary burn hospital. This study would consist of 170 burn patients with documented inhalation injury in 172 arms (control group and study group). Allocation ratio is171:171. Masking was not possible. The patients' primary outcome will be assessed for Lung Injury Scale and the patients' secondary outcome will be assessed for mortality, Coagulation tests (PT, PTT), ICU and hospital stay and duration of mechanical ventilation support. Duration of this study is about 132 months. ]. Available from: http://clinicaltrials.gov.
- 29.Desai MH, Brown M, Mlcak R, et al. Nebulization treatment of smoke-inhalation injury in sheep model with dimethylsulfoxide, heparin, combination and N-acetylcysteine. Critical Care Medicine. 1986;14(4):321–321. [Google Scholar]
- 30.Brown M, Desai M, Traber LD, et al. Dimethylsulfoxide with heparin in the treatment of smoke inhalation injury. J Burn Care Rehabil. 1988;9(1):22–25. doi: 10.1097/00004630-198801000-00007. [DOI] [PubMed] [Google Scholar]
- 31.Cindrick L, Schenarts P, Bone H, et al. Nebulization of a non-anticoagulant heparinoid (gm1892) attenuates lung lymph flow after acute lung injury in sheep. Faseb Journal. 1996;10(3) [Google Scholar]
- 32.Katahira J, Murakami K, Mlcak R, et al. Effect of heparin nebulization in burn and smoke inhalation injury in sheep. ATS 2001 - American Thoracic Society International Conference; 2001. [Google Scholar]
- 33.Suman OE, Mlcak RP, Katahira J, et al. Effects of nebulized heparin on pulmonary mechanics following smoke inhalation injury. ATS 2001 - American Thoracic Society International Conference; 2001. [Google Scholar]
- 34.Murakami K, Cox R, Hawkins H, et al. Recombinant antithrombin (RhAT) inhibits airway obstruction after smoke inhalation in sheep. Faseb Journal. 2002;16(4):A76–A76. [Google Scholar]
- 35.Thomas S, Suman OE, Enkhbaatar P, et al. The effect of tissue plasminogen activator on pulmonary diffusion capacity in a burn and smoke inhalation sheep model. ATS 2004 - American Thoracic Society International Conference; 2004. [Google Scholar]
- 36.Enkhbaatar P, Cox R, Morita N, et al. Role of airway fibrin formation in pathogenesis of acute lung injury. ATS 2005 - American Thoracic Society International Conference; 2005. [Google Scholar]
- 37.Nakano YY, Enkhbaatar P, Traber LD, et al. Effect of aerosolized heparin with recombinant human antithrombin (rhAT) supplementation in ovine septic shock. ATS 2006 - American Thoracic Society; 2006. [Google Scholar]
- 38.Enkhbaatar P, Cox RA, Traber LD, et al. Aerosolized anticoagulants ameliorate acute lung injury in sheep after exposure to burn and smoke inhalation. Crit Care Med. 2007;35(12):2805–2810. doi: 10.1097/01.ccm.0000291647.18329.83. [DOI] [PubMed] [Google Scholar]
- 39.Enkhbaatar P, Esechie A, Wang J, et al. Clinical science. 4. Vol. 114. London, England: 2008. Combined anticoagulants ameliorate acute lung injury in sheep after burn and smoke inhalation; pp. 321–329. 1979. [DOI] [PubMed] [Google Scholar]
- 40.Rehberg S, Yamamoto Y, Traber D, et al. Intravenous antithrombin III combined with nebulized heparin and tissue plasminogen activator improves pulmonary function following combined burn and smoke inhalation injury. Critical Care Medicine. 2009;37(12):A168. [Google Scholar]
- 41.Asmussen S, Yamamoto Y, Traber DL, et al. Combined anticoagulant and fibrinolytic therapy decreases the ventilator days in an ovine model of cutaneous burn and smoke inhalation injury. Critical Care Medicine. 2010;38:A94. [Google Scholar]
- 42.Rehberg S, Bartha E, Yamamoto Y, et al. Cardiovascular dysfunction induced by combined burn and smoke inhalation injury is attenuated by maintaining physiological plasma levels of antithrombin. Intensive Care Medicine. 2010;36:S320. [Google Scholar]
- 43.Rehberg S, Maybauer MO, Enkhbaatar P, et al. Pathophysiology, management and treatment of smoke inhalation injury. Expert Rev Respir Med. 2009;3(3):283–297. doi: 10.1586/ERS.09.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rehberg S, Sousse L, Yamamoto Y, et al. Interactions of nebulized heparin with intravenous antithrombin for combined therapy of acute lung injury. Critical Care. 2011;15:S72–S73. [Google Scholar]
- 45.Asmussen S, Yamamoto Y, Traber DL, et al. Therapy with recombinant human antithrombin, heparin and tissue plasminogen activator improves survival and reduces ventilation days in a long-term ovine model of cutaneous burn and smoke inhalation injury. Critical Care. 2011;15:S64. [Google Scholar]
- 46.Tasaki O, Mozingo DW, Dubick MA, et al. Effects of heparin and lisofylline on pulmonary function after smoke inhalation injury in an ovine model. Crit Care Med. 2002;30(3):637–643. doi: 10.1097/00003246-200203000-00024. [DOI] [PubMed] [Google Scholar]
- 47.Baxter CR. Fluid volume and electrolyte changes of the early postburn period. Clin Plast Surg. 1974;1(4):693–703. [PubMed] [Google Scholar]
- 48.Rivero A, Elamin E, Nguyen V, et al. Can nebulized heparin and N-acetylcysteine reduce acute lung injury after inhalation lung insult? Chest. 2007;132(4):565S–565S. [Google Scholar]
- 49.Yip LY, Lim YF, Chan HN. Safety and potential anticoagulant effects of nebulised heparin in burns patients with inhalational injury at Singapore General Hospital Burns Centre. Burns. 2011;37(7):1154–1160. doi: 10.1016/j.burns.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 50.Holt J, Saffle JR, Morris SE, et al. Use of inhaled heparin/N-acetylcystine in inhalation injury: does it help? Journal of burn care & research : official publication of the American Burn Association. 2008;29(1):192–195. doi: 10.1097/BCR.0b013e31815f596b. [DOI] [PubMed] [Google Scholar]
- 51.Hubbard GB, Langlinais PC, Shimazu T, et al. The morphology of smoke inhalation injury in sheep. The Journal of trauma. 1991;31(11):1477–1486. doi: 10.1097/00005373-199111000-00003. [DOI] [PubMed] [Google Scholar]
- 52.Thorning DR, Howard ML, Hudson LD, et al. Pulmonary responses to smoke inhalation: morphologic changes in rabbits exposed to pine wood smoke. Hum Pathol. 1982;13(4):355–364. doi: 10.1016/s0046-8177(82)80225-6. [DOI] [PubMed] [Google Scholar]
- 53.Cox RA, Burke AS, Soejima K, et al. Airway obstruction in sheep with burn and smoke inhalation injuries. Am J Respir Cell Mol Biol. 2003;29(3 Pt 1):295–302. doi: 10.1165/rcmb.4860. [DOI] [PubMed] [Google Scholar]
- 54.Linares HA, Herndon DN, Traber DL. Sequence of morphologic events in experimental smoke inhalation. J Burn Care Rehabil. 1989;10(1):27–37. doi: 10.1097/00004630-198901000-00005. [DOI] [PubMed] [Google Scholar]
- 55.Lee AS, Mellins RB. Lung injury from smoke inhalation. Paediatric respiratory reviews. 2006;7(2):123–128. doi: 10.1016/j.prrv.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 56.Cahalane M, Demling RH. Early respiratory abnormalities from smoke inhalation. JAMA. 1984;251(6):771–773. [PubMed] [Google Scholar]
- 57.Miller K, Chang A. Acute inhalation injury. Emergency medicine clinics of North America. 2003;21(2):533–557. doi: 10.1016/s0733-8627(03)00011-7. [DOI] [PubMed] [Google Scholar]
- 58.Rabinowitz PM, Siegel MD. Acute inhalation injury. Clinics in chest medicine. 2002;23(4):707–715. doi: 10.1016/s0272-5231(02)00025-4. [DOI] [PubMed] [Google Scholar]
- 59.Elamin E, Miller A. Impact of Nebulized Unfractionated Heparin and N-acetylcysteine in Management of Smoke Inhalation Injury. Critical Care. 2009;13(Suppl 1):P438. doi: 10.1097/BCR.0b013e318198a268. [DOI] [PubMed] [Google Scholar]
- 60.Fuchs HJ, Borowitz DS, Christiansen DH, et al. Effect of aerosolized recombinant human DNase on exacerbations of respiratory symptoms and on pulmonary function in patients with cystic fibrosis. The Pulmozyme Study Group The New England journal of medicine. 1994;331(10):637–642. doi: 10.1056/NEJM199409083311003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.