Abstract
Several dengue vaccines are under development, and some are expected to become available imminently. Concomitant with the anticipated release of these vaccines, vaccine allocation strategies for dengue endemic countries in Southeast Asia and Latin America are currently under development. We developed a model of dengue transmission that incorporates the age-specific distributions of dengue burden corresponding to those in Thailand and Brazil, respectively, to determine vaccine allocations that minimize the incidence of dengue hemorrhagic fever, taking into account limited availability of vaccine doses in the initial phase of production. We showed that optimal vaccine allocation strategies vary significantly with the demographic burden of dengue hemorrhagic fever. Consequently, the strategy that is optimal for one country may be sub-optimal for another country. More specifically, we showed that, during the first years following introduction of a dengue vaccine, it is optimal to target children for dengue mass vaccination in Thailand, whereas young adults should be targeted in Brazil.
Keywords: Dengue hemorrhagic fever, Dengue vaccine, Optimization, Mathematical modeling
Introduction
Dengue is a mosquito-borne Flavivirus disease and a growing public health problem in many tropical and sub tropical countries (1), with around 2.5 billion people worldwide at risk of infection (2, 3). It is estimated that 50 to 100 million cases of dengue and 12,000 deaths occur annually (2). Dengue is primarily transmitted by the mosquito vector Aedes aegypti, but Aedes albopictus and Aedes polynesiensis may also act as vectors (3). The disease is caused by a virus organized in four distinct, but closely related and co-circulating, serotypes: DENV-1, DENV-2, DENV-3, and DENV-4 (3). Infection with a serotype appears to provide life long immunity against reinfection with that serotype, but not against the others (4). The severity of the disease varies from asymptomatic infections to life-threatening dengue hemorrhagic fever (DHF). DHF is a potentially fatal complication of dengue due to plasma leaking, fluid accumulation, respiratory distress, severe bleeding, or organ impairment (3). DHF is the leading cause of viral hemorrhagic fever worldwide with more than 500,000 cases annually (5, 6). Importantly, severe disease, including DHF, is much more likely among individuals who have already recovered from a primary infection and are experiencing a secondary infection with a different serotype (4).
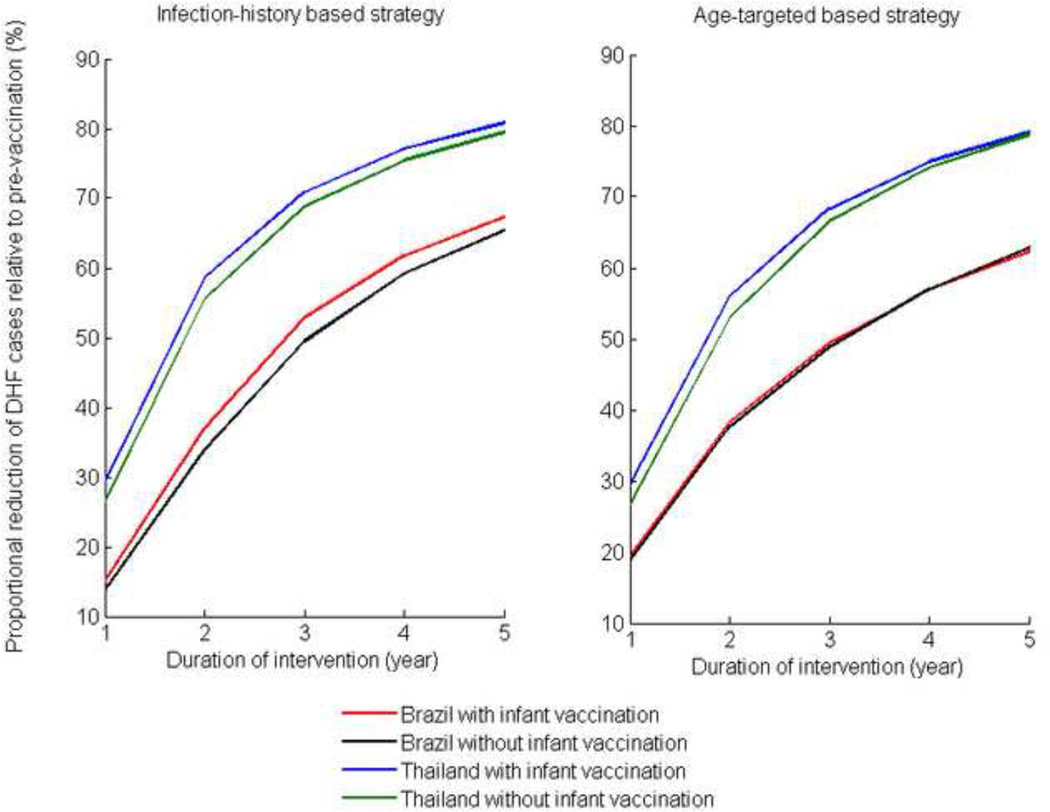
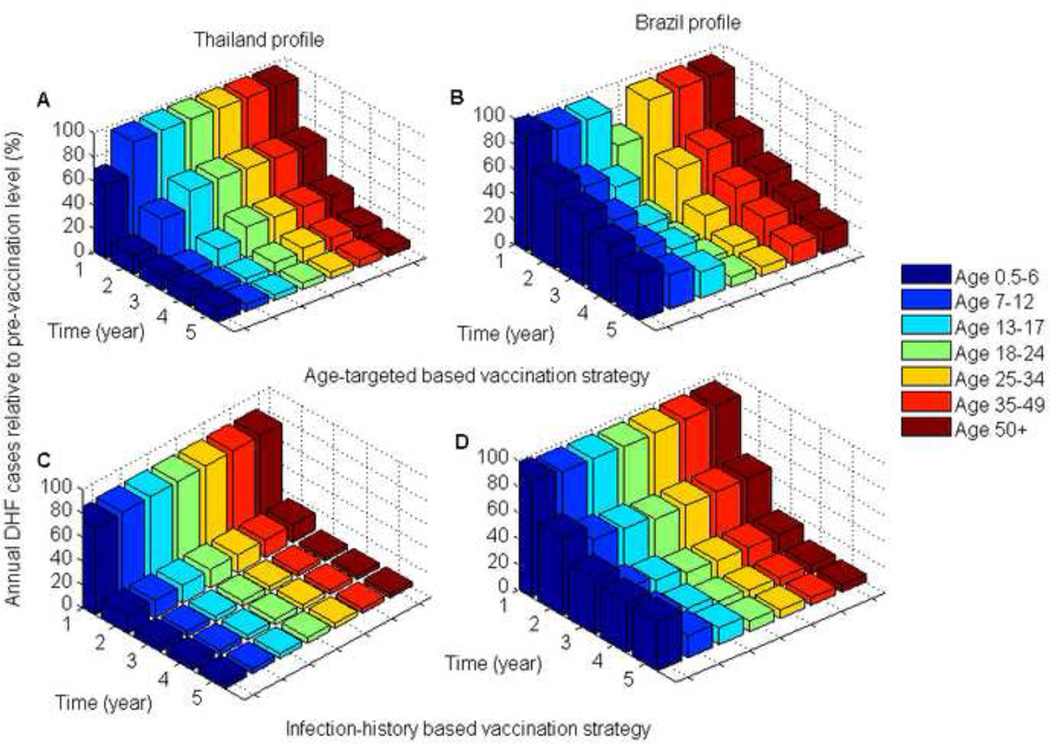
Figure 5. Reduction of DHF cases for the optimal vaccination strategies relative to pre-vaccination DHF cases for the two DHF epidemiological profiles over the duration of the vaccination program.
Epidemics of dengue fever were first reported in Southeast Asia in the 19th century (7). The four dengue serotypes have long been endemic in many Southeast Asian countries, including Thailand, which is one of the world’s most affected countries (7, 8). Starting as early as the 1960s, Thailand was implementing vector control programs through insecticide use and health education (8). In contrast to Thailand, dengue was reintroduced in Brazil in 1989 after an absence of over 20 years (9). Three serotypes, DENV-1,2, and 3, are currently endemic throughout Brazil with the four serotype emerging (9). Despite government efforts to promote surveillance and vectors control, dengue is continuing to spread in Brazil (9–11).
Vaccine development has been challenging by the need for vaccines conferring strong protection against all four serotypes to avoid the elevated risk of severe disease associated with secondary infections (12). However, several vaccine candidates are currently under development that target the four dengue serotypes (4, 12). A Sanofi Pasteur vaccine, which is currently undergoing phase 3 trials (13), is projected to become available by 2015–2020 (14, 15). It has been suggested that during the first years of roll-out, vaccination campaigns should focus on the routine vaccination of infants and catch-up mass vaccination for the remainder of the population (14–16). However, logistical and supply limitations are likely to make the vaccine scarce during the first years following its release (15). Thus, we determine the optimality of vaccine allocation strategies that most effectively minimize the incidence of dengue hemorrhagic fever.
The demographic burden of dengue differs geographically (17). For example, DHF is predominant among adults in Latin American countries such as, and most notably, Brazil where the dengue burden is high and continues to increase (9, 18, 19). By contrast, children are disproportionately affected by DHF in Southeast Asia, such as Thailand, burdened with the largest endemic prevalence (17, 20–22). As a consequence of the heavy dengue burden in Thailand and Brazil, these countries are likely to be among the first to introduce dengue vaccination (14, 23).
Several mathematical models have been developed to investigate issues related to dengue transmission dynamics, such as seasonality, infection-induced immunity, antibody-dependent enhancement of disease (24, 25), vector control (11), and vaccination (26). To our knowledge, ours is the first model to address the problem of optimal allocation of dengue vaccine during the early stage of vaccine availability. We extended a previous dengue transmission model (11, 27) to account for human demography, four dengue serotypes, and the different epidemiological profiles for DHF corresponding to Brazil and Thailand. Our dengue transmission model consists of a system of ordinary differential equations describing the rates at which humans and mosquitoes transition between different infection and immunity states (11). We used the dengue transmission model to identify the optimal vaccine allocation strategies for minimizing the overall number of DHF cases during the initial phase of a dengue vaccine roll-out. We found that optimal vaccine allocation strategies vary significantly with the profile of the age-specific dengue burden and with vaccine availability. Consequently, a mass vaccination strategy that is optimal in one country is not necessarily optimal in another. Specifically, we found that it is optimal to target children in Thailand and to target young adults in Brazil, when initial vaccine supplies are limited.
Methods
We constructed a deterministic, compartmental model capturing features known to affect the transmission dynamics of dengue: host-vector interactions; immunological interactions between the 4 dengue serotypes; population age-structure; and age specific levels of transmission. We assumed that all infants are born protected against dengue infection through maternal immunity (28). As maternal immunity wanes, individuals become susceptible to the four dengue serotypes. Infection with a serotype provides lifelong immunity against that specific serotype. We account for antibody-dependent enhancement by assuming that the probability of developing dengue hemorrhagic fever (DHF) after secondary/tertiary/quaternary infection is greater than that of primary infection. Each individual can develop up to 4 dengue infections during their lifetime. We assumed that vaccine recipients would have reduced susceptibility and infectiousness.
We calibrated our model to an endemic dengue incidence of 5% annually, which is consistent with epidemiological data from Thailand and Brazil under the assumption that approximately 80% of dengue infections are asymptomatic, also consistent with clinical data (19, 20, 22). We compared two epidemiological profiles for the demographic burden of dengue. The first profile was representative of empirical epidemiological profile of DHF incidence in Thailand, where children are disproportionately infected (20, 29, 30). The second profile was representative of empirical epidemiological profiles of DHF incidence in Brazil, where DHF is more common among adults (18, 31). To generate the different DHF epidemiological profiles, we parameterized an age-dependent probability of mosquito-to-human transmission. This probability was chosen because it is the main factor influencing the age-dependent DHF incidence in our model. We varied these probabilities to capture the patterns of empirical DHF incidence (Figure S2).
We optimized the age-targeted vaccination programs. Given that some vaccines are used inefficiently on recovered and immune individuals, we additionally optimize a vaccine program where individuals are vaccinated according to their infection history (never infected versus infected at least once). Information on individuals’ infection history can be obtained using commercially available serological tests such as immunoglobulin M and immunoglobulin G enzyme-linked immunosorbent assay (ELISA) (32). Although such a policy may be impractical (32), it gives an upper bound on the effectiveness of vaccination programs.
Given a number TVac of daily vaccine doses, we used an optimization routine (33, 34) to identify the age-specific allocation of vaccine doses that minimizes the incidence of DHF. We assumed that there were only enough doses to vaccinate 10% of the population (older than six months old) annually. Such a constraint in vaccine availability is likely to be observed during the first years of a dengue vaccine roll-out due to high demand from dengue endemic countries and limited production capacity of pharmaceutical companies (15). We also assumed that, in addition to age-targeted vaccination, routine vaccination of infants (at six months old) was independently carried out at a rate νvac. We assumed that infants younger than six months old are protected against dengue infection through maternal antibodies (28). Full model description is given in the Supplementary Materials.
To calculate the degree of vaccine coverage necessary to sustain herd immunity, we assumed a fraction νvac of infants receive the vaccine and calculated the equilibrium level of dengue infections while varying νvac from 0 to 1. We defined herd immunity as occurring when dengue incidence was lower than 1% of the pre-vaccination incidence.
Given that different values of the age-dependent probability of mosquito-to-human transmission could generate similar DHF profiles, we performed an uncertainty analysis to test the robustness of the optimal vaccine allocation strategies to the value of the age-dependent probabilities of mosquito-to-human transmission. We randomly sampled the age-dependent probability of mosquito-to-human transmission to identify different probability values that generated similar DHF profiles to those of Thailand and Brazil (see Supporting Materials for details). For each set of values of the age-dependent probability, we computed the optimal vaccine allocation strategy and compared its effectiveness (number of new DHF cases averted over 5 years) against that of the strategy that was found optimal for the base value (Table 1).
Table 1. Epidemiological parameters.
These parameters value were used in the analysis unless indicated otherwise.
| Symbol | Parameter | Value (references) | |
|---|---|---|---|
| bm | Birth rate of mosquitoes | *Calibrated for constant population* | |
| dO | Death rate of mosquitoes | 0.042 d−1 (44–48) | |
| bh | Birth rate of humans | *Calibrated for constant population* | |
| dh | Death rate of humans | 0.0000165 d−1 (49) | |
| χ | Proportion of dengue infections that are symptomatic | 0.2 (28, 50, 51) | |
| ε | Rate of loss of maternal antibodies | 0.0075 d−1 (52, 53) | |
| δ | Rate of loss of cross-immunity | 0.0055 d−1 (4, 24, 28) | |
| γ | Dengue fever (non-DHF) recovery rate | 0.1429 d−1 (28, 52, 53) | |
| η1 | Rate of developing DHF after primary infection | 0.002 d−1 (8) | |
| η2 | Rate of developing DHF after secondary/tertiary/quaternary infection | Age-dependent data (31) | |
| α | Risk of death from DHF | 0.01 (54) | |
| φ | DHF recovery rate | 0.2 d−1 (52) | |
| c | Biting rate (number of bites per mosquito per day) | 0.7 (55) | |
| σi | Relative probability of being susceptible to new infection after having i previous infections | ||
| β0 | Percentage of human infected per bite | 0.5 (53, 56) | |
| βm | Percentage of mosquito infected per bite | 0.9 (53, 56) | |
| Rate of aging out of age group a | Proportional to the length of age group a | ||
| Rate of aging into age group a | Proportional to the length of age group a−1 | ||
| πa | Age-dependent relative probability of mosquito-to-human transmission | Thailand: πa = 1; Brazil: π1 = 0.1, π2 = 0.2, π3 = 0.3, π4 = 0.8, πj = 1, j = 5,6,7. *Calibrated to fit DHF profiles* |
|
| Proportion of vaccinated individuals in age group a | Varied | ||
| Veff | Vaccine efficacy | 0.75 |
Results
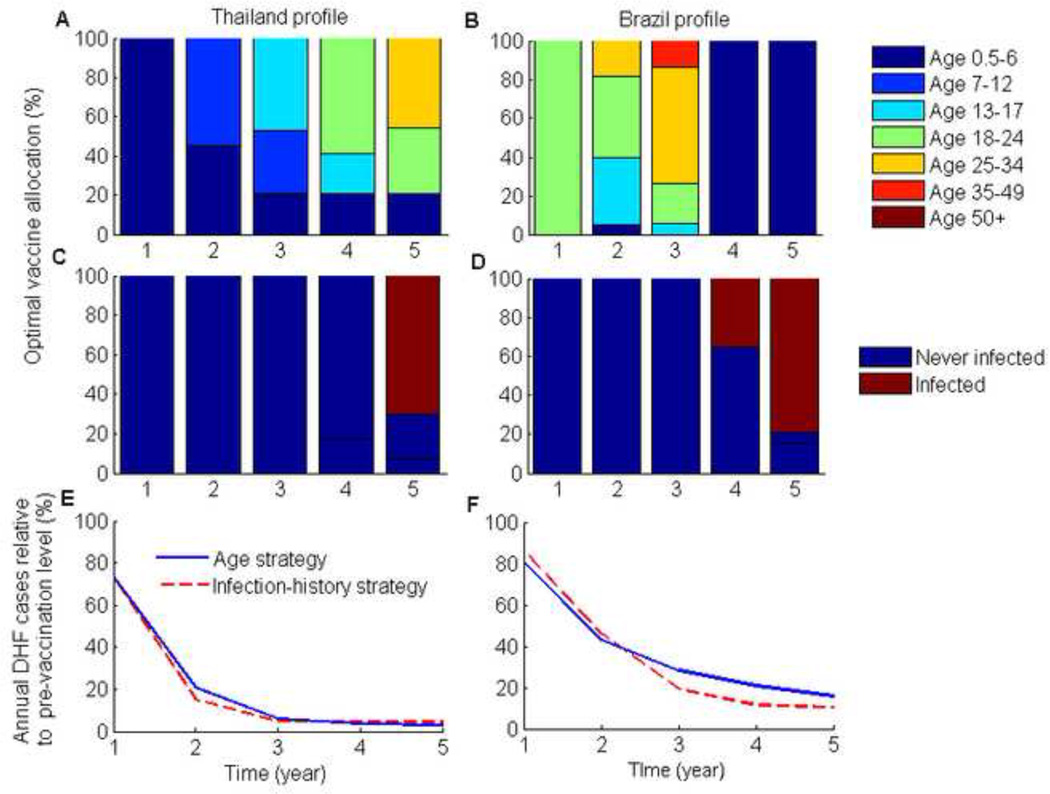
In the long term, we calculated that herd immunity may be achieved by routine infant coverage of 70% for both Thailand and Brazil. Throughout the initial intervention period, we found that when vaccination coverage in infants was at 70%, the remaining doses were optimally allocated primarily to children (aged 0.5–12 years old) and adolescents (aged 13–17 years old) for the Thailand DHF profile (Figure 1). Without infant vaccination the optimal age-targeted vaccination strategy gave priority first to the youngest age group and then progressively shifted toward the older age groups (Figure 3). For the Brazil DHF profile, vaccinating first the adults aged 18–34 years old was the most efficient use of vaccine to reduce the incidence of DHF, both in the presence and absence of a simultaneous policy of infant vaccination (Figures 1 & 3), contrasting with the Thailand DHF profiles where vaccines would be optimally first allocated to children.
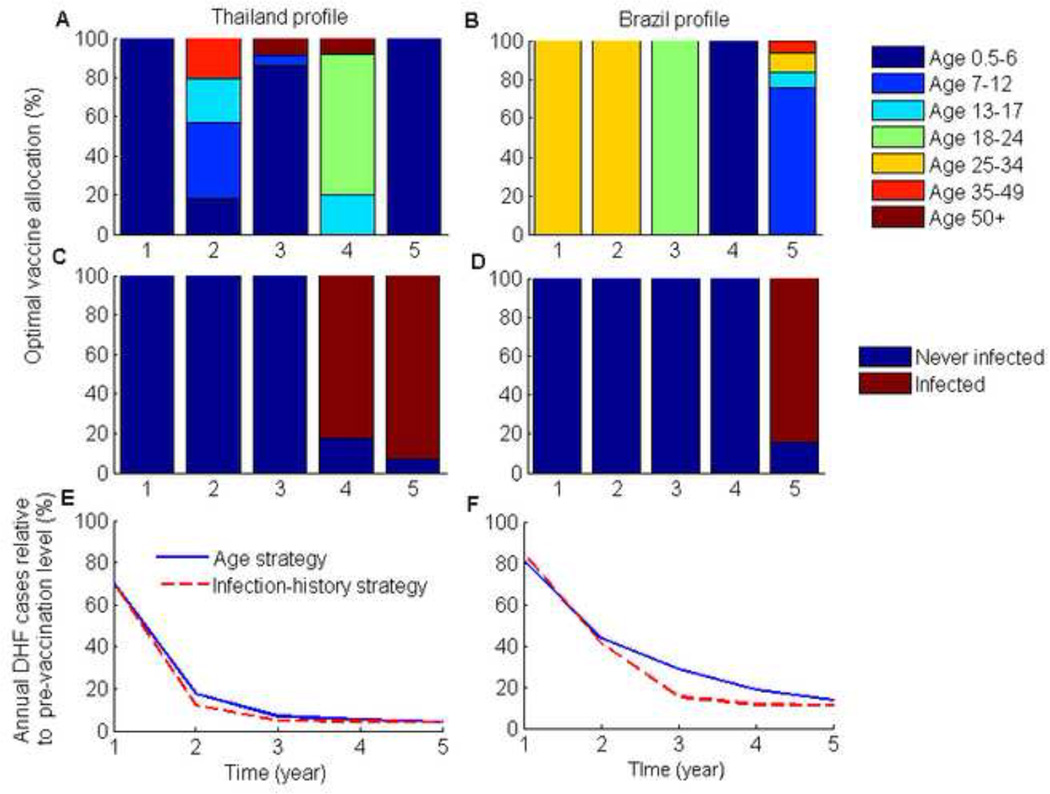
Figure 1. Optimal age- and infection-history- targeted vaccination strategies for a five-year intervention period with a 70% vaccination coverage of infants.
(A,B) The proportion of each annual vaccine optimally allocated to each age group for the two DHF epidemiological profiles, and (C,D) the optimal infection-history vaccine allocation strategies. (E,F) The number of DHF cases each year for the optimal vaccination strategies relative to the pre-vaccination number of DHF cases. 70% infant vaccination coverage was the vaccination threshold for disease elimination over the long-term.
Figure 3. Optimal age- and infection-history-targeted vaccination strategies for a five-year intervention period with no infant vaccination.
(A,B)The proportion of each annual vaccine optimally allocated to each age group for the two DHF epidemiological profiles, and (C,D) the optimal infection-history vaccine allocation strategies. (E,F) The number of DHF cases each year for the optimal vaccination strategies relative to the pre-vaccination number of DHF cases.
The optimal age-targeted strategy reduced the number of new DHF cases over 5 years by 78% for the Thailand profile and by 63% for the Brazil profile, with and without infant vaccination (Figure 5). Only a marginal increase in the reduction of DHF cases could be achieved by using information on individual infection history. The optimal infection-history-based vaccination strategy further reduced the number of new DHF cases by 2% for the Thailand profile and by 4% for the Brazil profile, when the infant vaccination coverage was 70% (Figure 5). With no infant vaccination, the optimal infection-history-based vaccination strategy further reduced the number of new DHF cases by 1% for the Thailand profile and by 2% for the Brazil profile (Figure 5).
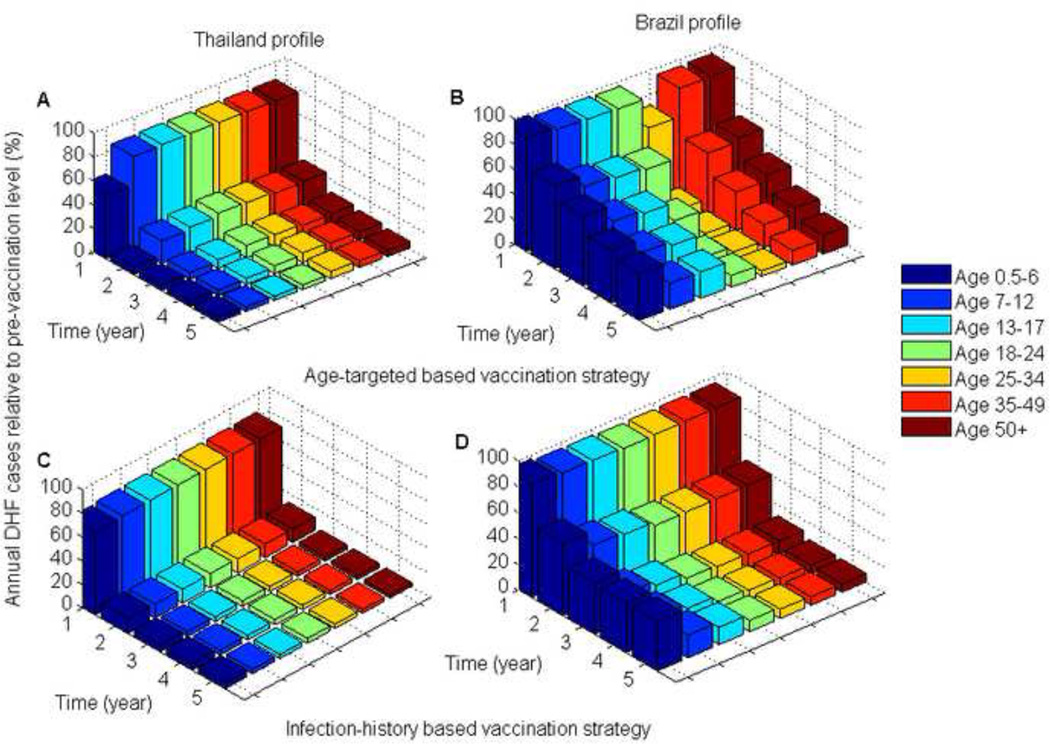
Our model showed that mass vaccination substantially shifted the age-distribution of dengue burden within the population (Figures 2 & 4). For the two DHF epidemiological profiles that were considered, the optimal vaccination strategies shifted the disease burden to children and adolescents (Figures 2 & 4). This was especially pronounced for the Brazil profile where the highest disease burden was shifted from young adults to children (Figures 2 & 4). Consequently, in Brazil vaccination programs initially targeted young adults and then children as the burden of disease shifted during the course of vaccine roll out.
Figure 2. Comparison of DHF cases for the optimal vaccination strategies relative to pre-vaccination DHF cases for the two DHF epidemiological profiles.
(A,B) The optimal age-targeted vaccination strategies; (C,D) The optimal infection-history-targeted vaccination strategies.
Figure 4. Comparison of DHF cases for the optimal vaccination strategies relative to pre-vaccination DHF cases for the two DHF epidemiological profiles.
(A,B) The optimal age-targeted vaccination strategies; (C,D) The optimal infection-history targeted vaccination strategies.
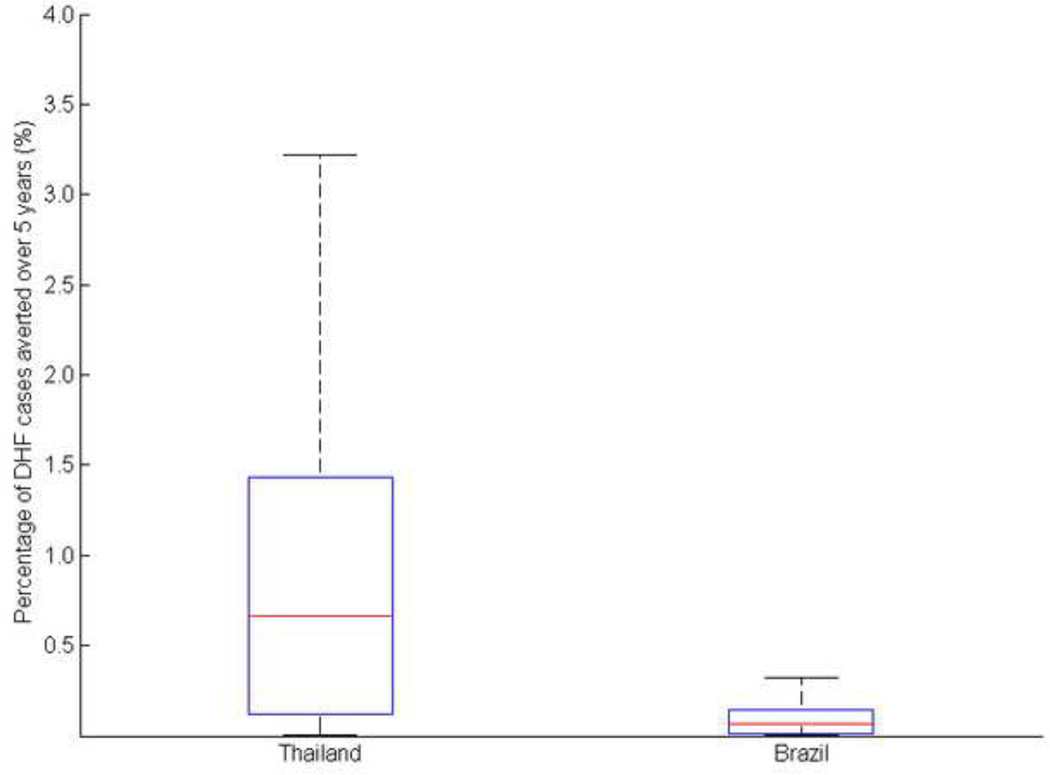
Given that different probabilities of mosquito-to-human transmission can generate similar DHF profiles, we evaluated the sensitivity of the age-targeted optimal vaccine allocation strategy to variation in the probability of mosquito-to-human transmission. More specifically, we compared the effectiveness of the strategy that was identified as optimal for the base value (presented above) with that of the optimal strategies obtained for different values of the probability of mosquito-to-human transmission that generate similar DHF profiles (Figure 6). We showed that for the Brazilian DHF profile, the proportional difference in the number of DHF cases averted between two strategies was less than 0.5%, while for Thailand is was less than 3.5% (Figure 6). We concluded that the probability of mosquito-to-human transmission has a marginal effect on the optimal strategy.
Figure 6. Comparison between the optimal age-targeted vaccination strategies derived from the base value with the optimal strategies obtained for different probabilities of mosquito-to-human transmission.
The red line represents the median, while the lower and upper bound of the boxplot represent the first and third quartiles.
Discussion
The optimal allocation of dengue vaccines within South-east Asia and Latin America is a pressing public health question which we addressed by applying optimization to a dynamic model for dengue 9 transmission between humans and mosquitoes. We identified the optimal age-specific vaccine allocation strategies that minimize the incidence DHF over the first 5 years following vaccine availability. We found that it is necessary to tailor the allocation of vaccines geographically. Specifically, we showed that for the Thailand DHF profile, initially vaccinating children was the most efficient strategy for reducing DHF cases. In contrast, for the Brazil DHF profile, initially vaccinating young adults was the most efficient strategy for reducing DHF cases. These results highlighted the importance of accounting for the demographic profile of dengue morbidity in the development of dengue vaccination strategies.
When infection history is available, the vaccination strategy marginally outperformed age-targeted vaccination. In addition, infection-history-based vaccination requires serological tests to be performed on potential vaccination recipients, raising further additional logistical and financial obstacles. An economic analysis is needed to determine if testing for infection history is a cost-effective strategy.
Recent dengue epidemics in Brazil have been characterized by a disproportionate increase in DHF cases among children (9, 18). Some studies have suggested that if this shift in age of DHF cases persists, the age specific DHF burden of Brazil could resemble that of Southeast Asia in the near future, with DHF occurring mainly in younger age groups (9, 10, 18, 35). If the Brazil DHF profile were to become similar to that of Thailand, we anticipate that the proposed optimal vaccine allocation strategy based on the current DHF in Brazil would no longer be optimal. Instead, the optimal vaccination strategy for this new Brazil DHF profile would more likely be similar to that of Thailand.
Previous studies on dengue vaccine allocation have focused on evaluating the effectiveness of predetermined vaccination strategies such as routine vaccination of infants (23, 36) or random allocation of vaccines to a proportion of the population (37). Optimization procedures, such as those we have applied here, make it possible to essentially evaluate the full range of vaccination policies within the parameters and constraints considered. Identifying optimal vaccine allocation strategies is an important factor for disease control, especially when resources are limited (38, 39). Here, we have extended these previous studies by identifying the optimal vaccine allocation strategies of a dengue vaccine, for different DHF profiles, during the first 5 years following the release of the vaccine.
Our model assumed that vaccine confers life-long immunity to dengue infection. This assumption can be relaxed to account for possible vaccine waning, but we anticipate that vaccine waning would not significantly affect our results, given that the analysis was conducted with a 5-year time horizon, while vaccine waning is likely much slower (40). Although the Phase 2b trial of the Sanofi Pasteur vaccine showed a surprisingly low efficacy of 30.2% (95% CI−13.4—56.6%) (13), further vaccine trials may refine this estimate and may reveal any sampling biases in the relatively small sample size of Phase 2b trial (41). Moreover, other vaccine candidates, which aim for have higher efficacy, are currently under development (12). Here we assumed a 75% vaccine efficacy. Our model provides an initial step toward understanding how dengue vaccination could be optimally implemented once a dengue vaccine becomes available. The current study focused on optimizing dengue vaccine allocation for a single objective, minimizing the overall number of DHF cases over 5 years. However, public-health decision making for vaccine allocation may combine several potentially competing objectives such as minimizing cases of dengue fever, cases of dengue hemorrhagic fever, deaths, and vaccination costs (42, 43). Our model can be extended to allow for multiple policy objectives of dengue vaccination.
It has been suggested that dengue vaccination should be introduced into the WHO Expanded Program on Immunization, the primary goal of which is to achieve high vaccination coverage of children worldwide (14, 16). Our findings indicate that, to maximize the benefits of dengue vaccination, regional vaccination programs should be carefully tailored to the demographic and infection history of the local population.
Supplementary Material
Highlights.
We model dengue transmission and incorporate age-specific distribution of dengue burden.
We identify optimal dengue vaccine allocations that minimize dengue hemorrhagic fever cases.
We showed that optimal vaccine allocation strategies vary with the demographic burden of dengue.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Beatty ME, Beutels P, Meltzer MI, Shepard DS, Hombach J, Hutubessy R, et al. Health economics of dengue: A systematic literature review and expert panel's assessment. The American journal of tropical medicine and hygiene. 2011;84(3):473–488. doi: 10.4269/ajtmh.2011.10-0521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Suaya JA, Shepard DS, Siqueira JB, Martelli CT, Lum LCS, Tan LH, et al. Cost of dengue cases in eight countries in the Americas and Asia: a prospective study. The American journal of tropical medicine and hygiene. 2009;80(5):846–855. [PubMed] [Google Scholar]
- 3.Halstead SB. Dengue. The Lancet. 2007;370(9599):1644–1652. doi: 10.1016/S0140-6736(07)61687-0. [DOI] [PubMed] [Google Scholar]
- 4.Murrell S, Wu SC, Butler M. Review of dengue virus and the development of a vaccine. Biotechnology advances. 2011;29(2):239–247. doi: 10.1016/j.biotechadv.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 5.Srikiatkhachorn A, Gibbons RV, Green S, Libraty DH, Thomas SJ, Endy TP, et al. Dengue Hemorrhagic Fever: The Sensitivity and Specificity of the World Health Organization Definition for Identification of Severe Cases of Dengue in Thailand, 1994–2005. Clinical Infectious Diseases. 2010 Apr 15;50(8):1135–1143. doi: 10.1086/651268. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whitehorn J, Farrar J. Dengue. British Medical Bulletin. 2010 Sep 1;95(1):161–173. doi: 10.1093/bmb/ldq019. 2010. [DOI] [PubMed] [Google Scholar]
- 7.Chareonsook O, Foy HM, Teeraratkul A, Silarug N. Changing epidemiology of dengue hemorrhagic fever in Thailand. Epidemiol Infect. 1999;122(1):161–166. doi: 10.1017/s0950268898001617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nagao Y, Koelle K. Decreases in dengue transmission may act to increase the incidence of dengue hemorrhagic fever. Proceedings of the National Academy of Sciences. 2008 Feb 12;105(6):2238–2243. doi: 10.1073/pnas.0709029105. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rodriguez-Barraquer I, Cordeiro MT, Braga C, de Souza WV, Marques ET, Cummings DAT. From re-emergence to hyperendemicity: the natural history of the dengue epidemic in Brazil. PLoS neglected tropical diseases. 2011;5(1):e935. doi: 10.1371/journal.pntd.0000935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Siqueira JB, Jr, M C, Coelho GE, da Rocha Simplício AC, Hatch DL. Dengue and dengue hemorrhagic fever, Brazil, 1981–2002. Emerg Infect Dis. 2005;11(1):48–53. doi: 10.3201/eid1101.031091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luz PM, Vanni T, Medlock J, Paltiel AD, Galvani AP. Dengue vector control strategies in an urban setting: an economic modelling assessment. The Lancet. 2011;377(9778):1673–1680. doi: 10.1016/S0140-6736(11)60246-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmitz J, Roehrig J, Barrett A, Hombach J. Next generation dengue vaccines: A review of candidates in preclinical development. Vaccine. 2011;29(42):7276–7284. doi: 10.1016/j.vaccine.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 13.Sabchareon A, Wallace D, Sirivichayakul C, Limkittikul K, Chanthavanich P, Suvannadabba S, et al. Protective efficacy of the recombinant, live-attenuated, CYD tetravalent dengue vaccine in Thai schoolchildren: a randomised, controlled phase 2b trial. The Lancet. 2012;(0) doi: 10.1016/S0140-6736(12)61428-7. [DOI] [PubMed] [Google Scholar]
- 14.Amarasinghe A, Mahoney RT. Estimating potential demand and supply of dengue vaccine in Brazil. Hum Vaccin. 2011 Jul;7(7):776–780. doi: 10.4161/hv.7.7.16255. PubMed PMID: 21734468. Pubmed Central PMCID: 3225768. Epub 2011/07/08. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Watson M. Dengue vaccine roll-out: getting ahead of the game. Bull World Health Organ. 2011;89:476–477. doi: 10.2471/BLT.11.030711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amarasinghe A, Wichmann O, Margolis HS, Mahoney RT. Forecasting dengue vaccine demand in disease endemic and non-endemic countries. Human vaccines. 2010;6(9):745. doi: 10.4161/hv.6.9.12587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Halstead SB. Dengue in the Americas and Southeast Asia: do they differ? Revista Panamericana de Salud Pública. 2006;20(6):407–415. doi: 10.1590/s1020-49892006001100007. [DOI] [PubMed] [Google Scholar]
- 18.San Martín JL, Brathwaite O, Zambrano B, Solórzano JO, Bouckenooghe A, Dayan GH, et al. The epidemiology of dengue in the Americas over the last three decades: a worrisome reality. The American journal of tropical medicine and hygiene. 2010;82(1):128–1235. doi: 10.4269/ajtmh.2010.09-0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shepard DS, Coudeville L, Halasa YA, Zambrano B, Dayan GH. Economic impact of dengue illness in the Americas. The American journal of tropical medicine and hygiene. 2011;84(2):200–207. doi: 10.4269/ajtmh.2011.10-0503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nisalak A, Endy TP, Nimmannitya S, Kalayanarooj S, SCOTT RM, BURKE DS, et al. Serotype-specific dengue virus circulation and dengue disease in Bangkok, Thailand from 1973 to 1999. The American journal of tropical medicine and hygiene. 2003;68(2):191–202. [PubMed] [Google Scholar]
- 21.Shekhar KC, Huat OL. Epidemiology of dengue/dengue hemorrhagic fever in Malaysia--a retrospective epidemiological study 1973–1987. Part I: Dengue hemorrhagic fever (DHF) Asia Pac J Public Health. 1992;6(2):15–25. doi: 10.1177/101053959300600203. PubMed PMID: 1308765. Epub 1992/01/01. eng. [DOI] [PubMed] [Google Scholar]
- 22.Endy TP, Chunsuttiwat S, Nisalak A, Libraty DH, Green S, Rothman AL, et al. Epidemiology of inapparent and symptomatic acute dengue virus infection: a prospective study of primary school children in Kamphaeng Phet, Thailand. American journal of epidemiology. 2002;156(1):40–51. doi: 10.1093/aje/kwf005. [DOI] [PubMed] [Google Scholar]
- 23.Lee BY, Connor DL, Kitchen SB, Bacon KM, Shah M, Brown ST, et al. Economic Value of Dengue Vaccine in Thailand. The American journal of tropical medicine and hygiene. 2011 May 5;84(5):764–772. doi: 10.4269/ajtmh.2011.10-0624. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johansson MA, Hombach J, Cummings DAT. Models of the impact of dengue vaccines: A review of current research and potential approaches. Vaccine. 2011;29(35):5860–5868. doi: 10.1016/j.vaccine.2011.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andraud M, Hens N, Marais C, Beutels P. Dynamic Epidemiological Models for Dengue Transmission: A Systematic Review of Structural Approaches. PLoS ONE. 2012;7(11):e49085. doi: 10.1371/journal.pone.0049085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chao DL, Halstead SB, Halloran ME, Longini IM., Jr Controlling Dengue with Vaccines in Thailand. PLoS Negl Trop Dis. 2012;6(10):e1876. doi: 10.1371/journal.pntd.0001876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Durham D, Ndeffo Mbah M, Medlock J, Luz P, Meyers L, Paltiel A, et al. Dengue dynamics and vaccine cost-effectiveness in Brazil. Vaccine. 2013;31(37):3957–3961. doi: 10.1016/j.vaccine.2013.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murphy BR WS. Immune Response to Dengue Virus and Prospects for a Vaccine Annual Review of Immunology. 2011;29(1):587–619. doi: 10.1146/annurev-immunol-031210-101315. [DOI] [PubMed] [Google Scholar]
- 29.Sriprom M, Pongsumpun P, Yoksan S, Barbazan P, Gonzalez J, Tang I. Dengue Haemorrhagic Fever in Thailand, 1998–2003: Primary or Secondary Infection. Dengue Bulletin. 2003;27:39–45. [Google Scholar]
- 30.Cummings DAT, Iamsirithaworn S, Lessler JT, McDermott A, Prasanthong R, Nisalak A, et al. The impact of the demographic transition on dengue in Thailand: insights from a statistical analysis and mathematical modeling. PLoS medicine. 2009;6(9):e1000139. doi: 10.1371/journal.pmed.1000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guzmán MG, Kouri G, Bravo J, Valdes L, Susana V, Halstead SB. Effect of age on outcome of secondary dengue 2 infections. International journal of infectious diseases. 2002;6(2):118–124. doi: 10.1016/s1201-9712(02)90072-x. [DOI] [PubMed] [Google Scholar]
- 32.Peeling RW, Artsob H, Pelegrino JL, Buchy P, Cardosa MJ, Devi S, et al. Evaluation of diagnostic tests: dengue. Nat Rev Micro. 2010;8:S30–S38. doi: 10.1038/nrmicro2459. [DOI] [PubMed] [Google Scholar]
- 33.Boggs PT, Tolle JW. Sequential quadratic programming for large-scale nonlinear optimization. Journal of Computational and Applied Mathematics. 2000;124(1):123–137. [Google Scholar]
- 34.Nocedal J, Wright S. Numerical optimization. Springer; 2006. [Google Scholar]
- 35.Teixeira MGCM, Coelho G, Barreto ML. Recent shift in age pattern of dengue hemorrhagic fever, Brazil. Emerg Infect Dis. 2008;14(10):1663. doi: 10.3201/eid1410.071164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Coudeville L, Garnett GP. Transmission Dynamics of the Four Dengue Serotypes in Southern Vietnam and the Potential Impact of Vaccination. PLoS ONE. 2012;7(12):e51244. doi: 10.1371/journal.pone.0051244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carrasco LR, Lee LK, Lee VJ, Ooi EE, Shepard DS, Thein TL, et al. Economic impact of dengue illness and the cost effectiveness of future vaccination programs in Singapore. PLoS neglected tropical diseases. 2011;5(12):e1426. doi: 10.1371/journal.pntd.0001426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ndeffo Mbah ML, Gilligan CA. Resource Allocation for Epidemic Control in Metapopulations. PLoS ONE. 2011;6(9):e24577. doi: 10.1371/journal.pone.0024577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Klepac P, Laxminarayan R, Grenfell BT. Synthesizing epidemiological and economic optima for control of immunizing infections. Proceedings of the National Academy of Sciences. 2011;108(34):14366–14370. doi: 10.1073/pnas.1101694108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chanthavanich P, Luxemburger C, Sirivichayakul C, Lapphra K, Pengsaa K, Yoksan S, et al. Immune response and occurrence of dengue infection in Thai children three to eight years after vaccination with live attenuated tetravalent dengue vaccine. The American journal of tropical medicine and hygiene. 2006;75(1):26–28. doi: 10.4269/ajtmh.2006.75.1.0750026. [DOI] [PubMed] [Google Scholar]
- 41.Halstead SB. Dengue vaccine development: a 75% solution? Lancet. 2012 Nov 3;380(9853):1535–1536. doi: 10.1016/S0140-6736(12)61510-4. PubMed PMID: 22975339. Epub 2012/09/15. eng. [DOI] [PubMed] [Google Scholar]
- 42.Hollingsworth TD, Klinkenberg D, Heesterbeek H, Anderson RM. Mitigation Strategies for Pandemic Influenza A: Balancing Conflicting Policy Objectives. PLoS computational biology. 2011;7(2):e1001076. doi: 10.1371/journal.pcbi.1001076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Group W-VDVM. Assessing the Potential of a Candidate Dengue Vaccine with Mathematical Modeling. PLoS Negl Trop Dis. 2012;6(3):e1450. doi: 10.1371/journal.pntd.0001450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Styer LM, Minnick SL, Sun AK, Scott TW. Mortality and reproductive dynamics of Aedes aegypti (Diptera: Culicidae) fed human blood. Vector-Borne and Zoonotic Diseases. 2007;7(1):86–98. doi: 10.1089/vbz.2007.0216. [DOI] [PubMed] [Google Scholar]
- 45.Scott TW, Naksathit A, Day JF, Kittayapong P, Edman JD. A fitness advantage for Aedes aegypti and the viruses it transmits when females feed only on human blood. The American journal of tropical medicine and hygiene. 1997;57(2):235–239. doi: 10.4269/ajtmh.1997.57.235. [DOI] [PubMed] [Google Scholar]
- 46.Harrington LC, Edman JD, Scott TW. Why do female Aedes aegypti (Diptera: Culicidae) feed preferentially and frequently on human blood? Journal of medical entomology. 2001;38(3):411–422. doi: 10.1603/0022-2585-38.3.411. [DOI] [PubMed] [Google Scholar]
- 47.Crovello TJ, Hacker CS. Evolutionary strategies in life table characteristics among feral and urban strains of Aedes aegypti (L.) Evolution. 1972:185–196. doi: 10.1111/j.1558-5646.1972.tb00186.x. [DOI] [PubMed] [Google Scholar]
- 48.Watts DM, Burke DS, Harrison BA, Whitmire RE, Nisalak A. Effect of temperature on the vector efficiency of Aedes aegypti for dengue 2 virus. DTIC Document. 1986 doi: 10.4269/ajtmh.1987.36.143. [DOI] [PubMed] [Google Scholar]
- 49.Economic UNDo. World population prospects: The 2004 Revision: Volume I: comprehensive tables: United Nations Publications. 2006 [Google Scholar]
- 50.Balmaseda A, Hammond SN, Tellez Y, Imhoff L, Rodriguez Y, Saborío SI, et al. High seroprevalence of antibodies against dengue virus in a prospective study of schoolchildren in Managua, Nicaragua. Tropical Medicine & International Health. 2006;11(6):935–942. doi: 10.1111/j.1365-3156.2006.01641.x. [DOI] [PubMed] [Google Scholar]
- 51.Honório NA, Nogueira RMR, Codeço CT, Carvalho MS, Cruz OG, Magalhães MdAFM, et al. Spatial evaluation and modeling of dengue seroprevalence and vector density in Rio de Janeiro, Brazil. PLoS neglected tropical diseases. 2009;3(11):e545. doi: 10.1371/journal.pntd.0000545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Whitehead SS, Blaney JE, Durbin AP, Murphy BR. Prospects for a dengue virus vaccine. Nature Reviews Microbiology. 2007;5(7):518–528. doi: 10.1038/nrmicro1690. [DOI] [PubMed] [Google Scholar]
- 53.Focks DA, Daniels E, Haile DG, Keesling JE. A simulation model of the epidemiology of urban dengue fever: literature analysis, model development, preliminary validation, and samples of simulation results. American Journal of Tropical Medicine and Hygiene. 1995;53(5):489–506. doi: 10.4269/ajtmh.1995.53.489. [DOI] [PubMed] [Google Scholar]
- 54.Stephenson JR. Understanding dengue pathogenesis: implications for vaccine design. Bulletin of the World Health Organization. 2005;83(4):308–314. [PMC free article] [PubMed] [Google Scholar]
- 55.Scott TW, Amerasinghe PH, Morrison AC, Lorenz LH, Clark GG, Strickman D, et al. Longitudinal studies of Aedes aegypti (Diptera: Culicidae) in Thailand and Puerto Rico: blood feeding frequency. Journal of medical entomology. 2000;37(1):89–101. doi: 10.1603/0022-2585-37.1.89. [DOI] [PubMed] [Google Scholar]
- 56.Hartley L, Donnelly C, Garnett G. The seasonal pattern of dengue in endemic areas: mathematical models of mechanisms. Transactions of the royal society of tropical medicine and hygiene. 2002;96(4):387–397. doi: 10.1016/s0035-9203(02)90371-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.