Abstract
Type 2 diabetes is associated with poor exercise tolerance and peak aerobic capacity (VO2peak) even when compared to obese non-diabetic peers. Exercise training studies have demonstrated improvements in VO2peak among T2D, yet there is a large amount of variability in this response. Recent evidence suggests that cardiac autonomic modulation may be an important factor when considering improvements in aerobic capacity.
Purpose
To determine the effects of a 16 wk aerobic exercise program on VO2peak in obese individuals, with and without T2D, who were classified as having either high or low cardiovagal modulation (HCVM or LCVM) at baseline.
Methods
Obese individuals (38 women/19 men; BMI = 36.1 kg/m2) were studied in the fasted state. ECG recordings were obtained while seated for 3 min, prior to and after 4 mo of exercise training (4 d/wk, 65% VO2peak). The ECG recording was analyzed for HRV in the spectral domain. Groups were split on a marker of CVM (normalized high frequency (HFnu)) at the 50th percentile, as either high (H) or low (L) CVM.
Results
VO2peak only increased with exercise training among those classified as having HCVM, regardless of diabetes status (T2D: HCVM 20.3 to 22.5 mL/kg/min, LCVM 24.3 to 25.0 mL/kg/min; Obese non-diabetics: HCVM 24.5 to 26.3 mL/kg/min, LCVM 23.1 to 23.7 mL/kg/min) (p<0.05). No change in VO2peak was observed for the LCVM group. Changes in weight do not explain the change in VO2peak among the HCVM group. Glucose tolerance only improved among the LCVM group with T2D.
Conclusion
Obese individuals, with or without T2D, when classified as having relatively HCVM prior to exercise training, have a greater propensity to improve VO2peak following a 16-week aerobic training program.
Keywords: Aerobic capacity, exercise training, cardiovagal modulation, heart rate variability, obesity, type 2 diabetes
Introduction
Exercise intolerance is common in individuals with type 2 diabetes (T2D), manifested as low levels of cardiovascular fitness (VO2peak) and described in a recent review by Reusch et al. (29). This low exercise capacity is associated with both cardiovascular and all-cause morbidity and mortality in this population (39). Despite a marked decrease in the prevalence of cardiovascular disease in the general population, diabetes-related cardiovascular mortality is 3–5 times higher than in populations without diabetes (29, 36). Despite aggressive risk factor reduction, higher mortality rates persist in individuals with T2D, which may in part be due to low cardiovascular fitness(29).
Low VO2peak levels are mediated by a number of factors (26, 29) including: insulin resistance, oxidative stress, metabolic dysfunction, endothelial dysfunction, diastolic dysfunction, low cardiac perfusion, and peripheral muscle dysfunction. In essence, in the presence of a disrupted metabolic environment, as is the case often with obesity and/or T2D, this leads to cardiac, endothelial and skeletal muscle dysfunction, all of which are important determinants of either oxygen utilization and/or delivery, thus contributing to reduced exercise capacity, even when groups are matched on physical activity/sedentary behavior and level of obesity (29).
Recent evidence suggests that cardiac autonomic regulation may play an important role in exercise tolerance (11, 12, 16). VO2peak is strongly associated with cardiac autonomic function in cross-sectional studies (2, 4, 5, 9, 28, 34). Endurance exercise training also improves cardiac autonomic control, as primarily manifested by increases in cardiovagal modulation (10). Heart rate variability (HRV) has also been successfully used to individually tailor the exercise prescription based on vagal modulation to produce greater improvements in VO2peak than traditional exercise prescriptions (16, 17). Additionally, baseline levels of vagal modulation are associated with improved training-induced benefits in athletes (12).
Individuals with T2D exhibit poor autonomic control (manifested a low vagal and high sympathetic modulation) compared to their non-diabetic peers and this is associated with poor cardiovascular fitness in this population (15, 38). Autonomic control is also negatively impacted by reduced glucose tolerance and insulin insensitivity (37), and both glucose tolerance and insulin insensitivity are associated with VO2peak (29). Furthermore, in a diabetic rat model, exercise training improved mitochondrial protein expression in control animals but not in diabetic rats (19). Considering individuals with T2D have low levels of cardiovascular fitness and HRV, they may have more difficulty improving fitness (as a result of reduced impact of training on mitochondria) compared to persons without T2D (29). Thus, it is possible that autonomic dysfunction, often manifested as low HRV, impacts fitness differently in obese persons with and without T2D. Consequently, a person with T2D, with low HRV at baseline, may not have the propensity to improve their aerobic capacity following a standard training program and therefore may not improve their risk profile to the same extent as someone with T2D with higher baseline HRV. Yet, to our knowledge it is unknown if baseline (e.g. pre-training) cardiac vagal modulation impacts the effect of an aerobic training program on VO2peak in persons with T2D. Therefore, understanding the impact of exercise training in groups of obese individuals with and without T2D is important to further our appreciation of factors related to exercise tolerance.
The purpose of this study was to determine the effects of a 16 wk aerobic exercise program on VO2peak in obese individuals with and without T2D who were classified as having either high or low cardiovagal modulation (HCVM or LCVM) at baseline. We hypothesized that: 1) VO2peak would not increase among diabetics classified as LCVM, but would increase among obese individuals classified as LCVM; 2) VO2peak would increase among individuals classified as HCVM, regardless of diabetic status.
Material and Methods
Participants
We conducted a retrospective analysis on the effects of training-induced changes in exercise tolerance, anthropometrics, and HRV in 59 obese individuals who completed the study (7). Training-induced changes were examined with individuals classified as having HCVM or LCVM. Two individuals were excluded as outliers (>3SD from the mean), thus 57 individuals were included in the current analyses (Table 1). Self-reported T2D status was confirmed with either a glucose tolerance test or prescribed medications for glucose control. Table 2 contains a list of medications. Goulopoulou et al. (2010) provides further details on the methodology presented below. All subjects were between 40 to 60 years of age and had a body mass index (BMI) greater than 30 kg/m2. Subjects self-reported a sedentary lifestyle for a minimum of 6 months before enrolling in this study. Peri-menopausal women were excluded and all premenopausal women were tested in the first 10 days of their menstrual cycle. Subjects were also excluded if they had self-reported overt cardiovascular disease or if they exhibited evidence of myocardial ischemia during the stress test. Subjects were also excluded if they self-reported peripheral neuropathy, tobacco use, insulin therapy, oral contraceptives, beta-blocker and glucocorticoids medications for chronic pulmonary, cardiac or other systemic diseases. Written informed consent was obtained from each volunteer prior to participation in the study. This study was approved by the Syracuse University and State University of New York at Upstate Medical University Institutional Review Boards.
Table 1.
Anthropometric, glucose tolerance, and heart rate variability measurements pre and post-training (16 wk aerobic exercise training) among either individuals with or without type 2 diabetes, classified retrospectively as either having low or high cardiovagal modulation (LCVM, HCVM).
| Type 2 Diabetes | Obese Non-Diabetes | ||||
|---|---|---|---|---|---|
|
| |||||
| Low CVM | High CVM | Low CVM | High CVM | ||
|
| |||||
| N | 10 | 11 | 17 | 19 | |
| Male/Female | 3/7 | 2/9 | 7/10 | 7/12 | |
| Age (yrs) | 50 ± 5 | 48 ± 4 | 50 ± 6 | 48 ± 5 | |
|
| |||||
|
Anthropometrics
| |||||
| Weight (kg) | Pre | 108.5 ± 26.0 | 106.0 ± 22.1 | 102.0 ± 13.5 | 99.8 ± 12.3 |
|
| |||||
| Post | 109.2 ± 25.8 | 103.9 ± 22.5* | 101.2 ± 13.8 | 98.6 ± 12.6* | |
|
| |||||
| BMI (kg/m2)** | Pre | 35.5 ± 6.3 | 39.4 ± 5.1 | 36.5 ± 4.5 | 35.0 ± 4.0 |
|
| |||||
| Post | 35.7 ± 6.4 | 38.6 ± 5.6* | 36.2 ± 4.5 | 34.0 ± 5.0* | |
|
| |||||
| Waist (cm) | Pre | 115.8 ± 12.0 | 124.8 ± 17.1 | 109.6 ± 12.6 | 109.3 ± 9.9 |
|
| |||||
| Post | 115.1 ± 12.6 | 118.2 ± 17.9* | 108.2 ± 10.9 | 106.6 ± 9.2* | |
|
| |||||
| Body Fat (%) | Pre | 37.7 ± 5.8 | 47.5 ± 6.0 | 42.6 ± 8.6 | 40.7 ± 8.1 |
|
| |||||
| Post | 37.8 ± 6.0 | 47.3 ± 6.8 | 42.1 ± 7.8 | 40.5 ± 8.3 | |
|
| |||||
|
Glucose Tolerance
| |||||
| Glucose AUC**(mmol/L/min) | Pre | 2702 ± 674 | 2349 ± 744 | 1418 ± 186 | 1389 ± 164 |
|
| |||||
| Post | 2396 ± 600† | 2382 ± 762 | 1370 ± 209 | 1378 ± 254 | |
|
| |||||
| Whole-body Insulin Sensitivity | Pre | 4.64 ± 3.1 | 3.45 ± 1.79 | 5.03 ± 3.12 | 5.27 ± 3.19 |
|
| |||||
| Post | 6.77 ± 4.30* | 5.40 ± 3.24† | 6.70 ± 3.70 * | 4.71 ± 2.89 | |
|
| |||||
|
Heart Rate Variability
| |||||
| LFln | Pre | 5.10 ± 1.20 | 4.71 ± 0.78 | 5.06 ± 0.77 | 5.26 ± 0.78 |
|
| |||||
| Post | 5.13 ± 1.04 | 4.82 ± 0.85 | 5.46 ± 0.76 | 5.36 ± 0.96 | |
|
| |||||
| HFln | Pre | 4.68 ± 1.66 | 5.63 ± 1.14 | 4.66 ± 0.96 | 6.20 ± 0.88 |
|
| |||||
| Post | 5.24 ± 0.87* | 5.27 ± 1.48 | 5.44 ± 0.72* | 6.27 ± 0.97 | |
|
| |||||
|
Peak Heart Rate
| |||||
| Heart rate (bpm) | Pre | 174 ± 12 | 167 ± 7 | 172 ± 12 | 174 ± 14 |
|
| |||||
| Post | 171 ± 12 | 167 ± 9 | 170 ± 13 | 172 ± 12 | |
Data are mean ± SD.
BMI = body mass index
ln = natural log transformation
bpm = beats per minute
p < 0.05, time effect (pre vs. post-training), within respective heart rate variability status
p<0.05, baseline differences between T2D and obese non-diabetic groups.
p < 0.05, time (pre vs. post-training) by group (T2D vs. obese non-diabetic) effect, within respective heart rate variability status.
Table 2.
Type and classification of medication with number of participants listed by category. Subjects may be counted more than once if they were prescribed more than one medication.
| Type 2 Diabetes | Obese Non-Diabetes | |||
|---|---|---|---|---|
| Low CVM | High CVM | Low CVM | High CVM | |
| Lipid lowering | ||||
| Statin | 4 | 1 | 4 | 4 |
| Phenofibrate | 0 | 0 | 1 | 0 |
| Ezetimibe | 0 | 1 | 0 | 0 |
| Glucose lowering | ||||
| Metformin | 9 | 6 | 0 | 0 |
| Thiazolidinedione | 4 | 2 | 0 | 0 |
| Sulfonamides | 2 | 0 | 0 | 0 |
| Antihypertensive | ||||
| ACE inhibitors | 2 | 3 | 0 | 1 |
| ARBs | 2 | 1 | 1 | 1 |
| Hydrochlorothiazides | 0 | 1 | 1 | 2 |
| Antidepressant | 2 | 3 | 2 | 1 |
| Others | 5 | 5 | 9 | 10 |
ACE—Angiotensin-converting enzyme inhibitors
ARBs—Angiotensin receptor blocker
CVM—Cardiovagal modulation
Study design
Subjects completed 2 testing visits prior to and 2 visits following the 16-wk aerobic exercise intervention. During visit 1, all subjects completed a physician supervised walking treadmill protocol to determine VO2peak. Visit 2 was completed within 2 weeks of their treadmill tests and subjects arrived at the laboratory at 0700h following a 12h overnight fast for measurements of resting HRV, as well as a glucose challenge test and body composition measurements. For visit 2, subjects refrained from caffeine for 12 h and alcohol and exercise for 24 h prior to testing. Subjects then participated in a 16-wk combination supervised and home-based aerobic exercise intervention. Following the training period, the same testing visits were repeated for post measurements. Medication use and dose was not changed during the study period for the subjects. Subjects were also instructed to maintain their normal dietary patterns.
Aerobic Exercise Intervention
Subjects participated in a 16-wk combined supervised/home-based aerobic exercise program. Subjects were instructed to walk 4 d/wk at 65% of VO2peak for 30 min. The duration of exercise was slowly increased over a 2 wk period at the half-way point (9 wk), so that all subjects were exercising 45 min/day during weeks 11–16. Out of the 4 days of prescribed walking, subjects reported to the lab one d/wk for their one-on-one supervised exercise session on a treadmill. The researchers were able to accurately monitor the subjects’ workload and make adjustments as necessary during the in-person visits. Heart rate and ratings of perceived exertion were utilized to monitor the intensity of each workout. Exercise logs were maintained and any exercise-related issues were discussed thoroughly on a weekly basis. Compliance was ~90% (14).
Heart Rate Variability
Following 20 min of supine rest, continuous R-R intervals were recorded (Biopac MP100, Santa Barbara, CA) during an additional 5 min of supine rest with a modified CM5 ECG lead at a sampling rate of 1000 Hz. To control for the respiratory influence on HRV, breathing was paced at 12 breaths/min using a metronome. The R-R intervals were analyzed using Heart Software (Oulu, Finland) as previously described (1, 7, 13). Low frequency (LF, range: 0.04–0.15 Hz) and high frequency (HF, range: 0.15–0.40 Hz) spectral power were determined using an autoregressive model (order of 10) following previous recommendations (33). Further, LF and HF were as log transformed values.
To classify our groups as having HCVM or LCVM at baseline, the HF component of the HRV analysis was used in determining the 50th percentile split (LCVM<50th percentile>HCVM; Table 1).
Blood Analyses
Glucose concentrations from the oral glucose tolerance test (75 g dextrose beverage, with blood sampling every 30 min over 4 h) were analyzed using whole blood samples on a YSI 2300 STAT PLUS (Yellow Springs, OH). Glycosylated hemoglobin (HbA1c) concentrations were analyzed by Diabetes Technologies, Inc. (Thomasville, GA) using the HPLC-BA analytical method. Plasma was assayed in duplicate for insulin concentrations using a radioimmunoassay (Diagnostics Products Corporation-DPC; Los Angeles, CA). The intra- and inter- assay coefficients of variation for the insulin assay were 7.6% and 8.9%, respectively. The whole-body insulin sensitivity index was determined as per Matsuda and DeFronzo’s calculation (21), with glucose concentrations presented as mg/dL and insulin concentrations presented as μU/L. Mean values are the average concentrations obtained at 0, 30, 60, 90 and 120 min during the oral glucose tolerance test. Lastly, glucose area under the curve was determined from the OGTT using GraphPad 5.0 (La Jolla, CA).
Anthropometrics and VO2peak
BMI was calculated and percent body fat was determined via the Bod Pod (Life Measurements, Concord, CA). Waist circumference (cm) was measured at the level of the umbilicus. VO2peak was determined by a walking treadmill protocol using indirect calorimetry (Quark b2, Cosmed, Rome, Italy) with a 12-lead ECG recorded at rest, during each stage of exercise, as well as into recovery (14).
Statistical Analysis
HRV and the whole body insulin sensitivity data were not normally distributed and were log transformed in order to meet the assumption of normality when using parametric statistical analyses. Back-transformed whole-body insulin sensitivity index data are presented in Table 1. A two-way analysis of variance (ANOVA) with repeated measures (between subject—T2D and obese non-diabetic; within subject—pre vs. post-training) was used to determine differences in VO2peak, anthropometrics, glucose tolerance and HRV within each HRV grouping (e.g. LCVM vs. HCVM). An analysis of covariance (ANCOVA) was conducted on differences in VO2peak in order to control for any potential effect of statin use. Correlations were conducted on the change in VO2peak with changes in body weight, glucose area under the curve, and whole-body insulin sensitivity using Pearson correlation coefficients. Appropriate post-hoc analyses were conducted if significant interactions were detected. Data are presented as means ± SD. Significance was set at α = 0.05.
Results
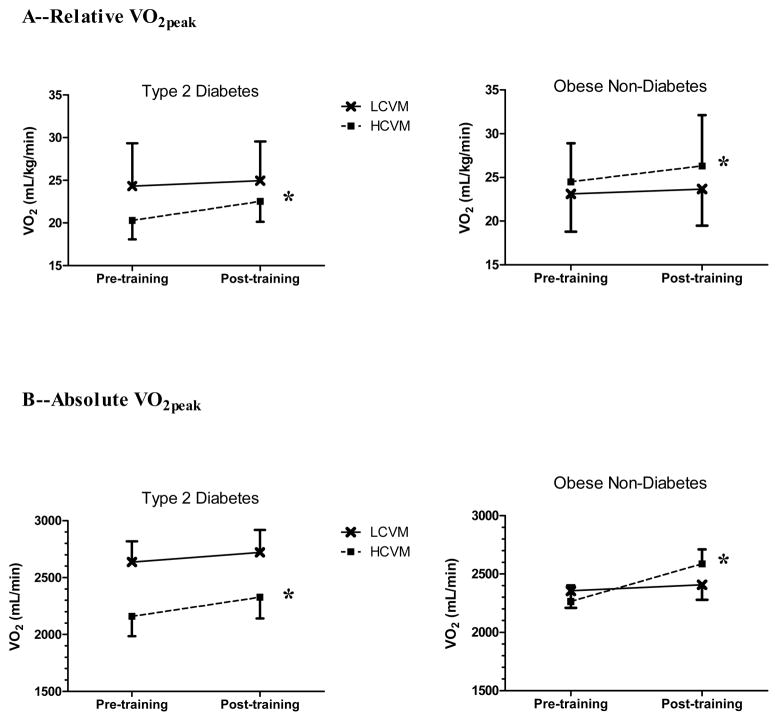
VO2peak is presented in Figure 1. Both relative (Panel A) and absolute (Panel B) VO2peak increased following the training period among individuals classified as HCVM (p<0.05), regardless of diabetes status. No change in VO2peak was observed among those classified as LCVM. VO2peak expressed relative to lean body mass did not alter any of our findings. Furthermore, statin use did not influence our results based on the ANCOVA.
Figure 1.
Peak aerobic capacity (VO2peak) levels pre and post-training (16-wk aerobic training period), among individuals with or without type 2 diabetes, classified as either having low or high cardiovagal modulation (LCVM or HCVM) at baseline. Panel A is VO2peak expressed in relative units (mL/kg/min) and panel B is VO2peak expressed in absolute values (mL/min). *p<0.05; time effect (pre vs. post-training) only among HCVM.
The pre-training VO2peak appeared to be different between the subjects in the HCVM and LCVM groups (Figure 1). This was further explored and there was a medium effect size associated with this difference, although statistical significance was not achieved (η2 = 0.071) (3).
Table 1 depicts anthropometric, glucose tolerance and HRV data. No baseline differences were observed in body weight, BMI, and percent body fat. The T2D group had a larger waist circumference and higher glucose area under the curve at baseline (p<0.05). Body weight, BMI and waist circumference decreased among those classified as HCVM following the training intervention (p<0.05), with no training effect observed in those classified as LCVM. Diabetic status did not affect these parameters. No effects were observed for percent body fat.
The glucose area under the curve decreased among individuals with T2D classified as LCVM (p<0.05), with no changes in the HCVM group (Table 1). The whole-body insulin sensitivity index increased with training in the LCVM group when considering T2D and obese non-diabetics together (p<0.05), but for individuals with T2D only those classified as HCVM increased the insulin sensitivity index (p<0.05) (Table 1).
By design, the HCVM groups were different from the LCVM groups (p<0.05), with no effect of diabetes status. HRV HF increased following the training period among those classified as LCVM (p<0.05), with no effect of diabetes status. No effect of training was observed for LF. Table 1.
Correlations
The change in VO2peak (expressed as either relative or absolute) was not associated with the change in body weight among the HCVM group (r = 0.20, 0.27, respectively), but the change in VO2peak (relative) was associated with the change in body weight among the LCVM group (r = 0.39, p<0.05). The change in VO2peak was also not associated with whole-body insulin sensitivity (HCVM r=−0.07; LCVM r= 0.11) or glucose area under the curve (HCVM r=−0.02; LCVM r=−0.15).
Discussion
The primary finding of this study is that in sedentary obese subjects, with and without T2D, cardiovagal modulation prior to the beginning of the training program appeared to impact the response to training. In particular, obese individuals with and without T2D who had high levels of vagal modulation prior to the commencement of an exercise program showed greater improvements in aerobic capacity compared to those with low cardiovagal modulation, as measured by HRV. Additionally, aerobic exercise improved body weight in the HCVM group to a greater extent than the LCVM group, but this change could not explain the improvements in aerobic capacity. Yet, the LCVM group demonstrated a significant relationship between weight change and VO2peak (relative, not absolute), which may suggest that it is more important for this group to experience weight loss to achieve improvements in exercise tolerance, even though aerobic capacity did not improve among the LCVM group overall. Further, statin use did not influence our findings, given recent evidence in humans that statins may negatively affect potential for increases in VO2peak (22). Thus, baseline autonomic function appears to be a significant contributor to beneficial exercise training induced changes in obese individuals with and without T2D.
Changes in fitness may not explain changes in other cardiovascular risk factors. Gibbs et al. (6) explored data from the Look AHEAD study, a large multi-year lifestyle intervention trial, and found that changes in fitness and body weight explained a relatively small amount of variability (0.1–9.3%) in 1-year change in traditional cardiovascular risk factors (e.g. fasting glucose, HbA1c, high density cholesterol, triglycerides, diastolic blood pressure). Thus, the change in cardiovascular risk factors did not contribute to substantial changes in fitness and body weight, suggesting additional parameters may explain changes inVO2peak. Hence factors such as cardiovagal modulation may play an important role in determining the responsiveness to a training program. Our data suggest that baseline parasympathetic modulation is an important factor contributing to improvements in aerobic capacity following endurance training. It is possible that HCVM at baseline allows for such changes in aerobic capacity, regardless of diabetes status, because HCVM may be protective against cardiac autonomic neuropathy, or that HCVM is related to better cholinergic control of peripheral circulation. However, these hypotheses warrant further investigation.
Glucose control, insulin resistance, endothelial dysfunction, peripheral oxygen utilization and central oxygen delivery have all been implicated in poor exercise capacity in T2D, as depicted in two reviews (26, 29). Our study resulted in a small improvement in glucose control among the T2D group with LCVM (e.g. glucose area under the curve), yet the LCVM groups did not increase VO2peak. Thus our exercise training data support the lack of association between glucose control and cardiovascular fitness described in studies of acute exercise and glucose control (18, 27, 31). Insulin resistance has also been associated with reduced VO2peak in T2D (18, 20, 27, 30, 32). We observed no significant association between glucose area under the curve and whole-body insulin sensitivity and changes in VO2peak, suggesting insulin sensitivity (or glucose tolerance) cannot explain the increase in VO2peak with exercise training. It is possible that the training intensity in our study was not high enough to induce significant changes in insulin sensitivity or possibly a more sensitive model for estimating insulin resistance (e.g. clamp models) would be needed detect such changes. Thus, these data support the importance of pre-training vagal modulation as an important contributor improvement in VO2peak independent of changes in glucose tolerance or insulin sensitivity.
Recently, Notarius et al. (24) showed that aerobic capacity was associated with neurovascular coupling in middle-aged healthy men, as measured with muscle sympathetic nerve activity (a marker of sympathetic outflow), during lower body negative pressure. They reported a higher correlation among low fit middle-aged men with sympathetic activity versus higher fit men (24), suggesting fitness status and autonomic function are interconnected. Generally, individuals with LCVM are likely to have a larger sympathetic component driving autonomic responses regardless of diabetic status. Thus, the prevailing existing evidence suggests that low fitness is associated with low parasympathetic modulation and high sympathetic output. Our data extend these observations showing that low baseline parasympathetic modulation prevented endurance training induced improvements in cardiovascular fitness. Furthermore, high levels of baseline parasympathetic modulation were required for fitness to improve, suggesting that autonomic status is an important contributor to fitness gains. In addition, it is important to note that our T2D subjects exhibited good glucose control (7.2% hemoglobin A1c, no change with training) and also did not report peripheral neuropathy. Future studies are needed to investigate the response to endurance training in T2D across a glucose control continuum.
No baseline differences between individuals with or without T2D were found for either LF or HF. By design, group differences for HF were observed between HCVM and LCVM groups, whereas these differences were unaffected by diabetes status. A training effect on HF was observed for individuals classified as LCVM, suggesting a level of plasticity in this marker of parasympathetic modulation. It is possible that a higher training stimulus (e.g. ~75% VO2peak or high intensity interval training) may impart larger changes in HRV (25), which may be coupled (or not coupled) with larger improvements in VO2peak. The role of pre-training parasympathetic modulation on the responsiveness to a higher intensity program still needs to be determined. It is possible that LF was unaffected due the current belief that LF is comprised of both sympathetic and parasympathetic influences and perhaps there was a cancellation affect. Interestingly, recent data suggests LF may in fact be indicative of baroreceptor sensitivity, however this needs to be explored further in this population (23). However, the improvement in HF in the LCVM group suggests that endurance training can change parasympathetic modulation in obese individuals. Thus, it is possible that a longer period of endurance training is needed to change VO2peak in LCVM individuals, since VO2peak may only after improve after parasympathetic modulation is improved. Previous reports have established the effectiveness of the mode, duration, and intensity of individuals exercise sessions on HRV in healthy young and older populations (10, 25, 35). Yet, the exact training-induced dose-response relationship in relation to autonomic function and aerobic capacity in obese individuals with and without T2D is unknown at this time. It is also possible that our data may be a reflection of a ‘regression towards the mean’ artifact; however, ANCOVA analyses does not statistically suggest this is occurring in our data, we cannot fully rule it out either.
This study has several limitations. We did not measure leisure time physical activity, and this may have an important impact on peak aerobic capacity (8); however, all subjects were instructed to maintain their leisure time physical activity. The lack of a healthy non-obese control group may be an additional limitation; however, the main objective of this study was to determine the influences of cardiovagal modulation on exercise training responsiveness in individuals with T2D. Since obesity is a common feature of T2D we chose to study obese individuals without T2D as our control. Finally, the exercise program consisted of mostly home-based exercise sessions; however, this was countered with regular laboratory-based supervised sessions to ensure adequate opportunity to adjust the training load.
In conclusion, this study demonstrates that obese individuals, with or without T2D, when classified as having HCVM prior to exercise training, have a greater propensity to improve VO2peak following a 16-week aerobic training program. While the improvements in VO2peak, body weight, BMI, and waist circumference are modest among the HCVM group, this study corroborates previous research (11, 12, 16) suggesting that the individual’s response to a training stimulus should be individualized for maximum benefit.
Acknowledgments
This study was supported in part by the National Institutes of Health (JAK) (R21DK063179).
The authors would like to thank all of our volunteers for their time and dedication as well as Roselynn Kingsbury, RN, NP for her assistance in the study.
Footnotes
Disclosure:
No author has any disclosures to report.
None of the authors have professional relationships with companies or manufacturers who will benefit from the results of the present study.
The results of the present study do not constitute endorsement by the American College of Sports Medicine.
References
- 1.Baynard T, Pitetti KH, Guerra M, Fernhall B. Heart rate variability at rest and during exercise in persons with down syndrome. Arch Phys Med Rehabil. 2004;85:1285–1290. doi: 10.1016/j.apmr.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 2.Buchheit M, Gindre C. Cardiac parasympathetic regulation: Respective associations with cardiorespiratory fitness and training load. Am J Physiol Heart Circ Physiol. 2006;291:H451–458. doi: 10.1152/ajpheart.00008.2006. [DOI] [PubMed] [Google Scholar]
- 3.Cohen J. Statistical power analysis for the behavior sciences. Routledge: 1988. [Google Scholar]
- 4.Davy KP, DeSouza CA, Jones PP, Seals DR. Elevated heart rate variability in physically active young and older adult women. Clin Sci (Lond) 1998;94:579–584. doi: 10.1042/cs0940579. [DOI] [PubMed] [Google Scholar]
- 5.Davy KP, Miniclier NL, Taylor JA, Stevenson ET, Seals DR. Elevated heart rate variability in physically active postmenopausal women: A cardioprotective effect? Am J Physiol. 1996;271:H455–460. doi: 10.1152/ajpheart.1996.271.2.H455. [DOI] [PubMed] [Google Scholar]
- 6.Gibbs BB, Brancati FL, Chen H, Coday M, Jakicic JM, Lewis CE, Stewart KJ, Clark JM. Effect of improved fitness beyond weight loss on cardiovascular risk factors in individuals with type 2 diabetes in the look ahead study. Eur J Prev Cardiol. doi: 10.1177/2047487312462823. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goulopoulou S, Baynard T, Franklin RM, Fernhall B, Carhart R, Jr, Weinstock R, Kanaley JA. Exercise training improves cardiovascular autonomic modulation in response to glucose ingestion in obese adults with and without type 2 diabetes mellitus. Metabolism. 2010;59:901–910. doi: 10.1016/j.metabol.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hautala A, Martinmaki K, Kiviniemi A, Kinnunen H, Virtanen P, Jaatinen J, Tulppo M. Effects of habitual physical activity on response to endurance training. J Sports Sci. 2012;30:563–569. doi: 10.1080/02640414.2012.658080. [DOI] [PubMed] [Google Scholar]
- 9.Hautala AJ, Kiviniemi AM, Makikallio TH, Tiinanen S, Seppanen T, Huikuri HV, Tulppo MP. Muscle sympathetic nerve activity at rest compared to exercise tolerance. Eur J Appl Physiol. 2008;102:533–538. doi: 10.1007/s00421-007-0618-1. [DOI] [PubMed] [Google Scholar]
- 10.Hautala AJ, Kiviniemi AM, Tulppo MP. Individual responses to aerobic exercise: The role of the autonomic nervous system. Neurosci Biobehav Rev. 2009;33:107–115. doi: 10.1016/j.neubiorev.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 11.Hautala AJ, Makikallio TH, Kiviniemi A, Laukkanen RT, Nissila S, Huikuri HV, Tulppo MP. Cardiovascular autonomic function correlates with the response to aerobic training in healthy sedentary subjects. Am J Physiol Heart Circ Physiol. 2003;285:H1747–1752. doi: 10.1152/ajpheart.00202.2003. [DOI] [PubMed] [Google Scholar]
- 12.Hedelin R, Bjerle P, Henriksson-Larsen K. Heart rate variability in athletes: Relationship with central and peripheral performance. Med Sci Sports Exerc. 2001;33:1394–1398. doi: 10.1097/00005768-200108000-00023. [DOI] [PubMed] [Google Scholar]
- 13.Kanaley JA, Baynard T, Franklin RM, Weinstock RS, Goulopoulou S, Carhart R, Jr, Ploutz-Snyder R, Figueroa A, Fernhall B. The effects of a glucose load and sympathetic challenge on autonomic function in obese women with and without type 2 diabetes mellitus. Metabolism. 2007;56:778–785. doi: 10.1016/j.metabol.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kanaley JA, Goulopoulou S, Franklin RM, Baynard T, Holmstrup ME, Carhart R, Jr, Weinstock RS, Fernhall B. Plasticity of heart rate signalling and complexity with exercise training in obese individuals with and without type 2 diabetes. Int J Obes (Lond) 2009;33:1198–1206. doi: 10.1038/ijo.2009.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karayannis G, Giamouzis G, Cokkinos DV, Skoularigis J, Triposkiadis F. Diabetic cardiovascular autonomic neuropathy: Clinical implications. Expert Rev Cardiovasc Ther. 2012;10:747–765. doi: 10.1586/erc.12.53. [DOI] [PubMed] [Google Scholar]
- 16.Kiviniemi AM, Hautala AJ, Kinnunen H, Nissila J, Virtanen P, Karjalainen J, Tulppo MP. Daily exercise prescription on the basis of hr variability among men and women. Med Sci Sports Exerc. 2010;42:1355–1363. doi: 10.1249/mss.0b013e3181cd5f39. [DOI] [PubMed] [Google Scholar]
- 17.Kiviniemi AM, Hautala AJ, Kinnunen H, Tulppo MP. Endurance training guided individually by daily heart rate variability measurements. Eur J Appl Physiol. 2007;101:743–751. doi: 10.1007/s00421-007-0552-2. [DOI] [PubMed] [Google Scholar]
- 18.Kjaer M, Hollenbeck CB, Frey-Hewitt B, Galbo H, Haskell W, Reaven GM. Glucoregulation and hormonal responses to maximal exercise in non-insulin-dependent diabetes. J Appl Physiol. 1990;68:2067–2074. doi: 10.1152/jappl.1990.68.5.2067. [DOI] [PubMed] [Google Scholar]
- 19.Knaub LA, McCune S, Chicco AJ, Miller M, Moore RL, Birdsey N, Lloyd MI, Villarreal J, Keller AC, Watson PA, Reusch JE. Impaired response to exercise intervention in the vasculature in metabolic syndrome. Diab Vasc Dis Res. 2013;10:222–238. doi: 10.1177/1479164112459664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laws A, Reaven GM. Effect of physical activity on age-related glucose intolerance. Clin Geriatr Med. 1990;6:849–863. [PubMed] [Google Scholar]
- 21.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: Comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22:1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 22.Mikus CR, Boyle LJ, Borengasser SJ, Oberlin DJ, Naples SP, Fletcher J, Meers GM, Ruebel M, Laughlin MH, Dellsperger KC, Fadel PJ, Thyfault JP. Simvastatin impairs exercise training adaptations. J Am Coll Cardiol. doi: 10.1016/j.jacc.2013.02.074. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moak JP, Goldstein DS, Eldadah BA, Saleem A, Holmes C, Pechnik S, Sharabi Y. Supine low-frequency power of heart rate variability reflects baroreflex function, not cardiac sympathetic innervation. Cleve Clin J Med. 2009;76 (Suppl 2):S51–59. doi: 10.3949/ccjm.76.s2.11. [DOI] [PubMed] [Google Scholar]
- 24.Notarius CF, Murai H, Morris BL, Floras JS. Effect of fitness on reflex sympathetic neurovascular transduction in middle-age men. Med Sci Sports Exerc. 2012;44:232–237. doi: 10.1249/MSS.0b013e31822a68a5. [DOI] [PubMed] [Google Scholar]
- 25.Okazaki K, Iwasaki K, Prasad A, Palmer MD, Martini ER, Fu Q, Arbab-Zadeh A, Zhang R, Levine BD. Dose-response relationship of endurance training for autonomic circulatory control in healthy seniors. J Appl Physiol. 2005;99:1041–1049. doi: 10.1152/japplphysiol.00085.2005. [DOI] [PubMed] [Google Scholar]
- 26.Regensteiner JG. Type 2 diabetes mellitus and cardiovascular exercise performance. Rev Endocr Metab Disord. 2004;5:269–276. doi: 10.1023/B:REMD.0000032416.13070.01. [DOI] [PubMed] [Google Scholar]
- 27.Regensteiner JG, Sippel J, McFarling ET, Wolfel EE, Hiatt WR. Effects of non-insulin-dependent diabetes on oxygen consumption during treadmill exercise. Med Sci Sports Exerc. 1995;27:875–881. [PubMed] [Google Scholar]
- 28.Rennie KL, Hemingway H, Kumari M, Brunner E, Malik M, Marmot M. Effects of moderate and vigorous physical activity on heart rate variability in a British study of civil servants. Am J Epidemiol. 2003;158:135–143. doi: 10.1093/aje/kwg120. [DOI] [PubMed] [Google Scholar]
- 29.Reusch JE, Bridenstine M, Regensteiner JG. Type 2 diabetes mellitus and exercise impairment. Rev Endocr Metab Disord. 2013;14:77–86. doi: 10.1007/s11154-012-9234-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reusch JE, Regensteiner JG, Watson PA. Novel actions of thiazolidinediones on vascular function and exercise capacity. Am J Med. 2003;115(Suppl 8A):69S–74S. doi: 10.1016/j.amjmed.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 31.Saltin B, Lindgarde F, Houston M, Horlin R, Nygaard E, Gad P. Physical training and glucose tolerance in middle-aged men with chemical diabetes. Diabetes. 1979;28 (Suppl 1):30–32. doi: 10.2337/diab.28.1.s30. [DOI] [PubMed] [Google Scholar]
- 32.Schneider SH, Khachadurian AK, Amorosa LF, Gavras H, Fineberg SE, Ruderman NB. Abnormal glucoregulation during exercise in type II (non-insulin-dependent) diabetes. Metabolism. 1987;36:1161–1166. doi: 10.1016/0026-0495(87)90243-5. [DOI] [PubMed] [Google Scholar]
- 33.TaskForce. Heart rate variability: Standards of measurement, physiological interpretation and clinical use. Task force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- 34.Ueno LM, Hamada T, Moritani T. Cardiac autonomic nervous activities and cardiorespiratory fitness in older men. J Gerontol A Biol Sci Med Sci. 2002;57:M605–610. doi: 10.1093/gerona/57.9.m605. [DOI] [PubMed] [Google Scholar]
- 35.Uusitalo AL, Laitinen T, Vaisanen SB, Lansimies E, Rauramaa R. Effects of endurance training on heart rate and blood pressure variability. Clin Physiol Funct Imaging. 2002;22:173–179. doi: 10.1046/j.1475-097x.2002.00414.x. [DOI] [PubMed] [Google Scholar]
- 36.Vamos EP, Millett C, Parsons C, Aylin P, Majeed A, Bottle A. Nationwide study on trends in hospital admissions for major cardiovascular events and procedures among people with and without diabetes in England, 2004–2009. Diabetes Care. 2012;35:265–272. doi: 10.2337/dc11-1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vinik AI, Maser RE, Ziegler D. Autonomic imbalance: Prophet of doom or scope for hope? Diabet Med. 2011;28:643–651. doi: 10.1111/j.1464-5491.2010.03184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vinik AI, Ziegler D. Diabetic cardiovascular autonomic neuropathy. Circulation. 2007;115:387–397. doi: 10.1161/CIRCULATIONAHA.106.634949. [DOI] [PubMed] [Google Scholar]
- 39.Wei M, Gibbons LW, Kampert JB, Nichaman MZ, Blair SN. Low cardiorespiratory fitness and physical inactivity as predictors of mortality in men with type 2 diabetes. Ann Intern Med. 2000;132:605–611. doi: 10.7326/0003-4819-132-8-200004180-00002. [DOI] [PubMed] [Google Scholar]