Abstract
Objective:
Observed associations between fluid balance and septic shock outcomes are likely confounded by initial mortality risk. We conducted a risk-stratified analysis of the association between post-intensive care unit (ICU) admission fluid balance and pediatric septic shock outcomes.
Design:
Retrospective analysis of an ongoing, multi-center pediatric septic shock clinical and biological database.
Setting:
Seventeen pediatric ICUs in the United States.
Patients:
Three hundred and seventeen children with septic shock.
Interventions:
None.
Measurements and Main Results:
We stratified subjects into three mortality risk categories (low, intermediate, and high) using a validated, biomarker-based stratification tool. Within each category, we assessed three fluid balance variables: total fluid intake/kg/day during the first 24 hours, percent positive fluid balance during the first 24 hours, and cumulative percent positive fluid balance up to seven days. We used logistic regression to estimate the effect of fluid balance on the odds of 28-day mortality, and on complicated course, defined as either death within 28 days or persistence of two or more organ failures at seven days. There were 40 deaths and 91 subjects had a complicated course. Increased cumulative percent positive fluid balance was associated with mortality in the low risk cohort (n = 204, OR 1.035, 95%CI 1.004 – 1.066), but not in the intermediate and high risk cohorts. No other associations with mortality were observed. Fluid intake, percent positive fluid balance in the first 24 hours, and cumulative percent positive fluid balance were all associated with increased odds of a complicated course in the low risk cohort, but not the intermediate and high risk cohorts.
Conclusions:
When stratified for mortality risk, increased fluid intake and positive fluid balance after ICU admission are associated with worse outcomes in pediatric septic shock patients with a low initial mortality risk, but not in patients at moderate or high mortality risk.
INTRODUCTION
Septic shock remains a major cause of morbidity and mortality in children (1). Over 20 years ago, Carcillo and colleagues reported that in children with septic shock, fluid resuscitation in excess of 40 ml/kg within the first hour of presentation was associated with improved survival, without increased risk of cardiogenic pulmonary edema or acute respiratory distress syndrome (2). Since then, aggressive fluid resuscitation has been a core intervention for the management of both pediatric and adult septic shock (3, 4), and the practice has been supported by subsequent observational and interventional studies (5-8).
While seemingly a fundamental tenet of septic shock management, aggressive fluid resuscitation for septic shock was recently criticized as being only weakly supported by evidence (9). Further, recent cohort studies have reported an association between positive fluid balance and increased mortality in adult and pediatric patients with sepsis, as well as other critical illnesses (10-19). Most recently, the Fluid Expansion as Supportive Therapy (FEAST) study compared fluid boluses of 20 to 40 ml/kg to no bolus in over 3,000 acutely ill African children, and reported significantly increased mortality in the group randomized to the fluid bolus arm (20). The FEAST study raises many questions regarding the efficacy of fluid resuscitation, even though the relevance for resource rich environments is unclear (4).
It is biologically and physiologically plausible that the association between a positive fluid balance and the risk of mortality is a result of confounding by illness severity. That is, positive fluid balance could be simply a marker of increased illness severity leading to increased vascular leak, increased third spacing of fluid, and increased fluid requirements, rather than a direct cause of increased mortality itself (21). Accordingly, associations between positive fluid balance and septic shock outcomes would be better interpreted in the context of reliable risk stratification.
We recently derived and validated a multibiomarker-based risk model called PERSEVERE (PEdiatRic SEpsis biomarkEr Risk modEl) that reliably predicts outcomes in heterogeneous cohorts of children with septic shock (22). PERSEVERE stratifies patients based on their risk of mortality. One potential application of PERSEVERE is to help adjust for illness severity in analysis of clinical data. In the current study, we have used PERSEVERE to conduct a risk-stratified analysis of the association between post intensive care unit admission positive fluid balance and outcomes in pediatric patients with septic shock.
METHODS
Study and data collection
Study subjects (n = 317) were participants in an ongoing, multi-center genomics database of children with septic shock. The study protocol was approved by the Institutional Review Boards of each participating institution (n = 17), and has been previously described in detail (23-34). Briefly, children ≤ 10 years of age admitted to the pediatric intensive care unit (PICU) and meeting pediatric-specific criteria for septic shock were eligible for enrollment (35). After informed consent from parents or legal guardians, blood samples were obtained within 24 hours of initial presentation to the PICU. Clinical and laboratory data were collected daily while in the PICU. Mortality was tracked for 28 days after enrollment, and organ failure was defined using pediatric-specific criteria (35). All subjects < 28 days of age (i.e. neonates) were full term and were admitted to the PICU with septic shock subsequent to being discharged to home after birth.
Fluid status variables
We calculated three different fluid balance parameters based on PICU admission weights. First, we calculated the total fluid intake (ml/kg/day) during the first 24 hours of PICU admission. Second, we calculated the percent positive fluid balance during the first day of PICU admission, and third we calculated the cumulative percent positive fluid balance up to 7 days from PICU admission. Percent positive fluid balance was calculated using the formula:
The fluid output variable included urine plus all other sources of fluid loss except insensible fluid loss. Fluid therapy decisions were not under protocol.
Stratification
Subjects were stratified by initial risk of 28-day mortality using the updated version of PERSEVERE (22). Two hundred and seventy nine (88%) of the current subjects were included in either the original PERSEVERE derivation cohort or the PERSEVERE test cohort, which originally consisted of 355 subjects. The 279 subjects were selected because they had complete fluid balance data available, whereas the remaining 76 subjects had incomplete fluid balance data and where therefore excluded from this analysis.
Data analysis
All statistical analyses were conducted using SigmaStat Software (Systat Software, Inc., San Jose, CA). Initially, data are described using medians, interquartile ranges, frequencies, and percents. Comparisons between study groups were made using the Mann-Whitney U-test, Chi-square, or Fisher’s Exact tests, as appropriate.
Associations between the fluid status variables and outcome were analyzed using logistic regression. Pearson’s correlation coefficient was used to test for associations between the fluid status variables; in the presence of moderate or high correlations between these predictor variables the regression coefficients may not be well estimated and a multivariable approach to modeling outcomes would not be informative. We considered two different outcome variables. First, we used all cause 28-day mortality. Second, we considered a composite endpoint with we termed “complicated course”, which is defined as either death within the 28-day study period, or persistence of two or more organ failures at 7 days after meeting criteria for septic shock, as previously described (36, 37).
RESULTS
Demographics and clinical characteristics
There were 317 children included. Table 1 compares the survivors and the non-survivors. The 40 non-survivors (12.6%) were significantly younger and were more likely to have septic shock-associated renal failure, compared to the 277 survivors. Non-survivors also had a higher Pediatric Risk of Mortality (PRISM) score, a higher probability of mortality based on PERSEVERE, and a greater proportion of subjects < 28 days of age. The median fluid intake per kilogram during the first 24 hours, percent positive fluid balance during the first 24 hours, and cumulative percent positive fluid balance up to 7 days were all significantly higher in the non-survivors compared to the survivors. The types of pathogens identified were not significantly different between survivors and non-survivors.
Table 1.
Clinical characteristics of non-survivors and survivors.
| Non-survivors (n = 40) | Survivors (n = 277) | p value | |
|---|---|---|---|
| Median age in years (IQR) | 1.3 (0.2 – 4.5) | 2.9 (1.1 – 6.7) | 0.007 |
| Number of males (%) | 26 (65) | 166 (59) | 0.225 |
| Median PRISM score | 28 (17 – 37) | 12 (7 – 18) | <0.001 |
| Number with renal failure (%) | 11 (27) | 23 (8) | <0.001 |
| Median fluid intake/kg in first 24 hours | 178 (134 – 227) | 138 (100 – 186) | 0.003 |
| %Positive fluid balance in first 24 hours | 10.6 (3.8 – 15.7) | 4.5 (1.3 – 9.8) | <0.001 |
| Cumulative %positive fluid balance | 19.5 (10.5 – 40.1) | 6.5 (−1.3 – 14.6) | <0.001 |
| PERSEVERE probability of mortality | 0.472 (0.222 – 0.472) | 0.011 (0.011 – 0.182) | <0.001 |
| # of subjects < 28 days of age (%) | 9 (23) | 22 (8) | 0.004 |
| # with gram positive infection (%) | 10 (25) | 66 (24) | 0.871 |
| # with gram negative infection (%) | 8 (20) | 54 (19) | 0.940 |
| # with viral infection (%) | 4 (10) | 15 (5) | 0.253 |
| # with fungal infection (%) | 0 (0) | 2 (1) | 0.590 |
| # with mixed infection (%) | 1 (3) | 10 (4) | 0.720 |
| # with no organism identified (%) | 17 (43) | 130 (47) | 0.599 |
Table 2 compares the 91 subjects with a complicated course to the 226 subjects without a complicated course. The pattern of differences was similar to that for the comparison between survivors and non-survivors, except that the proportion of subjects < 28 days of age was similar between the two groups. In addition, a greater proportion of subjects with a complicated course had mixed infections as the cause of septic shock, while a lower proportion of the subjects with complicated course had no causative organism isolated.
Table 2.
Clinical characteristics of patients with and without a complicated course (CC).
| CC (n = 91) | Non-CC (n = 226) | p value | |
|---|---|---|---|
| Median age in years (IQR) | 1.6 (0.6 – 5.2) | 3.0 (1.2 – 7.0) | 0.003 |
| Number of males (%) | 61 (67) | 131 (60) | 0.135 |
| Median PRISM score | 20 (11 – 30) | 12 (6 – 17) | <0.001 |
| Number with renal failure (%) | 26 (29) | 8 (4) | <0.001 |
| Median fluid intake/kg in first 24 hours | 177 (125 – 228) | 134 (99 – 172) | <0.001 |
| %Positive fluid balance in first 24 hours | 8.5 (3.7 – 15.2) | 3.8 (0.6 – 8.3) | <0.001 |
| Cumulative %positive fluid balance | 13.2 (4.3 – 29.6) | 5.9 (−1.8 – 13.6) | <0.001 |
| PERSEVERE probability of mortality | 0.222 (0.182 – 0.472) | 0.011 (0.011 – 0.025) | <0.001 |
| # of subjects < 28 days of age (%) | 13 (14) | 18 (8) | 0.087 |
| # with gram positive infection (%) | 25 (27) | 51 (23) | 0.355 |
| # with gram negative infection (%) | 20 (22) | 42 (19) | 0.491 |
| # with viral infection (%) | 8 (9) | 11 (5) | 0.183 |
| # with fungal infection (%) | 1 (1) | 1 (0) | 0.504 |
| # with mixed infection (%) | 7 (8) | 4 (2) | 0.009 |
| # with no organism identified (%) | 30 (33) | 117 (52) | 0.002 |
Association between fluid status and outcomes
Based on a histogram (not shown), the cohort was stratified according to the PERSEVERE-based mortality probabilities: low risk (mortality probability 0 to 2.5%), intermediate risk (mortality probability >2.5% to 26.7%), and high risk (mortality probability >26.7% to 62.5%). The three fluid status variables were moderately to highly correlated: fluid intake per kg during the first 24 hours vs. percent positive fluid balance during the first 24 hours (r = 0.670, p < 0.001); fluid intake per kg during the first 24 hours vs. cumulative percent positive fluid balance (r = 0.365, p < 0.001); and percent positive fluid balance during the first 24 hours vs. cumulative percent positive fluid balance (r = 0.505, p < 0.001). Because of this co-linearity, we used univariable logistic regression to analyze the association between the fluid status variables and outcome.
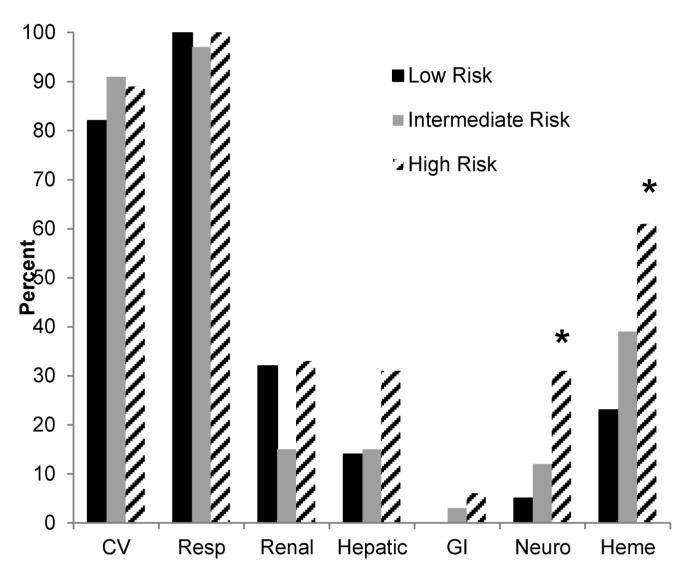
As shown in Tables 3 and 4, fluid status was not associated with mortality or a complicated course in the intermediate and high risk groups. Fluid intake and percent positive fluid balance during the first 24 hours were not associated with mortality in the low risk group. However, increased cumulative percent positive fluid balance was associated with mortality in the low risk group, and increases in all three fluid status variables were associated with increased odds of a complicated course in the low risk group. Figure 1 shows the distribution of organ failures for patients with a complicated course. A greater proportion of the high risk patients had neurologic and hematologic failure, compared to the low and intermediate risk patients. The rates of other organ failures were otherwise not significantly different between the three risk groups.
Table 3.
Univariable logistic regression results for mortality.
| Risk Group (n) | # of deaths |
Variable | Odds Ratio (95% C.I.) | p value |
|---|---|---|---|---|
| Low (204) | 3 | |||
| Fluid intake/kg in first 24 hrs | 0.991 (0.969 – 1.014) | 0.449 | ||
| %Pos. fluid balance in first 24 hrs | 1.020 (0.867 – 1.200) | 0.813 | ||
| Cumulative %pos. fluid balance | 1.035 (1.004 – 1.066) | 0.024 | ||
| Intermediate (68) | 12 | |||
| Fluid intake/kg in first 24 hrs | 1.004 (0.995 – 1.012) | 0.380 | ||
| %Pos. fluid balance in first 24 hrs | 1.023 (0.938 – 1.116) | 0.604 | ||
| Cumulative %pos. fluid balance | 1.045 (1.000 – 1.092) | 0.050 | ||
| High (45) | 25 | |||
| Fluid intake/kg in first 24 hrs | 1.000 (0.993 – 1.007) | 0.933 | ||
| %Pos. fluid balance in first 24 hrs | 0.999 (0.941 – 1.061) | 0.983 | ||
| Cumulative %pos. fluid balance | 0.933 (0.971 – 1.015) | 0.536 | ||
Table 4.
Univariable logistic regression results for complicated course (CC).
| Risk Group (n) | # with CC |
Variable | Odds Ratio (95% C.I.) | p value |
|---|---|---|---|---|
| Low (204) | 22 | |||
| Fluid intake/kg in first 24 hrs | 1.010 (1.004 – 1.016) | 0.002 | ||
| %Pos. fluid balance in first 24 hrs | 1.123 (1.054 – 1.197) | <0.001 | ||
| Cumulative %pos. fluid balance | 1.031 (1.011 – 1.052) | 0.003 | ||
| Intermediate (68) | 33 | |||
| Fluid intake/kg in first 24 hrs | 1.002 (0.995 – 1.008) | 0.636 | ||
| %Pos. fluid balance in first 24 hrs | 1.049 (0.975 – 1.129) | 0.202 | ||
| Cumulative %pos. fluid balance | 1.021 (0.987 – 1.056) | 0.228 | ||
| High (45) | 36 | |||
| Fluid intake/kg in first 24 hrs | 0.999 (0.990 – 1.007) | 0.739 | ||
| %Pos. fluid balance in first 24 hrs | 0.993 (0.922 – 1.070) | 0.851 | ||
| Cumulative %pos. fluid balance | 0.978 (0.951 – 1.005) | 0.108 | ||
Figure 1.
Distribution of organ failures for the subjects with a complicated course. *p < 0.05 vs. low and intermediate risk groups, Chi-square with 2 degrees of freedom.
In order to consider the interaction between fluid intake and positive fluid balance, we tested the interaction between fluid intake and percent positive fluid balance in the first 24 hours in each of the risk groups. The interaction term was significantly associated with a complicated course in the low risk group (p < 0.001). No other associations between the interaction term and outcome were observed.
Because fluid administration was not under protocol, we also considered the possibility that different fluid administration practices across the multiple contributing centers could affect outcome. We did not find an association between center and outcome (data not shown).
DISCUSSSION
We explored the potential association between post-PICU admission fluid balance and outcome in a large and heterogeneous cohort of children with septic shock drawn from seventeen centers in the U.S. When the cohort is stratified into three mortality risk groups, we detected associations between post-PICU admission fluid balance and worse outcomes only in the low risk group.
Our data diverge substantially from recent reports that suggest independent associations between a positive fluid balance and poor outcomes in adult and pediatric patients with sepsis or other forms of critical illness (10-19). This divergence is unlikely a reflection of an insufficient sample size, given the size of our cohort relative to previous pediatric studies (11, 14-19). We note that our study included only children ≤ 10 years of age with septic shock, whereas previous pediatric studies included a broader age range and a mixed population of critically ill children, including sepsis. Accordingly, our findings may not be generalizable beyond children ≤10 years of age with septic shock. Our findings in moderate to high risk patients are consistent with a recent study in adult patients with septic shock, which demonstrated no association between initial fluid status and mortality, although we did not replicate the association between higher fluid volumes and reduced mortality in patients with shock duration of three or more days (8).
In our analysis, stratification by risk was used to evaluate the association between post-ICU admission fluid balance and outcome while taking into account the problem of confounding by severity. Previous studies have attempted to adjust for such confounding by including physiology-based scoring systems into analyses (10-19). It has been proposed, however, that physiology-based scoring systems are poor surrogates for risk stratification and that they tend to perform poorly when applied to specific forms of critical illness, rather than a general critically ill population (38). PERSEVERE was derived and validated in children with septic shock and outperforms a physiology-based scoring system when risk stratifying children with septic shock (22). Furthermore, ongoing testing continues to demonstrate that it reliably risk stratifies children with septic shock (H. Wong, unpublished data).
Optimal fluid balance in septic shock is currently an area of much debate (9, 21). There are two inter-related, but distinct issues central to this debate. First, there is the issue surrounding the optimal amount and type of fluid administration during the early phase of resuscitation. The current study does not fully address this issue because we were unable to distinguish between fluids specifically administered for resuscitation and the obligate fluids associated with the general care process (e.g. fluids related to medications), nor were we able to specify the type of fluid used in resuscitation (i.e. crystalloid vs. colloid). In addition, we did not capture the amount of fluid administered prior to PICU admission, which is a major limitation of our study. These issues notwithstanding, our data suggest that in low risk patients, increased fluid administration post admission to the PICU may be detrimental, rather than beneficial. In addition, our data suggest that the optimal amount of initial fluid requirements for children with septic shock encompasses a relatively broad range. Ultimately, it is hoped that this issue will be clarified substantially by three ongoing, multi-center trials in the United Kingdom (ISRCTN36307479), Australia (NCT00975793), and the U.S. (NCT00510835), which are directly addressing this important question.
Second, there is the issue of positive fluid balance after the initial resuscitation period, which is more directly addressed by our current study. Addressing this analytically is challenging given the interaction and interdependence between the risk of a positive fluid balance and illness severity, and our stratified analysis helps address this. Intuitively, there should be a critical range at which a positive fluid balance beyond the initial resuscitation phase has negative consequences for outcome. Our data demonstrate that there is indeed a relationship between positive fluid balance and increased odds of a complicated course, but interestingly this is limited only to the low risk cohort. While not significant, the opposite trend is observed in the high risk cohort. This would suggest that a positive fluid balance might be beneficial in the more severely ill patients, but detrimental in lower risk patients. Whether these associations are causal or secondary to some other confounding factor requires exploration. Regardless, our data do not support the institution of aggressive fluid removal, through either medications or renal replacement therapies, at any specified state of positive fluid balance.
In conclusion, our current data indicate that positive fluid balance post admission to the PICU affects children with septic shock having an initial low mortality risk; increasing fluid intake and positive fluid balance in this cohort, but not in those at intermediate or high risk, are associated with increased odds of a worse outcome. The optimal fluid balance for children with septic shock seems to be quite broad, and is highly dependent on illness severity. The specific level of positive fluid balance that leads to poor outcomes is not evident from our data. Given the heterogeneity and complexities of septic shock, the decision to address therapeutically a positive fluid balance will likely continue to rely on clinical acumen and patient-specific context.
ACKNOWLEDGEMENTS
The authors thank the following research coordinators for their effort and dedication in enrolling study participants: Tasha Capozzi, Mary Ann De Liberto, Mercedes Galera-Perez, Kristin Greathouse, Lauren Hoadley, Katherine Luther, Stephanie Osborne, Amber Hughes-Schalk, Tonia Polanski, Julie Simon, Debra Spear, Lisa Steele, Naresh B. Talathoti, Tiffany Vertican, Monica Weber, Andrew A. Wiles, Trisha Williams, and Erin Zielinski
FUNDING SOURCE
Supported by National Institutes of Health Grants RC1HL100474, RO1GM064619, and RO1GM099773. Supported in part by an Institutional Clinical and Translational Science Award, NIH/NCRR 8UL1 TR000077.
Footnotes
AUTHOR COMPETING INTERESTS
Hector R. Wong: Dr. Wong and the Cincinnati Children’s Hospital Research Foundation have submitted a provisional patent application for PERSEVERE.
Christopher J. Lindsell: Dr. Lindsell is named as a co-inventor in the above patent application.
Drs. Wong, Cvijanovich, Allen, Hall, Freishtat, Sen, Shanley, Nowak, Quasney, Beckman, and Lindsell’s institutions received grant support from the National Institutes of Health. Dr. Meyer’s institution received grant support from NIH (specimen collection). Drs. Thomas, Abulebda, Bigham, Checchia, Shanley, Chopra, and Banschbach received grant support from NIH. Dr. Quasney received grant support from NIH (does not overlap with this project). Dr. Thomas received grant support from the FDA (R01 grant).
Drs. Wong, Cvijanovich, Allen, Anas, Sen, Shanley, Nowak, Quasney, Chopra, Beckman, and Lindsell received support for article research from NIH.
Drs. Wong, Thomas, Abulebda, Allen, Bigham, Hall, Freishtat, Sen, Meyer, Checchia, Shanley, Quasney, Beckman, and Lindsell disclosed a patent (CCHMCdescribed in manuscript).
Dr. Anas provided expert testimony and received royalties (past). Dr. Shanley received royalties from Springer. Dr. Cvijanovich is employed by CCCMG, Inc.
Dr. Thomas has Scientific Advisory Board membership with Discovery Labs. Dr. Shanley had SPR board and external advisory board memberships.
Dr. Weiss has disclosed that he does not have any potential conflicts of interest.
REFERENCES
- 1.Wong HR, Nowak JE, Standage S, de Oliveira CF. In: Sepsis and septic shock. Pediatric critical care medicine. 4th Fuhrman BP, Zimmerman JJ, editors. Mosby; St. Louis: 2011. pp. 1413–1429. [Google Scholar]
- 2.Carcillo JA, Davis AL, Zaritsky A. Role of early fluid resuscitation in pediatric septic shock. JAMA. 1991;266:1242–1245. [PubMed] [Google Scholar]
- 3.Brierley J, Carcillo JA, Choong K, et al. Clinical practice parameters for hemodynamic support of pediatric and neonatal septic shock: 2007 update from the american college of critical care medicine. Crit Care Med. 2009;37:666–688. doi: 10.1097/CCM.0b013e31819323c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dellinger RP, Levy MM, Rhodes A, et al. Surviving sepsis campaign: International guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41:580–637. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- 5.de Oliveira CF, de Oliveira DS, Gottschald AF, et al. ACCM/PALS haemodynamic support guidelines for paediatric septic shock: An outcomes comparison with and without monitoring central venous oxygen saturation. Intensive Care Med. 2008;34:1065–1075. doi: 10.1007/s00134-008-1085-9. [DOI] [PubMed] [Google Scholar]
- 6.Han YY, Carcillo JA, Dragotta MA, et al. Early reversal of pediatric-neonatal septic shock by community physicians is associated with improved outcome. Pediatrics. 2003;112:793–799. doi: 10.1542/peds.112.4.793. [DOI] [PubMed] [Google Scholar]
- 7.Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 8.Smith SH, Perner A. Higher vs. Lower fluid volume for septic shock: Clinical characteristics and outcome in unselected patients in a prospective, multicenter cohort. Crit Care. 2012;16:R76. doi: 10.1186/cc11333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hilton AK, Bellomo R. A critique of fluid bolus resuscitation in severe sepsis. Crit Care. 2012;16:302. doi: 10.1186/cc11154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boyd JH, Forbes J, Nakada TA, Walley KR, Russell JA. Fluid resuscitation in septic shock: A positive fluid balance and elevated central venous pressure are associated with increased mortality. Crit Care Med. 2011;39:259–265. doi: 10.1097/CCM.0b013e3181feeb15. [DOI] [PubMed] [Google Scholar]
- 11.Goldstein SL, Somers MJ, Baum MA, et al. Pediatric patients with multi-organ dysfunction syndrome receiving continuous renal replacement therapy. Kidney Int. 2005;67:653–658. doi: 10.1111/j.1523-1755.2005.67121.x. [DOI] [PubMed] [Google Scholar]
- 12.Murphy CV, Schramm GE, Doherty JA, et al. The importance of fluid management in acute lung injury secondary to septic shock. Chest. 2009;136:102–109. doi: 10.1378/chest.08-2706. [DOI] [PubMed] [Google Scholar]
- 13.Vincent JL, Sakr Y, Sprung CL, et al. Sepsis in european intensive care units: Results of the soap study. Crit Care Med. 2006;34:344–353. doi: 10.1097/01.ccm.0000194725.48928.3a. [DOI] [PubMed] [Google Scholar]
- 14.Sutherland SM, Zappitelli M, Alexander SR, et al. Fluid overload and mortality in children receiving continuous renal replacement therapy: The prospective pediatric continuous renal replacement therapy registry. Am J Kidney Dis. 2010;55:316–325. doi: 10.1053/j.ajkd.2009.10.048. [DOI] [PubMed] [Google Scholar]
- 15.Selewski DT, Cornell TT, Blatt NB, et al. Fluid overload and fluid removal in pediatric patients on extracorporeal membrane oxygenation requiring continuous renal replacement therapy. Crit Care Med. 2012;40:2694–2699. doi: 10.1097/CCM.0b013e318258ff01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hayes LW, Oster RA, Tofil NM, Tolwani AJ. Outcomes of critically ill children requiring continuous renal replacement therapy. J Crit Care. 2009;24:394–400. doi: 10.1016/j.jcrc.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 17.Arikan AA, Zappitelli M, Goldstein SL, Naipaul A, Jefferson LS, Loftis LL. Fluid overload is associated with impaired oxygenation and morbidity in critically ill children. Pediatr Crit Care Med. 2012;13:253–258. doi: 10.1097/PCC.0b013e31822882a3. [DOI] [PubMed] [Google Scholar]
- 18.Askenazi DJ, Goldstein SL, Koralkar R, et al. Continuous renal replacement therapy for children </=10 kg: A report from the prospective pediatric continuous renal replacement therapy registry. J Pediatr. 2012;162:587–592. doi: 10.1016/j.jpeds.2012.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Willson DF, Thomas NJ, Tamburro R, et al. The relationship of fluid administration to outcome in the pediatric calfactant in acute respiratory distress syndrom (cards) trial. Pediatr Crit Care Med. 2013 doi: 10.1097/PCC.0b013e3182917cb5. in press. [DOI] [PubMed] [Google Scholar]
- 20.Maitland K, Kiguli S, Opoka RO, et al. Mortality after fluid bolus in african children with severe infection. N Engl J Med. 2011;364:2483–2495. doi: 10.1056/NEJMoa1101549. [DOI] [PubMed] [Google Scholar]
- 21.Russell JA. How much fluid resuscitation is optimal in septic shock? Crit Care. 2012;16:146. doi: 10.1186/cc11393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wong HR, Salisbury S, Xiao Q, et al. The pediatric sepsis biomarker risk model. Crit Care. 2012;16:R174. doi: 10.1186/cc11652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Basu RK, Standage SW, Cvijanovich NZ, et al. Identification of candidate serum biomarkers for severe septic shock-associated kidney injury via microarray. Crit Care. 2011;15:R273. doi: 10.1186/cc10554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cvijanovich N, Shanley TP, Lin R, et al. Validating the genomic signature of pediatric septic shock. Physiol Genomics. 2008;34:127–134. doi: 10.1152/physiolgenomics.00025.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shanley TP, Cvijanovich N, Lin R, et al. Genome-level longitudinal expression of signaling pathways and gene networks in pediatric septic shock. Mol Med. 2007;13:495–508. doi: 10.2119/2007-00065.Shanley. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wong HR, Cvijanovich N, Allen GL, et al. Genomic expression profiling across the pediatric systemic inflammatory response syndrome, sepsis, and septic shock spectrum. Crit Care Med. 2009;37:1558–1566. doi: 10.1097/CCM.0b013e31819fcc08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wong HR, Cvijanovich N, Lin R, et al. Identification of pediatric septic shock subclasses based on genome-wide expression profiling. BMC Med. 2009;7:34. doi: 10.1186/1741-7015-7-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wong HR, Cvijanovich N, Wheeler DS, et al. Interleukin-8 as a stratification tool for interventional trials involving pediatric septic shock. Am J Resp Crit Care Med. 2008;178:276–282. doi: 10.1164/rccm.200801-131OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wong HR, Cvijanovich NZ, Allen GL, et al. Validation of a gene expression-based subclassification strategy for pediatric septic shock. Crit Care Med. 2011;39:2511–2517. doi: 10.1097/CCM.0b013e3182257675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wong HR, Cvijanovich NZ, Hall M, et al. Interleukin-27 is a novel candidate diagnostic biomarker for bacterial infection in critically ill children. Crit Care. 2012;16:R213. doi: 10.1186/cc11847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wong HR, Freishtat RJ, Monaco M, Odoms K, Shanley TP. Leukocyte subset-derived genomewide expression profiles in pediatric septic shock. Pediatr Crit Care Med. 2010;11:349–355. doi: 10.1097/PCC.0b013e3181c519b4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wong HR, Shanley TP, Sakthivel B, et al. Genome-level expression profiles in pediatric septic shock indicate a role for altered zinc homeostasis in poor outcome. Physiol Genomics. 2007;30:146–155. doi: 10.1152/physiolgenomics.00024.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wong HR, Wheeler DS, Tegtmeyer K, et al. Toward a clinically feasible gene expression-based subclassification strategy for septic shock: Proof of concept. Crit Care Med. 2010;38:1955–1961. doi: 10.1097/CCM.0b013e3181eb924f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wynn JL, Cvijanovich NZ, Allen GL, et al. The influence of developmental age on the early transcriptomic response of children with septic shock. Mol Med. 2011;17:1146–1156. doi: 10.2119/molmed.2011.00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goldstein B, Giroir B, Randolph A. International pediatric sepsis consensus conference: Definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med. 2005;6:2–8. doi: 10.1097/01.PCC.0000149131.72248.E6. [DOI] [PubMed] [Google Scholar]
- 36.Mickiewicz B, Vogel HJ, Wong HR, Winston BW. Metabolomics as a novel approach for early diagnosis of pediatric septic shock and mortality. Am J Resp Crit Care Med. 2013;187:967–976. doi: 10.1164/rccm.201209-1726OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xiao W, Mindrinos MN, Seok J, et al. A genomic storm in critically injured humans. J Exp Med. 2011;208:2581–2590. doi: 10.1084/jem.20111354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vincent JL, Opal SM, Marshall JC. Ten reasons why we should not use severity scores as entry criteria for clinical trials or in our treatment decisions. Crit Care Med. 2010;38:283–287. doi: 10.1097/CCM.0b013e3181b785a2. [DOI] [PubMed] [Google Scholar]