Abstract
Purpose
To compare qualitative and quantitative ovarian response in idiopathic infertile women treated with low-dose-aspirin (LDA) during in-vitro-fertilization (IVF) cycles (pl) versus untreated ones.
Methods
We conducted an observational-cohort-study on normo-responders patients aged between 25 and 45 years referred to Assisted-Reproductive Unit - University of Padua – in order to evaluate the ovarian response effects (both qualitative and quantitative) after LDA administration. In detail we aim to assess if LDA administration could improve ovarian response, reducing the gonadotropin administration, and if its administration could increase the amount of follicles greater than 16 mm at pick-up, the amount and quality of oocytes retrieved, the amount and quality of embryos, the chance to achieve a pregnancy and to carry it on.
Results
One hundred six LDA-treated patients (Group-A) and 100 not-treated ones (Group-B) were homogeneous for age and BMI. The Group-A, compared to Group-B, showed higher gonadotropin request, higher number of ovarian follicles at pick-up, more follicles bigger than 16 mm in diameter and more retrieved oocytes (despite higher number of immature and at germinal vesicle stage oocytes) but lower quality of obtained embryos. The comparison between two Groups in term of retrieved oocytes /number of follicles, mature oocytes/retrieved oocytes, fertilized oocytes/mature oocytes and good embryos quality/mature oocytes showed a strongly advantageous ratio for Group-B. For each considered outcome, we found a dose-related effect.
Conclusions
It is mandatory to define which patients could benefit from LDA administration and the adequate timing to administer it since the empirical administration could negatively affect both oocyte and embryo quality during IVF cycles.
Keywords: IVF outcome, Low dose aspirin administration, Oocyte quality, Embryo quality, Ovarian response
Introduction
Infertile couples undergoing in-vitro fertilization techniques (IVF) are often extremely receptive to any intervention aimed to boost their fertility, even without any scientific bases [1].
Testing new pharmacological strategies thought to be useful, is mandatory to assess their potential and risks. Therapeutic protocols and clinical decisions should be based on the best available evidences, often investigated by meta-analyses and systematic reviews [2].
Unfortunately, the results of such works are not always clinically acceptable, as for the actual debate on the use of low-dose aspirin (LDA) during IVF cycle.
Aspirin (acetylsalicylic acid) is well known for its analgesic, anti-inflammatory and antipyretic properties. It acts as irreversible inhibitor of the cyclo-oxygenase enzyme (COX), resulting in the direct inhibition of the biosynthesis of prostaglandins (PG) and thromboxanes from arachidonic acid [3, 4].
Despite the amount of studies performed on this topic, the role of aspirin in infertile women is still controversial and the evidences reported are inconsistent [5].
Traditionally, LDA is thought to increase uterine blood flow thus improving implantation in early pregnancy. Haapsamo et al. demonstrated that LDA reduces utero-placental vascular impedance in early and mid-pregnancy in unselected subjects who carried out assisted reproductive techniques (ARTs) when therapy is started along with controlled ovarian hyperstimulation. They postulated that it may be due to previous improved trophoblastic invasion and spiral arteries remodeling [6].
This model comes from the hypothesis that an adequate uterine blood supply (thanks to LDA anti-inflammatory, vasodilatory and platelet aggregation inhibition properties) appears to be an important determinant of endometrial receptivity during both spontaneous conception and ARTs [7].
From 2007 many reviews and meta-analysis were performed to assess the LDA effect on the likelihood of a pregnancy in women undergoing ARTs. The Authors concluded that not significant differences were found between patients who received LDA and those who received placebo or no treatment [4, 8–11].
Unfortunately all analyzed studies lack of informations about when to start and to stop aspirin therapy, and how long aspirin should be administered during IVF cycle.
Given that LDA could have a positive effects on endometrial receptivity and implantation, as recently demonstrated also in mice [12], the absence of clear advantages in aspirin users during IVF should lead to speculate if aspirin intake during controlled ovarian hyperstimulation could worsen the overall ovarian responsiveness, both quantitatively and qualitatively.
The aim of this study is to evaluate whether LDA administration in idiopathic infertile women undergone to IVF cycles affects the success of the treatments. We compared two cohorts of patients, treated and not treated with LDA, evaluating the impact of treatment in term of amount of retrieved oocytes and their maturation degree, oocytes fertilization rate, embryos quality and subsequent pregnancy rate.
Patients and methods
We performed an observational study on 206 women undergoing their second or third fresh non-donor IVF cycle for idiopathic primary infertility. The work was conducted in the Assisted Reproductive Unit of Gynecologic and Obstetrics Clinic - Department of Woman and Child Health of Padua University from January 2010 to December 2012.
All enrolled patients were properly informed about the aim of the study and consented to the use of their data according to Italian Law for Privacy 675/96.
Our Study was defined exempt from IRB after consultation of the local ethical committee. Approval from the local institutional review board for health sciences is not required for observational studies in which clinical and surgical management is not modified. By the way, written informed consent was obtained from all patients at the enrollement.
Our attention was focused on a cohort of normo-responders patients aged between 25 and 45 years with idiopathic primary infertility and referred to second or third IVF treatment (Intracytoplasmic sperm injection - ICSI) after a previous cycle failure.
All considered patients were classified as normo-responders on the base of both ovarian reserve test before stimulation and ovarian response during previous IVF cycle.
All patients were accurately informed about the lack of secure evidences on the efficacy of aspirin administration in improving ART success rate and was offered them the chance whether to choose or not this option.
All eligible patients were admitted in Group-A when they opted for 100 mg/daily aspirin take, starting from the first gonadotropin stimulation day until hCG administration, and in Group-B when they did not go for LDA.
The Group-A was further divided into Sub-Group-A1 and Sub-Group-A2 according to total dose of aspirin taken in the interval time between stimulation and induction of ovulation: Sub-Group-A1 included patients treated with a total of ≤700 mg aspirin and Sub-Group-A2 patients treated with a total dose >700 mg.
As exclusion criteria we considered history of smoking (for both the partners) in the previous six months, acquired or inherited thrombophilia, history of platelet dysfunction, thrombocytopenia, gastrointestinal ulcers, recurrent gastritis, aspirin hypersensitivity, previous chemo and/or radio treatment for neoplasia, untreated uterine diseases (such as endometrial polyps, sub mucous myomas, intrauterine synechiae and/or uterine septus), severe qualitative and quantitative alteration in semen (according to World Health Organization guidelines) [13].
We excluded all cases of ovarian hyperstimulation syndrome and/or spontaneous ovulation that required the interruption of the cycle. Finally, according to our local Protocol, we excluded all patients with abnormal karyotype and/or mutations of the cystic fibrosis gene.
All eligible patients underwent long-protocol stimulation according to our Units Protocol. All stimulation cycles were performed with the gonadotropin-releasing hormone agonist (Decapeptyl, Ipsen, Paris, France), 0.1 mg daily started in the mild-luteal phase of previous cycle for hypothalamic inhibition, human menopausal gonadotrophin (hMG) (Menopur, Ferring, Saint-Prex, Switzerland) or recombinant follicle-stimulating hormone (rFSH) (Puregon, Organon, Oss, The Netherlands or Gonal-F, Merck-Serono, Geneva, Switzerland) 150–300 IU daily started after the check of correct hypothalamic inhibition.
The choice of gonadotropin was made by the clinician, although the majority of patients were treated with rFSH. The starting dose and subsequent adjustments were decided by the clinician too according to the biochemical and ultrasound features of ovarian response.
When an adequate number of follicles (at least three follicles bigger than 18 mm in diameter) was found at transvaginal sonography (TVS), we administrated rhCG 250 ugr (Ovitrelle -Merck-Serono, Geneva, Switzerland) for ovulation’s induction.
Oocyte retrieval took place 35 h after hCG administration. The oocytes were fertilized by ICSI technique.
One to three embryos were transferred on days 2–3 following oocyte retrieval. The number of embryos transferred was based on the age of the patient and on embryo quality.
As luteal support, we administered vaginal progesterone 400 mg daily (Progeffik – Effik Italia - Milano - Italy) until day 14 after pick-up, stopping the treatment in case of negative βhCG serum test.
Pregnancy was confirmed by the increasing β-hCG concentrations 2 weeks after embryo transfer (ET) and with the sonographic evidence of an intrauterine gestational sac 3–4 weeks after ET. On-going pregnancy was defined as embryo heart beat detected with TVS.
For all patients we collected data about: age, BMI, total dose of aspirin administration, total dose of gonadotropin administration, number of total follicles at pick up, number of follicles with diameter greater than 16 mm, total number of retrieved oocytes, even according to maturation stage (mature (metaphase II - MII), immature (metaphase I - MI) or at germinal vesicle stage), number of fertilized oocytes, number of obtained embryos, quality of obtained embryos and number of transferred embryos.
We also collected data about pregnancy rate (βhCG positivity) reporting the on-going pregnancy rate (single or multiple) and all the cases of pregnancy loss.
According to Son et al. and Khoudja at al., we defined embryos of good, intermediate or poor quality on the base of the blastomeres number, their difference in size and degree of fragmentation [14, 15].
In details, the embryos quality was defined good when showed 4–8 cells - I degree; intermediate when showed 4–8 cells - II degree; poor when showed 2 cells - all degree, 4–8 cells - III and IV degree and odd number of cells at any degree. We reported separately the cases of embryo aneuploidy.
Statistical analysis was performed by SPSS (Chicago, IL) software for Windows version 19, using parametric and nonparametric tests, where appropriate. We performed the Kolmogorov–Smirnov to test normality of distribution. Continuous data have been tested with the t test, and categorical variables have been tested with the χ2 test or Fisher’s exact test, where appropriate. The results obtained from the data collection were expressed in absolute numbers, percentages for discrete variables and in means ± standard deviations for continuous variables.
In order to demonstrate the effect and the dose-dependent effect of aspirin intake on number of ovarian follicles, number of retrieved oocytes and their quality, number of embryos and their quality, we performed Kaplan-Meir curves. Statistical significance was defined as p < 0.05.
Results
Among all the infertile women referred to our Centre during the time of study, 206 patients resulted eligible. Of these, 106 patients were included in Group-A (78 patients Sub-Group-A1 and 28 patients Sub-Group-A2) and 100 patients in Group-B.
Data about age, BMI, total dose of aspirin administration, total dose of gonadotropin administration, number of total follicles at pick up, number of follicles with diameter greater than 16 mm, total number of oocytes retrieved, number of mature oocytes (metaphase II), immature (metaphase I) or at germinal vesicle stage, number of fertilized oocytes, number of obtained embryos, quality of obtained embryos and number of transferred embryos were reported in Table 1.
Table 1.
Comparison between Group-A versus Group-B in term of general features and ART cycle outcomes
| Item | GROUP-A (Mean ± Standard Deviation) | GROUP-B (Mean ± Standard Deviation) | P value |
|---|---|---|---|
| BMI | 22.08 ± 1.83 | 21.91 ± 1 .89 | n.s. |
| Age | 37.35 ± 3.92 | 37.04 ± 4.52 | n.s. |
| Total dose of gonadotropins used (IU) | 3027.24 ± 1256.12 | 2411.13 ± 1126.82 | <0.001 |
| Total number of ovarian follicles | 12.67 ± 5.82 | 9.98 ± 4.96 | <0.001 |
| Numbers of follicles larger than 16 mm | 6.79 ± 3.17 | 5.17 ± 2.83 | <0.001 |
| Numbers of follicles smaller than 16 mm | 5.86 ± 4.43 | 4.81 ± 3.21 | 0.05 |
| Total number of retrieved oocytes | 8.01 ± 5.08 | 5.47 ± 3.77 | <0.001 |
| Number of mature oocytes (MII) | 5.07 ± 3.29 | 4.23 ± 2.69 | 0.05 |
| Number of non-mature oocytes (MI) | 2.04 ± 1.21 | 0.76 ± 0.94 | <0.001 |
| Number of oocytes at germinal stage | 0.91 ± 1.54 | 0.48 ± 1.02 | 0.02 |
| Number of fertilized oocytes | 3.47 ± 2.17 | 3.68 ± 2.30 | <0.05 |
| Good quality embryos | 1.67 ± 1.04 | 2.65 ± 1.49 | <0.001 |
| Intermediate quality embryos | 1.06 ± 0.72 | 0.72 ± 0.57 | <0.001 |
| Poor quality embryos | 0.75 ± 0.58 | 0.31 ± 0.46 | <0.001 |
| Number of tranferred embryos | 2.25 ± 0.76 | 2.24 ± 0.83 | n.s. |
From the comparison between the two Cohorts, Group-A and Group-B resulted homogeneous for age and BMI.
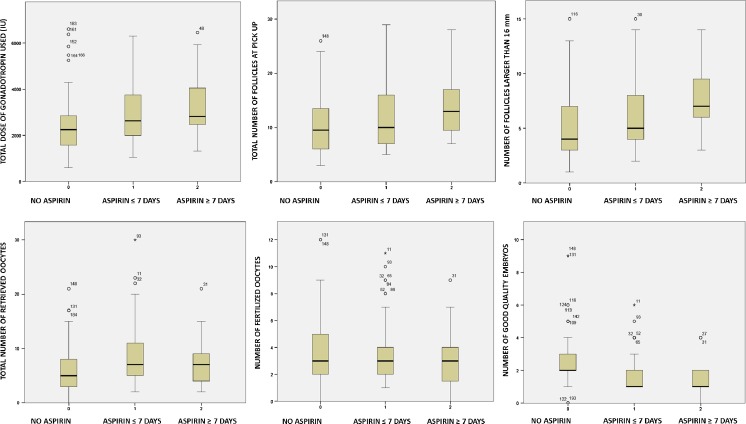
Compared to Group-B, Group-A showed higher dose of gonadotropin administration [p < 0.001], higher number of total ovarian follicles at pick-up [p < 0.001], higher number of follicles bigger than 16 mm of diameter [p < 0.001] higher number of total retrieved oocytes [p < 0.001] and higher number of retrieved mature oocytes [p < 0.05]. No difference was found between the Groups in term of number of follicles smaller than 16 mm of diameter at pick-up [p = 0.05].
Anyway, Group-A showed both a higher number of immature oocytes [p < 0.001] and a higher number of germinal vesicle stage oocytes [p = 0.02] compared to Group-B.
The ratio between mature oocytes and total number of retrieved oocytes showed statistical significant differences between the Groups.
Despite the high number of MII oocytes, Group-A was characterized by a significant low fertilization rate [p < 0.05]. Similarly, this group presented a significant low number of good quality embryos and a significant high number of intermediate and poor quality embryos [p < 0.001]. The detailed data are reported in Table 1 and Fig. 1.
Fig. 1.
Comparison among SubGroup-A1, SubGroup-A2 and Group B in term of ART cycles outcomes
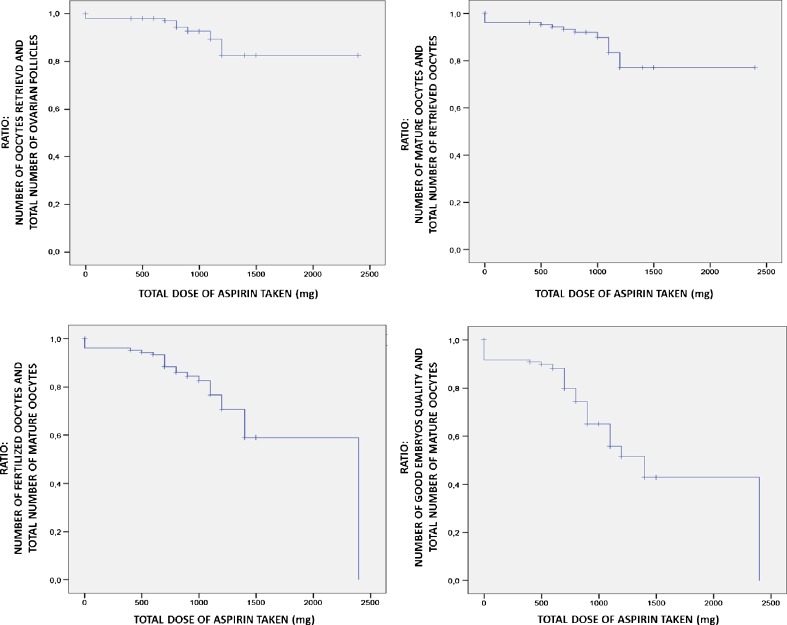
Between the two groups differences emerged in term of number of oocytes retrieved/total number of follicles (0.64 ± 0.21 versus 0.55 ± 0.25; p < 0.05), number of mature oocytes/total number of retrieved oocytes (0.63 ± 0.15 versus 0.78 ± 0.23; p < 0.001), fertilized oocytes/mature oocytes (0.44 ± 0.11 versus 0.70 ± 0.23; p < 0.001), good embryos quality/mature oocytes (0.39 ± 0.23 versus 0.66 ± 0.22; p < 0.001) ratios. No difference was detected in term of number of mature oocytes/total number of follicles ratio (0.40 ± 0.17 versus 0.43 ± 0.21; p = n.s.). All detailed data are reported in Fig. 2.
Fig. 2.
Kaplan-Meir curves on cumulative LDA dose-effect impact in ART outcomes: data obtained using effects ratio
A strongly dose-related LDA effect was assessed by the post-hoc analysis (Bonferroni test).
From the comparison between the Sub-Group-A1 and Sub-Group-A2, better outcomes in term of number of oocytes retrieved/total number of follicles (0.66 ± 0.20 versus 0.51 ± 0.19), mature oocytes/total number of follicles (0.43 ± 0.17 versus 0.31 ± 0.15), number of mature oocytes/total number of oocytes retrieved (0.65 ± 0.15 versus 0.60 ± 0.16), fertilized oocytes/mature oocytes (0.46 ± 0.10 versus 0.40 ± 0.12) and good embryos quality/mature oocytes (0.39 ± 0.23 versus 0.30 ± 0.24) ratios [p < 0.01] were registered (Fig. 2).
All embryo-transfers were performed in the third day after pick-up. No differences were found between the two groups in term of number of intra-uterine transferred embryos. (2.25 ± 0.76 versus 2.24 ± 0.83, respectively).
We collected an overall pregnancy rate of 26.2 % (54 patients). An on-going pregnancy developed in 38 patients. In the remaining 16 cases, biochemical pregnancies (13/16), miscarriages (2/16) and tubal pregnancies (1/16) were reported.
The comparison between Group-A and Group-B showed no differences in term of pregnancies (26.4 % versus 26 %), on-going pregnancies (17 % versus 20 %) and pregnancy failure rates (9.4 % versus 6.0 %).
No case of surgical complications after oocytes pick-up nor cases of oocyte aneuploidia were reported.
Discussion
Despite every year increases the number of couples which refers to the ARTs, pregnancy and delivery rates after IVF remains relatively stable if compared to the previous years [16].
Beside the beneficial effects on cardiovascular system, LDA appears to improve the outcome of pregnancy in women with various pathological conditions such as antiphospholipid antibodies, idiopathic recurrent miscarriages, history of pre-eclampsia, preterm birth, fetal or neonatal death and small-for-gestational age babies [3].
This led to the hypothesis that LDA may improve uterine and ovarian perfusion and that aspirin might enhance endometrial receptivity and ovarian responsiveness as well, which could result in better implantation and pregnancy rates in patients undergoing ARTs [17]. This hypothesis, along with the lower cost, the high availability and the minimal side effects of LDA, led the clinicians to perform several studies on women undergoing IVF/ICSI cycle but, unfortunately, all failed to demonstrate any advantage in its use, that is currently not recommended.
Haapsamo et al. demonstrated that LDA therapy in unselected IVF/ICSI treatment (when started along with controlled ovarian stimulation) could reduce both the incidence of non-optimal uterine haemodynamics at embryo-transfer and vascular impedance in early and mid-pregnancy [6, 18]. Khairy et al. reported that LDA treatment in unselected IVF/ICSI women improve the uterine artery pulsatility index without increasing the overall fertility rate. This unexpected and disappointing result seems to be related to the fact that LDA, inhibiting COX (both one and two isoforms), reduces vasoconstriction via COX1 inhibition and could negatively interfere with decidualization via COX2 inhibition [9].
The first critical step in human reproduction, as well as in all mammalians, is the ovarian follicular development and subsequent ovulation that depends largely upon the harmonic effects of pituitary gonadotropins, FSH and LH [18]. Nevertheless, how gonadotropins regulate this complex reproductive process is not completely clear, although some effects are mediated by known mediators and, among these, PGs play a crucial role.
Our clinical results permit us to hypothesize that LDA administration could negatively interfere with physiological ovarian follicular growth, oocyte maturation, oocyte fertilization and subsequent embryo quality via inhibition of COX activity, particularly the COX-2.
Tokuyama et al. first demonstrated that, in humans, COX-2 protein begins to be expressed at the secondary follicle stage and its expression is withdrawn just before exposure to LH raise [19]. This findings suggest that COX-2 and its metabolites might be involved in follicular development, including oocyte cumulus complex maturation.
On this basis, LDA administration via COX inhibition could negatively interfere with follicle response to gonadotropins stimulation process. Our data showed how the treated group required an higher total dose of exogenous FSH administration to reach the oocytes pick-up if compared to the untreated one.
In 2005 Duffy et al. first demonstrated that, in primate, during peri-ovulatory period follicular PGs levels increase up to 100-fold in response to ovarian gonadotropin stimulation, thanks to arachidonic acid mobilization in granulosa cells [20].
The importance of PGs in induction follicular growth in response to FSH stimulation was recently demonstrated in humans by Velthut et al. Authors proved how a high follicular oxidative stress PG-mediated via COX2 determined a raise of follicle stimulation sensitivity and, as a consequence, the necessity of a lower dose of FSH per retrieved oocyte [21].
Tokuyama et al. demonstrated the linear correlation between COX-2 intrafollicular concentrations and serum E2 level, suggesting COX-2 concentration in follicular fluid as a novel biomarker of granulosa cells function [19].
However, the PGs cascade is probably one of the most complex process in the follicle growth, oocyte maturation and subsequent fertilization process. Interestingly, Hizaki et al. underlined how in rats PGE2 elicits expansion by itself and also enhances the FSH-induced one. It still remains unclear whether endogenous PGE2 truly contributes to these steps. The authors concluded that PGE2 cooperates (but it is not the only one involved) with gonadotropins to complete cumulus expansion for successful fertilization, due the fact that Indomethacin indeed seems to fail to inhibit FSH-induced expansion [22].
Our data showed that the LDA treated patients, although requiring a higher dose of exogenous FSH administration, achieve a significant higher number of ovarian follicles, follicles larger than 16 mm and retrieved oocytes, leading to conclude that COX2 inhibition partially interfere with FSH mediated follicles growth.
Both the bigger number of antral follicles recruited and of follicles larger than 16 mm obtained in treated patients led to hypothesize that low-dose aspirin increases ovarian blood flow by changing the balance between the thromboxane-mediated vasoconstriction and prostacyclin-mediated vasodilation. Ovarian blood flow has been reported related with ovarian response despite it is not clear how long it must be increased to influence ovarian response [23].
Shimada et al. demonstrated that LH peak, inducing PGE2 and progesterone dependent pathways in granulosa cells, could mediate critical events during the ovulation process, as the reprogramming of granulosa and cumulus cell gene expression during the ovulatory cascade that influence cumulus expansion and oocyte maturation [24].
The fact that, in our study, the treated group showed an higher number of non-mature oocytes without significant differences in total number of mature oocytes lead to hypothesize that LDA could negatively interfere with hCG induced LH-dependent cascade of oocytes maturation process (that mimics the LH peak) by previous cumulus COX activity inhibition (particularly COX2 activity). The LDA negative impact on oocyte maturation could be perpetuated subsequently during fertilization steps until embryo development. In fact, our data suggested that the ratio of good quality embryo per retrieved oocytes was strongly unbalanced between the two groups with a clear advantage for the untreated group and, for the treated one, at lower dose of LDA.
Early experiments on rodents, showed that oocytes obtained from COX-2 deficient mice presented a severely compromised fertilization rate as EP2 (a PGE2 receptor subtype) deficient ones [19].
Another hypothesis regards the effects of aspirin on the luteal phase. Currently there are clear and largely accepted evidences about intra-luteal production of PGs in several species and under a variety of experimental conditions [25].
In general, secretion of PGs appears to be elevated in the early corpus luteum and during the period of luteolysis. Regulation of intra-luteal PGs production is due to a variety of factors and is important to initiate and amplify physiological signals to the corpus luteum. It may be important in specific aspects of luteal physiology, particularly during luteal regression [25, 26].However, our evidences do not resolve the debate about theoretical LDA negative effect on luteal phase, since all patients received a pharmacological luteal function support (by progesterone administration) after pick-up.
Our work presents many limits. Patients were in exiguous number and not randomized into the distinct groups, data about both follicular PGs concentrations and COX1-2 activity [19, 27], as well as possible effects of smoking and diet habitus lack [28, 29]. An ultrasound investigation of ovarian blood flow has not been performed [17]. Another limitation of the study is a potential affection of the implantation rate due to the execution of embryo-transfer on the second rather than the third day after pick-up, despitethe percentage of embryo transfer on the second day resulted very low (8.4 % in Group-A and 6 % in Group-B).
Further perspective studies on this field are desirable to confirm the LDA ability to interfere with follicular COX-2 activity, to explain the dose-dependent effects, to clarify the possible differences linked to the timing of drug administration.
Finally, according to recent evidences, it is mandatory to define the endometrial effects of LDA. Despite it is generally accepted that an adequate endometrial blood supply is required to improve the implantation rate following embryo transfer, recent data demonstrated that a relative endometrial ischemia and hypoxia are associated with the periovulatory fall of estradiol and that this process is one of the most important determinant for the endometrial receptivity [30].
A multicenter registry about effect of LDA administration in IVF cycle should be mandatory to facilitate the clarification of its exact mechanism of action and its influence on ovarian responsiveness, oocyte maturation, fertilization rate, embryo quality and endometrial receptivity. In absence of clear evidences, it is not possible to remove the aspirin from the register of “drugs with orphan indications in IVF” [2, 5, 23]. Performing all the necessary tests would represent a cost, but ignorance would be more expensive and arguably immoral [31].
It is therefore urgent to identify which patients could benefit from such treatment, when to start it and how long the treatment should be continued, in order to prevent the clinicians from unconsciously worsening IVF outcomes of their patients.
Acknowledgments
Conflict of interests
All Authors declare no conflicts of interest.
Funding
All Authors declare they have no funding.
Footnotes
Capsule Empirical LDA administration during IVF cycles could potentially affect both qualitative and quantitative ovarian response via COX-2 activity inhibition.
References
- 1.Akhtar MA, Eljabu H, Hopkisson J, et al. Aspirin and heparin as adjuvants during IVF do not improve live birth rates in unexplained implantation failure. Reprod Biomed Online. 2013;26(6):586–594. doi: 10.1016/j.rbmo.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 2.Lambers MJ, Mijatovic V, Hompes PG. Low dose aspirin and IVF: ‘Is it time for a meta-analysis’? Continued: the consequences of the choices made. Hum Reprod Update. 2009;15(2):262–263. doi: 10.1093/humupd/dmn051. [DOI] [PubMed] [Google Scholar]
- 3.Dirckx K, Cabri P, Merien A, et al. Does low-dose aspirin improve pregnancy rate in IVF/ICSI? A randomized double-blind placebo controlled trial. Hum Reprod. 2009;24(4):856–860. doi: 10.1093/humrep/den476. [DOI] [PubMed] [Google Scholar]
- 4.Dentali F, Ageno W, Rezoagli E, et al. Low-dose aspirin for in vitro fertilization or intracytoplasmic sperm injection: a systematic review and a meta-analysis of the literature. J Thromb Haemost. 2012;10(10):2075–2085. doi: 10.1111/j.1538-7836.2012.04886.x. [DOI] [PubMed] [Google Scholar]
- 5.Siristatidis CS, Dodd SR, Drakeley AJ. Aspirin is not recommended for women undergoing IVF. Hum Reprod Update. 2012;18(3):233. doi: 10.1093/humupd/dmr049. [DOI] [PubMed] [Google Scholar]
- 6.Haapsamo M, Martikainen H, Räsänen J. Low-dose aspirin reduces uteroplacental vascular impedance in early and mid gestation in IVF and ICSI patients: a randomized, placebo-controlled double-blind study. Ultrasound Obstet Gynecol. 2008;32(5):687–693. doi: 10.1002/uog.6215. [DOI] [PubMed] [Google Scholar]
- 7.Schisterman EF, Gaskins AJ, Whitcomb BW. Effects of low-dose aspirin in in-vitro fertilization. Curr Opin Obstet Gynecol. 2009;21(3):275–278. doi: 10.1097/GCO.0b013e32832a0673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gelbaya TA, Kyrgiou M, Li TC, et al. Low-dose aspirin for in vitro fertilization: a systematic review and meta-analysis. Hum Reprod Update. 2007;13(4):357–364. doi: 10.1093/humupd/dmm005. [DOI] [PubMed] [Google Scholar]
- 9.Khairy M, Banerjee K, El-Toukhy T, et al. Aspirin in women undergoing in vitro fertilization treatment: a systematic review and meta-analysis. Fertil Steril. 2007;88(4):822–831. doi: 10.1016/j.fertnstert.2006.12.080. [DOI] [PubMed] [Google Scholar]
- 10.Poustie VJ, Dodd S, Drakeley AJ. Low-dose aspirin for in vitro fertilisation. Cochrane Database Syst Rev. 2007;4 doi: 10.1002/14651858.CD004832.pub2. [DOI] [PubMed] [Google Scholar]
- 11.Ruopp MD, Collins TC, Whitcomb BW, et al. Evidence of absence or absence of evidence? A reanalysis of the effects of low-dose aspirin in in vitro fertilization. Fertil Steril. 2008;90:71–76. doi: 10.1016/j.fertnstert.2007.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao M, Chang C, Liu Z, et al. Treatment with low-dose aspirin increased the level LIF and integrin β3 expression in mice during the implantation window. Placenta. 2010;31(12):1101–1105. doi: 10.1016/j.placenta.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 13.WHO laboratory manual for the examination of human semen and sperm-cervical mucus interaction. Cambridge: Cambridge University Press; 1999. [Google Scholar]
- 14.Son WY, Chung JT, Henderson S, et al. Fertilization and embryo development with spermatozoa obtained from testicular sperm extraction into oocytes generated from human chorionic gonadotropin-primed in vitro maturation cycles. Fertil Steril. 2013;100:989–993. doi: 10.1016/j.fertnstert.2013.05.037. [DOI] [PubMed] [Google Scholar]
- 15.Khoudja R, Li T, Ding C, et al. Effect of co-incubation of oocytes with a decreasing number of spermatozoa on embryo quality. Reprod Biomed Online. 2013;26(4):353–359. doi: 10.1016/j.rbmo.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 16.Ferraretti AP, Goossens V, Kupka M, The European IVF-monitoring (EIM); Consortium, for The European Society of Human Reproduction and Embryology (ESHRE) et al. Assisted reproductive technology in Europe, 2009: results generated from European registers by ESHRE. Hum Reprod. 2013;28:2318–2331. doi: 10.1093/humrep/det278. [DOI] [PubMed] [Google Scholar]
- 17.Rubinstein M, Marazzi A, Polak de Fried E. Low-dose aspirin treatment improves ovarian responsiveness, uterine and ovarian blood flow velocity, implantation, and pregnancy rates in patients undergoing in vitro fertilization: a prospective, randomized, double-blind placebo controlled assay. Fertil Steril. 1999;71:825–829. doi: 10.1016/S0015-0282(99)00088-6. [DOI] [PubMed] [Google Scholar]
- 18.Haapsamo M, Martikainen H, Räsänen J. Low-dose aspirin and uterine haemodynamics on the day of embryo transfer in women undergoing IVF/ICSI: a randomized, placebo-controlled, double-blind study. Hum Reprod. 2009;24(4):861–866. doi: 10.1093/humrep/den489. [DOI] [PubMed] [Google Scholar]
- 19.Tokuyama O, Nakamura Y, Musoh A, et al. Expression and distribution of cyclooxygenase-2 in human ovary during follicular development. Osaka City Med J. 2003;49(1):39–47. [PubMed] [Google Scholar]
- 20.Duffy DM, Seachord CL, Dozier BL. An ovulatory gonadotropin stimulus increases cytosolic phospholipase A2 expression and activity in granulosa cells of primate periovulatory follicles. J Clin Endocrinol Metab. 2005;90(10):5858–5865. doi: 10.1210/jc.2005-0980. [DOI] [PubMed] [Google Scholar]
- 21.Velthut A, Zilmer M, Zilmer K, et al. Elevated blood plasma antioxidant status is favourable for achieving IVF/ICSI pregnancy. Reprod Biomed Online. 2013;26(4):345–352. doi: 10.1016/j.rbmo.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 22.Hizaki H, Segi E, Sugimoto Y, et al. Abortive expansion of the cumulus and impaired fertility in mice lacking the prostaglandin E receptor subtype EP(2) Proc Natl Acad Sci U S A. 1999;96(18):10501–10506. doi: 10.1073/pnas.96.18.10501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meldrum DR, Chang RJ, de Ziegler D, et al. Adjuncts for ovarian stimulation: when do we adopt “orphan indications” for approved drugs? Fertil Steril. 2009;92(1):13–18. doi: 10.1016/j.fertnstert.2009.03.081. [DOI] [PubMed] [Google Scholar]
- 24.Shimada M, Hernandez-Gonzalez I, Gonzalez-Robayna I, et al. Paracrine and autocrine regulation of epidermal growth factor-like factors in cumulus oocyte complexes and granulosa cells: key roles for prostaglandin synthase 2 and progesterone receptor. Mol Endocrinol. 2006;20(6):1352–1365. doi: 10.1210/me.2005-0504. [DOI] [PubMed] [Google Scholar]
- 25.Kowalewski MP, Fox B, Gram A, et al. Prostaglandin E2 functions as a luteotrophic factor in the dog. Reproduction. 2013;145(3):213–226. doi: 10.1530/REP-12-0419. [DOI] [PubMed] [Google Scholar]
- 26.Wiltbank MC, Ottobre JS. Regulation of intraluteal production of prostaglandins. Reprod Biol Endocrinol. 2003;1:91. doi: 10.1186/1477-7827-1-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mendonça LL, Khamashta MA, Nelson-Piercy C, et al. Non-steroidal anti-inflammatory drugs as a possible cause for reversible infertility. Rheumatology (Oxford) 2000;39(8):880–882. doi: 10.1093/rheumatology/39.8.880. [DOI] [PubMed] [Google Scholar]
- 28.Fréour T, Dessolle L, Lammers J, et al. Comparison of embryo morphokinetics after in vitro fertilization-intracytoplasmic sperm injection in smoking and nonsmoking women. Fertil Steril. 2013;99(7):1944–1950. doi: 10.1016/j.fertnstert.2013.01.136. [DOI] [PubMed] [Google Scholar]
- 29.Twigt JM, Bolhuis ME, Steegers EA, et al. The preconception diet is associated with the chance of ongoing pregnancy in women undergoing IVF/ICSI treatment. Hum Reprod. 2012;27(8):2526–2531. doi: 10.1093/humrep/des157. [DOI] [PubMed] [Google Scholar]
- 30.Raine-Fenning N. The role of three-dimensional ultrasound in assisted reproduction treatment. Ultrasound Obstet Gynecol. 2004;23(4):317–322. doi: 10.1002/uog.1028. [DOI] [PubMed] [Google Scholar]
- 31.Clark DA. Aspirin and heparin to improve live birth rate in IVF for unexplained implantation failure? Reprod Biomed Online. 2013;26(6):538–541. doi: 10.1016/j.rbmo.2013.03.007. [DOI] [PubMed] [Google Scholar]