Abstract
Purpose
We hypothesised that varying native oocyte-secreted factor (OSF) exposure or using different recombinant OSF peptides would have differential effects on post-in vitro maturation (IVM) embryo and fetal development.
Methods
Mouse cumulus oocyte complexes (COCs) were treated with the purified mature domain of GDF9 and/or BMP15 or were co-cultured with denuded oocytes (DOs) from 0 h or 3 h of IVM. DOs were matured for 3 h as either intact COCs+/-FSH before denuding, or as DOs + FSH. COCs were fertilised and blastocyst development was assessed on days 5 and 6, and either differentially stained for ICM numbers or vitrified/warmed embryos were transferred to recipients to assess implantation and fetal rates.
Results
No improvement in embryo development was observed with the addition of GDF9 and/or BMP15 to IVM. In contrast, embryos derived from COCs co-cultured with DOs had significantly improved blastocyst rates and ICM numbers compared to controls (P < 0.05). The highest response was obtained when DOs were first added to COCs at 3 h of IVM, after being pre-treated (0–3 h) as COCs + FSH. Compared to control, co-culture with DOs from 3 h did not affect implantation rates but more than doubled fetal yield (21 % vs 48 %; P < 0.05). GDF9 Western blot analysis was unable to detect any differences in quantity or form of GDF9 (17 and 65 kDa) in extracts of DO at 0 h or 3 h.
Conclusions
This study provides new knowledge on means to improve oocyte quality in vitro which has the potential to significantly aid human infertility treatment and animal embryo production technologies.
Keywords: Oocyte in vitro maturation (IVM), GDF9, BMP15, Oocyte-secreted factor (OSFs)
Introduction
In vitro maturation (IVM) of oocytes is an alternative system used in assisted reproductive technology for generating embryos in vitro, which reduces or eliminates the need to administer gonadotrophins to patients. In addition, IVM is widely used in the domestic animal sector to further advanced-breeding technologies. However, there is a discrepancy in the success rate of conventional in vitro matured oocytes compared to in vivo matured oocytes. In women, it is reported that the pregnancy rate post-IVM is less than half of the pregnancy rate post-IVF (in vitro fertilisation) [1]. To overcome this challenge, it is important to understand factors that regulate the maturation and development of oocytes which can be implemented in clinical and veterinary scenarios to improve the success rate of IVM [2].
Oocytes acquire developmental competence in the ovarian follicle. There are numerous molecules, proteins and cellular processes involved in the bi-directional communication axis between the oocyte and follicular somatic cells [3]. The oocyte regulates this communication network to a significant degree. The oocyte secretes soluble factors (OSFs) which are transmitted to cumulus cells via paracrine signalling to regulate a multitude of important cumulus cell processes, including; proliferation [4, 5], apoptosis [6], differentiation [7], luteinisation [8], metabolism [9–12] and expansion [13, 14]. Another type of communication between the oocyte and cumulus cells is via gap junctional signalling, mediated by cumulus trans-zonal cytoplasmic projections that abut the oocyte membrane, enabling transport of cAMP, purines/pyrimidines, amino acids and other small regulatory molecules [15–18]. Cumulus cells provide signals and molecules to the oocyte to regulate meiosis and promote developmental competence [19, 20]. Removal of cumulus cells during maturation has a significant detrimental effect on nuclear maturation, normal fertilization and developmental competence of oocytes [21, 22]. Follicle-stimulating hormone (FSH) is typically added to IVM medium to stimulate cumulus expansion [17] and increase normal fertilization rates and fetal development of IVM oocytes [23, 24].
Growth differentiation factor-9 (GDF9) and bone morphogenetic protein-15 (BMP15) are two well-known soluble growth factors derived from oocytes that are important for normal cumulus cell function and hence normal oocyte development [19]. In mouse oocytes, the expression level of GDF9 mRNA is much higher compared to BMP15 [25], reflecting the relative importance of their roles in folliculogenesis. Homozygous GDF9 mutant mice are sterile due to a block in follicular development beyond the primary follicle stage [26], whereas BMP15 null mice demonstrate only a mild reduction in ovulation and fertilization rates [27]. Homozgous GDF9 or BMP15 mutant sheep derived either by natural mutation or active immunization are sterile, however, heterozygous carriers of mutations in either GDF9 or BMP15 have an increased ovulation rate and multiple pregnancies [28–30]. GDF9 and BMP15 also play an important role in human fertility, where several mutations of GDF9 may be potentially associated with polycystic ovary syndrome [31] and rare variants of GDF9 contribute to dizygotic twinning [32]. Moreover, rare mutations of GDF9 and BMP15 are associated with premature ovarian failure [33–35].
GDF9 and BMP15 in the proteolytically processed form consist of two structural parts: the pro-region and the mature region [36]. GDF9 and BMP15 are processed and secreted as non-covalent complexes of the pro- and mature regions, the mature region being the bioactive receptor binding region, whereby the pro-region plays a vital and species-specific role in regulating bioactivity [37–39]. Immunizing mice against the pro-regions of GDF9 or BMP15 caused ovary abnormalities and smaller litter sizes [40].
However, the exact forms of GDF9 and BMP15 secreted by oocytes in vivo and in vitro are unclear. Mouse in vitro matured oocytes secrete GDF9 as a mixture of the unprocessed pro-protein and mature domain [41]. Rat IVM oocytes secrete the mature domains of GDF9 and BMP15 [42]. Sheep follicular fluid contains GDF9 and BMP15 proteins in the unprocessed pro-protein form only [43], whereas sheep oocytes secrete the mature domains of GDF9 and BMP15 during IVM [42]. To our knowledge, the only commercially available forms of GDF9 and BMP15 produced in a recombinant mammalian cell expression system are from R&D Systems and these consist of purified mature domains only.
IVM COCs have abberant gene expression and altered matrix protein profiles in cumulus cells compared to in vivo matured oocytes [44], and in vitro matured oocytes also have notably decreased expression of BMP15 compared to in vivo matured oocytes (Gilchrist et al., unpublished). Currently, studies show that addition of exogenous native OSFs during IVM significantly improves subsequent goat [45], cow [20, 46, 47] and pig [48] embryo development, and recombinant GDF9 and BMP15 in their pro-mature form improve in vitro matured cattle [12, 46, 47] and mouse oocytes [49]. In addition, differing degrees of improved bovine oocyte competence were observed depending on the temporal pattern of addition of OSFs during IVM [46]. The current study explores in more detail the effects of different forms of OSFs added during IVM on oocyte developmental competence. We examine the effects of commercially available forms of recombinant GDF9 and BMP15 and of native OSFs. We also explore factors that affect the production of native OSFs by mouse oocytes, such as the timing of addition of denuded oocytes (DOs) and the presence of cumulus cells and FSH before denuding COCs and addition to IVM. Outcomes examined were embryo development, cryotolerance of blastocysts and fetal measures following embryo transfer.
Materials and methods
All chemicals and media were purchased from Sigma Chemical (St Louis, MO, USA) unless indicated otherwise.
Isolation and maturation of COCs
Mice used in this study were maintained in the Animal House, Medical School, University of Adelaide. This study was approved by the local animal ethics committee and conducted in accordance with the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes.
Twenty-one to twenty-eight day-old SV129 mice were injected intraperitoneally with 5 IU equine chorionic gonadotrophin (eCG; Folligon, Intervet, Castle Hill, Australia), and ovaries were collected 46–48 hours (h) later. Ovaries were cleaned of any connective tissues and placed in HEPES-buffered αMEM (handling medium, GIBCO, USA) supplemented with 3 mg/ml fatty-acid free bovine serum albumin (FAF BSA, MP Biomedicals, USA), 1 mg/ml fetuin and 50 μmol 3-isobutyl-1-methylxanthine (IBMX). All antral follicles were punctured with 27-gauge needles and immature COCs collected in handling medium, then washed twice with handling medium minus IBMX. Only COCs with compact cumulus cells (CCs) were taken. The rationale behind the use of IBMX was to maintain all COCs in the same nuclear stage before transfer into maturation medium or being denuded. For the control group, 20 COCs were cultured in 50 μl IVM drops [(αMEM supplemented with 3 mg/ml FAF BSA, 1 mg/ml fetuin and 50 mIU/ml FSH (Puregon, Organon, Oss, Netherlands)] overlaid with mineral oil in 60 mm Petri dishes (Falcon, Becton Dickinson, USA) for 17–18 h at 37 °C in a humidified atmosphere of 5 % CO2 in air. For treatment groups, 20 COCs were cultured with 50 DOs in 50 μl IVM drops (1 DO/μl). In addition, the effect of exogenous recombinant human BMP15 (Catalogue No: 739-G9, R&D Systems, Minnaepolis, MN, USA) and recombinant mouse GDF9 (Catalogue No: 5096-BM, R&D Systems) in mouse IVM was examined. Both of these growth factors are supplied as homodimers of the mature region. COCs were exposed to graded doses (50 ng/ml, 100 ng/ml, and 200 ng/ml) of exogenous BMP15 and GDF9, as well as a combination of both proteins.
Generation of denuded oocytes as a source of native OSFs
DOs were generated by vortexing COCs for ~2 min in αMEM handling medium in order to remove the CC.
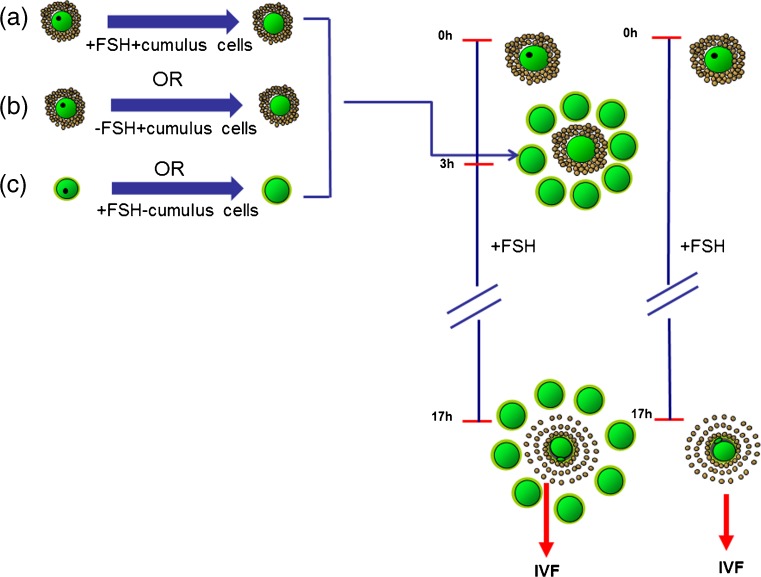
There were then a number of different processing methods used to produce native OSFs before co-culture with immature COCs (see Table 1 and Fig. 1):
COCs were denuded at 0 h before co-culture of 50 DOs with 20 COCs in IVM medium containing FSH. For COCs + DOs, IBMX was not present in the handling medium, and therefore >90 % of DOs were at the GVBD stage. This group was utilized to assess the benefits of DOs as a source of native OSFs and will be referred to as COCs + DOs. By contrast, for COCs + DOs (0 h [GV]), IBMX was present in the handling medium and therefore these DOs were at the GV stage.
COCs were matured first for 3 h in separate IVM medium containing FSH. After 3 h of IVM, COCs were denuded and 50 DOs were co-cultured with 20 COCs for another 14–15 h in IVM medium containing FSH (Fig. 1a). This group is based on the design by Hussein et al. (2011), and was utilized to examine temporal, cumulus cell and FSH effects on the production of native OSFs. This group is referred to as COCs + DOs at 3 h + FSH + CC.
Intact COCs were matured first for 3 h in separate IVM medium without FSH (Fig. 1b). After 3 h of IVM, COCs were denuded and 50 DOs were co-cultured with 20 COCs for another 14–15 h in IVM medium containing FSH. This group assessed the production of native OSFs in the absence of FSH during the first 3 h of maturation and is referred to as COCs + DOs at 3 h-FSH + CC.
COCs were denuded at 0 h and then matured for 3 h as DOs in separate IVM medium containing FSH, 50 of these DOs were co-cultured with 20 COCs for another 14–15 h in IVM medium containing FSH (Fig. 1c). This group is referred to as COCs + DOs at 3 h + FSH-CC and was designed to assess the effect of CCs on production of native OSFs.
Table 1.
Summary of methods used to generate differing native OSFs
| Treatments | Pre-treatment of OSFs | Explanation |
|---|---|---|
| Control | Not applicable | 20 COCs were cultured in 50 μl IVM drops |
| COC + DO | Not applicable | 20 COCs were cultured with 50 DOs in 50 μl IVM drops. DOs were added at 0 h at the GVBD stage. |
| COC + DO | 0h [GV] | 20 COCs were cultured with 50 DOs in 50 μl IVM drops. DOs were added at 0 h at the GV stage, as IBMX was present in the handling medium and during denuding. |
| COC + DO | 3h + FSH + CCs | COCs were pre-cultured for 3 h in separate IVM medium containing FSH. After 3 h, these COCs were denuded and 50 resultant DOs were then co-cultured with 20 COCs for another 14–15 h (Fig. 1a). |
| COC + DO | 3h - FSH + CCs | COCs were pre-cultured for 3 h in separate IVM medium without FSH, before denuding and co-culture with 20 COCs for another 14–15 h (Fig. 1b). |
| COC + DO | 3h + FSH - CC | COCs were denuded at 0 h and then cultured for 3 h as DOs in separate IVM medium containing FSH, then 50 resultant DOs were co-cultured with 20 COCs for another 14–15 h (Fig. 1c). |
Fig. 1.
a Schematic illustration of methods of producing native OSFs based on pretreatment of oocytes with and without cumulus cells and FSH. Intact COCs cultured for 3 h in separate IVM medium containing FSH. After 3 h, COCs were denuded of CCs and the resultant DOs were then co-cultured with a separate cohort of COCs + FSH for 14–15 h, before the COCs underwent standard IVF and embryo culture (3h + FSH + CC) b COCs pre-cultured for 3 h in separate IVM medium without FSH, before denuding and co-cultured with a separate cohort of COCs + FSH for 14–15 h (3 h-FSH + CC) c COCs were denuded at 0h and then pre-cultured for 3 h in separate IVM medium containing FSH, then these DOs were co-cultured with a separate cohort of COCs + FSH for 14–15 h (3 h + FSH-CC)
Oocyte nuclear maturation
An experiment was performed to assess whether adding native OSFs affects the timing of oocyte nuclear maturation. At 12 h and 15 h of IVM, COCs were denuded and fixed in paraformaldehyde for 30 min, then stained with DAPI. The presence of the first polar body was determined using an upright microscope (Nikon, TE 2000-E) under UV light.
In vitro fertilization
Sperm from CBA F1 male mice aged 6–24 weeks and of proven fertility were incubated for 1 h in IVF medium (COOK® IVF, Research medium, Catalogue No: K-RVWA- 50, Australia) under 5 % CO2 at 37 °C for capacitation. Post-IVM, COCs were transferred into a 90 μl equilibrated IVF drop overlayed with mineral oil and then 10 μl of capacitated sperm were added into the drops. Three hours post-fertilization under 5 % CO2 at 37 °C, the presumptive zygotes were denuded of sperm and cumulus cells. Insemination day was counted as day 1 (0 h).
In vitro culture of embryos
Five to ten presumptive zygotes were cultured in 20 μl drops of equilibrated culture media (COOK® IVC, Research medium, Catalogue No: K-RVCL-50, Australia) overlayed with mineral oil under 6 % CO2, 5 % O2 and 89 % N2 atmosphere. Embryos were cultured for 6 days, with cleavage rates assessed on day 2 (24 h post-fertilization) and blastocysts assessed on day 5 (96–100 h post-fertilization) and day 6 (120–24 h post-fertilization).
Differential staining of blastocysts
Differential staining was performed on day 6 blastocysts to assess inner cell mass (ICM) and trophectoderm (TE) cell. Briefly, expanded, hatching and hatched blastocysts were placed into pronase (5 mg/ml) at 37 °C until the zona dissolved. The zona-free blastocysts were incubated in 10 mM trinitrobenzene sulfonic acid (TNBS) in 0.4 % polyvinyl alchol (PVA) in phosphate-buffered saline (PBS) at 4 °C for 10 min then washed twice before being transferred into 0.1 mg/ml anti dinitrophenol-BSA antibody at 37 °C for 10 min. Embryos were then incubated in 10 μg/ml propidium iodide for 5–10 min at 37 °C, followed by 4 μg/ml Hoechst 33,342 in 96 % ethanol at 4 °C overnight. The blastocysts were transferred onto a glass microscope side, covered by a cover slip and assessed immediately under UV fluorescence using an upright microscope (Nikon, TE 2000-E, excitation, 340–380 nm; emission, 440–480 nm), where ICM cells appeared blue and TE cells appeared pink.
Vitrification and warming of blastocysts
Hatching and expanded blastocysts on day 6 were vitrified and then within 1–2 weeks those blastocysts were warmed and assessed for cryotolerance, prior to embryo transfer. Vitrification and warming solutions were prepared in HEPES-buffered αMEM. The vitrification solution consisted of 2.3 M dimethylsulfoxide (DMSO) + 3 M ethylene glycol supplemented with 0.75 M sucrose. The corresponding equilibration solution contained half the concentration of cryoprotectants of the vitrification solution and no sucrose. Vitrification steps were performed at 37 °C. Blastocysts were placed in equilibration solution for 3 min then transferred to vitrification solution, with minimal transfer of equilibration solution (less than 3 μl), placed on a fibre-plugTM (CVM kit, Cryologic, Victoria, Australia) and touched on a pre-cooled steel block in liquid nitrogen, and then sealed in a pre-cooled straw (CVM kit, Cryologic) for storage in liquid nitrogen. The time taken from leaving equilibration solution to vitrification on the block was 30–40 s. Vitrified blastocysts were warmed at 37 °C and sequentially passed through 3 solutions of sucrose (0.3 M, 0.25 M and 0.15 M) in HEPES-buffered αMEM for 5 min each. Blastocysts were held in culture medium for 2–3 h and then blastocyst morphology was assessed before being transferred into foster mothers. The criteria used for blastocyst cryosurvival were as follows [50]; normal: blastocysts expanded with no signs of lysis, collapse or degeneration; abnormal: blastocysts with signs of lysis, collapse and degeneration.
Embryo transfer and assessment of fetal outcomes
Vitrified/warmed blastocysts assessed as normal were transferred into pseudopregnant recipients (Swiss female mice aged 12–20 weeks, mated with vasectomised F1 CBA males). On day 3.5 of pseudopregnancy, recipients were anaesthetized with 2 % Avertin (0.015 ml/g body weight) prior to embryo transfer. For each recipient, six normal expanded or hatching blastocysts post-vitrification/warming were transferred to each uterine horn, with control embryos in one horn and treatment embryos in the other. Eight replicates were performed with a total of 96 blastocysts transferred into 8 recipients. The number of implantation sites, fetuses, fetal and placental weights and fetal crown to rump lengths were assessed on day 17 post-embryo transfer.
Immunodetection of oocyte GDF9
DOs and oocyte-conditioned media were collected for western blot analysis of the form and quantity of GDF9 protein. Briefly, COCs were collected and denuded at 0 h and suspended at a ratio of 8 DOs/μl in IVM medium in Eppendorf tubes. DOs were matured for 17–18 h under 5 % CO2 at 37 °C. Post-IVM, DOs were pelleted by centrifugation and oocyte-conditioned medium was transferred to a separate tube and snap frozen in PBS. Oocytes were washed once in PBS, after which the PBS was removed and the cells snap frozen. Oocytes and oocyte-conditioned medium were also collected from the 3 h + FSH + CC group (Fig. 1a). COCs were matured first for 3 h in IVM medium, then denuded and matured for an extra 14–15 h in fresh IVM medium. Post-IVM, oocytes and oocyte-conditioned medium were processed as above. Samples (30–60 DOs or media equivalent) were boiled for 3 min in 1 × LDS reducing buffer [15ul, 1 × Novex NuPAGE LDS sample buffer (NP0009)] with mercaptoethanol (Novex NuPAGE NP0007, Carlsbad, USA) and centrifuged. The supernatant was fractionated by SDS-PAGE for 1 h at RT on 4–12 % Bis-Tris pre-cast gel (BioRad, Hercules, Ca, USA, Cat#345-0125) in MES buffer (Novex, NuPAGE, NP0002) followed by electrotransfer at 30 V overnight at 4 °C onto a nitrocellulose membrane (Hybond-C Extra, GE Healthcare Life Sciences, Bucks, UK). The membrane was incubated with biotinylated GDF9- monoclonal antibody 53 (Oxford Brookes University, Oxford, UK) at 1:2,000 for 1 h at RT then washed, followed by a further incubation with horseradish peroxidase-conjugated anti-mouse IgG (SNN2004, Lot No 892329B, Biosource), at 1:10,000 for 1 h at RT. Immunoreactive proteins were detected using Lumilight chemiluminesence reagents (Roche Diagnostics, GmbH Mannheim, Germany). Membranes were scanned on the BioRad ChemiDoc MP system and the images analysed using Image J software (Biorad). Recombinant mGDF9 (17 k) was used as a reference protein. Biotinylated molecular wt protein standards (Cat No. MW001) were obtained from R&D Systems, (Minneapolis, USA). Parallel controls (blanks) were undertaken with oocyte extracts in the absence of biotinylated antibody but in the presence of HRP-IgG antibody.
Statistical analyses
All proportional data for embryo development were arcsine transformed and analysed using either an independent t-test or multivariate ANOVA with least significant difference (LSD) post-hoc tests. Log transformation was performed for blastocyst cell number when data were not normally distributed, and analysed using an independent t-test or one way ANOVA with either LSD or Dunnet’s T3 post-hoc tests, depending on whether the data did or did not pass the homogeneity of variance test, respectively. Fetal measurements were analysed with independent t-tests with LSD post-hoc tests. Oocyte nuclear maturation, survival rate post-warming of blastocysts and post-embryo transfer data for fetal survival and implantation rates were assessed using a Chi-square test. All statistical analyses were performed using SPSS version 13 (SPSS Inc, Chicago, IL, USA). Differences were considered statistically significant at P < 0.05. Data are expressed as means ± SEM.
Results
Effect of co-culture of intact mouse COCs with native OSFs during IVM
Exposure of intact COCs to exogenous native OSFs from DOs throughout IVM significantly (P < 0.05) increased the cleavage rate compared to COCs cultured alone. ICM cell numbers were also significantly increased in blastocysts derived from COCs exposed to DOs, whereas TE and total cell numbers were not affected (Table 2).
Table 2.
Effect of native OSFs during IVM on subsequent embryo development
| Treatments | Number of oocytes | Cleavage1 | Blastocyst on day 52 | Blastocyst on day 63 | Hatching blastocyst on day 64 | ICM5 | TE5 | TCN5 |
|---|---|---|---|---|---|---|---|---|
| Control | 224 | 90.6 ± 1.8a | 52.8 ± 8.9 | 77.3 ± 4.6 | 58.8 ± 8.6 | 18.5 ± 0.8a | 55.8 ± 2.1 | 74.3 ± 2.6 |
| COC + DO6 | 233 | 96.1 ± 0.8b | 60.1 ± 4.9 | 74.3 ± 2.4 | 68.0 ± 1.9 | 25.3 ± 1.5b | 55.5 ± 2.5 | 80.8 ± 3.5 |
Values with different superscripts within a column are statistically different (P < 0.05)
Data are presented as means ± SEM of four replicate experiments. Each replicate experiment consisted of 53–60 oocytes
1Percentage of cleaved embryos per total oocytes
2Percentage of blastocysts generated 96–100 h post-fertilization per cleaved embryo
3Percentage of blastocysts generated 100–125 h post-fertilization per cleaved embryo
4Percentage of hatching blastocysts generated 100–125 h post fertilization per cleaved embryo
5Mean blastocyst cell numbers following differential staining of 16–18 blastocysts
6GVBD-stage denuded oocytes (DO) added from t = 0 h of IVM
Effect of mature form of GDF9 and BMP15 (R&D Systems) on mouse oocyte developmental competence
In contrast to the effect of native OSFs, improvements in embryo development were not observed with the addition of mature domain GDF9 (Table 3) or mature BMP15 (Table 4) to IVM medium at 50 ng/ml, 100 ng/ml or 200 ng/ml, or a combination of both proteins at the same doses (Table 5).
Table 3.
Effect of graded doses of recombinant GDF9 during IVM on subsequent embryo development
| Treatment | GDF9 (ng/ml) | Number of oocytes | Cleavagea | Blastocyst on day 6b | Hatching blastocyst on day 6c | ICMd | TEd | TCNd |
|---|---|---|---|---|---|---|---|---|
| Control | 0 | 152 | 82.9 ± 3.3 | 81.7 ± 4.4 | 62.6 ± 8.6 | 16.0 ± 0.8 | 53.0 ± 2.4 | 69.0 ± 2.8 |
| GDF9 | 50 | 118 | 74.6 ± 5.2 | 72.6 ± 8.9 | 61.8 ± 8.9 | 16.8 ± 1.1 | 54.2 ± 3.6 | 71.0 ± 4.4 |
| GDF9 | 100 | 118 | 74.8 ± 9.6 | 82.6 ± 7.8 | 59.1 ± 7.6 | 16.7 ± 0.9 | 49.2 ± 2.6 | 65.9 ± 3.3 |
| GDF9 | 200 | 147 | 86.1 ± 4.7 | 82.5 ± 8.3 | 64.3 ± 7.1 | 17.9 ± 0.7 | 56.8 ± 3.5 | 74.8 ± 3.9 |
Data are presented as means ± SEM of five replicate experiments. Each replicate experiment consisted of 10–50 oocytes
aPercentage of cleaved embryos per total oocytes
bPercentage of blastocysts generated 100–125 h post-fertilization per cleaved embryo
cPercentage of hatching blastocysts generated 100–125 h post fertilization per cleaved embryo
dMean blastocyst cell numbers following differential staining of 28–51 blastocysts
Table 4.
Effect of graded doses of BMP15 during IVM on subsequent embryo development
| Treatment | BMP15 (ng/ml) | Number of oocytes | Cleavagea | Blastocyst on day 6b | Hatching blastocyst on day 6c | ICMd | TEd | TCNd |
|---|---|---|---|---|---|---|---|---|
| Control | 0 | 241 | 66.6 ± 8.6 | 64.8 ± 8.0 | 44.3 ± 8.2 | 16.2 ± 1.0 | 50.7 ± 2.8 | 66.9 ± 3.4 |
| BMP15 | 50 | 193 | 77.7 ± 4.7 | 56.4 ± 8.8 | 36.5 ± 9.5 | 16.4 ± 0.7 | 48.3 ± 2.4 | 64.8 ± 3.0 |
| BMP15 | 100 | 200 | 75.8 ± 8.5 | 65.1 ± 7.9 | 45.0 ± 9.1 | 17.6 ± 1.1 | 49.1 ± 2.4 | 66.7 ± 3.1 |
| BMP15 | 200 | 187 | 76.3 ± 7.1 | 63.4 ± 8.1 | 43.1 ± 8.8 | 18.9 ± 0.8 | 55.1 ± 2.1 | 74.0 ± 2.5 |
Data are presented as means ± SEM of seven replicate experiments. Each replicate experiment consisted of 14–75 oocytes
aPercentage of cleaved embryos per total oocytes
bPercentage of blastocysts generated 100–125 h post-fertilization per cleaved embryo
cPercentage of hatching blastocysts generated 100–125 h post fertilization per cleaved embryo
dNumber of blastocysts staining in each treatment group 38–47
Table 5.
Effect of graded doses of GDF9 and BMP15 during IVM on subsequent embryo development
| Treatment | GDF9 + BMP15 (ng/ml) | Number of oocytes | Cleavagea | Blastocyst on day 6b | Hatching blastocyst on day 6c | ICMd | TEd | TCNd |
|---|---|---|---|---|---|---|---|---|
| Control | 0 + 0 | 78 | 92.0 ± 1.3 | 85.4 ± 5.7 | 84.2 ± 6.9 | 12.9 ± 0.8 | 42.8 ± 1.7 | 55.7 ± 1.9 |
| BMP15 + GDF9 | 50 + 50 | 88 | 91.5 ± 2.7 | 82.6 ± 9.5 | 80.5 ± 11.5 | 12.7 ± 0.7 | 43.5 ± 2.6 | 56.2 ± 2.9 |
| BMP15 + GDF9 | 100 + 100 | 86 | 93.2 ± 0.4 | 78.6 ± 10.8 | 75.2 ± 11.3 | 12.9 ± 1.0 | 37.9 ± 2.1 | 50.8 ± 2.1 |
| BMP15 + GDF9 | 200 + 200 | 85 | 89.7 ± 5.2 | 72.3 ± 7.1 | 67.0 ± 7.6 | 11.8 ± 1.0 | 45.5 ± 2.1 | 57.3 ± 2.7 |
Data are presented as means ± SEM of three replicate experiments. Each replicate experiment consisted of 12–40 oocytes
aPercentage of cleaved embryos per total oocytes
bPercentage of blastocysts generated 100–125 h post-fertilization per cleaved embryo
cPercentage of hatching blastocysts generated 100–125 h post fertilization per cleaved embryo
dNumber of blastocysts staining in each treatment group 14–19
Temporal effect of co-culture of intact mouse COCs with native OSFs during IVM
A previous study by [46] demonstrated that there is a temporal effect of native OSFs on bovine oocyte developmental competence. Based on this result, we assessed mouse oocyte developmental competence following co-culture of COCs with DOs in a temporal manner. COCs were co-cultured with DOs from 0 h (0 h [GV] stage) or with DOs precultured as COCs for 3 h + FSH + CC (refer to Fig. 1a). Following co-culture of COCs with DOs from 0 to 17 h (0 h [GV] stage), a significant increase was observed in cleavage rate compared to controls, however there were no significant differences in blastocyst rates or ICM number (Table 6). By contrast, treating COCs with DOs from 3 h (3 h + FSH + CC) significantly (P < 0.05) increased blastocyst and hatching blastocyst rates measured at day 6 and subsequent ICM cell numbers, compared to the control group.
Table 6.
Effects of exogenous native OSFs during IVM on subsequent embryo development
| Treatments | Pre-treatment of OSFs1 | Number of oocytes | Cleavage2 | Blastocyst on day 53 | Blastocyst on day 64 | Hatching blastocyst on day 65 | ICM6 | TE6 | TCN6 |
|---|---|---|---|---|---|---|---|---|---|
| Control | N/A | 152 | 87.5 ± 1.6a | 52.2 ± 7.2 | 67.4 ± 6.0a | 53.3 ± 5.6a | 18.7 ± 1.9a | 51.1 ± 3.4 | 69.8 ± 4.3 |
| COC + DO | 0h [GV] | 155 | 95.5 ± 2.2b | 57.5 ± 2.6 | 77.2 ± 4.4a,b | 66.9 ± 2.8a,b | 22.0 ± 1.7a,b | 52.4 ± 2.4 | 74.4 ± 3.5 |
| COC + DO | 3h + FSH + CCs | 153 | 94.8 ± 1.1a,b | 65.5 ± 1.7 | 83.4 ± 2.6b | 74.5 ± 2.8b | 24.8 ± 1.3b | 55.7 ± 2.7 | 80.5 ± 3.4 |
Values with different superscripts within a column are statistically different (P < 0.05)
Data are presented as means ± SEM of four replicate experiments. Each replicate experiment consisted of 37–40 oocytes
1See Fig. 1 and Table 1 for explanation of experimental design
2Percentage of cleaved embryos per total oocytes
3Percentage of blastocysts generated 96–100 h post-fertilization per cleaved embryo
4Percentage of blastocysts generated 100–125 h post-fertilization per cleaved embryo
5Percentage of hatching blastocysts generated 100–125 h post fertilization per cleaved embryo
6Mean blastocyst cell numbers following differential staining of 13–22 blastocysts
Effect of exposure of COCs to native OSFs on nuclear maturation
Native OSFs regulate CC cGMP levels [51] which affects meiosis and, according to [20], co-culture of COCs with DOs increased nuclear maturation rate in bovine oocytes. Since the highest competence treatment group from the previous experiment was COCs co-cultured with DOs from 3 h (3 h + FSH + CC), we hypothesised that an effect on oocyte nuclear maturation could account for this enhanced oocyte quality in mice. However, in the present experiment, there was no significant difference in the maturation rates of control oocytes or COCs exposed to DOs (3 h + FSH + CC) at 12 h or 15 h of IVM (Table 7).
Table 7.
Effect of native OSFs added at 3 h on oocyte nuclear maturation
| Treatments | Pre-treatment of OSFsa | Maturation time (h) | n | MII oocytes (%) |
|---|---|---|---|---|
| Control | N/A | 12 | 111 | 92.8 |
| 15 | 74 | 91.9 | ||
| COC + DO | 3h + FSH + CCs | 12 | 113 | 93.8 |
| 15 | 78 | 93.6 |
Temporal effects on oocyte GDF9 levels
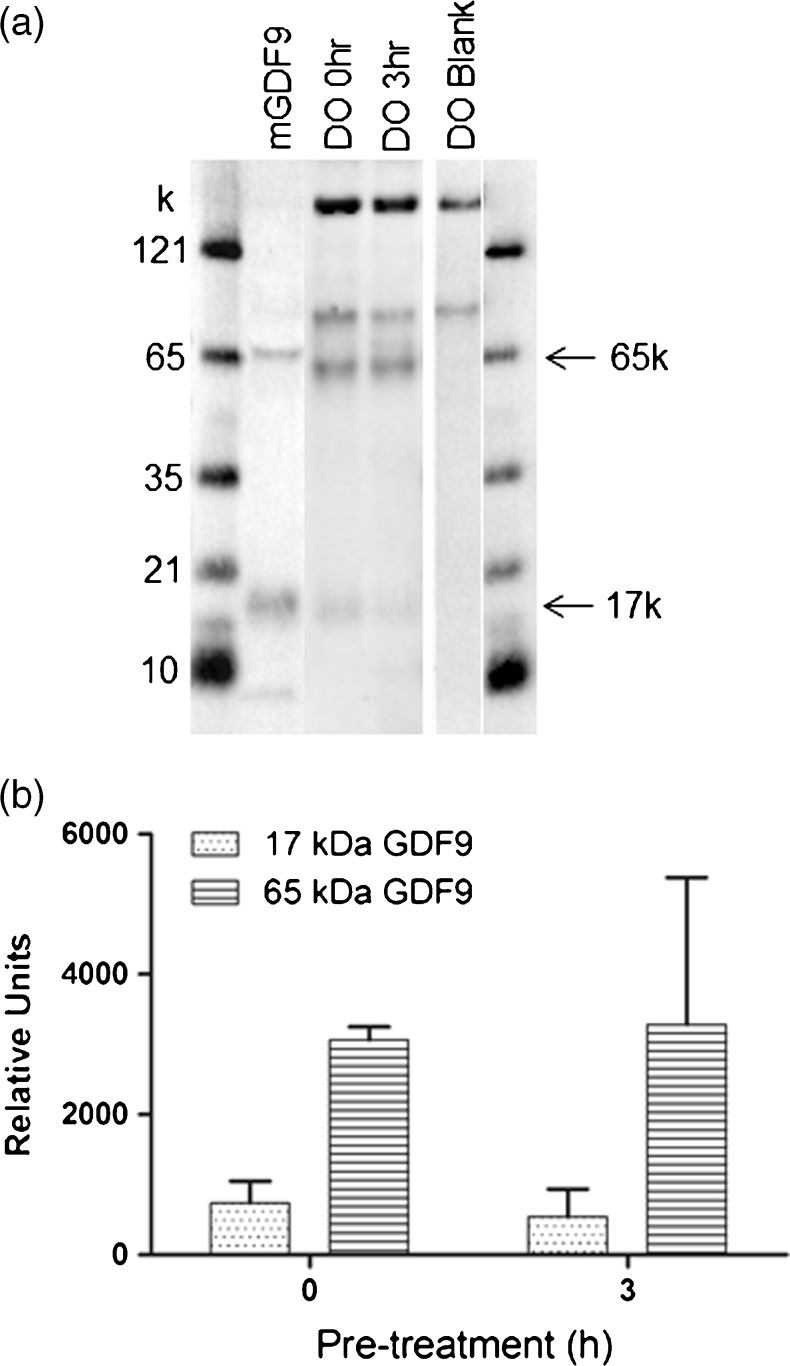
Given the beneficial effects of pre-culture of oocytes with their CCs for 3 h with FSH on OSF-stimulated oocyte developmental competence (Table 6), it was attempted to quantify the amount and form of GDF9 expressed within and secreted by oocytes. The band for the un-processed pro-protein was observed at ~65 kDa and the mature protein at ~17 kDa, with the mature protein less abundant in oocyte extracts (Fig. 2). Three hours of pre-culture (3 h + FSH + CC) had no effect on the total quantity or the proportion of pro- versus mature-GDF9 in oocytes. No GDF9 bands were observed in the DO conditioned media.
Fig. 2.
a GDF9 Western blot comparison of denuded oocyte (DO) extracts at 0h and 3 h (3 h + FSH + CC) of pretreatment. mGDF9 refers to recombinant mGDF9 standard. Blank refers to DO sample in the absence of the GDF9 primary antibody. The 65 kDa band corresponds to the unprocessed GDF9 pro-protein while the 17 kDa band corresponds to the mature domain. The 90 kDa–180 kDa bands seen in all samples are attributed to the non-specific presence of biotinylated proteins in the cell extracts b Quantitative analysis of Western blot denuded oocyte extracts at 0 and 3h (3 h + FSH + CC) of pretreatment from 5 separate experiments. The Western blot image was quantified using BioRad ChemiDoc MP system and analysed using Image J software (Biorad)
The role of FSH and cumulus cells in the production of native OSFs
We hypothesized that FSH and cumulus cells are two factors that affect the production of native OSFs. In this experiment, DOs were generated using different methods (see Fig. 1). Following co-culture of COCs with precultured DOs (3 h + FSH + CC; see Fig. 1a), a significantly increased blastocyst rate on day 5 was observed compared to control, which contributed to an improvement in ICM number compared to control (Table 8). DOs that were pre-cultured as intact COCs but without FSH (3 h-FSH + CC, Fig. 1b) did not stimulate developmental competence in their co-cultured COCs; these exhibited embryo developmental parameters equivalent to the control and ICM numbers significantly lower than those stimulated with FSH (3 h + FSH + CC). Interestingly, there was a significant improvement in blastocyst rate on day 6 from COCs co-cultured with DOs that were without CCs from 0–3 h (3 h + FSH-CC, see Fig. 1c) compared to the control. Overall, all treatment groups co-cultured with DOs tended to show an improvement in embryo development.
Table 8.
Effects of cumulus cells and FSH on native OSF efficacy at enhancing post-IVM embryo development
| Treatments | Pre-treatment OSFs1 | Number of oocytes | Cleavage2 | Blastocyst on day 53 | Blastocyst on day 64 | Hatching Blastocyst on d65 | ICM6 | TE6 | TCN6 |
|---|---|---|---|---|---|---|---|---|---|
| Control | N/A | 192 | 92.7 ± 2.9 | 52.5 ± 3.4a | 76.6 ± 3.0a | 60.8 ± 3.3 | 11.9 ± 0.6a | 43.1 ± 2.2 | 55.0 ± 2.4 |
| COC + DO | 3h + FSH + CCs | 191 | 93.6 ± 1.9 | 65.2 ± 5.6b | 83.2 ± 2.0a,b | 69.0 ± 3.4 | 13.8 ± 0.5b | 43.6 ± 1.5 | 57.4 ± 1.6 |
| COC + DO | 3h - FSH + CCs | 190 | 94.7 ± 1.9 | 61.0 ± 3.6a,b | 79.5 ± 2.5a,b | 66.7 ± 4.0 | 11.7 ± 0.5a | 46.4 ± 1.9 | 58.2 ± 2.0 |
| COC + DO | 3h + FSH - CCs | 191 | 93.7 ± 2.5 | 63.8 ± 3.5a,b | 86.2 ± 2.9b | 70.4 ± 2.7 | 12.2 ± 0.7a,b | 44.1 ± 2.0 | 56.3 ± 2.4 |
Values with different superscripts within a column are statistically different (P < 0.05)
Data are presented as means ± SEM of five replicate experiments. Each replicate experiment consisted of 36–39 oocytes
1See Fig. 1 and Table 1 for explanation of experimental design
2Percentage of cleaved embryos per total oocytes
3Percentage of blastocysts generated 96–100 h post-fertilization per cleaved embryo
4Percentage of blastocysts generated 100–125 h post-fertilization per cleaved embryo
5Percentage of hatching blastocysts generated 100–125 h post fertilization per cleaved embryo
6Mean blastocyst cell numbers following differential staining of 33–42 blastocysts
Effect of exposure of COCs to native OSFs during IVM on cryo-survival of blastocysts
With respect to cryopreserved blastocysts, the blastocyst re-expansion rate post-vitrification/warming (normal morphology of blastocysts) did not differ significantly from control for blastocysts derived from COCs exposed to native OSFs (COCs + DOs at 3 h + FSH + CC; Table 9). Therefore exposure of COCs to native OSFs did not measurably alter the cryotolerance of blastocysts.
Table 9.
Effect of native OSFs during IVM on survival rate post-warming of blastocysts
| Treatments | Pre-treatment OSFsa | Numbers of blastocysts | Normal (%) | Abnormal (%) |
|---|---|---|---|---|
| Control | N/A | 90 | 73 (81.1) | 17 (18.9) |
| COC + DO | 3 h + FSH + CC | 92 | 74 (80.4) | 18 (19.6) |
Effect of exposure of COCs to native OSFs on pregnancy rates and fetal outcomes
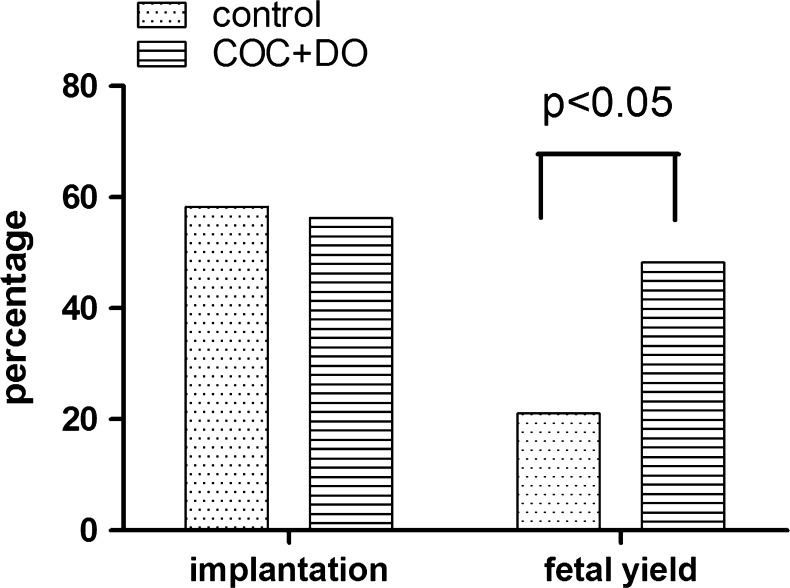
After 2 h of warming, the morphologically normal blastocysts were transferred into pseudo pregnant recipients. There was no difference in implantation rates between the control and treatment group (COCs co-cultured with DOs [3 h + FSH + CC]). However, the proportion of fetuses that developed to day 17 per implantation site, from COCs co-cultured with native OSFs, was more than double that of the control (21 % vs 48 %, P < 0.05, Fig. 3). Moreover, based on gross morphological criteria, those fetuses were normal, with no significant differences between treatments in fetal or placental weight or fetal crown-to-rump length (Table 10).
Fig. 3.
Effect of treatment with native OSFs during IVM on subsequent pregnancy outcomes. Blastocysts were derived from standard IVM (control) or from COCs exposed to native OSFs during IVM (COC + DO; 3 h + FSH + CC pretreatment). Control and treatment blastocysts were vitrified on day 6 and then later warmed and transferred to pseudo pregnant recipients. Six control and six treatment blastocysts were transferred into each uterine horn of a recipient (n = 8). Pregnancy outcomes were analysed on day 17. Implantation rate; implantation sites/embryos transferred (n = 48 embryos transferred/treatment). Fetal yield; day 17 fetuses/implantation sites
Table 10.
Effect of native OSFs during IVM on subsequent fetal and placental weight and crown-rump length
| Treatments | Pre-treatment OSFsa | Placental weight (g) | Fetal weight (g) | Crown to rump length (mm) |
|---|---|---|---|---|
| Control | N/A | 0.2 ± 0.01 | 1.8 ± 0.1 | 24.5 ± 1.9 |
| COC + DO | 3 h + FSH + CC | 0.2 ± 0.01 | 1.7 ± 0.1 | 25.6 ± 0.8 |
Discussion
Our results show that treatment of COCs during IVM with native OSFs, enhances oocyte quality, as measured by subsequent embryo cleavage, blastocyst and fetal survival rates. These results are supported by previous studies which have shown that exogenous native OSFs (obtained by co-culture with DOs), when used as supplements in oocyte IVM, significantly improve embryo development in cattle [20, 46, 47], pigs [48] and goats [45]. In contrast, addition of recombinant mature homodimers of GDF9 and/or BMP15 (R&D Systems) in IVM media did not improve mouse embryo development (current study). However, previous results from our laboratory using a complex of pro- and mature-domains of GDF9 and BMP15 (pro-mature protein) showed a positive effect on bovine as [12, 46, 47] well as mouse [49] oocyte developmental competence.
From this study, we suggest that there are a number of factors which influence the capacity of exogenous OSFs to improve mouse embryo and fetal development post-IVM namely; the form of recombinant OSFs, the capacity of cumulus cells and FSH to regulate native OSF production, and the temporally regulated potency of the native OSF pool. Supplementation of IVM with OSFs has the potential to dramatically improve the efficiency of IVM and therefore its applicability in human and veterinary clinics [2], as we saw more than a doubling in fetal yield using this approach. However, this was only achieved using native and not recombinant OSFs, and while this illustrates the principal clearly, co-culture with denuded oocytes is not practical in a clinical scenario. Even though the exact identity of growth factors secreted by denuded oocytes in vitro remains poorly characterised, the principal OSFs are thought to be GDF9, BMP15, BMP6, fibroblast growth factor 8 (FGF8) and FGF10, with widely differing roles for individual growth factors between species [11, 26, 28–30, 41, 42, 52, 53]. Notably, the only commercially available recombinant preparations of GDF9 and BMP15 produced in mammalian cells, had no effect on post-IVM embryo development, despite the extensive dose range tested. By contrast, recombinant FGF10 added during bovine IVM enhances subsequent embryo development [52]. We hypothesize that the failure of these preparations of GDF9 and BMP15 to improve mouse IVM is because these proteins are supplied as isolated homodimers of their mature regions, lacking their prodomains. For the same reason we would predict that the GDF9 and BMP15 preparations produced in E.coli would be ineffective in IVM. GDF9 and BMP15 are likely to form a heterodimer, although so far the production of a recombinant preparation has proved controversial [54, 55] and it has not yet been tested in IVM.
When full length recombinant GDF9 and BMP15 are expressed in mammalian cells such as human embryonic kidney 293H cells, the proteins are processed (pro and mature domains are proteolytically cleaved) and secreted into the medium as dimers or monomers of pro- and mature regions, that are non-covalently bound as pro-mature complexes [36]. Our in-house produced GDF9 and BMP15 preparations [41, 56], which contain both the pro- and mature domains, when added to IVM, significantly improve the quality of the blastocysts generated (as measured by ICM cell number) and thus improve fetal survival rates in mouse [49], as well as increase embryo development in cattle [12, 46, 47].
The current results demonstrated that blastocyst development and fetal survival rates were further improved when COCs were co-cultured with DOs from 3 h (versus control), if those DOs were matured as COCs for the first 3 h in the presence of FSH, then subsequently denuded and added to IVM. This result supports previous findings in bovine IVM oocytes, which suggested a temporal effect on secretion of OSFs [46]. It has been demonstrated that even a short exposure of COCs to FSH significantly increases subsequent bovine blastocyst development, via protein kinase C activation [23]. Hence, the FSH-treated CCs may in some manner improve the quantity and/or quality of factors which are secreted by the resultant DO. During the 3 h maturation, the oocyte and cumulus cells communicate with each other through gap junctional communication and via paracrine factors, which presumably provides enough time for the oocyte to obtain the beneficial effect of FSH through the receptors in cumulus cells. This effect may be mediated more by paracrine factors rather than gap junctional communication. After meiotic resumption, gap junctional communication between the oocyte and cumulus cells is lost [18]. The action of OSFs can be observed even without direct contact between cumulus cells and oocytes, suggesting that they are acting in a paracrine manner [6]. In the present study, the cleavage rate of mouse DOs post co-culture with COCs was significantly higher compared to DOs cultured alone (result not shown). In cattle, the developmental competence of DOs co-cultured with COCs is significantly improved compared to DOs cultured alone [20, 47].
Based on the notable improvement in oocyte developmental competence from the 3 h + FSH + CC group, we anticipated that COCs matured first for 3 h in medium containing FSH would produce different quantities and/or forms of GDF9, compared to oocytes denuded at 0 h. However, unfortunately we were unable to detect any GDF9 in oocyte-conditioned medium, unlike the recent study by Lin et al. (2012). This may be due to our oocyte-conditioning medium procedures or the western blot being insufficiently sensitive to detect actual secreted forms. Also, we could not observe any effect of the 3 h pre-culture with FSH treated cumulus cells on the GDF9 produced within the oocyte. It showed that there was no difference in the amount or the proportion of either the pro-protein or the mature form of GDF9 in DOs at 0 h or 3 h. While GDF9 is the principal OSF produced by mouse oocytes [25, 41], other OSFs such as BMP15 [57], BMP6 [53], FGF10 [52] and FGF8 [11] may be processed and interact differently in DOs at 0 h and 3 h.
Overall, we have shown that there is an obvious advantage to treatment of COCs with exogenous OSFs during IVM. Mouse exogenous OSFs did not affect oocyte nuclear maturation, in contrast with bovine oocytes matured in vitro, where co-culture with DOs improves nuclear maturation [20]. Clearly exogenous OSFs have major effects on key aspects governing the developmental program of oocytes, although it is not yet clear how and by which exact mechanisms this is achieved. Other groups have shown that native OSFs play a significant role in; the activity of hyaluronic acid synthase 2 (HAS2), which correlates with mucification and expansion of CCs [13], increased GPX1 gene expression (related to the production of glutathione peroxidise as an antioxidant in oocytes), and enhanced expression of steroidogenic acute regulatory protein (STAR), which contributes to oocyte nuclear and cytoplasmic maturation [20]. Bovine oocytes matured with the mature form of BMP15 showed a significant increase in glucose uptake [9], and those matured with pro-mature BMP15 exhibited increased oxidative phosphorylation activity in the oocyte [12].
The results of this current study confirm that OSFs notably improve embryo development and fetal survival in mice, and extend this knowledge by demonstrating for the first time that the effect of OSFs on mouse oocytes depends on timing, presence of FSH and cumulus cells. The observation that recombinant GDF9 and BMP15 in their mature form do not improve mouse embryo development leads us to the conclusion that the mature forms of those growth factors is not the functional form that improves the quality of IVM mouse oocytes. The beneficial effect of native OSFs on oocyte quality and subsequent embryo development can be applied in the veterinary clinic; however, application of co-culture of COCs with DOs is not possible in human IVF clinics since it is unethical and not feasible to generate supernumerary DOs simply for OSF supplementation of media. Thus, the next step is to identify and purify the proteins that are secreted by oocytes, such as GDF9 and BMP15, into a functional form that can be added into IVM medium, to improve the quality of IVM oocytes and subsequent embryo development and pregnancy rates.
Acknowledgments
J.S. was financially supported by an International Postgraduate Research Scholarship (IPRS) from The University of Adelaide. This study was also supported by research grants and fellowships from the National Health and Medical Research Council of Australia (1008137, 1017484, 169201, 494802, APP1023210, APP627007), by grants from Cook Medical and by the Victorian Government’s Operational Infrastructure Support Program. The Prince Henry’s Institute Data Audit number is 13:32.
Conflict of interest
The University of Adelaide owns a patent family on the applications of GDF9 and BMP15 in oocyte in vitro maturation. RBG and JGT are inventors.
Footnotes
Capsule Oocyte developmental competence and pregnancy rates from in vitro matured oocytes can be notably improved by the addition of exogenous oocyte-secreted factors. Isolated mature domains of recombinant GDF9 and BMP15 were ineffective, whereas addition of native oocyte-secreted factors improved IVM outcomes.
References
- 1.Gremeau AS, Andreadis N, Fatum M, Craig J, Turner K, McVeigh E, et al. In vitro maturation or in vitro fertilization for women with polycystic ovaries? A case-control study of 194 treatment cycles. Fertil Steril. 2012;98:355–360. doi: 10.1016/j.fertnstert.2012.04.046. [DOI] [PubMed] [Google Scholar]
- 2.Gilchrist RB. Recent insights into oocyte-follicle cell interactions provide opportunities for the development of new approaches to in vitro maturation. Reprod Fertil Dev. 2011;23:23–31. doi: 10.1071/RD10225. [DOI] [PubMed] [Google Scholar]
- 3.Gilchrist RB, Ritter LJ, Armstrong DT. Oocyte-somatic cell interactions during follicle development in mammals. Anim Reprod Sci. 2004;82–83:431–446. doi: 10.1016/j.anireprosci.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 4.Gilchrist RB, Ritter LJ, Myllymaa S, Kaivo-Oja N, Dragovic RA, Hickey TE, et al. Molecular basis of oocyte-paracrine signalling that promotes granulosa cell proliferation. J Cell Sci. 2006;119:3811–3821. doi: 10.1242/jcs.03105. [DOI] [PubMed] [Google Scholar]
- 5.Vanderhyden BC, Telfer EE, Eppig JJ. Mouse oocytes promote proliferation of granulosa cells from preantral and antral follicles in vitro. Biol Reprod. 1992;46:1196–1204. doi: 10.1095/biolreprod46.6.1196. [DOI] [PubMed] [Google Scholar]
- 6.Hussein TS, Froiland DA, Amato F, Thompson JG, Gilchrist RB. Oocytes prevent cumulus cell apoptosis by maintaining a morphogenic paracrine gradient of bone morphogenetic proteins. J Cell Sci. 2005;118:5257–5268. doi: 10.1242/jcs.02644. [DOI] [PubMed] [Google Scholar]
- 7.Li R, Norman RJ, Armstrong DT, Gilchrist RB. Oocyte-secreted factor(s) determine functional differences between bovine mural granulosa cells and cumulus cells. Biol Reprod. 2000;63:839–845. doi: 10.1095/biolreprod63.3.839. [DOI] [PubMed] [Google Scholar]
- 8.Salustri A, Yanagishita M, Hascall VC. Mouse oocytes regulate hyaluronic acid synthesis and mucification by FSH-stimulated cumulus cells. Dev Biol. 1990;138:26–32. doi: 10.1016/0012-1606(90)90173-G. [DOI] [PubMed] [Google Scholar]
- 9.Caixeta E, Sutton-McDowall M, Gilchrist RB, Thompson JG, Price C, Machado MF, et al. Bone morphogenetic protein 15 and fibroblast growth factor 10 enhance cumulus expansion, glucose uptake and expression of genes in the ovulatory cascade during in vitro maturation of bovine cumulus-oocyte complexes. Reproduction. 2013. [DOI] [PubMed]
- 10.Su YQ, Sugiura K, Wigglesworth K, O’Brien MJ, Affourtit JP, Pangas SA, et al. Oocyte regulation of metabolic cooperativity between mouse cumulus cells and oocytes: BMP15 and GDF9 control cholesterol biosynthesis in cumulus cells. Development. 2008;135:111–121. doi: 10.1242/dev.009068. [DOI] [PubMed] [Google Scholar]
- 11.Sugiura K, Su YQ, Diaz FJ, Pangas SA, Sharma S, Wigglesworth K, et al. Oocyte-derived BMP15 and FGFs cooperate to promote glycolysis in cumulus cells. Development. 2007;134:2593–2603. doi: 10.1242/dev.006882. [DOI] [PubMed] [Google Scholar]
- 12.Sutton-McDowall ML, Mottershead DG, Gardner DK, Gilchrist RB, Thompson JG. Metabolic differences in bovine cumulus-oocyte complexes matured in vitro in the presence or absence of follicle-stimulating hormone and bone morphogenetic protein 15. Biol Reprod. 2012;87:87. doi: 10.1095/biolreprod.112.102061. [DOI] [PubMed] [Google Scholar]
- 13.Dragovic RA, Ritter LJ, Schulz SJ, Amato F, Armstrong DT, Gilchrist RB. Role of oocyte-secreted growth differentiation factor 9 in the regulation of mouse cumulus expansion. Endocrinology. 2005;146:2798–2806. doi: 10.1210/en.2005-0098. [DOI] [PubMed] [Google Scholar]
- 14.Dragovic RA, Ritter LJ, Schulz SJ, Amato F, Thompson JG, Armstrong DT, et al. Oocyte-secreted factor activation of SMAD 2/3 signaling enables initiation of mouse cumulus cell expansion. Biol Reprod. 2007;76:848–857. doi: 10.1095/biolreprod.106.057471. [DOI] [PubMed] [Google Scholar]
- 15.Eppig JJ, Downs SM. Chemical signals that regulate mammalian oocyte maturation. Biol Reprod. 1984;30:1–11. doi: 10.1095/biolreprod30.1.1. [DOI] [PubMed] [Google Scholar]
- 16.Salustri A, Siracusa G. Metabolic coupling, cumulus expansion and meiotic resumption in mouse cumuli oophori cultured in vitro in the presence of FSH or dcAMP, or stimulated in vivo by hCG. J Reprod Fertil. 1983;68:335–341. doi: 10.1530/jrf.0.0680335. [DOI] [PubMed] [Google Scholar]
- 17.Salustri A, Yanagishita M, Hascall VC. Synthesis and accumulation of hyaluronic acid and proteoglycans in the mouse cumulus cell-oocyte complex during follicle-stimulating hormone-induced mucification. J Biol Chem. 1989;264:13840–13847. [PubMed] [Google Scholar]
- 18.Thomas RE, Armstrong DT, Gilchrist RB. Bovine cumulus cell-oocyte gap junctional communication during in vitro maturation in response to manipulation of cell-specific cyclic adenosine 3′,5′-monophosophate levels. Biol Reprod. 2004;70:548–556. doi: 10.1095/biolreprod.103.021204. [DOI] [PubMed] [Google Scholar]
- 19.Gilchrist RB, Lane M, Thompson JG. Oocyte-secreted factors: regulators of cumulus cell function and oocyte quality. Hum Reprod Update. 2008;14:159–177. doi: 10.1093/humupd/dmm040. [DOI] [PubMed] [Google Scholar]
- 20.Dey SR, Deb GK, Ha AN, Lee JI, Bang JI, Lee KL, et al. Coculturing denuded oocytes during the in vitro maturation of bovine cumulus oocyte complexes exerts a synergistic effect on embryo development. Theriogenology. 2012;77:1064–1077. doi: 10.1016/j.theriogenology.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 21.Wongsrikeao P, Kaneshige Y, Ooki R, Taniguchi M, Agung B, Nii M, et al. Effect of the removal of cumulus cells on the nuclear maturation, fertilization and development of porcine oocytes. Reprod Domest Anim. 2005;40:166–170. doi: 10.1111/j.1439-0531.2005.00576.x. [DOI] [PubMed] [Google Scholar]
- 22.Zhang L, Jiang S, Wozniak PJ, Yang X, Godke RA. Cumulus cell function during bovine oocyte maturation, fertilization, and embryo development in vitro. Mol Reprod Dev. 1995;40:338–344. doi: 10.1002/mrd.1080400310. [DOI] [PubMed] [Google Scholar]
- 23.Ali A, Sirard MA. Protein kinases influence bovine oocyte competence during short-term treatment with recombinant human follicle stimulating hormone. Reproduction. 2005;130:303–310. doi: 10.1530/rep.1.00387. [DOI] [PubMed] [Google Scholar]
- 24.Merriman JA, Whittingham DG, Carroll J. The effect of follicle stimulating hormone and epidermal growth factor on the developmental capacity of in-vitro matured mouse oocytes. Hum Reprod. 1998;13:690–695. doi: 10.1093/humrep/13.3.690. [DOI] [PubMed] [Google Scholar]
- 25.Crawford JL, McNatty KP. The ratio of growth differentiation factor 9: bone morphogenetic protein 15 mRNA expression is tightly co-regulated and differs between species over a wide range of ovulation rates. Mol Cell Endocrinol. 2012;348:339–343. doi: 10.1016/j.mce.2011.09.033. [DOI] [PubMed] [Google Scholar]
- 26.Dong J, Albertini DF, Nishimori K, Kumar TR, Lu N, Matzuk MM. Growth differentiation factor-9 is required during early ovarian folliculogenesis. Nature. 1996;383:531–535. doi: 10.1038/383531a0. [DOI] [PubMed] [Google Scholar]
- 27.Yan C, Wang P, DeMayo J, DeMayo FJ, Elvin JA, Carino C, et al. Synergistic roles of bone morphogenetic protein 15 and growth differentiation factor 9 in ovarian function. Mol Endocrinol. 2001;15:854–866. doi: 10.1210/mend.15.6.0662. [DOI] [PubMed] [Google Scholar]
- 28.Galloway SM, McNatty KP, Cambridge LM, Laitinen MP, Juengel JL, Jokiranta TS, et al. Mutations in an oocyte-derived growth factor gene (BMP15) cause increased ovulation rate and infertility in a dosage-sensitive manner. Nat Genet. 2000;25:279–283. doi: 10.1038/77033. [DOI] [PubMed] [Google Scholar]
- 29.Hanrahan JP, Gregan SM, Mulsant P, Mullen M, Davis GH, Powell R, et al. Mutations in the genes for oocyte-derived growth factors GDF9 and BMP15 are associated with both increased ovulation rate and sterility in Cambridge and Belclare sheep (Ovis aries) Biol Reprod. 2004;70:900–909. doi: 10.1095/biolreprod.103.023093. [DOI] [PubMed] [Google Scholar]
- 30.Juengel JL, Hudson NL, Whiting L, McNatty KP. Effects of immunization against bone morphogenetic protein 15 and growth differentiation factor 9 on ovulation rate, fertilization, and pregnancy in ewes. Biol Reprod. 2004;70:557–561. doi: 10.1095/biolreprod.103.023333. [DOI] [PubMed] [Google Scholar]
- 31.Wang B, Zhou S, Wang J, Liu J, Ni F, Yan J, et al. Identification of novel missense mutations of GDF9 in Chinese women with polycystic ovary syndrome. Reprod Biomed Online. 2010;21:344–348. doi: 10.1016/j.rbmo.2010.04.013. [DOI] [PubMed] [Google Scholar]
- 32.Palmer JS, Zhao ZZ, Hoekstra C, Hayward NK, Webb PM, Whiteman DC, et al. Novel variants in growth differentiation factor 9 in mothers of dizygotic twins. J Clin Endocrinol Metab. 2006;91:4713–4716. doi: 10.1210/jc.2006-0970. [DOI] [PubMed] [Google Scholar]
- 33.Dixit H, Rao LK, Padmalatha V, Kanakavalli M, Deenadayal M, Gupta N, et al. Mutational screening of the coding region of growth differentiation factor 9 gene in Indian women with ovarian failure. Menopause. 2005;12:749–754. doi: 10.1097/01.gme.0000184424.96437.7a. [DOI] [PubMed] [Google Scholar]
- 34.Chand AL, Ponnampalam AP, Harris SE, Winship IM, Shelling AN. Mutational analysis of BMP15 and GDF9 as candidate genes for premature ovarian failure. Fertil Steril. 2006;86:1009–1012. doi: 10.1016/j.fertnstert.2006.02.107. [DOI] [PubMed] [Google Scholar]
- 35.Wang TT, Ke ZH, Song Y, Chen LT, Chen XJ, Feng C, et al. Identification of a mutation in GDF9 as a novel cause of diminished ovarian reserve in young women. Hum Reprod. 2013. [DOI] [PubMed]
- 36.McIntosh CJ, Lun S, Lawrence S, Western AH, McNatty KP, Juengel JL. The proregion of mouse BMP15 regulates the cooperative interactions of BMP15 and GDF9. Biol Reprod. 2008;79:889–896. doi: 10.1095/biolreprod.108.068163. [DOI] [PubMed] [Google Scholar]
- 37.Al-Musawi SL, Walton KL, Heath D, Simpson CM, Harrison CA. Species differences in the expression and activity of bone morphogenetic protein 15. Endocrinology. 2013;154:888–899. doi: 10.1210/en.2012-2015. [DOI] [PubMed] [Google Scholar]
- 38.Mottershead DG, Pulkki MM, Muggalla P, Pasternack A, Tolonen M, Myllymaa S, et al. Characterization of recombinant human growth differentiation factor-9 signaling in ovarian granulosa cells. Mol Cell Endocrinol. 2008;283:58–67. doi: 10.1016/j.mce.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 39.Simpson CM, Stanton PG, Walton KL, Chan KL, Ritter LJ, Gilchrist RB, et al. Activation of latent human GDF9 by a single residue change (Gly 391 Arg) in the mature domain. Endocrinology. 2012;153:1301–1310. doi: 10.1210/en.2011-1632. [DOI] [PubMed] [Google Scholar]
- 40.McIntosh CJ, Lawrence S, Smith P, Juengel JL, McNatty KP. Active immunization against the proregions of GDF9 or BMP15 alters ovulation rate and litter size in mice. Reproduction. 2012;143:195–201. doi: 10.1530/REP-11-0336. [DOI] [PubMed] [Google Scholar]
- 41.Gilchrist RB, Ritter LJ, Cranfield M, Jeffery LA, Amato F, Scott SJ, et al. Immunoneutralization of growth differentiation factor 9 reveals it partially accounts for mouse oocyte mitogenic activity. Biol Reprod. 2004;71:732–739. doi: 10.1095/biolreprod.104.028852. [DOI] [PubMed] [Google Scholar]
- 42.Lin JY, Pitman-Crawford JL, Bibby AH, Hudson NL, McIntosh CJ, Juengel JL, et al. Effects of species differences on oocyte regulation of granulosa cell function. Reproduction. 2012;144:557–567. doi: 10.1530/REP-12-0267. [DOI] [PubMed] [Google Scholar]
- 43.McNatty KP, Lawrence S, Groome NP, Meerasahib MF, Hudson NL, Whiting L, et al. Meat and livestock association plenary lecture 2005. Oocyte signalling molecules and their effects on reproduction in ruminants. Reprod Fertil Dev. 2006;18:403–412. doi: 10.1071/RD05104. [DOI] [PubMed] [Google Scholar]
- 44.Dunning KR, Lane M, Brown HM, Yeo C, Robker RL, Russell DL. Altered composition of the cumulus-oocyte complex matrix during in vitro maturation of oocytes. Hum Reprod. 2007;22:2842–2850. doi: 10.1093/humrep/dem277. [DOI] [PubMed] [Google Scholar]
- 45.Romaguera R, Morato R, Jimenez-Macedo AR, Catala M, Roura M, Paramio MT, et al. Oocyte secreted factors improve embryo developmental competence of cocs from small follicles in prepubertal goats. Theriogenology. 2010. [DOI] [PubMed]
- 46.Hussein TS, Sutton-McDowall ML, Gilchrist RB, Thompson JG. Temporal effects of exogenous oocyte-secreted factors on bovine oocyte developmental competence during IVM. Reprod Fertil Dev. 2011;23:576–584. doi: 10.1071/RD10323. [DOI] [PubMed] [Google Scholar]
- 47.Hussein TS, Thompson JG, Gilchrist RB. Oocyte-secreted factors enhance oocyte developmental competence. Dev Biol. 2006;296:514–521. doi: 10.1016/j.ydbio.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 48.Gomez MN, Kang JT, Koo OJ, Kim SJ, Kwon DK, Park SJ, et al. Effect of oocyte-secreted factors on porcine in vitro maturation, cumulus expansion and developmental competence of parthenotes. Zygote. 2012;20:135–145. doi: 10.1017/S0967199411000256. [DOI] [PubMed] [Google Scholar]
- 49.Yeo CX, Gilchrist RB, Thompson JG, Lane M. Exogenous growth differentiation factor 9 in oocyte maturation media enhances subsequent embryo development and fetal viability in mice. Hum Reprod. 2008;23:67–73. doi: 10.1093/humrep/dem140. [DOI] [PubMed] [Google Scholar]
- 50.Gautam SK, Verma V, Palta P, Chauhan MS, Manik RS. Effect of type of cryoprotectant on morphology and developmental competence of in vitro-matured buffalo (Bubalus bubalis) oocytes subjected to slow freezing or vitrification. Reprod Fertil Dev. 2008;20:490–496. doi: 10.1071/RD07203. [DOI] [PubMed] [Google Scholar]
- 51.Wigglesworth K, Lee KB, O’Brien MJ, Peng J, Matzuk MM, Eppig JJ. Bidirectional communication between oocytes and ovarian follicular somatic cells is required for meiotic arrest of mammalian oocytes. Proc Natl Acad Sci U S A. 2013;110:E3723–E3729. doi: 10.1073/pnas.1314829110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang K, Hansen PJ, Ealy AD. Fibroblast growth factor 10 enhances bovine oocyte maturation and developmental competence in vitro. Reproduction. 2010;140:815–826. doi: 10.1530/REP-10-0190. [DOI] [PubMed] [Google Scholar]
- 53.Solloway MJ, Dudley AT, Bikoff EK, Lyons KM, Hogan BL, Robertson EJ. Mice lacking Bmp6 function. Dev Genet. 1998;22:321–339. doi: 10.1002/(SICI)1520-6408(1998)22:4<321::AID-DVG3>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 54.Peng J, Li Q, Wigglesworth K, Rangarajan A, Kattamuri C, Peterson RT, et al. Growth differentiation factor 9: bone morphogenetic protein 15 heterodimers are potent regulators of ovarian functions. Proc Natl Acad Sci U S A. 2013;110:E776–E785. doi: 10.1073/pnas.1218020110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mottershead DG, Harrison CA, Mueller TD, Stanton PG, Gilchrist RB, McNatty KP. Growth differentiation factor 9: bone morphogenetic protein 15 (GDF9:BMP15) synergism and protein heterodimerization. Proc Natl Acad Sci U S A. 2013;110:E2257. doi: 10.1073/pnas.1303459110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hickey TE, Marrocco DL, Amato F, Ritter LJ, Norman RJ, Gilchrist RB, et al. Androgens augment the mitogenic effects of oocyte-secreted factors and growth differentiation factor 9 on porcine granulosa cells. Biol Reprod. 2005;73:825–832. doi: 10.1095/biolreprod.104.039362. [DOI] [PubMed] [Google Scholar]
- 57.Yoshino O, McMahon HE, Sharma S, Shimasaki S. A unique preovulatory expression pattern plays a key role in the physiological functions of BMP-15 in the mouse. Proc Natl Acad Sci U S A. 2006;103:10678–10683. doi: 10.1073/pnas.0600507103. [DOI] [PMC free article] [PubMed] [Google Scholar]