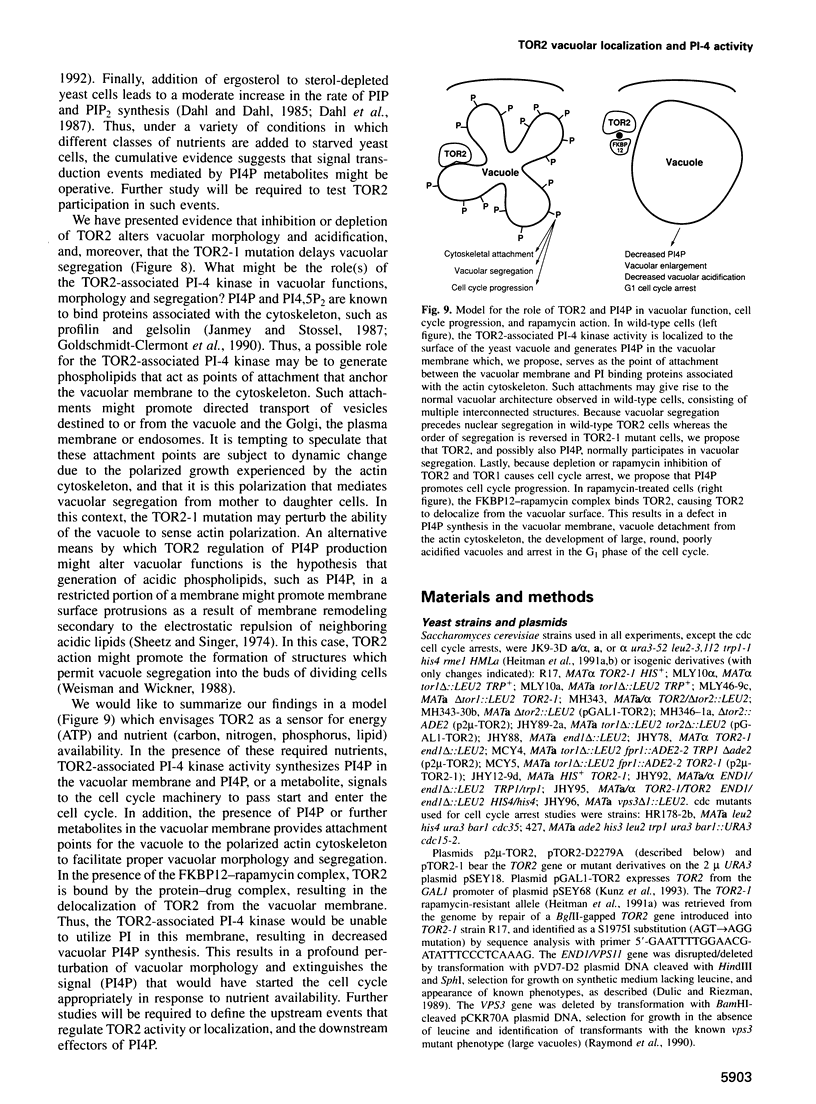
Abstract
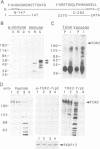
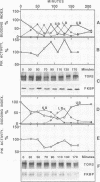
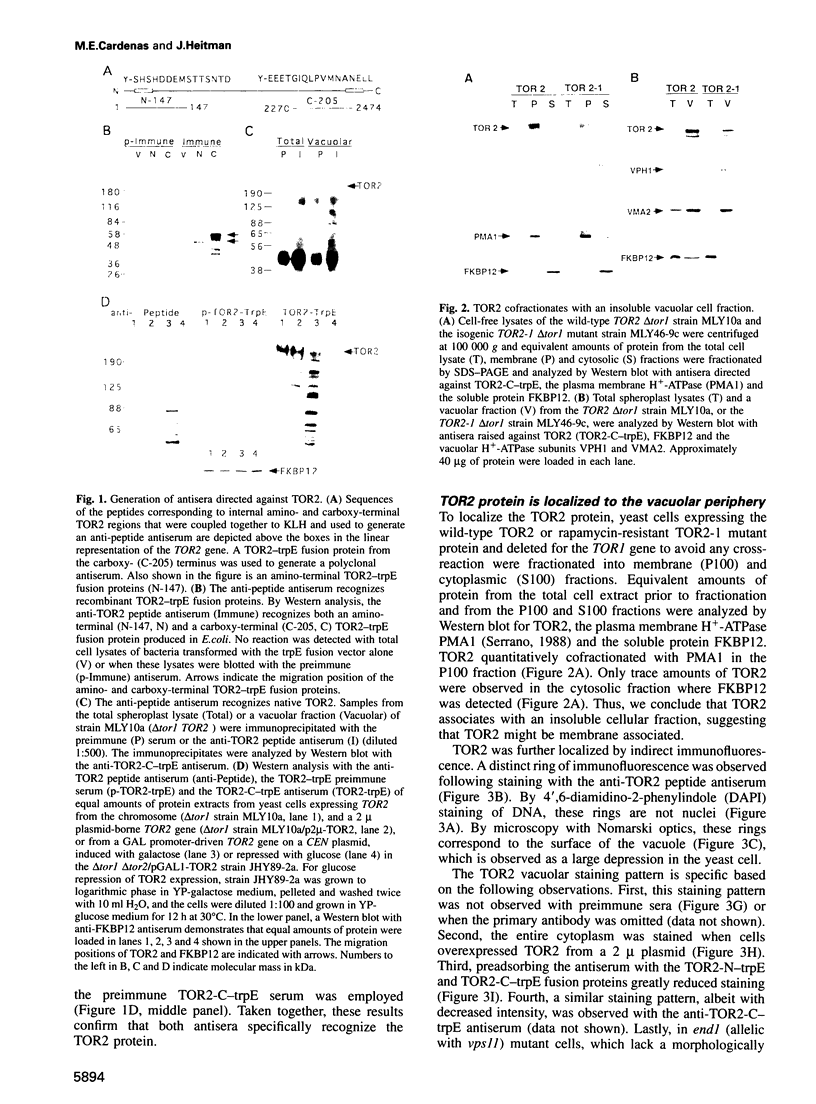
In complex with the immunophilin FKBP12, the natural product rapamycin inhibits signal transduction events required for G1 to S phase cell cycle progression in yeast and mammalian cells. Genetic studies in yeast first implicated the TOR1 and TOR2 proteins as targets of the FKBP12-rapamycin complex. We report here that the TOR2 protein is membrane associated and localized to the surface of the yeast vacuole. Immunoprecipitated TOR2 protein contains readily detectable phosphatidylinositol-4 (PI-4) kinase activity attributable to either a TOR2 intrinsic activity or to a PI-4 kinase tightly associated with TOR2. Importantly, we find that rapamycin stimulates FKBP12 binding to wild-type TOR2 but not to a rapamycin-resistant TOR2-1 mutant protein. Surprisingly, FKBP12-rapamycin binding does not markedly inhibit the PI kinase activity associated with TOR2, but does cause a delocalization of TOR2 from the vacuolar surface, which may deprive the TOR2-associated PI-4 kinase activity of its in vivo substrate. Several additional findings indicate that vacuolar localization is important for TOR2 function and, conversely, that TOR2 modulates vacuolar morphology and segregation. These studies demonstrate that TOR2 is an essential, highly conserved component of a signal transduction pathway regulating cell cycle progression conserved from yeast to man.
Full text
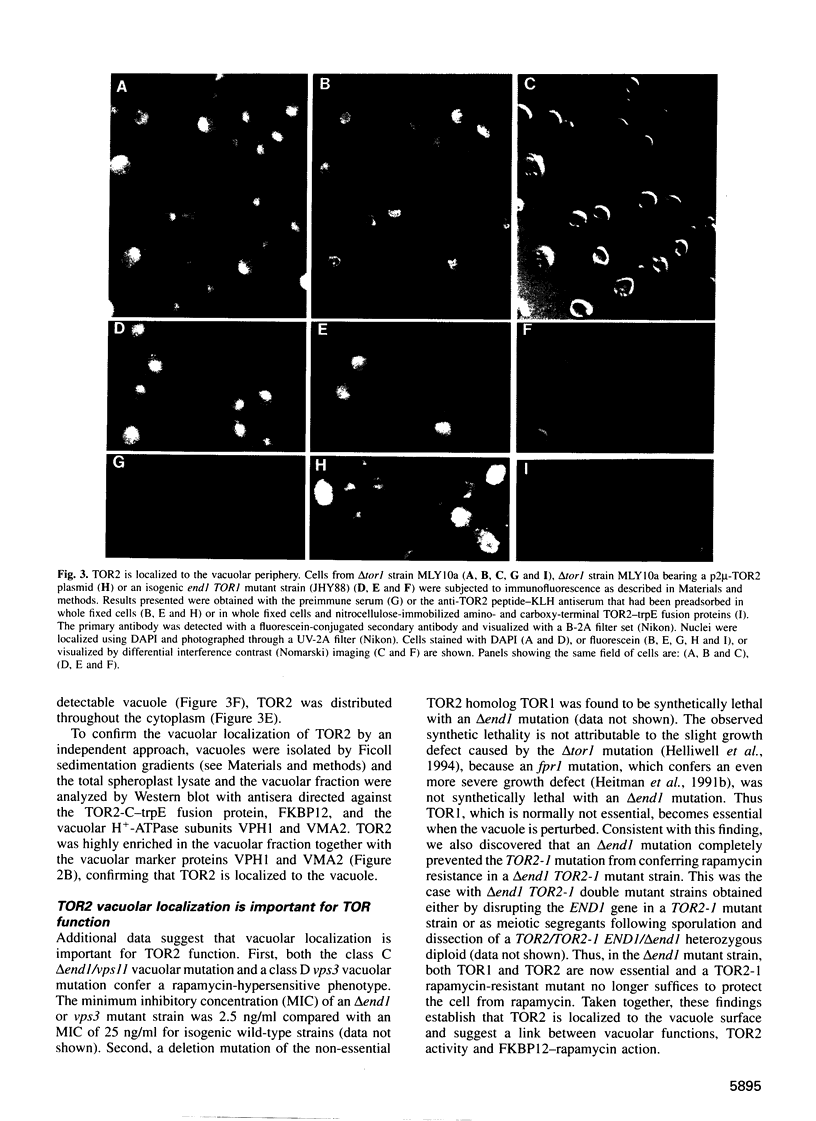
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aitken J. F., van Heusden G. P., Temkin M., Dowhan W. The gene encoding the phosphatidylinositol transfer protein is essential for cell growth. J Biol Chem. 1990 Mar 15;265(8):4711–4717. [PubMed] [Google Scholar]
- Albers M. W., Williams R. T., Brown E. J., Tanaka A., Hall F. L., Schreiber S. L. FKBP-rapamycin inhibits a cyclin-dependent kinase activity and a cyclin D1-Cdk association in early G1 of an osteosarcoma cell line. J Biol Chem. 1993 Oct 25;268(30):22825–22829. [PubMed] [Google Scholar]
- Bankaitis V. A., Aitken J. R., Cleves A. E., Dowhan W. An essential role for a phospholipid transfer protein in yeast Golgi function. Nature. 1990 Oct 11;347(6293):561–562. doi: 10.1038/347561a0. [DOI] [PubMed] [Google Scholar]
- Becker G. W., Lester R. L. Changes in phospholipids of Saccharomyces cerevisiae associated with inositol-less death. J Biol Chem. 1977 Dec 10;252(23):8684–8691. [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and calcium signalling. Nature. 1993 Jan 28;361(6410):315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol phosphates and cell signalling. Nature. 1989 Sep 21;341(6239):197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Bierer B. E., Mattila P. S., Standaert R. F., Herzenberg L. A., Burakoff S. J., Crabtree G., Schreiber S. L. Two distinct signal transmission pathways in T lymphocytes are inhibited by complexes formed between an immunophilin and either FK506 or rapamycin. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9231–9235. doi: 10.1073/pnas.87.23.9231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boronenkov I. V., Anderson R. A. The sequence of phosphatidylinositol-4-phosphate 5-kinase defines a novel family of lipid kinases. J Biol Chem. 1995 Feb 17;270(7):2881–2884. doi: 10.1074/jbc.270.7.2881. [DOI] [PubMed] [Google Scholar]
- Breuder T., Hemenway C. S., Movva N. R., Cardenas M. E., Heitman J. Calcineurin is essential in cyclosporin A- and FK506-sensitive yeast strains. Proc Natl Acad Sci U S A. 1994 Jun 7;91(12):5372–5376. doi: 10.1073/pnas.91.12.5372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown E. J., Albers M. W., Shin T. B., Ichikawa K., Keith C. T., Lane W. S., Schreiber S. L. A mammalian protein targeted by G1-arresting rapamycin-receptor complex. Nature. 1994 Jun 30;369(6483):756–758. doi: 10.1038/369756a0. [DOI] [PubMed] [Google Scholar]
- Cafferkey R., Young P. R., McLaughlin M. M., Bergsma D. J., Koltin Y., Sathe G. M., Faucette L., Eng W. K., Johnson R. K., Livi G. P. Dominant missense mutations in a novel yeast protein related to mammalian phosphatidylinositol 3-kinase and VPS34 abrogate rapamycin cytotoxicity. Mol Cell Biol. 1993 Oct;13(10):6012–6023. doi: 10.1128/mcb.13.10.6012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas M. E., Hemenway C., Muir R. S., Ye R., Fiorentino D., Heitman J. Immunophilins interact with calcineurin in the absence of exogenous immunosuppressive ligands. EMBO J. 1994 Dec 15;13(24):5944–5957. doi: 10.1002/j.1460-2075.1994.tb06940.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas M. E., Muir R. S., Breuder T., Heitman J. Targets of immunophilin-immunosuppressant complexes are distinct highly conserved regions of calcineurin A. EMBO J. 1995 Jun 15;14(12):2772–2783. doi: 10.1002/j.1460-2075.1995.tb07277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu M. I., Katz H., Berlin V. RAPT1, a mammalian homolog of yeast Tor, interacts with the FKBP12/rapamycin complex. Proc Natl Acad Sci U S A. 1994 Dec 20;91(26):12574–12578. doi: 10.1073/pnas.91.26.12574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung J., Kuo C. J., Crabtree G. R., Blenis J. Rapamycin-FKBP specifically blocks growth-dependent activation of and signaling by the 70 kd S6 protein kinases. Cell. 1992 Jun 26;69(7):1227–1236. doi: 10.1016/0092-8674(92)90643-q. [DOI] [PubMed] [Google Scholar]
- Clipstone N. A., Crabtree G. R. Identification of calcineurin as a key signalling enzyme in T-lymphocyte activation. Nature. 1992 Jun 25;357(6380):695–697. doi: 10.1038/357695a0. [DOI] [PubMed] [Google Scholar]
- Conradt B., Shaw J., Vida T., Emr S., Wickner W. In vitro reactions of vacuole inheritance in Saccharomyces cerevisiae. J Cell Biol. 1992 Dec;119(6):1469–1479. doi: 10.1083/jcb.119.6.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyert M. S., Kunisawa R., Kaim D., Thorner J. Yeast has homologs (CNA1 and CNA2 gene products) of mammalian calcineurin, a calmodulin-regulated phosphoprotein phosphatase. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7376–7380. doi: 10.1073/pnas.88.16.7376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl C., Biemann H. P., Dahl J. A protein kinase antigenically related to pp60v-src possibly involved in yeast cell cycle control: positive in vivo regulation by sterol. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4012–4016. doi: 10.1073/pnas.84.12.4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl J. S., Dahl C. E. Stimulation of cell proliferation and polyphosphoinositide metabolism in Saccharomyces cerevisiae GL7 by ergosterol. Biochem Biophys Res Commun. 1985 Dec 31;133(3):844–850. doi: 10.1016/0006-291x(85)91211-2. [DOI] [PubMed] [Google Scholar]
- Dilling M. B., Dias P., Shapiro D. N., Germain G. S., Johnson R. K., Houghton P. J. Rapamycin selectively inhibits the growth of childhood rhabdomyosarcoma cells through inhibition of signaling via the type I insulin-like growth factor receptor. Cancer Res. 1994 Feb 15;54(4):903–907. [PubMed] [Google Scholar]
- Dulić V., Riezman H. Characterization of the END1 gene required for vacuole biogenesis and gluconeogenic growth of budding yeast. EMBO J. 1989 May;8(5):1349–1359. doi: 10.1002/j.1460-2075.1989.tb03515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont F. J., Melino M. R., Staruch M. J., Koprak S. L., Fischer P. A., Sigal N. H. The immunosuppressive macrolides FK-506 and rapamycin act as reciprocal antagonists in murine T cells. J Immunol. 1990 Feb 15;144(4):1418–1424. [PubMed] [Google Scholar]
- Flanagan C. A., Schnieders E. A., Emerick A. W., Kunisawa R., Admon A., Thorner J. Phosphatidylinositol 4-kinase: gene structure and requirement for yeast cell viability. Science. 1993 Nov 26;262(5138):1444–1448. doi: 10.1126/science.8248783. [DOI] [PubMed] [Google Scholar]
- Foor F., Parent S. A., Morin N., Dahl A. M., Ramadan N., Chrebet G., Bostian K. A., Nielsen J. B. Calcineurin mediates inhibition by FK506 and cyclosporin of recovery from alpha-factor arrest in yeast. Nature. 1992 Dec 17;360(6405):682–684. doi: 10.1038/360682a0. [DOI] [PubMed] [Google Scholar]
- Garcia-Bustos J. F., Marini F., Stevenson I., Frei C., Hall M. N. PIK1, an essential phosphatidylinositol 4-kinase associated with the yeast nucleus. EMBO J. 1994 May 15;13(10):2352–2361. doi: 10.1002/j.1460-2075.1994.tb06519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gietz R. D., Sugino A. New yeast-Escherichia coli shuttle vectors constructed with in vitro mutagenized yeast genes lacking six-base pair restriction sites. Gene. 1988 Dec 30;74(2):527–534. doi: 10.1016/0378-1119(88)90185-0. [DOI] [PubMed] [Google Scholar]
- Goldschmidt-Clermont P. J., Machesky L. M., Baldassare J. J., Pollard T. D. The actin-binding protein profilin binds to PIP2 and inhibits its hydrolysis by phospholipase C. Science. 1990 Mar 30;247(4950):1575–1578. doi: 10.1126/science.2157283. [DOI] [PubMed] [Google Scholar]
- Hawkins P. T., Stephens L. R., Piggott J. R. Analysis of inositol metabolites produced by Saccharomyces cerevisiae in response to glucose stimulation. J Biol Chem. 1993 Feb 15;268(5):3374–3383. [PubMed] [Google Scholar]
- Hay J. C., Fisette P. L., Jenkins G. H., Fukami K., Takenawa T., Anderson R. A., Martin T. F. ATP-dependent inositide phosphorylation required for Ca(2+)-activated secretion. Nature. 1995 Mar 9;374(6518):173–177. doi: 10.1038/374173a0. [DOI] [PubMed] [Google Scholar]
- Heitman J., Movva N. R., Hall M. N. Proline isomerases at the crossroads of protein folding, signal transduction, and immunosuppression. New Biol. 1992 May;4(5):448–460. [PubMed] [Google Scholar]
- Heitman J., Movva N. R., Hall M. N. Targets for cell cycle arrest by the immunosuppressant rapamycin in yeast. Science. 1991 Aug 23;253(5022):905–909. doi: 10.1126/science.1715094. [DOI] [PubMed] [Google Scholar]
- Heitman J., Movva N. R., Hiestand P. C., Hall M. N. FK 506-binding protein proline rotamase is a target for the immunosuppressive agent FK 506 in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1948–1952. doi: 10.1073/pnas.88.5.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helliwell S. B., Wagner P., Kunz J., Deuter-Reinhard M., Henriquez R., Hall M. N. TOR1 and TOR2 are structurally and functionally similar but not identical phosphatidylinositol kinase homologues in yeast. Mol Biol Cell. 1994 Jan;5(1):105–118. doi: 10.1091/mbc.5.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiles I. D., Otsu M., Volinia S., Fry M. J., Gout I., Dhand R., Panayotou G., Ruiz-Larrea F., Thompson A., Totty N. F. Phosphatidylinositol 3-kinase: structure and expression of the 110 kd catalytic subunit. Cell. 1992 Aug 7;70(3):419–429. doi: 10.1016/0092-8674(92)90166-a. [DOI] [PubMed] [Google Scholar]
- Ho S. N., Hunt H. D., Horton R. M., Pullen J. K., Pease L. R. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989 Apr 15;77(1):51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- Janmey P. A. Phosphoinositides and calcium as regulators of cellular actin assembly and disassembly. Annu Rev Physiol. 1994;56:169–191. doi: 10.1146/annurev.ph.56.030194.001125. [DOI] [PubMed] [Google Scholar]
- Janmey P. A. Protein regulation by phosphatidylinositol lipids. Chem Biol. 1995 Feb;2(2):61–65. doi: 10.1016/1074-5521(95)90276-7. [DOI] [PubMed] [Google Scholar]
- Janmey P. A., Stossel T. P. Modulation of gelsolin function by phosphatidylinositol 4,5-bisphosphate. Nature. 1987 Jan 22;325(6102):362–364. doi: 10.1038/325362a0. [DOI] [PubMed] [Google Scholar]
- Jayaraman T., Marks A. R. Rapamycin-FKBP12 blocks proliferation, induces differentiation, and inhibits cdc2 kinase activity in a myogenic cell line. J Biol Chem. 1993 Dec 5;268(34):25385–25388. [PubMed] [Google Scholar]
- Kapeller R., Cantley L. C. Phosphatidylinositol 3-kinase. Bioessays. 1994 Aug;16(8):565–576. doi: 10.1002/bies.950160810. [DOI] [PubMed] [Google Scholar]
- Kato R., Ogawa H. An essential gene, ESR1, is required for mitotic cell growth, DNA repair and meiotic recombination in Saccharomyces cerevisiae. Nucleic Acids Res. 1994 Aug 11;22(15):3104–3112. doi: 10.1093/nar/22.15.3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klionsky D. J., Herman P. K., Emr S. D. The fungal vacuole: composition, function, and biogenesis. Microbiol Rev. 1990 Sep;54(3):266–292. doi: 10.1128/mr.54.3.266-292.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knighton D. R., Zheng J. H., Ten Eyck L. F., Ashford V. A., Xuong N. H., Taylor S. S., Sowadski J. M. Crystal structure of the catalytic subunit of cyclic adenosine monophosphate-dependent protein kinase. Science. 1991 Jul 26;253(5018):407–414. doi: 10.1126/science.1862342. [DOI] [PubMed] [Google Scholar]
- Koerner T. J., Hill J. E., Myers A. M., Tzagoloff A. High-expression vectors with multiple cloning sites for construction of trpE fusion genes: pATH vectors. Methods Enzymol. 1991;194:477–490. doi: 10.1016/0076-6879(91)94036-c. [DOI] [PubMed] [Google Scholar]
- Koltin Y., Faucette L., Bergsma D. J., Levy M. A., Cafferkey R., Koser P. L., Johnson R. K., Livi G. P. Rapamycin sensitivity in Saccharomyces cerevisiae is mediated by a peptidyl-prolyl cis-trans isomerase related to human FK506-binding protein. Mol Cell Biol. 1991 Mar;11(3):1718–1723. doi: 10.1128/mcb.11.3.1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz J., Henriquez R., Schneider U., Deuter-Reinhard M., Movva N. R., Hall M. N. Target of rapamycin in yeast, TOR2, is an essential phosphatidylinositol kinase homolog required for G1 progression. Cell. 1993 May 7;73(3):585–596. doi: 10.1016/0092-8674(93)90144-f. [DOI] [PubMed] [Google Scholar]
- Liu J., Albers M. W., Wandless T. J., Luan S., Alberg D. G., Belshaw P. J., Cohen P., MacKintosh C., Klee C. B., Schreiber S. L. Inhibition of T cell signaling by immunophilin-ligand complexes correlates with loss of calcineurin phosphatase activity. Biochemistry. 1992 Apr 28;31(16):3896–3901. doi: 10.1021/bi00131a002. [DOI] [PubMed] [Google Scholar]
- Liu J. FK506 and cyclosporin, molecular probes for studying intracellular signal transduction. Immunol Today. 1993 Jun;14(6):290–295. doi: 10.1016/0167-5699(93)90048-P. [DOI] [PubMed] [Google Scholar]
- Liu J., Farmer J. D., Jr, Lane W. S., Friedman J., Weissman I., Schreiber S. L. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell. 1991 Aug 23;66(4):807–815. doi: 10.1016/0092-8674(91)90124-h. [DOI] [PubMed] [Google Scholar]
- Liu Y., Ishii S., Tokai M., Tsutsumi H., Ohki O., Akada R., Tanaka K., Tsuchiya E., Fukui S., Miyakawa T. The Saccharomyces cerevisiae genes (CMP1 and CMP2) encoding calmodulin-binding proteins homologous to the catalytic subunit of mammalian protein phosphatase 2B. Mol Gen Genet. 1991 May;227(1):52–59. doi: 10.1007/BF00260706. [DOI] [PubMed] [Google Scholar]
- Majerus P. W., Ross T. S., Cunningham T. W., Caldwell K. K., Jefferson A. B., Bansal V. S. Recent insights in phosphatidylinositol signaling. Cell. 1990 Nov 2;63(3):459–465. doi: 10.1016/0092-8674(90)90442-h. [DOI] [PubMed] [Google Scholar]
- Mattila P. S., Ullman K. S., Fiering S., Emmel E. A., McCutcheon M., Crabtree G. R., Herzenberg L. A. The actions of cyclosporin A and FK506 suggest a novel step in the activation of T lymphocytes. EMBO J. 1990 Dec;9(13):4425–4433. doi: 10.1002/j.1460-2075.1990.tb07893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T., Liu Y., Hirata D., Namba H., Harada S., Hirokawa T., Miyakawa T. Protein phosphatase type 2B (calcineurin)-mediated, FK506-sensitive regulation of intracellular ions in yeast is an important determinant for adaptation to high salt stress conditions. EMBO J. 1993 Nov;12(11):4063–4071. doi: 10.1002/j.1460-2075.1993.tb06090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickels J. T., Jr, Carman G. M. Photoaffinity labeling of the 45-kDa and 55-kDa forms of phosphatidylinositol 4-kinase from the yeast Saccharomyces cerevisiae. J Biol Chem. 1993 Nov 15;268(32):24083–24088. [PubMed] [Google Scholar]
- Nikawa J., Kodaki T., Yamashita S. Primary structure and disruption of the phosphatidylinositol synthase gene of Saccharomyces cerevisiae. J Biol Chem. 1987 Apr 5;262(10):4876–4881. [PubMed] [Google Scholar]
- Novick P., Osmond B. C., Botstein D. Suppressors of yeast actin mutations. Genetics. 1989 Apr;121(4):659–674. doi: 10.1093/genetics/121.4.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Keefe S. J., Tamura J., Kincaid R. L., Tocci M. J., O'Neill E. A. FK-506- and CsA-sensitive activation of the interleukin-2 promoter by calcineurin. Nature. 1992 Jun 25;357(6380):692–694. doi: 10.1038/357692a0. [DOI] [PubMed] [Google Scholar]
- Price D. J., Grove J. R., Calvo V., Avruch J., Bierer B. E. Rapamycin-induced inhibition of the 70-kilodalton S6 protein kinase. Science. 1992 Aug 14;257(5072):973–977. doi: 10.1126/science.1380182. [DOI] [PubMed] [Google Scholar]
- Pringle J. R., Preston R. A., Adams A. E., Stearns T., Drubin D. G., Haarer B. K., Jones E. W. Fluorescence microscopy methods for yeast. Methods Cell Biol. 1989;31:357–435. doi: 10.1016/s0091-679x(08)61620-9. [DOI] [PubMed] [Google Scholar]
- Raymond C. K., Howald-Stevenson I., Vater C. A., Stevens T. H. Morphological classification of the yeast vacuolar protein sorting mutants: evidence for a prevacuolar compartment in class E vps mutants. Mol Biol Cell. 1992 Dec;3(12):1389–1402. doi: 10.1091/mbc.3.12.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond C. K., O'Hara P. J., Eichinger G., Rothman J. H., Stevens T. H. Molecular analysis of the yeast VPS3 gene and the role of its product in vacuolar protein sorting and vacuolar segregation during the cell cycle. J Cell Biol. 1990 Sep;111(3):877–892. doi: 10.1083/jcb.111.3.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts C. J., Raymond C. K., Yamashiro C. T., Stevens T. H. Methods for studying the yeast vacuole. Methods Enzymol. 1991;194:644–661. doi: 10.1016/0076-6879(91)94047-g. [DOI] [PubMed] [Google Scholar]
- Sabatini D. M., Erdjument-Bromage H., Lui M., Tempst P., Snyder S. H. RAFT1: a mammalian protein that binds to FKBP12 in a rapamycin-dependent fashion and is homologous to yeast TORs. Cell. 1994 Jul 15;78(1):35–43. doi: 10.1016/0092-8674(94)90570-3. [DOI] [PubMed] [Google Scholar]
- Sabers C. J., Martin M. M., Brunn G. J., Williams J. M., Dumont F. J., Wiederrecht G., Abraham R. T. Isolation of a protein target of the FKBP12-rapamycin complex in mammalian cells. J Biol Chem. 1995 Jan 13;270(2):815–822. doi: 10.1074/jbc.270.2.815. [DOI] [PubMed] [Google Scholar]
- Schomerus C., Küntzel H. CDC25-dependent induction of inositol 1,4,5-trisphosphate and diacylglycerol in Saccharomyces cerevisiae by nitrogen. FEBS Lett. 1992 Aug 3;307(3):249–252. doi: 10.1016/0014-5793(92)80688-d. [DOI] [PubMed] [Google Scholar]
- Schreiber S. L. Chemistry and biology of the immunophilins and their immunosuppressive ligands. Science. 1991 Jan 18;251(4991):283–287. doi: 10.1126/science.1702904. [DOI] [PubMed] [Google Scholar]
- Schreiber S. L., Crabtree G. R. The mechanism of action of cyclosporin A and FK506. Immunol Today. 1992 Apr;13(4):136–142. doi: 10.1016/0167-5699(92)90111-J. [DOI] [PubMed] [Google Scholar]
- Schu P. V., Takegawa K., Fry M. J., Stack J. H., Waterfield M. D., Emr S. D. Phosphatidylinositol 3-kinase encoded by yeast VPS34 gene essential for protein sorting. Science. 1993 Apr 2;260(5104):88–91. doi: 10.1126/science.8385367. [DOI] [PubMed] [Google Scholar]
- Serrano R. Structure and function of proton translocating ATPase in plasma membranes of plants and fungi. Biochim Biophys Acta. 1988 Feb 24;947(1):1–28. doi: 10.1016/0304-4157(88)90017-2. [DOI] [PubMed] [Google Scholar]
- Serunian L. A., Auger K. R., Cantley L. C. Identification and quantification of polyphosphoinositides produced in response to platelet-derived growth factor stimulation. Methods Enzymol. 1991;198:78–87. doi: 10.1016/0076-6879(91)98010-4. [DOI] [PubMed] [Google Scholar]
- Sheetz M. P., Singer S. J. Biological membranes as bilayer couples. A molecular mechanism of drug-erythrocyte interactions. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4457–4461. doi: 10.1073/pnas.71.11.4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikorski R. S., Boeke J. D. In vitro mutagenesis and plasmid shuffling: from cloned gene to mutant yeast. Methods Enzymol. 1991;194:302–318. doi: 10.1016/0076-6879(91)94023-6. [DOI] [PubMed] [Google Scholar]
- Stack J. H., Herman P. K., Schu P. V., Emr S. D. A membrane-associated complex containing the Vps15 protein kinase and the Vps34 PI 3-kinase is essential for protein sorting to the yeast lysosome-like vacuole. EMBO J. 1993 May;12(5):2195–2204. doi: 10.1002/j.1460-2075.1993.tb05867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvia V., Curtin G., Norman J., Stec J., Busbee D. Activation of a low specific activity form of DNA polymerase alpha by inositol-1,4-bisphosphate. Cell. 1988 Aug 26;54(5):651–658. doi: 10.1016/s0092-8674(88)80009-6. [DOI] [PubMed] [Google Scholar]
- Uno I., Fukami K., Kato H., Takenawa T., Ishikawa T. Essential role for phosphatidylinositol 4,5-bisphosphate in yeast cell proliferation. Nature. 1988 May 12;333(6169):188–190. doi: 10.1038/333188a0. [DOI] [PubMed] [Google Scholar]
- Walsh J. P., Caldwell K. K., Majerus P. W. Formation of phosphatidylinositol 3-phosphate by isomerization from phosphatidylinositol 4-phosphate. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):9184–9187. doi: 10.1073/pnas.88.20.9184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisman L. S., Wickner W. Intervacuole exchange in the yeast zygote: a new pathway in organelle communication. Science. 1988 Jul 29;241(4865):589–591. doi: 10.1126/science.3041591. [DOI] [PubMed] [Google Scholar]
- Wong K., Cantley L. C. Cloning and characterization of a human phosphatidylinositol 4-kinase. J Biol Chem. 1994 Nov 18;269(46):28878–28884. [PubMed] [Google Scholar]
- York J. D., Saffitz J. E., Majerus P. W. Inositol polyphosphate 1-phosphatase is present in the nucleus and inhibits DNA synthesis. J Biol Chem. 1994 Aug 5;269(31):19992–19999. [PubMed] [Google Scholar]
- Yoshida S., Ohya Y., Goebl M., Nakano A., Anraku Y. A novel gene, STT4, encodes a phosphatidylinositol 4-kinase in the PKC1 protein kinase pathway of Saccharomyces cerevisiae. J Biol Chem. 1994 Jan 14;269(2):1166–1172. [PubMed] [Google Scholar]
- de Groot R. P., Ballou L. M., Sassone-Corsi P. Positive regulation of the cAMP-responsive activator CREM by the p70 S6 kinase: an alternative route to mitogen-induced gene expression. Cell. 1994 Oct 7;79(1):81–91. doi: 10.1016/0092-8674(94)90402-2. [DOI] [PubMed] [Google Scholar]