Abstract
Purpose
To determine if microRNAs are differentially expressed in the follicular fluid of women with PCOS compared to fertile oocyte donors and identify associated altered gene expression.
Methods
Women undergoing IVF who met Rotterdam criteria for PCOS or who were fertile oocyte donors were recruited from a private IVF center. Individual follicle fluid was collected at the time of oocyte retrieval. MicroRNA analysis was performed using microarray and validated using real-time PCR on additional samples. Potential gene targets were identified and their expression analyzed by real time PCR.
Results
Microarray profiling of human follicular fluid revealed expression of 235 miRNAs, 29 were differentially expressed between the groups. Using PCR validation, 5 miRNAs (32, 34c, 135a, 18b, and 9) showed significantly increased expression in the PCOS group. Pathway analysis revealed genes involved in insulin regulation and inflammation. Three potential target genes were found to have significantly decreased expression in the PCOS group (interleukin 8, synaptogamin 1, and insulin receptor substrate 2).
Conclusions
MicroRNAs are differentially expressed in the follicular fluid of women with PCOS when compared to fertile oocyte donors. There is also altered expression of potential target genes associated with the PCOS phenotype.
Keywords: microRNAs, Follicular fluid, Polycystic ovary syndrome, Oocyte donors
Introduction
Polycystic ovary syndrome (PCOS) is a clinical syndrome with known phenotypic, hormonal, and metabolic abnormalities [1]. PCOS is the most common endocrine disorder in women [2] with a prevalence ranging from 6.1 to 19.9 %, depending on the criteria used for diagnosis [3]. Regardless of criteria used, the diagnosis of PCOS is associated with a two-fold increase in metabolic syndrome [3].
Women with PCOS have lower fecundability and higher infertility than women without PCOS [4]. Many factors may play a role in the subfertility associated with PCOS including obesity, endocrine abnormalities, and altered milieu in the developing follicle [1]. Two miRNAs (miR-132 and miR-320) were shown to have decreased expression in the follicular fluid of women with PCOS compared to women undergoing in vitro fertilization (IVF) for male factor infertility [5].
MicroRNAs (miRNA) are single-stranded noncoding RNA molecules composed of 20–24 nucleotides [6]. They are involved in the normal functioning of eukaryotic cells, including mammalian cells [7] and in abnormal functioning, or disease states, including cancers [8], heart disease [9], and infertility [7, 10, 11]. MicroRNAs regulate genes positively or negatively through a variety of mechanisms at the posttranscriptional level [12].
Recent research revealed miRNAs are present in the follicular fluid of humans [5] mares [13]. Two hundred miRNAs were found to be present in human follicular fluid both in microsomes and in free form [5]. Twenty-five miRNAs were found to be expressed in microsomes, exosomes, and in free form in the follicular fluid of mares [13]. In addition to follicular fluid, miRNAs have been identified in other human bodily fluids [14] including serum [15], urine [15], and saliva [16]. A commercially available miRNA panel is being used clinically in oncology as a disease biomarker and miRNAs are being investigated as a therapeutic target in many disease states [17].
The objectives of this study were to describe miRNAs in follicular fluid of women with PCOS compared to fertile oocyte donors (OD) and to identify target genes of the miRNAs associated with the PCOS phenotype.
Materials and methods
Participants
Consecutive patients undergoing IVF at a single center were recruited for participation in the study. Participants were approached if they met Rotterdam criteria for PCOS [18] or were fertile oocyte donors with no known infertility diagnoses. Informed consent was obtained and the protocol was IRB approved.
Ovarian stimulation
Ovarian stimulation and all other aspects of the IVF cycle were managed according to routine care, independent of this study. The majority of patients from both groups received an antagonist protocol (PCOS 92 % and OD 85 %, p = 0.6), receiving both FSH and LH for ovarian stimulation and GnRH antagonist per clinical protocol. Peak estradiol levels did not differ between the groups (PCOS 4,620 ± 1,925 vs. OD 4,463 ± 1,419 pg/ml, p = 0.8).
Fluid collection
At the time of oocyte retrieval, fluid from the first punctured follicle was collected from each ovary. The fluid was centrifuged for 5 min at 1,500 × g to pellet any blood and debris. The supernatant was then removed and cryopreserved using liquid nitrogen.
RNA isolation
Nine hundred microliters of individual follicle follicular fluid from each participant was used for RNA isolation using the mirVana™PARIS™Kit (Life Technologies, USA) according to manufacturer’s instructions. Briefly, RNA was separated from DNA and other organics using Acid-Phenol:Chloroform and then purified by binding to a glass-fiber filter where RNA was washed several times and then eluted with 100ul of water. Purified RNA was concentrated to an 8ul volume and then treated with a 2ul DNase I solution (Sigma-Aldrich, USA) to remove any contaminating DNA. After incubating at room temperature for 15 min, 1ul of Stop Solution was added to each sample and incubated at 70 °C for 10 min to prevent hydrolysis of the RNA. Quantification was determined using the NanaDrop Spectrophotometer (Thermo Scientific, USA).
Reverse transcription and miRNA pre-amplification
Seventy-five nanogram of each sample was reverse transcribed using the Megaplex™ Human Primer Pools A and the Taqman® MicroRNA Reverse Transcription Kit (Life Technologies, USA). 4.5ul of a master mix containing 20 mM dNTPs, 75U reverse transcriptase, 2U ribonuclease inhibitor, 22.5 mM MgCl2, and 1X RT primers, was combined with 3ul of each sample and incubated using the following thermal-cycling conditions: 40 cycles at 16 °C for 2 min, 42 °C for 1 min, and 50 °C for 1 s followed by a 5 min hold at 85 °C. cDNA targets were then preamplified using Taqman® PreAmp Master Mix and Megaplex™ PreAmp Human Pools A (Life Technologies, USA) to increase the amount of desired cDNA. 20ul of this master mix was combined with 5ul of cDNA for each sample and incubated as follows: 95 °C for 10 min, 55 °C for 2 min, 72 °C for 2 min, 12 cycles at 95 °C for 15 s and 60 °C for 4 min, and a final hold for 10 min at 99.9 °C. 75ul of a 0.1X TE solution was added to each sample after amplification and stored at −20 °C until analysis was performed.
Reverse transcription for miRNA validation
Additional samples were isolated and reverse transcribed without pre-amplification for array validation. 5 ng of purified RNA was reverse transcribed using the High Capacity cDNA Reverse Transcription Kit (Life Technologies, USA). 20ul of a master mix containing 1X dNTPs, 2.5U reverse transcriptase, 1U ribonuclease inhibitor, and 1X primers was combined with 20ul of each sample and incubated at 25 °C for 10 min followed by 37 °C for 2 h. The resulting cDNA was diluted 10-fold for all real-time PCR reactions.
Real-time PCR
For microRNA analysis using the Taqman® Array Human MicroRNA A Card, a master mix containing 1X Taqman® Universal PCR Master Mix, No AmpErase® UNG (Life Technologies, USA) was combined with 9ul of diluted PreAmp product for a final volume of 900ul. 100ul of this PCR reaction mix was added to each of the 8 ports of the array card and run on the ABI 7900HT Fast Real-Time PCR System (Life Technologies, USA) using the following thermal-cycling conditions: 50 °C for 2 min, 94.5 °C for 10 min, and 40 cycles of 97 °C for 30 s and 59.7 °C for 1 min. Ct curves were analyzed using the RQ Manager 1.2.1 (Life Technologies, USA). Statistical analysis for PCR data was performed using the internal constant housekeeping miRNAs and REST 2009 software with bootstrap randomization techniques (Qiagen, USA). Significance was defined at p < 0.05. For microRNA array validation, a master mix containing 1X Taqman® Universal PCR Master Mix, No AmpErase® UNG and 1X Taqman® Assay (Life Technologies, USA) was combined with 7 ng cDNA for a final volume of 20ul. Each sample was run in duplicate on the 7300 Real-Time PCR System (Life Technologies, USA) for 10 min at 95 °C, followed by 40 cycles at 95 °C for 15 s and 60 °C for 1 min. Expression of 10 miRNA probe sets (hsa-miR-32, hsa-miR −34c-5p, hsa-miR-135a, hsa-miR-138, hsa-miR-142-5p, hsa-miR-18b, hsa-miR-489, hsa-miR-627, hsa-miR-888, and hsa-miR-9) were analyzed relative to the internal constant expression of the miRNA control probe U6snRNA in each of the individual follicle fluid samples. Currently, there is no standard for an internal control for miRNAs. We chose U6snRNA because it has been reported as an internal control in prior studies [19, 20], including in follicular fluid [5]. Standard curves were calculated for each probe set using 10-fold serial dilutions of reference miRNA (Life Technologies, USA).
For expression of target genes, Power SYBR® Green PCR Master Mix (Life Technologies, USA) was combined with 2.5uM of each primer set and 7 ng of cDNA for a final volume of 25ul. All primer set were designed by Primer Express Version 3.0 (Life Technologies, USA). The real-time PCR reaction was carried out on the ABI 7300 Real-Time PCR machine under the following thermal cycling conditions: 95 °C for 10 min, 40 cycles at 95 °C for 15 s, and 60 °C for 1 min, and a dissociation step at 95 °C for 15 s, 60 °C for 1 min, 95 °C for 15 s, and 60 °C for 15 s. Quantification of 8 target genes (CCL5, DUSP5, IL8, IRS2, FOXN3, UGP2, SYT1, and SYTL4) was calculated relative to the internal constant housekeeping gene, PPIA. Several potential internal housekeeping genes were tested and PPIA was found to be constant in all of our follicular fluid samples. All reactions were performed in duplicate relative to a standard curve of input reference RNA which contained 4, 10-fold serial dilutions of known concentration. The PCR reaction efficiency recorded R2 values ≥0.9 and the correlation coefficient was calculated >0.99.
Statistical analysis
Statistical analysis for PCR was performed using Relative Expression Software Tool (REST©) 2009 software using bootstrap randomization techniques (Qiagen, USA). This tool incorporates the variability of the from the control miRNA (u6snRNA) and the test miRNAs or the housekeeping gene (PPIA) and the study genes to calculate statistical significance [21]. Clinical characteristics of the groups were compared using t tests or Mann–Whitney as appropriate using Analyze-it® for Excel. Significance was defined at p < 0.05.
Target gene analysis
Pathway Studio (Elsevier) was used to investigate predicted target genes and pathways of these 5 significantly increased miRNAs in PCOS samples compared to OD controls (p < 0.05). Key pathways identified included insulin regulation, cell signaling, cell cycle, and DNA repair. From this analysis, predicted target genes were chosen from pathways involved in insulin regulation and inflammation due to their association with the PCOS phenotype.
Results
miRNA profiling
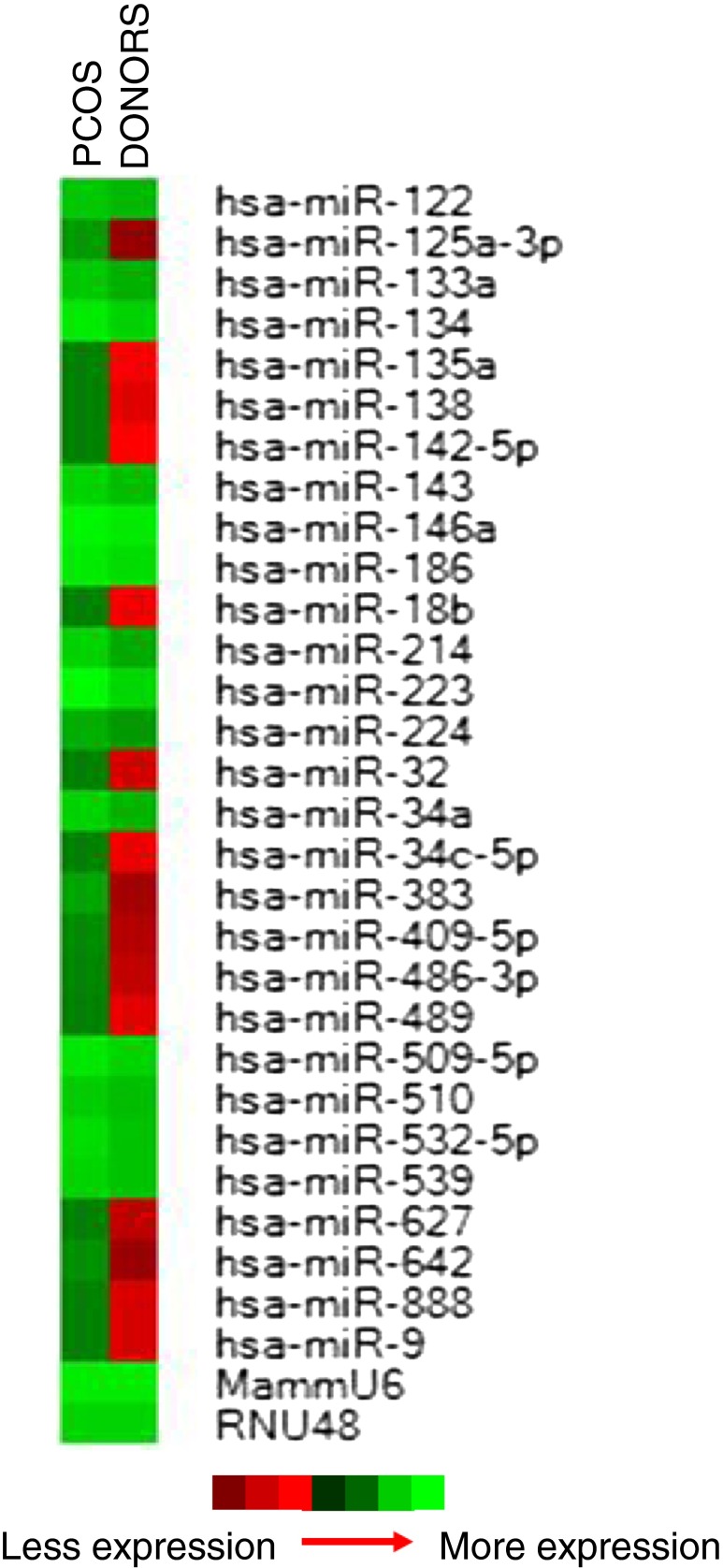
miRNA profiling of individual human follicle fluid (n = 4 per group) revealed the expression of 235 (62.3 %) miRNAs expressed in all samples out of the total 377 miRNAs present on the array. Statistical analysis using the REST 2009 software identified 29 miRNAs (12.3 %) to be differentially expressed between PCOS and OD samples (p < 0.05). These 29 differentially expressed miRNAs were expressed in all samples and are displayed in a heat map highlighting significantly increased expression in PCOS samples relative to OD controls (p < 0.05) (Fig. 1).
Fig. 1.
Heatmap of the 29 miRNAs that were differentially expressed between PCOS and Oocyte donor samples (p < 0.05). The left column is the PCOS group and the right column is the oocyte donor group. Red to green equates to an increase in miRNA expression
miRNA validation
The 4 samples used for miRNA profiling and 8 additional individual follicle fluid samples (total n = 12 per group) were isolated and reverse transcribed without pre-amplification for miRNA validation. A subset of the10 miRNAs displaying the largest differential expression was chosen from the 29 differentially expressed miRNAs. Expression of the 10 miRNAs (hsa-miR-32, hsa-miR −34c-5p, hsa-miR-135a, hsa-miR-138, hsa-miR-142-5p, hsa-miR-18b, hsa-miR-489, hsa-miR-627, hsa-miR-888, and hsa-miR-9) were analyzed relative to the expression of the miRNA control probe U6snRNA in each of the 12 individual follicle fluid samples. All 10 miRNAs showed expression in all samples. Increased expression in PCOS samples compared to OD controls was observed in concordance with the original miRNA profiling data (Table 1). However, only five (50 %) of these upregulated miRNAs (hsa-miR-9, 18b, 32, 34c, and 135a) displayed a significant increase in expression in the PCOS group compared to OD controls (p < 0.05) (Table 1).
Table 1.
MicroRNA validation raw cycle threshold (Ct) and fold change relative to U6snRNA (control probe) using RT-PCR
| miRNA | Raw Ct PCOS |
Raw Ct OD |
Fold change |
|---|---|---|---|
| U6snRNA | 20.1 | 20.1 | 1 |
| miR-32 | 28.0 | 30.9 | 7.5* |
| miR-34c | 26.3 | 32.2 | 59.7* |
| miR-135a | 28.2 | 30.7 | 5.7* |
| miR-138 | 29.5 | 32.1 | 6.1 |
| miR-142 | 32.7 | 35.6 | 7.5 |
| miR-18b | 27.5 | 30.0 | 5.7* |
| miR-489 | 20.4 | 21.6 | 2.3 |
| miR-627 | 16.8 | 18.6 | 3.5 |
| miR-888 | 24.6 | 27.3 | 6.5 |
| miR-9 | 31.8 | 38.5 | 104.0* |
* p < 0.05
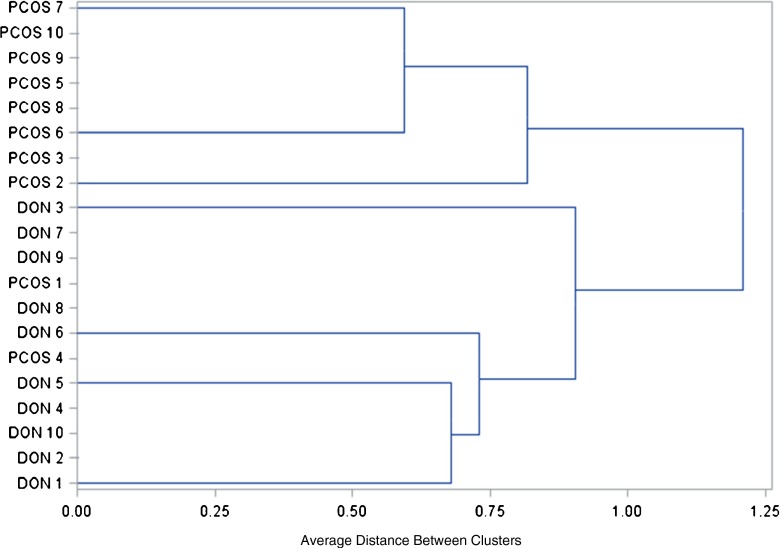
Unsupervised hierarchical clustering classified the miRNA profiles of individual follicular fluid samples into two main tree branches (Fig. 2). The first branch contained only PCOS follicular fluid samples, while the second branch included all the OD control samples along with two PCOS follicular fluid samples (Fig. 2). These results reflect underlying fundamental differences in miRNA expression profiles between the two groups as well as the presence of biological heterogeneity within group samples.
Fig. 2.
Unsupervised hierarchical clustering analysis of individual follicular fluid samples based on miRNA expression profiles. Two main tree branches are identified, the first branch contains only PCOS follicular fluid samples and the second branch includes all the OD control samples and two PCOS follicular fluid samples
Target gene analysis
miRNA target gene analysis was performed to demonstrate altered mRNA expression in association with aberrant miRNA profiles in PCOS samples. Target gene analysis was performed using Pathway Studio (Elsevier). Predicted target genes were chosen for further investigation based on their association with the PCOS phenotype. Quantitative real-time PCR was performed on additional individual follicle fluid samples (n = 10 samples per group) generating an mRNA expression profile for the following predicted genes; CCL5, IL8, FOXN3, UGP2, SYT1, DUSP5, SYTL4 and IRS2. The PCR efficiency of each reaction was very high (correlation coefficient >0.99) (data not shown). Quantification was performed for each gene relative to the expression of the internal constant housekeeping gene, PPIA (Table 2). Results showed that three predicted genes, IL8, SYT1 and IRS2, had significantly decreased expression in the PCOS group compared to OD controls (p < 0.05) (Table 2). This result was observed in all samples tested. A significant increase in miRNA expression (Table 1), in conjunction with a significant decrease in predicted gene expression (Table 2), was observed in these PCOS samples compared to controls, therefore suggesting a repressive type of miRNA regulatory mechanism.
Table 2.
Target gene analysis raw cycle threshold (Ct) and fold change relative to PPIA (internal housekeeping gene) using RT-PCR
| Target Genes | Raw Ct PCOS |
Raw Ct OD |
Fold change |
|---|---|---|---|
| PPIA | 26.3 | 26.4 | 1.0 |
| CCL5 | 29.0 | 29.2 | 1.1 |
| IL8 | 31.6 | 31.2 | 0.7* |
| FOXN3 | 30.4 | 30.6 | 1.1 |
| UGP2 | 31.3 | 31.4 | 1.0 |
| SYT1 | 31.6 | 30.9 | 0.6* |
| DUSP5 | 30.1 | 30.1 | 0.9 |
| STYL4 | 31.6 | 31.4 | 0.8 |
| IRS2 | 32.6 | 32.1 | 0.7* |
* p < 0.05
Clinical and medical parameters of study participants
Table 2 shows the clinical and medical parameters for study participants. The PCOS group was significantly older than the OD controls (PCOS 33.1 ± 4.4 vs. OD 27.1 ± 3.6, p = 0.001) (Table 3). The groups did not differ with respect to day 3 FSH or estradiol but LH, anti-müllerian hormone (AMH), and antral follicle count were each significantly greater in the PCOS group compared to OD controls (Table 3). The groups did not differ with respect to body mass index (BMI) (Table 3).
Table 3.
Clinical and medical parameters of PCOS and oocyte donors
| PCOS | Oocyte donors | p= | |
|---|---|---|---|
| Age (years) | 33.1 ± 4.4a | 27.1 ± 3.6 | 0.0001 |
| Body Mass Index (m/kg2) | 25.6 ± 6.3 | 23.8 ± 2.9 | 0.28 |
| Day 3 FSH (IU/L) | 6.0 ± 1.5 | 6.0 ± 1.3 | 0.96 |
| Day 3 LH (IU/L) | 8.1 (5.6, 15.2)b | 4.9 (3.7, 5.6) | 0.0013 |
| Day 3 Estradiol (pg/ml) | 34.0 (29.7, 42.3) | 37.0 (32.8, 57.2) | 0.19 |
| Anti-Müllerian Hormone (ng/dl) | 6.5 (3.6, 8.4) | 2.9 (1.9, 3.9) | 0.002 |
| Antral follicle count | 37.9 ± 13.5 | 27.7 ± 8.8 | 0.012 |
Reported as amean ± standard deviation or bmedian (25%ile, 75%ile)
Discussion
Recent studies have illustrated that miRNAs are abundant in follicular fluid in mares [13] and in humans [5]. Our study confirmed this finding, with the detection of 235 miRNAs in the follicular fluid of women undergoing IVF. Similar to the results of a study by Sang et al., we found that miRNAs are differentially expressed in women with PCOS compared to a control group [5]. In association with the altered miRNA expression seen in our PCOS samples, we identified 3 potential target genes with significantly decreased expression in the women with PCOS. The 3 genes identified (insulin receptor substrate 2 (IRS2), synaptogamin 1(SYT1), and interleukin 8 (IL8)) have functions related to the PCOS phenotype including roles in carbohydrate metabolism and beta cell function [22], cell-cell communication [13], and steroid synthesis [23].
We identified 5 miRNAs (hsa-miR-9, 18b, 32, 34c, and 135a) with significant overexpression in our PCOS group versus the OD control group. Sang et al. investigated a subset of 7 miRNAs (miR-132, 320, 24, 520c-3p, 193b, 483-5p, and 222) in the follicular fluid of women with PCOS versus a control group with male factor infertility [5]. The 7 miRNAs were chosen secondary to their association with steroidogenesis and 2 were found to have significantly decreased expression in the PCOS group (miR-132 and 320) [5]. In contrast, we did not identify differential expression of either miR-132 or 320. This may be secondary to race/ethnicity or PCOS phenotype differences as Sang et al.’s study was performed in China and our study was performed in the United States [5]. It may also reflect underlying differences in control groups. We used fertile oocyte donors for a control group whereas Sang et al. used infertile couples with male factor infertility [5]. Sang et al. used genome-wide analysis in addition to microarray analysis to identify miRNAs so methodological differences could also explain different results [5].
Although we identified differential expression of miRNAs, our study groups differed by age; the PCOS group was significantly older than the oocyte donor group. The groups differed statistically by age but this difference may not be clinically significant in terms of reproduction. Both groups were relatively young and had excellent ovarian reserve parameters. There is some data on altered microRNA expression associated with ovarian aging in mares [13] and in women with premature ovarian failure (POF) [24]. In the aging mares, 3 microRNAs showed increased expression (MIR181A, MIR375, MIR513A-3P) [13] and in the women with POF, 10 microRNAs showed increased expression (miR-202, miR-146a, miR-125b-2*, miR-139-3p, miR-654-5p, miR-27a, miR-765, miR-23a, miR-342-3p, miR-126) and 2 showed decreased expression (let-7c and miR-144) [24]. None of the microRNAs associated with ovarian aging in these 2 studies showed differential expression in our analysis, decreasing the likelihood that the differences we found are related to age.
Similarly to Sang et al. [5], we identified potential miRNA targets using bioinformatic analysis. We then carried our analysis further and investigated the expression of several potential target genes associated with the PCOS phenotype and identified 3 (IRS2, SYT1, and IL8) with significantly decreased expression in the PCOS group compared with the OD controls. The decreased expression found in the PCOS group is important as all the differentially expressed miRNAs showed overexpression in the PCOS group, suggesting an inhibitory role of the miRNAs on the potential target genes.
The 3 potential target genes have functions associated with the PCOS phenotype. IRS2 is a gene that encodes for the insulin receptor substrate 2 which mediates the effects of insulin and IGF-1 and is critical for peripheral carbohydrate metabolism and beta-cell function [22]. Mice lacking IRS2 demonstrate dysregulation of the estrous cycle, anovulation, infertility, and insulin resistance, similar to women with PCOS [25]. SYT1 is a gene that encodes for synaptogamin 1, a calcium binding synaptic vesicle protein [26]. It plays an important role in calcium triggered exocytosis throughout the body [26] and is required for exocytosis of insulin in the pancreatic beta cells [27, 28]. IL8 is the gene that encodes for interleukin 8, a chemokine and mediator of the inflammatory response, a chemo-attractant, and angiogenic factor [29]. IL8 is implicated in steroid synthesis in the late follicular and ovulatory follicle in both bovine [30] and humans [23].
The identification of these potential target genes is intriguing as all 3 have clear links with the PCOS phenotype, however, the associations observed in this study are limited. Currently, there is no gold standard for miRNA gene target analysis [31]. Each miRNA can have hundreds of gene targets and the gene targets may be different based on cell type and the presence or absence of other miRNAs [31]. Additionally, the impact of a miRNA on gene expression may be physiologically important but difficult to identify statistically [32].
Currently there is no diagnostic test for PCOS, only somewhat divergent diagnostic criteria from 3 different entities (Rotterdam criteria [18], NIH criteria [33], and Androgen Excess and PCOS Society [34]). Consequently, diagnosing PCOS is dependent on the criteria used [3]. Although PCOS is the most common endocrine disorder in women [2], few susceptibility genes have been repeatedly associated with the syndrome [35]. miRNAs are already being used commercially as disease biomarkers in oncology [17]. There is evidence for the use miRNAs as biomarkers in a wide range of diseases including prostate cancer [36], cardiac disease [37], acute kidney injury [38], and Alzheimer’s disease [39]. If we could identify miRNAs that could be used as biomarkers for PCOS, the diagnosis and treatment of PCOS would be revolutionized. PCOS is likely a wide spectrum of disease currently classified as one disease. Identifying specific miRNA biomarkers may allow for more precise diagnosis of the pathophysiology and allow personalized treatment for the endocrine, cardio-metabolic, and infertility impacts of the disease. Our study is only the second study we could identify that investigates miRNAs in the follicular fluid of women with PCOS [5] and only the third study we could identify that investigates miRNAs in PCOS in humans [40]. The field of miRNAs and PCOS is young and we, as a scientific community, are not yet at the point of identifying miRNAs that could be used as biomarkers but there is certainly potential for it as the field continues to grow.
The findings from this study provide further insight into the pathophysiology of PCOS. The differences in microRNA and gene expression may help to explain the aberrant follicular development and subfertility seen in women with PCOS [1]. Increasing our understanding of PCOS and identifying pathways associated with this phenotype may also improve clinical management of PCOS patients in regards to choices for infertility treatment based on observed aberrant follicular development.
Acknowledgments
We would like to acknowledge Andrew P. Bradford for his assistance with manuscript editing.
Conflict of interest
LWR received Clinical Research Fellowship and Mentor Award Supported by Pfizer, Inc. for research presented at ENDO 2012 and an ASRM Corporate Member Council In-training Travel Award for the IFFS/ASRM 2013. BM, RA, WBS, DM, and MGKJ have nothing to disclose.
Funding
This study was self-funded by the Colorado Center for Reproductive Medicine and the National Foundation for Fertility Research.
Footnotes
Capsule Follicular fluid from PCOS patients and oocyte donors was compared for microRNA and target gene expression. Five microRNAs were overexpressed and 3 target genes were decreased in the PCOS group.
References
- 1.Fauser BC, Tarlatzis BC, Rebar RW, Legro RS, Balen AH, Lobo R, Fauser BC, Tarlatzis BC, Rebar RW, Legro RS, Balen AH, Lobo R, et al. Fauser BC, Tarlatzis BC, Rebar RW, Legro RS, Balen AH, Lobo R. Fertil Steril. 2012;97(1):28–38. doi: 10.1016/j.fertnstert.2011.09.024. [DOI] [PubMed] [Google Scholar]
- 2.Health and fertility in World Health Organization group 2 anovulatory women. Hum Reprod Update. 2012;18(5):586-99. doi:10.1093/humupd/dms019. [DOI] [PubMed]
- 3.Yildiz BO, Bozdag G, Yapici Z, Esinler I, Yarali H. Prevalence, phenotype and cardiometabolic risk of polycystic ovary syndrome under different diagnostic criteria. Hum Reprod. 2012 doi: 10.1093/humrep/des232. [DOI] [PubMed] [Google Scholar]
- 4.Koivunen R, Pouta A, Franks S, Martikainen H, Sovio U, Hartikainen AL, et al. Fecundability and spontaneous abortions in women with self-reported oligo-amenorrhea and/or hirsutism: Northern Finland Birth Cohort 1966 Study. Hum Reprod. 2008;23(9):2134–9. doi: 10.1093/humrep/den136. [DOI] [PubMed] [Google Scholar]
- 5.Sang Q, Yao Z, Wang H, Feng R, Zhao X, Xing Q, et al. Identification of microRNAs in human follicular fluid: characterization of microRNAs that govern steroidogenesis in vitro and are associated with polycystic ovary syndrome in vivo. J Clin Endocrinol Metab. 2013 doi: 10.1210/jc.2013-1715. [DOI] [PubMed] [Google Scholar]
- 6.Ambros V. microRNAs: tiny regulators with great potential. Cell. 2001;107(7):823–6. doi: 10.1016/S0092-8674(01)00616-X. [DOI] [PubMed] [Google Scholar]
- 7.McCallie B, Schoolcraft WB, Katz-Jaffe MG. Aberration of blastocyst microRNA expression is associated with human infertility. Fertil Steril. 2010;93(7):2374–82. doi: 10.1016/j.fertnstert.2009.01.069. [DOI] [PubMed] [Google Scholar]
- 8.Barbarotto E, Schmittgen TD, Calin GA. MicroRNAs and cancer: profile, profile, profile. Int J Cancer. 2008;122(5):969–77. doi: 10.1002/ijc.23343. [DOI] [PubMed] [Google Scholar]
- 9.Tatsuguchi M, Seok HY, Callis TE, Thomson JM, Chen JF, Newman M, et al. Expression of microRNAs is dynamically regulated during cardiomyocyte hypertrophy. J Mol Cell Cardiol. 2007;42(6):1137–41. doi: 10.1016/j.yjmcc.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hawkins SM, Creighton CJ, Han DY, Zariff A, Anderson ML, Gunaratne PH, et al. Functional microRNA involved in endometriosis. Mol Endocrinol. 2011;25(5):821–32. doi: 10.1210/me.2010-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang C, Yang C, Chen X, Yao B, Zhu C, Li L, et al. Altered profile of seminal plasma microRNAs in the molecular diagnosis of male infertility. Clin Chem. 2011;57(12):1722–31. doi: 10.1373/clinchem.2011.169714. [DOI] [PubMed] [Google Scholar]
- 12.Osman A. MicroRNAs in health and disease–basic science and clinical applications. Clin Lab. 2012;58(5–6):393–402. [PubMed] [Google Scholar]
- 13.da Silveira JC, Veeramachaneni DN, Winger QA, Carnevale EM, Bouma GJ. cell-secreted vesicles in equine ovarian follicular fluid contain miRNAs and proteins: a possible new form of cell communication within the ovarian follicle. Biol Reprod. 2012;86(3):71. doi: 10.1095/biolreprod.111.093252. [DOI] [PubMed] [Google Scholar]
- 14.Weber JA, Baxter DH, Zhang S, Huang DY, Huang KH, Lee MJ, et al. The microRNA spectrum in 12 body fluids. Clin Chem. 2010;56(11):1733–41. doi: 10.1373/clinchem.2010.147405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci USA. 2008;105(30):10513–8. doi: 10.1073/pnas.0804549105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gallo A, Tandon M, Alevizos I, Illei GG. The majority of microRNAs detectable in serum and saliva is concentrated in exosomes. PLoS One. 2012;7(3):e30679. doi: 10.1371/journal.pone.0030679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ajit SK. Circulating microRNAs as biomarkers, therapeutic targets, and signaling molecules. Sensors (Basel). 2012;12(3):3359–69. doi: 10.3390/s120303359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuwabara Y, Ono K, Horie T, Nishi H, Nagao K, Kinoshita M, et al. Increased microRNA-1 and microRNA-133a levels in serum of patients with cardiovascular disease indicate myocardial damage. Circ Cardiovasc Genet. 2011;4(4):446–54. doi: 10.1161/CIRCGENETICS.110.958975. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y, Jia Y, Zheng R, Guo Y, Wang Y, Guo H, et al. Plasma microRNA-122 as a biomarker for viral-, alcohol-, and chemical-related hepatic diseases. Clin Chem. 2010;56(12):1830–8. doi: 10.1373/clinchem.2010.147850. [DOI] [PubMed] [Google Scholar]
- 20.Pfaffl MW, Horgan GW, Dempfle L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002;30(9):e36. doi: 10.1093/nar/30.9.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burks DJ. Font de Mora J, Schubert M, Withers DJ, Myers MG, Towery HH et al. IRS-2 pathways integrate female reproduction and energy homeostasis. Nature. 2000;407(6802):377–82. doi: 10.1038/35030105. [DOI] [PubMed] [Google Scholar]
- 22.Runesson E, Ivarsson K, Janson PO, Brannstrom M. Gonadotropin- and cytokine-regulated expression of the chemokine interleukin 8 in the human preovulatory follicle of the menstrual cycle. J Clin Endocrinol Metab. 2000;85(11):4387–95. doi: 10.1210/jcem.85.11.6954. [DOI] [PubMed] [Google Scholar]
- 23.Zhou Y, Zhu Y, Zhang S, Wang H, Wang S, Yang X. MicroRNA expression profiles in premature ovarian failure patients and its potential regulate functions. Chinese journal of birth health and heredity. 2011;19:20–2. [Google Scholar]
- 24.Neganova I, Al-Qassab H, Heffron H, Selman C, Choudhury AI, Lingard SJ, et al. Role of central nervous system and ovarian insulin receptor substrate 2 signaling in female reproductive function in the mouse. Biol Reprod. 2007;76(6):1045–53. doi: 10.1095/biolreprod.106.059360. [DOI] [PubMed] [Google Scholar]
- 25.Tucker WC, Chapman ER. Role of synaptotagmin in Ca2 + -triggered exocytosis. Biochem J. 2002;366(Pt 1):1–13. doi: 10.1042/BJ20020776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lang J, Fukuda M, Zhang H, Mikoshiba K, Wollheim CB. The first C2 domain of synaptotagmin is required for exocytosis of insulin from pancreatic beta-cells: action of synaptotagmin at low micromolar calcium. EMBO J. 1997;16(19):5837–46. doi: 10.1093/emboj/16.19.5837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakajima-Nagata N, Sugai M, Sakurai T, Miyazaki J, Tabata Y, Shimizu A. Pdx-1 enables insulin secretion by regulating synaptotagmin 1 gene expression. Biochem Biophys Res Commun. 2004;318(3):631–5. doi: 10.1016/j.bbrc.2004.04.071. [DOI] [PubMed] [Google Scholar]
- 28.Nyhlen K, Gautam C, Andersson R, Srinivas U. Modulation of cytokine-induced production of IL-8 in vitro by interferons and glucocorticosteroids. Inflammation. 2004;28(2):77–88. doi: 10.1023/B:IFLA.0000033023.76110.51. [DOI] [PubMed] [Google Scholar]
- 29.Shimizu T, Kaji A, Murayama C, Magata F, Shirasuna K, Wakamiya K, et al. Effects of interleukin-8 on estradiol and progesterone production by bovine granulosa cells from large follicles and progesterone production by luteinizing granulosa cells in culture. Cytokine. 2012;57(1):175–81. doi: 10.1016/j.cyto.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 30.Donadeu FX, Schauer SN, Sontakke SD. Involvement of miRNAs in ovarian follicular and luteal development. J Endocrinol. 2012;215(3):323–34. doi: 10.1530/JOE-12-0252. [DOI] [PubMed] [Google Scholar]
- 31.Berezikov E. Evolution of microRNA diversity and regulation in animals. Nat Rev Genet. 2011;12(12):846–60. doi: 10.1038/nrg3079. [DOI] [PubMed] [Google Scholar]
- 32.DA Zawadski JK. Diagnostic for polycystic ovary syndrome: Towards a rational approach. Polycystic Ovary Syndrome (Current Issues in Endocrinology and Metabolism) Boston: Blackwell Scientific Inc.; 1992. [Google Scholar]
- 33.Azziz R, Carmina E, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Futterweit W, et al. The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: the complete task force report. Fertil Steril. 2009;91(2):456–88. doi: 10.1016/j.fertnstert.2008.06.035. [DOI] [PubMed] [Google Scholar]
- 34.Abbott DH, Bacha F. Ontogeny of polycystic ovary syndrome and insulin resistance in utero and early childhood. Fertil Steril. 2013;100(1):2–11. doi: 10.1016/j.fertnstert.2013.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Srivastava A, Goldberger H, Dimtchev A, Ramalinga M, Chijioke J, Marian C, et al. MicroRNA Profiling in Prostate Cancer - The Diagnostic Potential of Urinary miR-205 and miR-214. PLoS One. 2013;8(10):e76994. doi: 10.1371/journal.pone.0076994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roncarati R, Anselmi CV, Losi MA, Papa L, Cavarretta E, Costa Martins PD, et al. Circulating miR-29a, Among Other Upregulated microRNAs, is the Only Biomarker for Both Hypertrophy and Fibrosis in Patients with Hypertrophic Cardiomyopathy. J Am Coll Cardiol. 2013 doi: 10.1016/j.jacc.2013.09.041. [DOI] [PubMed] [Google Scholar]
- 37.Aguado-Fraile E, Ramos E, Conde E, Rodriguez M, Liano F, Garcia-Bermejo ML. microRNAs in the kidney: Novel biomarkers of Acute Kidney Injury. Nefrologia. 2013 doi: 10.3265/Nefrologia.pre2013.Aug.12198. [DOI] [PubMed] [Google Scholar]
- 38.Kiko T, Nakagawa K, Tsuduki T, Furukawa K, Arai H, Miyazawa T. MicroRNAs in Plasma and Cerebrospinal Fluid as Potential Markers for Alzheimer’s Disease. J Alzheimers Dis. 2013 doi: 10.3233/JAD-130932. [DOI] [PubMed] [Google Scholar]
- 39.Chen YH, Heneidi S, Lee JM, Layman LC, Stepp DW, Gamboa GM, et al. miRNA-93 inhibits GLUT4 and is overexpressed in adipose tissue of polycystic ovary syndrome patients and women with insulin resistance. Diabetes. 2013;62(7):2278–86. doi: 10.2337/db12-0963. [DOI] [PMC free article] [PubMed] [Google Scholar]