Abstract
Purpose
This study was designed to evaluate the protective effects of vitamin E (VitE) and testosterone on varicocele (VCL)-induced damage in testis and sperm parameters and their effects on Hsp70-2 chaperone expression and on antioxidant status.
Methods
Wistar rats were divided into five groups: control-sham, VCL-induced, VitE-treated varicocelized (150 mg/kg, orally), testosterone-administrated varicocelized (400 μg/kg, intraperitoneally) and VitE + testosterone-received VCL-induced rats. The sperm count, DNA integrity, motility, viability and histone-protamine transition were evaluated after 60 days. The antioxidant status was analyzed by determining testicular malondialdehyde (MDA), total antioxidant capacity (TAC), superoxide desmutase (SOD) and glutathione peroxidase (GSH-Px). Endocrine status of the testicular tissue was estimated by evaluating the Leydig cells steroidogenic activity using fluorescent analyses for cytoplasmic steroid foci and by determination of serum testosterone. The expression of Hsp70-2 protein was analyzed using imunohistochemical and western blot analyses. RNA damage of the germinal cells was examined with epi-fluorescent examination.
Results
VitE and testosterone administration ameliorated the varicocele-reduced Leydig cell and testosterone level. In addition, co-administration of these compounds recovered the VCL-induced reduction of TAC, SOD, and GSH-px and lowered significantly (P < 0.05) the VCL-elevated content of MDA. The treated animals revealed with a significant (P < 0.05) up-regulation of the VCL-reduced expression of Hsp70-2 protein. Moreover, VitE and testosterone significantly (P < 0.05) inhibited the VCL-increased RNA damage in germinal cells.
Conclusion
Our data suggest that the protective effects of VitE and testosterone on VCL-induced derangements may depend on enhancing testicular antioxidant status and up-regulating endocrine activities, which enhanced the Hsp70-2 chaperone expression.
Keywords: Varicocele, Hsp70-2, Oxidative stress, Testosterone, RNA damage
Introduction
Varicocele (VCL) is characterized by abnormal tortuosity of the vein of the pampinifom plexus that drains the testis. About 15 %–20 % of the general population and more than 25 %–40 % of infertile couples with male factor infertility are reported to suffer from VCL [6, 20]. The Semen quality including; sperm count, motility, DNA integrity and viability decrease in men with VCL [1, 33]. VCL results in significant decline in serum level of testosterone in men as well as animal models [23, 25, 34]. More analyses were performed on animal models in order to clarify the reasons that may result in remarkable reduction in free testosterone level in men with VCL. Observations revealed significant decline in testosterone synthesis and severe reduction in Leydig cells distribution per 1 mm2 of the interstitial connective tissue in animal models [23, 25]. Moreover, the VCL-increased oxidative stress enhances the cellular DNA damage both in testis and in semen [6, 24, 28, 32]. Indeed, the damaged germinal epithelium and abnormal spermatozoa in VCL patients have been reported as main sources for generating reactive oxygen species (ROS) [27, 28, 31]. Moreover, the VCL down-regulates the total antioxidant capacity (TAC) in testis and semen of both fertile and infertile patients, which ultimately leads to pathologic ROS generation. The last impairment enhances the ROS-dependent damages in testicular tissue [31].
Several members of HSP70 and HSP90 families play crucial roles in cellular division and development during spermatogenesis process. Hsp70 proteins act as chaperones, which help infolding and assembling of proteins in cytoplasm, mitochondria and endoplasmic reticulum [9, 14]. The majority of functions which occur in meiotic prophase in spermatocytes including chromosome condensation and pairing of homologous chromosomes are largely depended on Hsp70-2expression [14, 16].
Previous studies on animal models showed that the absence of Hsp70-2 in germinal epithelium of mice resulted in triggering of apoptosis through P53-dependent mechanism [5, 15]. Beside these findings, it was noted that the Hsp70-2 proteins involvement in different cellular division processes (especially in meiosis) largely depends on androgens [2]. Synthesis of several proteins such as disulfide-isomers (P4hb1/Pdia1, Pdia3 and Pdia6) and heat shock proteins alters depending on testosterone deprivation. It has been shown that these proteins are involved in different cellular stresses [2, 3, 12]. Considering the fact that decreased testosterone level in varicocele negatively impacts the spermatogenesis process, here in present study we aimed to administrate the exogenous testosterone. The administration of testosterone may ameliorate the testicular Hsp70-2 protein expression and subsequently may inhibit the VCL-induced stresses at protein levels.
On the other hand, it has been shown that following oxidative stress the germinal cells produce high levels of stress proteins in order to maintain their physiologic homeostasis. In this regard the heat shock proteins (hsp families) have received considerable attentions [29]. The vitamin E (VitE), is the primary antioxidant component of the spermatozoa and it is one of the major membrane protective agents against the ROS and lipid peroxidation attacks [2, 8, 12]. The VitE, because of its lipid solubility, appears to be the first line of defense against peroxidation of poly-unsaturated fatty acids contained in the cellular and sub-cellular membrane phospholipids [3]. Therefore, the VitE was administrated alone and simultaneously with testosterone in order to evaluate its protective effect on VCL-reduced antioxidant status as well as analyzing the ameliorative effect of VitE-regulated antioxidant capacity on Hsp70-2 chaperone expression. For this purpose, the biochemical changes of superoxide desmutase (SOD), glutathione peroxidase (GSH-Px) in testicular tissue andtesticular total antioxidant capacity (TAC) were analyzed.
Materials and methods
Animals
Thirty two mature male Wistar rats, 10 weeks old and weighing between 180 and 200 g were used.
The rats were obtained from the Animal House of Faculty of Veterinary Medicine, Urmia University (Iran) and were acclimatized in an environmentally controlled room (temperature, 20–22 °C with 12 h light/12 h dark). Food and water were given ad libitum. In this study all experiments were conducted in accordance with the Urmia University guidelines for research on laboratory animals. Following week acclimatization, the animals were assigned into five groups (n = 6) as control-sham and test groups. The test group subdivided into four groups of VCL-induced (VCL), VitE-treated VCL-induced, testosterone-treated VCL-induced and VitE + testosterone-treated VCL-induced groups.
Varicocele induction
In test groups left varicocele was induced as previously reported (Sofikitis and Miyagawa 1992).
In brief, following induction of anesthesia with ketamine 5 % (Razak, Iran), 40 mg kg-1, intraperitoneally andxylazine 2 % (Trritau, Germany) 5 mg kg-1, intraperitoneally, the diameter of renal vein was reduced to 1 mm. Left renal vein ligation was performed at a direct medial to the junction of the adrenal andspermatic veins. Then, the anastomotic branch between the left testicular vein and the left common iliac vein was ligated. The animals in control-sham group were anesthetized and only underwent to a simple laparatomy and no vein ligation was performed on these animals.
Testicular weight determination
Following 8 weeks the animals were weighted for total body weight and the testes were dissected out and weighted on a MattlerBasbal scale (Delta Range, Tokyo). The total testicular weightgains of left testes on total body weight gain were evaluated.
Experiment design and administration of compounds
A vehicle dose of 0.5 mL of saline (0.85 % w/v) was administered to control-sham group by oral gavage and intraperitoneal route (n = 4 for each route of administration). Following 1 week after VCL induction, the animals in VitE- and VitE + testosterone-treated groups received150 mg/kg−1b.wt. of VitE (Sigma Chemical Co. st Louis MO), by oral gavages for 60 days [38]. The testosterone was administrated at the dose of 400μgr/kg−1[17], intraperitoneally, for 60 days in testosterone-and-VitE + testosterone-administrated groups.
Histological analyses
The animals were euthanized after 60 days. The left testicular tissues were dissected out andwashed with normal saline. Half of the tissues were fixed in Bouin’s fixative for histological investigations and subsequently embedded in paraffin. Sections (5–6 μm) were stained with Iron-Weigert (PajoheshAsia,Iran) for detection of germinal cell nuclei in the testis. The histological slides were analyzed using light microscope at two magnifications (×400 and × 1000). The tubular differentiation (TDI) and repopulation (RI) indexes were evaluated from 20 sections of each sample. The results for percentage of tubules with positive TDI (seminiferous tubules with more than three to four cellular layers) and RI (Ratio of active spermatogonia type B to inactive spermatogonia type A) reported.
Fluorescent analyses for RNA damage
The RNA damage was assessed using the acridine-orange NO dye (Sigma Aldrich, Germany) according to von Bertalanffy and Bickismethod [36]. In brief, the testes were washed out with ether alcohol and cut by cryostat (8 μm). The prepared sections were fixed by different degrees of alcoholfor 15 min. Then the sections briefly were rinsed in acetic acid, 1 % aqueous, followed by washing in distilled water. The specimens then were stained in acridine-orange for 3 min and distained in phosphate buffer, were followed for fluorescent colors differentiation in calcium chloride. The degenerated cells were characterized by loss of RNA and/or with faint red stained RNA. The normal cells were marked with bright red RNA at the apex of the nucleolus. In order to reducethe bias problems for staining density, 20 sections for each sample were investigated. The percentage of tubules with RNA damage was reported for all groups.
Assessment of steroidogenic foci (unsaturated fatty acid accumulation) in Leydig cells and serum level of testosterone
For this purpose the commercially available kit for fluorescent assay of Leydig cells intra-cytoplasmic steroid droplets Pajoheshasia (FLP, IUO100) was used. In brief; the frozen section prepared slides were dehydrated and stained with hematoxylin. The slides were washed with running water for 3–5 min and were stained in special fluorescent dye (FITC-conjugated1-anilinonaphthalene-8-sulphonate) for steroids and rinsed in distilled water. Then, the slides were dehydrated in 95 and absolute isopropanol and mounted in Harleco fluorescent mountant. In order to evaluate the serum level of testosterone the blood samples were collected directly from heart and serum samples were prepared with centrifugation (3000 g for 5 min), and the serum level of testosterone was assessed by using a competitive chemiluminescent immunoassay kit (Pishtaz Teb, Iran).
Immunohistochemcal analyses for Hsp70-2
Tissue section slides were heated at 60 °C for approximately 25 min in a hot air oven (Venticell, MMM, Einrichtungen, Germany). The tissue sections were de-paraffinized in xylene and rehydrated using an alcohol gradient. The antigen retrieval process was performed in 10 mM sodium citrate buffer. Immunohistochemical staining was conducted according to the manufacturer’s protocol (Biocare, USA). Briefly, endogenous peroxidase was blocked in aperoxidase blocking solution (0.03 % hydrogen peroxide containing sodium azide) for 5 min. Tissue sections were washed gently with washing buffer and subsequently incubated with Hsp70(1:500) biotinylated primary antibodies for 15 min. The sections were rinsed gently with washing buffer and placed in a buffer bath. The slides were then placed in a humidified chamber with asufficient amount of streptavidin–HRP (streptavidin conjugated to horseradish peroxidase inphosphate-buffered saline (PBS) containing an anti-microbial agent). The slides were incubated for 15 min. Subsequently, the tissue sections were rinsed gently in washing buffer and placed in a buffer bath. A diaminobenzidine-substrate-chromogen was added to the tissue sections andincubated for 5 min, followed by washing and counterstaining with hematoxylin for 5 s. The sections were then dipped in weak ammonia (0.037 M/L) 10 times, rinsed with distilled water and cover slipped. Positive immunohistochemical staining was observed as brown stains under alight microscope.
Western blot analysis for Hsp70-2
Western blot analysis was performed with a mouse monoclonal antibody specific for hsp70 (C92F3A-5; StressGen, Victoria, BC, Canada). The testicular tissues were immediately frozen inliquid nitrogen, homogenized, and centrifuged. After determination of protein concentration with the bicinchoninic acid method, 100 mg of proteins in each sample was loaded onto a 7.5 % SDSPAGE system. The blots were transferred onto a PVDF membrane (Bio-Rad Laboratories, Richmond, CA) and incubated in Tris-buffered saline-Tween 20 (20.0 mMTris-HCl, pH 7.5,150.0 mM NaCl, 0.05 % Tween 20) containing 2 % skim milk to block nonspecific binding sites. The membrane was immunoreacted with a 1:5,000 dilution of MA3-009 or a 1:1,000 dilution of SPA-810. Either membrane was then incubated with a 1:1,000 dilution of alkaline phosphatase–conjugated goat anti–mouse IgG antibody (Organon Teknika Corp., Durham, NC). Nitro bluetetrazolium and 5-bromo-4-chloro-3-indolyl phosphate were used as substrates for visualizationof the reaction product. The degree of HSP70 expression was semiquantitatively evaluated withc omputed densitometry (NIH Image; Macintosh; Apple Computers, Cupertino, CA) [29].
Determination of tissue malondialdehyde (MDA), SOD, GSH-Px and TAC
For the biochemical evaluation of oxidant-antioxidant system, the testicular tissue washed three times with 0.9 % NaCl solution and 1.15 % KCl was liquidified to amount of 9 ml for each tissue. The homogenate of the tissues was prepared with the teflon end on homogenizator (Elvenjempotter, Newton CT) and were centrifuged at 4000 rpm. The MDA content was measured by using the thiobarbituric acid (TBA) reaction as described previously [21]. The tissue SOD and GSH-px activities were evaluated by using the measurement kits of RAN-SOD and RANSOL (Rondaxlab.,Crumlin, BT 29, UK). We assessed the tissue TAC status based on the ferric reduction antioxidant power (FRAP) assay [13, 21].
Sperm preparation and DNA damage assessment
Epididymal tissues were separated carefully from the testicle under a ten time magnification provided by Stereo Zoom Microscope (TL2, Olympus Co., Tokyo). The left epididymis was divided into three segment; caput, corpus and cauda. The epididymal cauda was trimmed and minced in 5 ml Ham’s F10 medium (sigma co., USA) for 30 min, 6 % CO2, 36.5oC in CO2 culture device (LEEC Co., England). After centrifugation the sperm pellet was re-suspended in 0.5 mL of sperm Prep medium (Ham’s F10). A small aliquot (20 1 l) of sperm suspension was glass smeared. According to Tejada et al. [35] the slides were air dried and then fixed overnight in Carnoy’s solution (methanol/acetic acid, 3: 1). Once rinsed and air dried, the slides were stained for 5 min with freshly prepared acridine-orange stain (AO). After washing and drying, the slides were examined using a fluorescent microscope (Leitz,Germany; excitation of 450–490 nm). After AO staining, the samples were immediately observed. Each field was observed for some seconds under the fluorescence microscope. On each slide an average of 100 spermatozoa were counted. The percentage of spermatozoa with single-stranded DNA was calculated from the ratio of spermatozoa with red, orange, or yellow fluorescence to all spermatozoa counted per sample.
Sperm motility and viability
The sperm motility was examined based on the WHO [1999] standard method for manual examination of sperm motility. Briefly, the sperm samples were diluted 1:8 in Ham’s F10 before examination. A 20 μl of sperm sample was placed on sperm examination area and examined under 10× magnification loop. Only the motile sperm with forward progression counted within10 boxes and recorded. Finally, motility was evaluated based on the following equation:
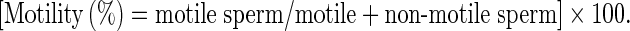 |
The eosin-nigrosin staining was performed for sperm viability assay [37]. In brief, 50 μl of epididymal sperm was mixed with 20 μl of eosin in sterile test tube. After 5 s 50 μl of nigrosin was added and mixed thoroughly. The mixture of stained sperm was smeared on the slide and examined under bright field microscope (1,000× magnification, Olympus, Germany). The colorless sperm were considered as live and stained sperm were marked as dead spermatozoa. The sperm viability, motility, and morphology were reported in percentage [37]. The sperm count was performed according to standard hemocytometric method as described previously by Pant and Srivastava [22].
Statistical analyses
For the measured parameters, mean and standard deviations were calculated. Results were analyzed by using Graph Pad Prism software (Version 2.01. Graph Pad software Inc. San Diego, California, USA). The comparisons between groups were made by analysis of variance (two way ANOVA) followed by Bonferroni post-hoc test. A P value <0.05 was considered significant.
Results
The testosterone and VitE administration significantly increased testicular weight
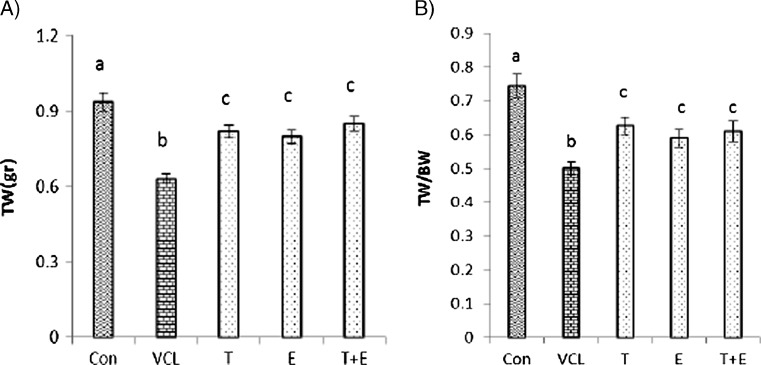
After 8 weeks from VCL induction, body weight gain did not change statistically (P > 0.05) in the VCL-induced and treated groups compared to the control-sham group. The testicular to body weight ratio in the VCL group showed a remarkable decline compared to the control-sham group, while VitE and testosterone co-administration resulted in a significant (P < 0.05) improvement in testicular weight (Fig. 1a, b).
Fig. 1.
Effect of VitE (150 mg/kg−1) and testosterone (400μgr/kg−1) on VCL-reduced testicular weight (a) and on the ratio of testicular weight to body weight (TW/BW). a, b, c are indicating significant differences between control-sham group with non-treated VCL-induced group and between all treated groups with each other and with VCL-induced group (N = 6 rats for each group). All data are presented in mean ± SD. Note; Con control-sham, VCL varicocele-induced, T testosterone-treated, E Vitamin E-received, T + E testosterone + vitamin E-administrated
VitE and testosterone protect against VCL-induced cellular damages
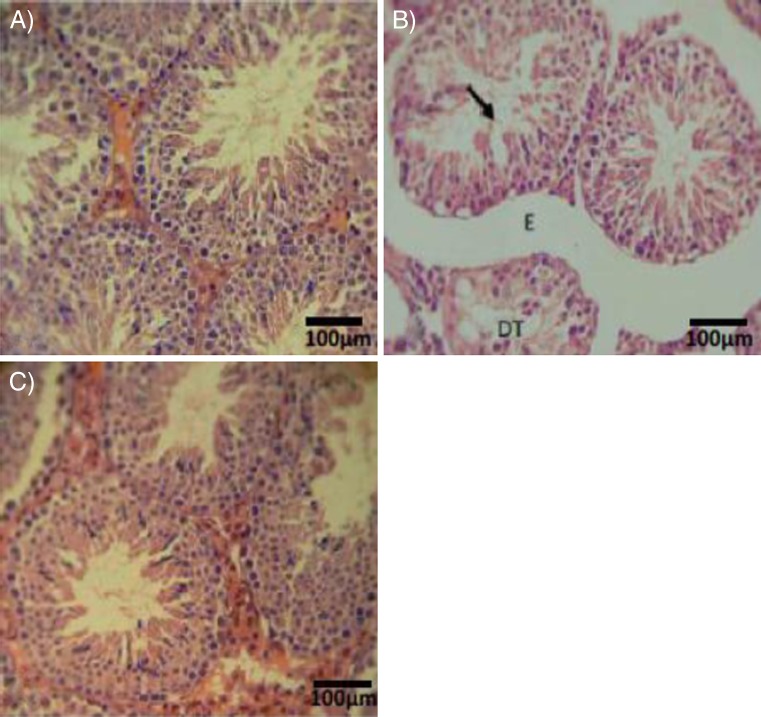
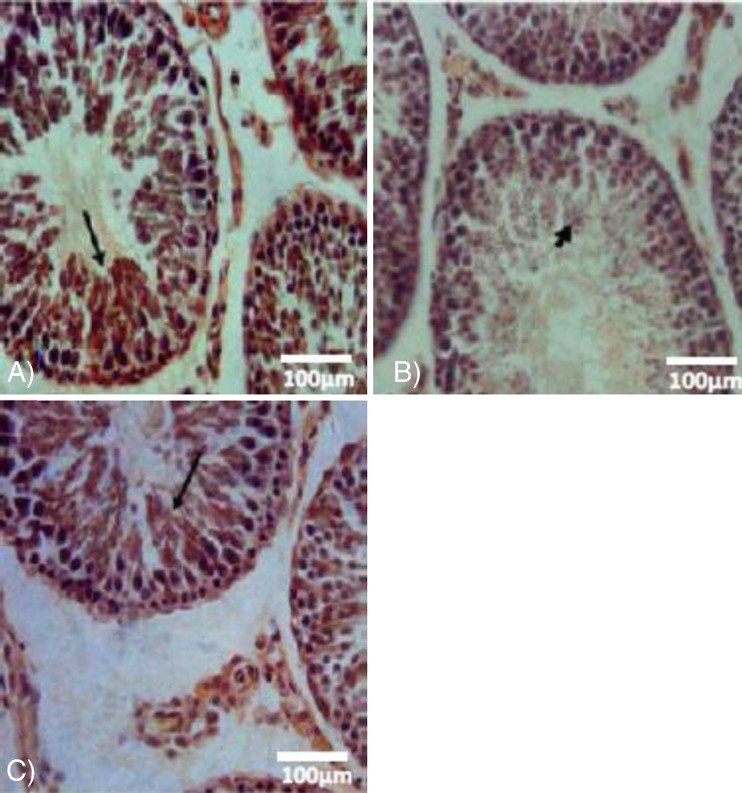
Histopathological investigations demonstrated that the rats in which VCL was induced showed highly degenerated testes with remarkable atrophy of seminiferous tubules and edema, while VitE and testosterone antagonized the atrophy and edema in interstitial tissue (Fig. 2a, b, c). Histomorphometric analyses showed a significant decrease in cell layers of germinal epithelium, indicating negative TDI and RI in seminiferous tubules of the VCL-induced group. By contrast, those animals that received VitE and testosterone (especially in simultaneous form) showed significantly more cell layers (Table 1).
Fig. 2.
Cross section from testicular tissue; a control-sham group with no histological changes, (b) VCL-induced group, VCL caused remarkable atrophy of seminiferous tubules and a severe edema in the interstitial connective tissue (E). Note the tubule with negative TDI (arrow) and depleted germinal cells (DT) in non-treated VCL-induced testis. c VitE + testosterone-received group, the tubules are apparently recovered and are presented with positive TDI. Iron-Weigert staining technique (×400)
Table 1.
Effect of VitE and testosterone on VCL-induced histomorphometric changes in testes (N = 6 rats for each group)
| Control-sham | VCL | VCL + E | VCL + T | VCL + E + T | |
|---|---|---|---|---|---|
| STD (μm) | 348.00 ± 18.53a | 234.60 ± 12.11a | 284.40 ± 7.23a | 279.60 ± 10.80a | 324.60 ± 30.08a |
| GEH (μm) | 131.75 ± 17.09a | 85.78 ± 4.20a | 96.56 ± 2.69a | 104.29 ± 5.80a | 122.46 ± 7.14a |
| TDI (%) | 86.73 ± 2.09a | 34.33 ± 3.36a | 56.40 ± 6.41a | 57.00 ± 7.12a | 70.79 ± 3.30a |
| RI (%) | 80.38 ± 5.97a | 36.18 ± 3.80a | 53.07 ± 5.27a | 55.48 ± 6.21a | 69.09 ± 3.40a |
All data are presented in mean ± SD
VCL varicocelized, E vitamin E (150 mg/kg/b.w.), T testosterone (400 μg/kg/b,w.), STD seminiferous tubule diameter, GEH germinal epithelium height, TDI tubular differentiation index, RI repopulation index
aare indicated the significant differences between data in the same row. P < 0.05 was considered as significant difference
VitE and testosterone co-administration lowered RNA damage and protected Leydig cells
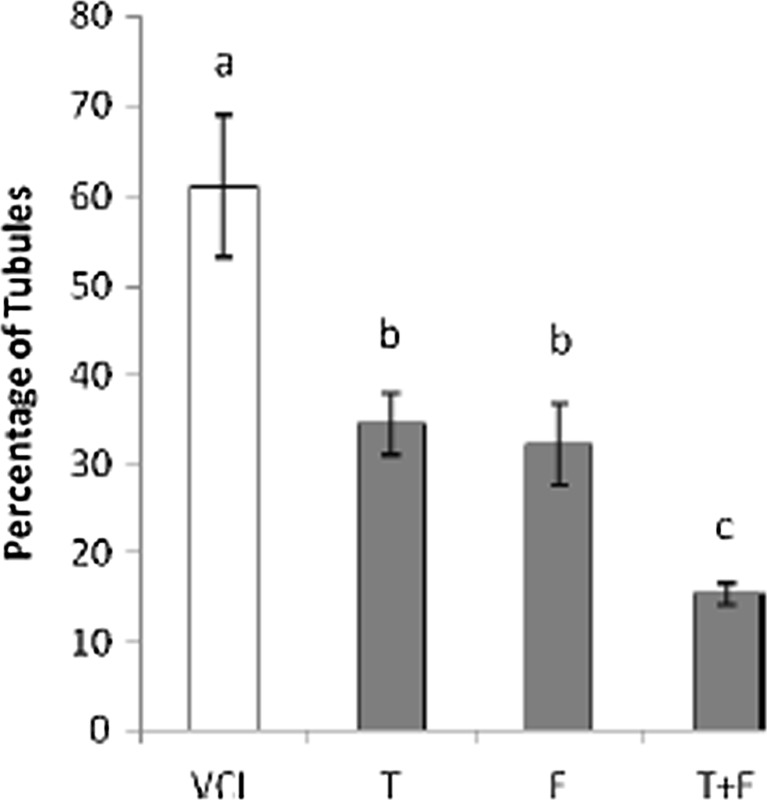
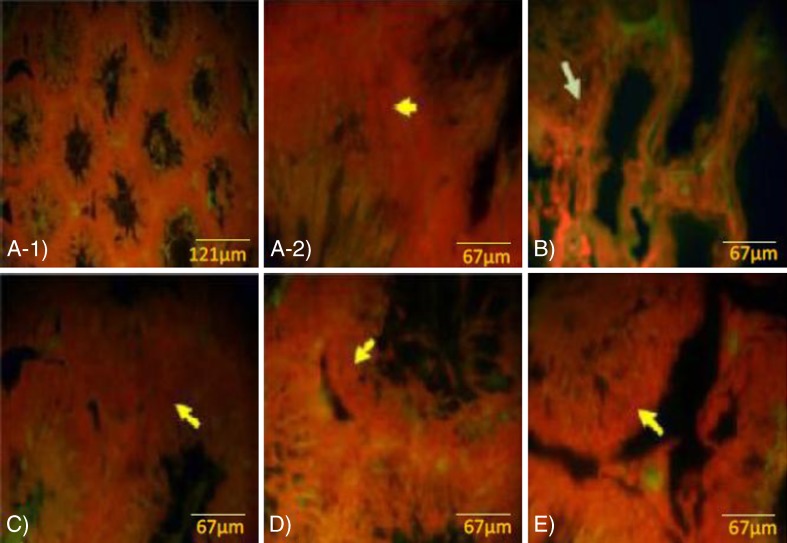
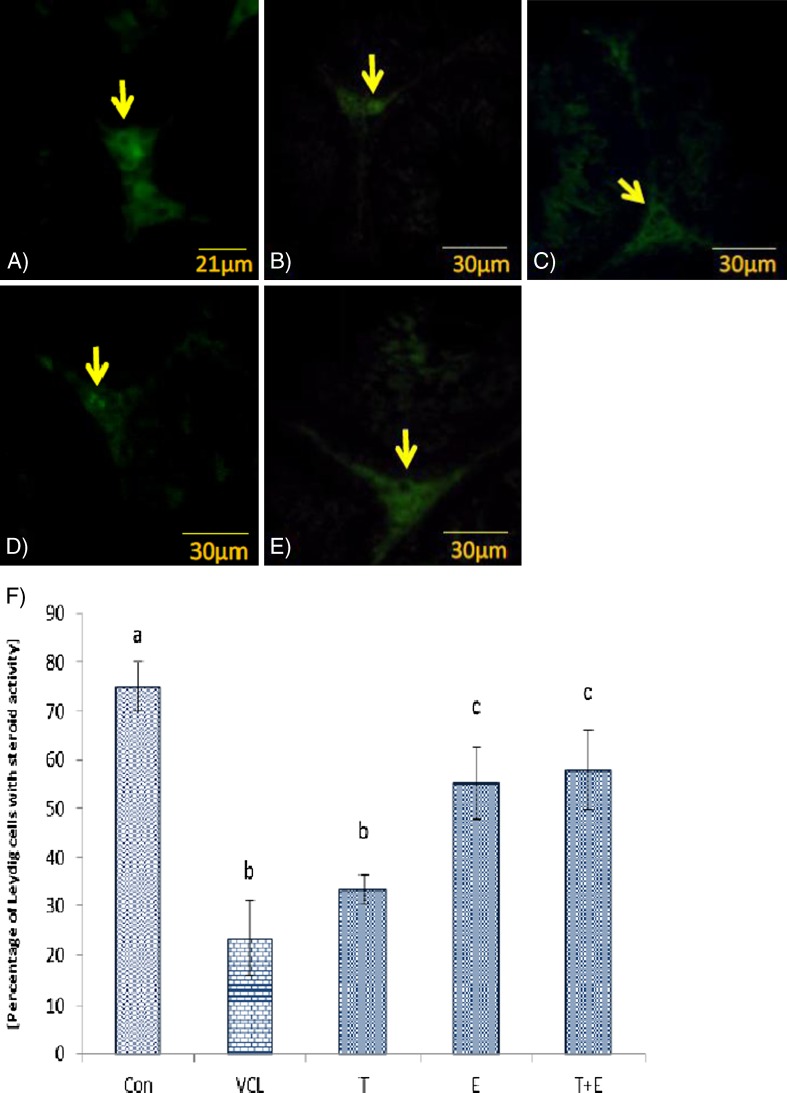
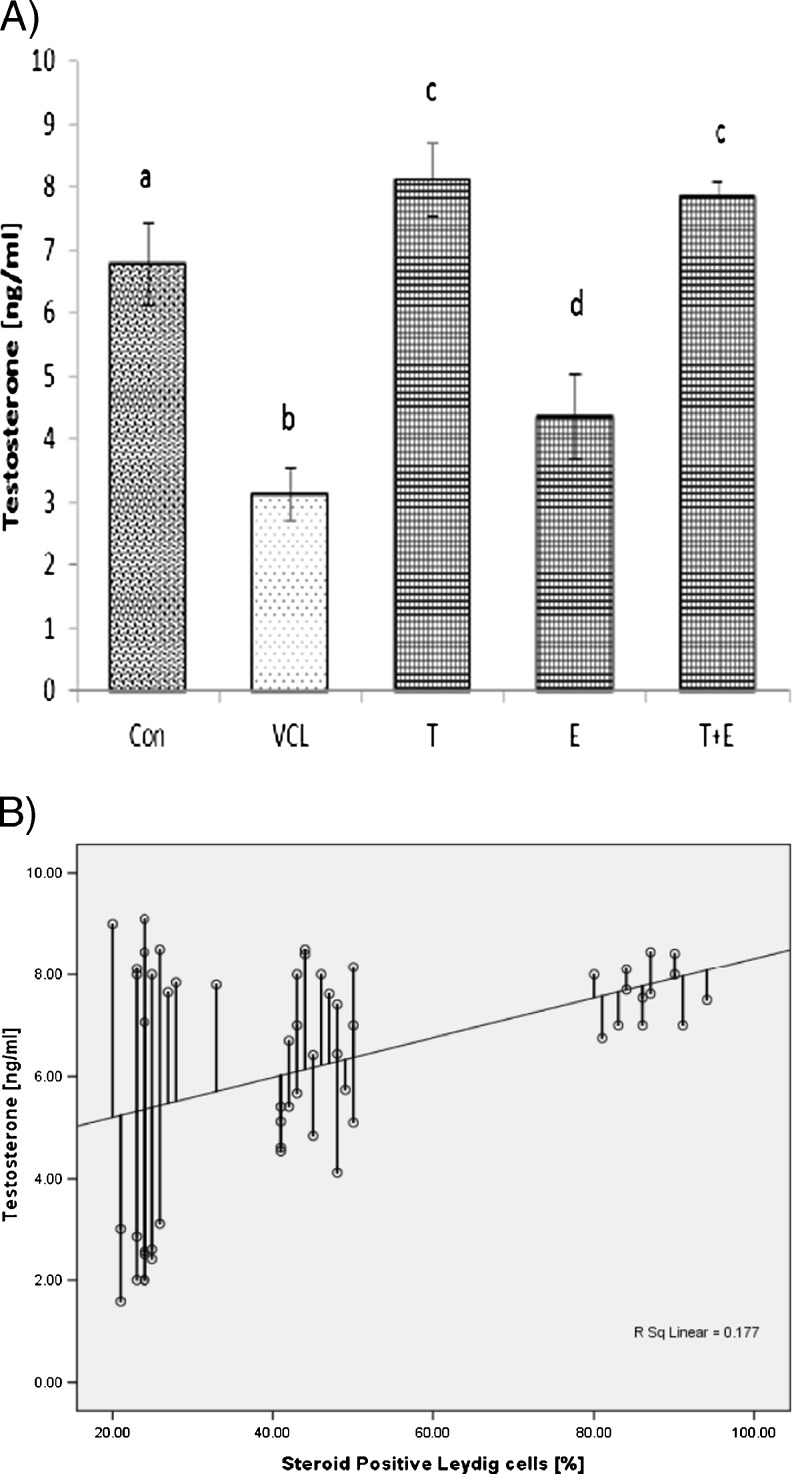
The fluorescent analyses for germinal cells RNA damage showed that the VCL-induced testes underwent to a severe (P < 0.05) RNA damage in germinal cells. Nevertheless, the percentage of seminiferous tubules with damaged RNA in VitE + testosterone-treated animals were lower (P < 0.05) than the VCL-induced group (Fig. 3). The control-sham testes were observed with no RNAdamage in germinal cells (Fig. 4a, b, c, d, e). Light microscopic analyses revealed that the Leydig cells number per 1 mm2 of the connective tissue remarkably (P < 0.05) decreased in VCL-exposed group and the percentage of hypertrophied Leydig cells enhanced in these animals. Meanwhile, the VitE and testosterone co-administration attenuated the Leydig cells degeneration and resulted in a significantly (P < 0.05) higher Leydig cells survival. Accordingly, the treated groups showed remarkably (P < 0.05) higher number of Leydig cells and considerably (P < 0.05) lower percentage of hypertrophied cells per 1 mm2of the interstitial tissue (Table 2). The fluorescent investigations showed that VitE administration increased VCL-reduced steroidogenic reactivity in Leydig cells (Fig. 5a, b, c, d, e, f). Accordingly, the animals in VitE alone and VitE + testosterone-received group showed more than 60–70 % of Leydig cells with steroid in cytoplasm versus approximately 20–30 % in the VCL-induced and 70–80 % in control-sham animals. However, the testosterone alone-received animals showed 30–40 % of Leydig cells with steroid activity. The biochemical analyses revealed that in the VCL-induced animals, serum testosterone declined remarkably, while the VitE and testosterone treated groups showed significantly (P < 0.05) higher levels of testosterone in comparison to the non-treated VCL-induced animals (Fig. 6a). Statistical analyses showedthat there was a positive correlation between the steroid-positive Leydig cell percentages per mm2 with serum levels of testosterone in different groups (Fig. 6b).
Fig. 3.
Effect of VitE (150 mg/kg−1) and testosterone (400μgr/kg−1) on VCL-induced RNA damage in germinal epithelium. a, b, c are indicating significant differences between control-sham group with non-treated VCL-induced group and between all treated groups with each other and with VCL-induced group (N = 6 rats for each group). All data are presented in mean percentage ± SD. Note; Con control-sham, VCL varicocele-induced, T testosterone-treated, E vitamin E received, T + E testosterone plus vitamin E-administrated
Fig. 4.
Fluorescent photomicrograph for RNA; a- 1 and a- 2 control group, the germinal cells in different layers are presented with remarkably higher normal mRNA levels (arrow in A-2), b VCL group, severe RNA damage has been marked with faint yellowish red appearance (arrow), c VitE-treated and d testosterone-received and e VitE + testosterone-administrated groups, simultaneous administration of VitE and testosterone remarkably inhibited mRNA damage (arrow). Fluorescent staining for mRNA ( a -1: ×100, a -2:×600, b, c, d, e : ×400)
Table 2.
Effect of VitE and testosterone on VCL-induced derangements on leydig cells (N = 6 rats for each group)
| Leydig cells (NO/one mm2) | Hypertrophied Leydig cells (NO/one mm2) | |
|---|---|---|
| Control-sham | 32.50 ± 4.91a | 0.62 ± 0.47a |
| VCL | 17.25 ± 2.23a | 9.30 ± 0.85a |
| VCL + E | 26.25 ± 3.86a | 5.50 ± 1.20a |
| VCL + T | 25.00 ± 1.41a | 6.75 ± 0.95a |
| VCL + E + T | 26.25 ± 1.70a | 5.32 ± 0.62a |
All data are presented in mean ± SD
VCL varicocelized, E vitamin E (150 mg/kg/b.w.), T testosterone (400 μg/kg/b,w.)
a are indicated the significant differences between data in the same column. P < 0.05 was considered as significant difference
Fig. 5.
Fluorescent photomicrograph for steroid foci in Leydig cells; a Control-sham group, the Leydig cells are marked with intensive intacytoplasmic steroid (arrow), b Non-treated VCL group, c testosterone-received group, the Leydig cells are presented with faint reaction for steroid (arrows), d VitE-treated and e VityE + testosterone-administrated group, note the improved steroidogenesis in these groups. Fluorescent staining for Leydig cells steroid foci (×400). f Mean ± SD for percentage of Leydig cells with estroidogenesis per 1 mm2 of the interstitial connective tissue, a, b, c are indicating significant differences between control-sham group with non-treated VCL-induced group and between all treated groups with each other and with VCL-induced group (N = 6 rats for each group). P < 0.05 was considered as significantly different. Note; Con control-sham, VCL varicocele-induced, T testosterone-treated, E vitamin E-received, T + E testosterone + vitamin E-administrated
Fig. 6.
a Effect of VitE (150 mg/kg−1) and testosterone (400μgr/kg−1) administration on VCL-reduced serum level of testosterone (N = 6 rats for each group), a,b,c,d are indicating significant differences between control-sham group with non-treated VCL-induced group and between all treated groups with each other and with VCL-induced group. All data are presented in mean ± SD. b positive correlation between the percentages of Leydig cells with steroidogenesis with serum level of testosterone (r2 = 0.177, P < 0.05). Note; Con control-sham, VCL varicocele-induced, T testosterone-treated, E vitamin E received, T + E testosterone + vitamin E-administrated
VitE and testosterone regulated the VCL-reduced Hsp70-2 expression
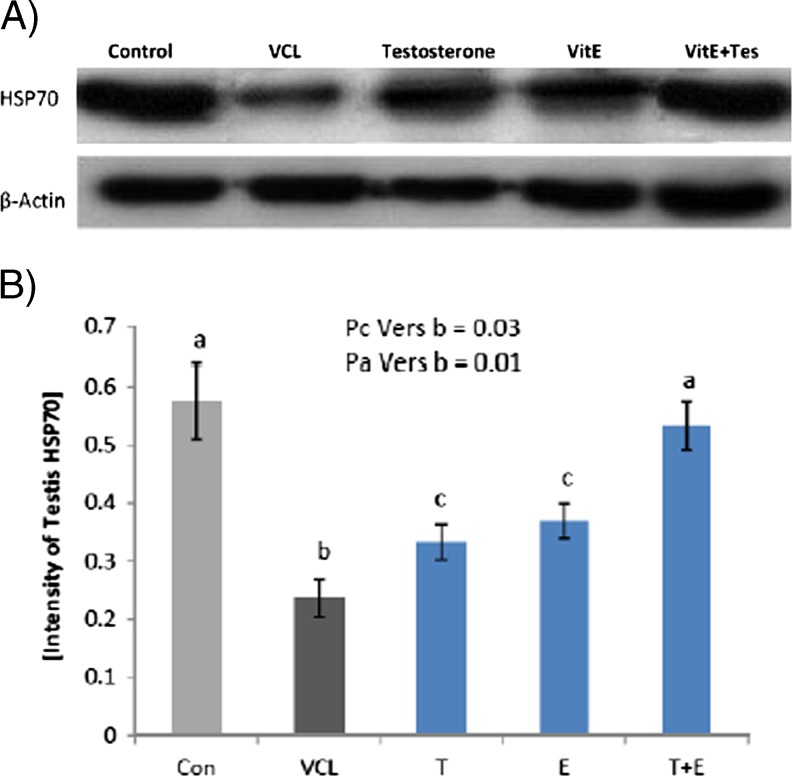
The immunohistochemical technique was used to show the changes in expression of Hsp70-2 protein in different germinal cells lineages. The optical density for Hsp70-2 expression in VCL-induced rats compared to control-sham germinal cells. Observations revealed that the expression of this protein significantly decreased in VCL-induced animals versus control-sham group. Meanwhile, co-administration of VitE and testosterone ameliorated the reduction in Hsp70-2 expression. Accordingly, the Hsp70-2 expression at protein level was back to control-sham level in VitE + testosterone-treated group, coincident with apparent seminiferous tubules recovery (Fig. 7a, b, c). The western bolt analyses confirmed the microscopic findings, as the tissue extract of VitE and testosterone-received groups exhibited significantly (P < 0.05) higher protein levels ofHsp70-2 in comparison to non-treated VCL-induced animals (Fig. 8a, b).
Fig. 7.
Immunohistochemical analyses for Hsp70-2; a control-sham, note Hsp70-2 expression in spermiogenesis cell lineages (arrow), b VCL-induced group, the germinal cells are exhibited with remarkable reduction in Hsp70-2 expression, c VitE + testosterone-treated testis, the Hsp70-2 expression was back to control condition, which the spermatocyte and spermatids are presented with up regulated Hsp70-2 expression. Immunohistochemical staining for Hsp70-2 (×600)
Fig. 8.
Western blot analyze for Hsp70-2 protein expression in testicular extract. The VitE and testosterone alone-and simultaneous-administration enhanced the Hsp70-2 protein expression as there is no significant difference (P > 0.05) between VitE + testosterone-treated animals with control-sham group. a, b, c indicate significant differences between VCL-induced group with control-sham and between treated groups with non-treated VCL-induced group
VitE and testosterone elevated VCL-reduced TAC, SOD and GSH-px levels
Biochemical results indicated that all TAC, SOD and GSH-px levels significantly (P < 0.05) decreased afterVCL induction, while a remarkable (P < 0.05) increase following co-treatment with VitE, testosterone and VitE + testosterone was obtained. Interestingly the elevation in antioxidantstatus after the simultaneous treatment with VitE + testosterone was more pronounced than that of the VitE and testosterone alone treatments. The testicular MDA significantly (p < 0.05) increased in the VCL-induced animals (8.21 ± 0.26 nmol/mg) in comparison to the control-sham (1.14 ± 0.50 nmol/mg) and VitE + testosterone-treated group (3.76 ± 0.30 nmol/mg). The data for antioxidant status and MDA level are presented in Table 3.
Table 3.
Effect of VitE and testosterone on VCL-induced changes in antioxidant status and MDA level
| GSH-PX (U/mg protein) | SOD (U/mg protein) | TAC (mmol/mg protein) | MDA (nmol/mg protein) | |
|---|---|---|---|---|
| Control-sham | 35.00 ± 4.60a | 700.66 ± 15.04a | 0.51 ± 0.013a | 1.14 ± 0.50a |
| VCL | 10.46 ± 2.11a | 174.53 ± 22.50a | 0.21 ± 0.015a | 8.21 ± 0.26a |
| VCL + E | 28.22 ± 1.35a | 586.64 ± 12.23a | 0.42 ± 0.012a | 4.70 ± 0.34a |
| VCL + T | 26.71 ± 3.08a | 579.33 ± 18.48a | 0.32 ± 0.020a | 5.84 ± 0.41a |
| VCL + E + T | 34.16 ± 2.80a | 645.33 ± 32.71a | 0.48 ± 0.031a | 3.76 ± 0.30a |
All data are presented in mean ± SD
VCL varicocelized, E vitamin E (150 mg/kg/b.w.), T testosterone (400 μg/kg/b,w.), SOD superoxide dismutase, GSH-px glutathione peroxidase, TAC total antioxidant capacity, MDA malondialdehyde
a are indicated the significant differences between data in the same column. P < 0.05 was considered as significant difference
Sperm parameters improved after VitE and testosterone administration
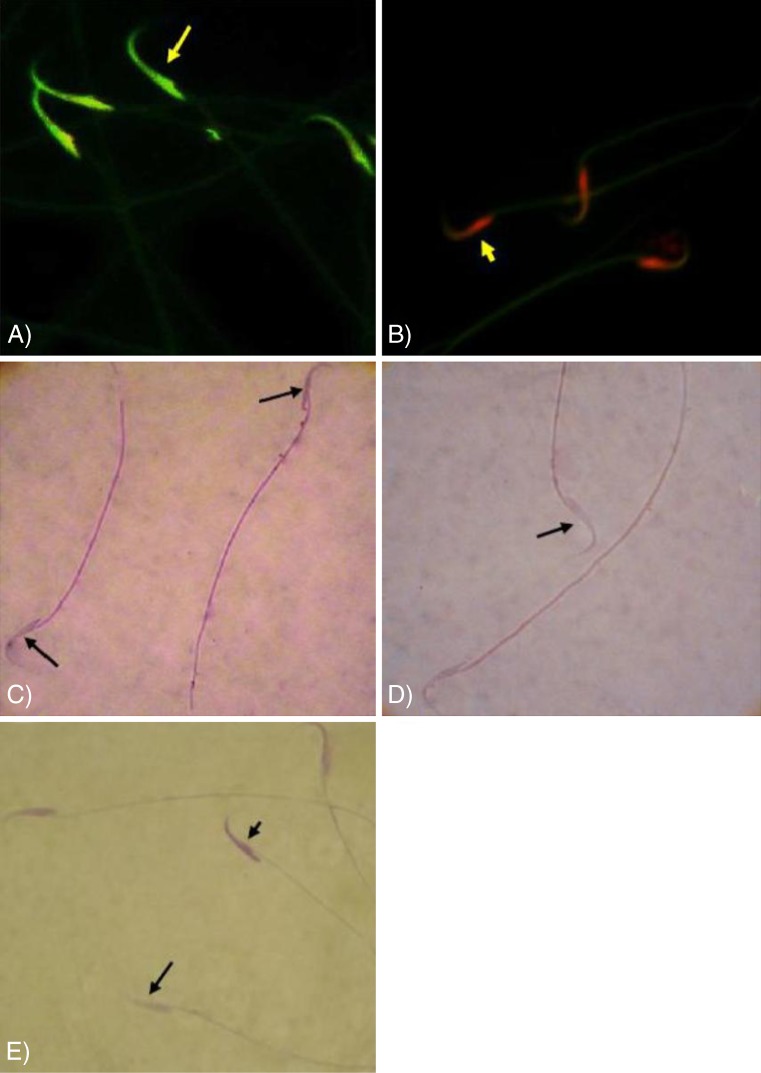
The sperm parameters are summarized in Table 4. The VCL-induced animals showed asignificant (P < 0.05) decrease in sperm count and viability in comparison to the VitE and testosterone-treated groups. No changes in sperm count and viability were observed in the control-sham animals. Fluorescent staining for DNA damage indicated that VCL-induced sperm DNA damage (71.00 ± 8.41 %) shown in Fig. 9, was significantly (P < 0.05) attenuated by VitE (35.00 ± 4.60 %), testosterone (50.11 ± 5.13 %) and VitE + testosterone (30.11 ± 3.66 %) administration. The eosin-nigrosin staining for sperm viability showed that the percentage of dead sperm was significantly (P < 0.05) reduced in the treated groups and that VitE and testosterone-treatment also attenuated the effects of VCL on sperm mobility (Fig. 9a, b, c, d, e).
Table 4.
Regulating effect of VitE and testosterone on VCL-changed sperm parameters
| Control-sham | VCL | VCL + E | VCL + T | VCL + E + T | |
|---|---|---|---|---|---|
| Count (×106) | 65.33 ± 3.09a | 14.35 ± 3.17a | 47.75 ± 3.18a | 41.50 ± 1.86a | 54.00 ± 4.12a |
| Motility (%) | 92.57 ± 3.00a | 19.75 ± 2.74a | 77.76 ± 3.23a | 55.94 ± 13.10a | 71.84 ± 16.10a |
| DNA damage (%) | 6.41 ± 1.42a | 71.00 ± 8.41a | 35.00 ± 4.60a | 37.12 ± 3.61a | 30.11 ± 3.66a |
| Chromatin condensation (%) | 89.42 ± 6.12a | 18.06 ± 2.10a | 58.41 ± 65.42a | 56.75 ± 8.47a | 75.91 ± 3.15a |
All data are presented in mean ± SD
VCL varicocelized, E vitamin E (150 mg/kg/b.w.), T testosterone (400 μg/kg/b,w.)
a are indicated the significant differences between data in the same row. P < 0.05 was considered as significant difference
Fig. 9.
a and b fluorescent micrograph of the sperms the sperms with normal DNA integrity are presented green fluorescent DNA (arrow) and the sperms with breaked double strand are marked with red fluorescent (head arrow). c Special staining for sperm viability; the dead sperms are presented with stained cytoplasm (head arrow) and the vital sperms are marked with unstained head (arrow) pieces in figure d. e Special staining for chromatin condensation; note the nucleuses of the sperms with condensed chromosome are stained lightly (arrow) and the sperms with uncondensed chromatin are marked with dense stained DNA (head arrow). a and b acridine-orange staining, b and c eosin-nigrosin staining and d aniline-blue staining, (×1000)
Discussion
Current study showed that the VitE and testosterone reduced the VCL-induced damages in testicular tissue and remarkably improved the sperm parameters. Accordingly, the animals in VitE + testosterone-treated group showed enhanced TDI and RI ratio associated with meliorated sperm DNA integrity, motility and viability. The treated groups exhibited improved antioxidant status and significantly decreased MDA level. Moreover, the down-regulated Hsp70-2 protein level in VCL-induced animals appeared regulated in treated groups. Additionally, the vitE and testosterone-received animals revealed with increased Leydig cells steroidogenic activities (revealed with increased inra-cytoplasmic steroid foci and elevated level of testosterone) and with improved RNA damage in germinal cells as basic essential factors in a healthy testicular tissue.
The Hsp70 heat shock proteins are molecular chaperones, which help other necessary proteins in their folding, transport and assembly into complexes during spermatogenesis [2, 15]. According to previous findings, the lack of Hsp70-2, results in developmental arrest and apoptosis of the spermatocytes at the transition from G2 to M phase of the cell cycle [29]. Therefore, it was suggested that Hsp70-2 provides the protein kinase activity by enabling Cdc2 that promotes cells transit from G2 to M phase [4, 38]. Our histological analyses for germinal cells differentiation and repopulation indexes showed that co-administration of VitE and testosterone improved the VCL-induced negative TDI and RI from more than 50 % of tubules into approximately 20 %. Interestingly, the Hsp70-2 protein expression up regulated in spermatocyte cells of treated testes. Considering the Hsp70-2 essential role in spermatocyte cells transition, it can be suggested that VitE and testosterone inhibited the VCL-induced spermatogenesis arrest partly by enhancing the Hsp70-2 expression.
To understand how these compounds elevated the Hsp70-2 expression, one should know that the Hsp70-2 protein synthesis manipulates depending on free radicals generation and androgens withdrawal [4, 11, 26, 38]. It was noted thatthe testosterone suppression associates with severe damages at germinal cells level, which in turn promote different cellular stresses such as oxidative stress [17, 25]. Therefore, reduced testosterone secretion indirectly results in remarkable changes in different cytoplasmic proteins such as CSH-px and SOD enzymes and pathologically impacts the proteins such as Hsp70 families [3]. In this regard, the serum level of testosterone, Leydig cells cytoplasmic steroid supplement and Leydig cells number per 1 mm2 of the connective tissue were analyzed. Observations showed a remarkable elevation in serum level of testosterone associated with increased cytoplasmic steroid accumulation and significant enhancement in Leydig cells distribution in treated groups. The VitE-treated animals showed significantly better results for Leydig cells versus the animals in VCL + testosterone alone group. However, the animals in Testosterone-treated group showed significantly higher serum level of testosterone in comparison to those in VCL + VitE alone-treated group. Thus, we can conclude that VitE and testosterone inhibited the VCL-induced damages completely via different mechanisms.
More biochemical assessments showed that the tissue levels of GSH-PX, SOD and TAC increased in treated animals. Interestingly, the animals in VitE-received groups (VitE alone-administrated and VitE + testosterone-treaed) revealed significantly higher antioxidant status compared to testosterone alone-administrated animals. Therefore, it can be suggested that VitE inhibited the VCL-induced damages on Leydig and germinal cells partly by enhancing the antioxidant content both at enzymatic and non-enzymatic levels. However, the exogenously administrated testosterone compensated the lack of physiologic testosterone synthesis thereby ameliorated the normal spermatogenesis process.
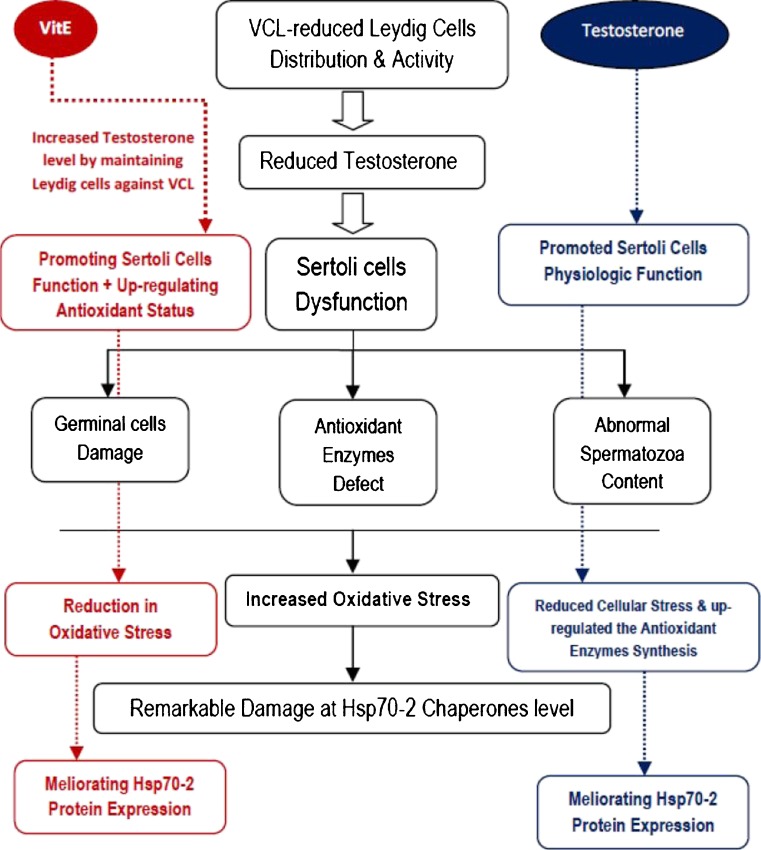
VCL-induced oxidative stress accomplished with reduced antioxidant capacity influences the germinal cells at DNA, lipids and proteins levels [2, 18, 28]. On the other hand, under different stresses expression of Hsp70-2 enhances in germinal cells in order to maintain the physiological hemostasis [29]. But beside this issue, the produced oxidative stress can affect the HSP70-2 chaperone protein structure. In this regard, the non-treated VCL-induced animals exhibited a significant reduction in Hsp70-2 expression both in immunohistocehmical and western blot analyses. Therefore, it would be more logic to conclude that the VCL-induced free radicals could damage the Hsp70-2 proteins and VitE by enhancing the antioxidant content could maintain the Hsp70-2, since VitE-treated animals showed significantly higher Hsp70-2 protein levels. However, the exogenously administrated testosterone via completely different mechanism, limited the VCL-induced damages by enhancing the endocrine status, which in turn resulted in lower ROS generation and up-regulated Hsp70-2 expression versus non-treated VCL-induced animals (note Fig. 10).
Fig. 10.
The protective mechanisms for VitE and testosterone are summarized; the VitE ameliorated the VCL-induced damages on Leydig cells by promoting the antioxidant status and regulated the interaction between Leydig and Sertoli cells. Therefore, the Sertoli cells-dependent cellular damages reduced, which in turn resulted in controlled oxidative stress. Finally the chaperones protected from reactive oxygen species impact. On the other hand the administrated testosterone up-regulated the Sertoli cells physiologic function. Ultimately, by reducing the cascade of evidences involved in generating oxidative stress could protect the heat shock proteins
The DNA strand break is able to trigger the p53-dependent apoptosis both in sperm cells and in pachytene spermatocytes [30]. On the other hand, any derangement in physiologic activity of Hsp70-2chaperones leads to incomplete recovery of DNA and incorrect proteins folding, orassembling, which may result in disrupting the balance between inhibitors and inducers ofcellular apoptosis. The VCL has been known to increase the sperm DNA damage and pachytene spermatocytes apoptosis [19, 25, 30]. Considering this fact, the elevated Hsp70-2 protein expression in testicular tissue can inhibit the VCL-induced DNA damage in sperm as well as germinal cells. This theory was proofed with our analyses. Accordingly, the VitE + testosterone-treated groups revealed with a significant decrease in percentage of sperms with DNA disintegrity. This situation suggests that co-administration of VitE + testosterone reduced the sperm DNA damage partly by elevating Hsp70-2 chaperone expression.
It has been showed that the mRNA remains stable for up to 7 days in haploid cells. The special RNA-binding proteins are involved in maintaining the stability of mRNA through recognition of a specific nucleotide sequences [7, 10]. The assembling and folding of these proteins are largely dependent on Hsp70-2 expression in haploid cells [7]. Our analyses showed that in contrast to VitE and testosterone-treated animals, the VCL-induced animals were manifested with severe RNA damage in germinal cells. Thus, the VitE and testosterone-enhanced Hsp70-2 expression could protect the germinal cells RNA content may be via maintaining RNA-binding proteins. The protected RNA contents are so essential in order to synthesis of crucial proteins such asantioxidant enzymes and the proteins involved in cell cycle. Moreover, the chromosome core proteins, the nuclear proteins such as transition proteins 1 and2, and protamines 1 and 2 present only during the post meiotic phases [39]. There is a positive correlation between these proteins expression and transition in cytoplasm with the Hsp proteins family synthesis. The significant increase in sperm chromatin condensation after administration of VitE and testosterone suggest that these two compounds may be by elevating Hsp70-2expression enhanced the core and protamin proteins synthesis and consequently improvedchromatin packing system. Considering these two findings, the VitE and testosterone administration reduced the DNA and RNA damages by two different mechanisms including; a) reducing free radicals impact on DNA via promoting antioxidant status, b) by improving chromatin condensation through maintaining the protamines stability and assembling and c) via up-regulating the Hsp70-2 chaperone expression and synthesis.
Conclusion
The data from present study declared that the VitE and testosterone alone and/or simultaneous administration could clearly inhibit the VCL-induced damages in testicular tissue and improved the sperm parameters. Comparing the co-and-alone administration of the VitE and testosterone clarified that the simultaneous form of application exhibited better results in Hsp70-2 protein expression and down-regulated the VCL-induced RNA and DNA damages. Moreover, mechanisms of action for VitE and testosterone may be attributed to their ameliorative impact on antioxidant status and testicular endocrine function, which resulted in protecting the Hsp70-2 proteins against the VCL-induced damages. Whereof, the treated animals revealed with up-regulated synthesis of enzymes involved in antioxidant defense and with elevated number of Leydig cells with steroidogenic activity.
Acknowledgments
We wish to thank Mr. Ali Karimi, the staffs of Histology laboratory, for his kind technical support. Also the authors wish to thank the Urmia University for financial supports.
Footnotes
Capsule Vitamin E and Testosterone Up-regulated varicocele-reduced Hsp70-2 protein expression by enhancing antioxidant status and intratesticular endocrine activity, respectively.
References
- 1.Agarwal A, Sharma RK, Desai NR, et al. Role of Oxidative Stress in Pathogenesis of Varicocele and Infertility. Urology. 2009;73:461–469. doi: 10.1016/j.urology.2008.07.053. [DOI] [PubMed] [Google Scholar]
- 2.Baek HY, Lim JW, Kim H, et al. Oxidative-stress related proteome changes in Helicobacterpylori-infected human gastric mucosa. Biochem J. 2004;379:291–299. doi: 10.1042/BJ20031208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chainy GB, Samantaray S, Samanta L. Testosterone-induced changes in testicular antioxidantsystem. Andrologia. 1997;29:343–349. doi: 10.1111/j.1439-0272.1997.tb00328.x. [DOI] [PubMed] [Google Scholar]
- 4.Chapman DL, Wolgemuth DJ. Regulation of M-phase promoting factor activity during development of mouse germ cells. Dev Biol. 1994;165:500–506. doi: 10.1006/dbio.1994.1270. [DOI] [PubMed] [Google Scholar]
- 5.Eddy EM. Role of heat shock protein HSP70-2 in spermatogenesis. Rev Reprod. 1999;4:23–30. doi: 10.1530/ror.0.0040023. [DOI] [PubMed] [Google Scholar]
- 6.French DB, Desai NR, Agarwal A. Varicocele repair: Does it still have a role in infertilitytreatment? Curr Opin Obstet Gynecol. 2008;20:269–274. doi: 10.1097/GCO.0b013e3282fcc00c. [DOI] [PubMed] [Google Scholar]
- 7.Hecht WB. Post transcriptional regulation of post meiotic gene expression. In: Hansson V, Levy FO, Tasken K, editors. Signal Transduction in Testicular Cells. Berlin: Springer Verlag; 1996. pp. 123–140. [Google Scholar]
- 8.Jaarin K, Gapor MT, Nafeeza MI, Fauzee AM. Effect of various doses of palm vitamin E andtocopherol on aspirin-induced gastric lesions in rats. Int J Exp Pathol. 2002;83:295–302. doi: 10.1046/j.1365-2613.2002.00242.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaur P, Bansal MP. Effect of oxidative stress on spermatogenenic process and Hsp70 expression in mice testis. Indian J Biochem Biophysics. 2003;40:246–251. [PubMed] [Google Scholar]
- 10.Kwon YK, Hecht NB. Cytoplasmic protein binding to highly conserved sequences in the3′ untranslated region of mouse protamine 2 mRNA, a translationally regulated transcript of male germ cells. Proc Natl Acad Sci. 1991;88:3584–3588. doi: 10.1073/pnas.88.9.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lazebnik YA, Kaufmann SH, Desnoyers S, et al. Characterization of the necrotic cleavageof poly (ADP-ribose) polymerase (PARP-1): implication of lysosomal proteases. Nature (London) 1994;371:346–347. doi: 10.1038/371346a0. [DOI] [PubMed] [Google Scholar]
- 12.Lee YH, Lee JC, Moon HJ, et al. Differential effect of oxidative stress on the apoptosis ofearly and late passage human diploid fibroblasts: implication of heat shock protein 60. Cell Biochem Funct. 2008;26:502–508. doi: 10.1002/cbf.1473. [DOI] [PubMed] [Google Scholar]
- 13.Lowry OH, Rosebrough NJ, Farr AL, et al. Protein measurement with the Folinphenol reagent. J Biol Chem. 1995;93:265–275. [PubMed] [Google Scholar]
- 14.Maekawa M, O’Brien DA, Allen RL, et al. Heat-shock cognate protein (hsc71) and related proteins in mouse spermatogenic cells. Biol Reprod. 1989;40:843–852. doi: 10.1095/biolreprod40.4.843. [DOI] [PubMed] [Google Scholar]
- 15.Marmar JL. Varicocele and male infertility Part II; the pathophysiology of varicocele in the light of current molecular and genetic information. Human Reprod Update. 2001;7:461–472. doi: 10.1093/humupd/7.5.461. [DOI] [PubMed] [Google Scholar]
- 16.Matsumoto M, Fujimoto H. Cloning of a hsp70-related gene expressed in mouse spermatid. Biochem Biophys Res Commun. 1990;166:43–49. doi: 10.1016/0006-291X(90)91909-C. [DOI] [PubMed] [Google Scholar]
- 17.Minerly AE, Russo SJ, Kemen LM et al. Tstosterone Plays A limited role in Cocain-induced conditioned place preference and locomotor activity in male rats. Etnicity disease. 2008;18:200–204. [PubMed]
- 18.Moshtagion SM, Malekinejad H, Razi M. Silymarin protects from varicocele-induced damages in testis and improves sperm quality: evidence for E2f1 involvement. Systems Biol Reprod Medicine Early online. 2013; doi:10.3109/19396368.2013.794253. [DOI] [PubMed]
- 19.Naughton CK, Nangia AK, Agarwal A. Pathophysiology of varicoceles in male infertility. Hum Reprod Update. 2001;7:473–481. doi: 10.1093/humupd/7.5.473. [DOI] [PubMed] [Google Scholar]
- 20.Niehaus WG, Samuelsson JRB. Formation of malonaldehyde from phospholipid arachidonate during microsomal lipid peroxidation. Eur J Biochem. 1968;6:126–130. doi: 10.1111/j.1432-1033.1968.tb00428.x. [DOI] [PubMed] [Google Scholar]
- 21.Pant N, Srivastava SP. Testicular and spermatotoxic effect of quinaphos in rats. J Appl Toxicol. 2003;23:271–274. doi: 10.1002/jat.919. [DOI] [PubMed] [Google Scholar]
- 22.Rajfer J, Turner TT, Rivera F, et al. Inhibition of testicular testosterone biosynthesisfollowing experimental varicocele in rats. Biol Reprod. 1987;36:933–937. doi: 10.1095/biolreprod36.4.933. [DOI] [PubMed] [Google Scholar]
- 23.Razi M, Malekinejad H, Sadrkhanloo RA. Histological Impact of long term varicocele-induction on right and left testes in rat (evidence for the reduction of sperm quality andmating abilities) Vet Research Forum. 2011;2:189–201. [Google Scholar]
- 24.Razi M, Sadrkhanloo RA, Malekinejad H. Varicocele time-dependently affects DNA integrity of sperm cells: evidence for lower in vitro fertilization rate in varicocele-positiverats. Inter J Fertil Steril. 2011;5:174–185. [PMC free article] [PubMed] [Google Scholar]
- 25.Ricci JE, Munoz-Pinedo C, Fitzgerald P, et al. Disruption of mitochondrial function during apoptosis is mediated by caspase cleavage of the p75 subunit of complex I of the electrontransport chain. Cell. 2004;117:773–786. doi: 10.1016/j.cell.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 26.Saalu LC, Oguntola JA, Babalola OS, Oyewopo AO. Reversal of experimental varicocele induced testicular toxicity by L-ascorbate in rats. African J Biotech. 2009;8:965–970. [Google Scholar]
- 27.Saleh RA, Agarwal A, Sharma RK, et al. Evaluation of nuclear DNA damage in spermatozoafrom infertile men with varicocele. Fertil Steril. 2003;80:1431–1436. doi: 10.1016/S0015-0282(03)02211-8. [DOI] [PubMed] [Google Scholar]
- 28.Sarge KD, Park-Sarge OK, Kirby JD, et al. Expression of Heat Shock Factor 2 in MouseTestis: Potential Role as a Regulator of Heat-Shock Protein Gene Expression during spermatogenesis. Biol Reprod. 1994;50:1334–1343. doi: 10.1095/biolreprod50.6.1334. [DOI] [PubMed] [Google Scholar]
- 29.Schwartz D, Goldfinger N, Rotter V. Expression of p53 protein in spermatogenesis isconfined to the tetraploid pachytene primary spermatocytes. Oncogene. 1993;8:1487–1494. [PubMed] [Google Scholar]
- 30.Sharma RK, Pasqualotto FF, Nelson DR, et al. The reactive oxygen speciestotal antioxidant capacity score is a new measure of oxidative stress to predict male infertility. Hum Reprod. 1999;14:2801–2807. doi: 10.1093/humrep/14.11.2801. [DOI] [PubMed] [Google Scholar]
- 31.Smith R, Kaune H, Parodi D, et al. Increased sperm DNA damage in patients with varicocele relationship with seminal oxidative stress. Hum Reprod. 2006;21:986–993. doi: 10.1093/humrep/dei429. [DOI] [PubMed] [Google Scholar]
- 32.Sofikitis N, Miyagawa I. Experimental models for the study of varicocele: A selected review. Jpn J Fertil Steril. 1992;38:168–177. [Google Scholar]
- 33.Tanrikut C, Mcquaid JW, Goldstein M. The impact of varicocele and varicocele repair on serum testosterone. Curr Opin Obstet Gynecol. 2011;23:227–231. doi: 10.1097/GCO.0b013e328348a3e2. [DOI] [PubMed] [Google Scholar]
- 34.Tejada RI, Mitchel JC, Norman A. A test for the practical evaluation of male infertility byacridine orange (AO) fluorescence. Fertil Steril. 1984;42:87–91. doi: 10.1016/s0015-0282(16)47963-x. [DOI] [PubMed] [Google Scholar]
- 35.Von Bertalanffy L, Bickis I. Identification of Cytoplasmic Basophilia (RibonucleicAcid) by Fluorescence Microscopy. J Histochem Cytochem. 1956;4:481. doi: 10.1177/4.5.481. [DOI] [PubMed] [Google Scholar]
- 36.WHO laboratory manual for the examination of human semen and sperm cervical mucus interaction. Cambridge: Cambridge University Press; 1999. [Google Scholar]
- 37.Yousef MI, Abdallah GA, Kamel KI. Effect of ascorbic acid and vitamin E supplementation onsemen quality and biochemical parameters of male rabbits. Anim Reprod Sci. 2003;76:99–111. doi: 10.1016/S0378-4320(02)00226-9. [DOI] [PubMed] [Google Scholar]
- 38.Zhu D, Dix DJ, Eddy EM. HSP70-2 is required for CDC2 kinase activity in meiosis I ofmouse spermatocytes. Development. 1997;124:3007–3014. doi: 10.1242/dev.124.15.3007. [DOI] [PubMed] [Google Scholar]