Abstract
Sleep disturbances are common in many neurodegenerative diseases and may include altered sleep duration, fragmented sleep, nocturia, excessive daytime sleepiness, and vivid dreaming experiences, with occasional parasomnias. Although representing the “gold standard,” polysomnography is not always cost-effective or available for measuring sleep disturbance, particularly for screening. Although numerous sleep-related questionnaires exist, many focus on a specific sleep disturbance (e.g., restless legs, REM Behavior Disorder) and do not capture efficiently the variety of sleep issues experienced by such patients. We developed and administered the 12-item Neurodegenerative Disease Sleep Questionnaire (NDSQ) and the Epworth Sleepiness Scale to 145 idiopathic Parkinson’s disease patients. Principal component analysis using eigenvalues greater than 1 suggested five separate components: sleep quality (e.g., sleep fragmentation), nocturia, vivid dreams/nightmares, restless legs symptoms, and sleep-disordered breathing. These results demonstrate construct validity of our sleep questionnaire and suggest the NDSQ may be a useful screening tool for sleep disturbances in at least some types of neurodegenerative disorders.
Keywords: sleep questionnaire, principal components analysis, nocturia, sleep disordered breathing, dreaming, restless legs syndrome, neurodegenerative disease, Parkinson’s disease
1. Introduction
Sleep disturbances in patients with neurodegenerative disease are common [1,2]. For example, Parkinson’s disease is associated with poor quality and highly disrupted sleep, presence of periodic leg movements, phasic muscle activity, vivid dreaming with occasional dream enactment, nocturia, and excessive daytime sleepiness [3]. These conditions are ideally measured with polysomnography, which, although providing objective verification of the sleep pattern in question, may be difficult to use on a routine basis because of cost, accessibility to sleep diagnostic services, interpretive complexity of the electroencephalogram, and/or patient unwillingness. Questionnaires offer a more cost effective means to screen sleep related symptoms in such patients, but no clear consensus exists on the preferred questionnaire, partially because scales often focus on one aspect of the sleep disturbance. Some questionnaires have inquired about signs and symptoms of sleep apnea [4], others focus on dream enactment behavior [5], and yet others examine overall quality of sleep [6], but there have been few attempts to assess many different kinds of symptoms in a single questionnaire [7]. In this study we describe our experience in using a very brief Neurodegenerative Disease Sleep Questionnaire (NDSQ), which we conceptualized [8] to encompass six structures (Table 1): sleep quality, nocturia, dreams/nightmares, restless legs, snoring, and daytime somnolence. We used principal component analysis to test these hypotheses.
Table 1.
Sleep questions were drawn from existing studies, which have assessed different aspects of sleep.
| Sleep Question | Scale | Predicted Component | Reference |
|---|---|---|---|
| 1. How many hours do you usually get at night? | Sleep quality | [26] | |
| 2. How often do you have trouble falling asleep? | 1–5 | Sleep quality | [27] |
| 3. How often do you have trouble waking up during the night? | 1–5 | Sleep quality | |
| 4. How often do you have trouble waking up too early and not being able to fall asleep again? | 1–5 | Sleep quality | |
| 5. When you awaken during the night, how often do you urinate? | 1–4 | Nocturia | [28] |
| 6. How often do you snore in any way? | 1–5 | Snoring | [11] |
| 7. What time do you typically go to bed at night? | Sleep quality | [29] | |
| 8. What time do you typically wake up in the morning? | Sleep quality | ||
| 9. How often do you have a night full of intense, vivid dreams? | 1–5 | Dreams/Nightmares | [17] |
| 10. How often do you have nightmares (frightening dreams)? | 1–5 | Dreams/Nightmares | |
| 11. At bedtime, how often does restlessness in your legs delay your falling asleep? | 1–4 | Restless Legs | [30,31] |
| 12. When you wake up during the night, how often do you feel unpleasant sensations in the leg muscles that require you to move your legs or walk in order to be comfortable? | 1–4 | Restless Legs | |
| [Epworth Sleepiness Scale] Rate how likely you are to doze or fall asleep (in 8 daily situations) | 0–24 | Daytime Sleepiness | [10] |
2. Methods
2.1 Patients
Idiopathic Parkinson’s disease (PD) patients (N=145; Age: M=64.74, SD=8.95; Gender: 34.5% female; Disease duration in years: M=8.03, SD=4.44; Unified Parkinson’s Disease Rating Scale motor subscore (part 3): M=17.97, SD=7.99) meeting United Kingdom Brain Bank diagnostic criteria [9] were evaluated and diagnosed by a board-certified neurologist with extensive experience in movement disorders. Patients were predominantly (92.41%) classified as Hoehn and Yahr stages I–II. The study was approved by the Emory University Institutional Review Board and all patients provided informed consent.
2.2 Neurodegenerative Disease Sleep Questionnaire (NDSQ)
The NDSQ is available in the Online Supplementary Material. The NDSQ, presented in Table 1, is an amalgam of questions from different research studies. These studies focused on various aspects of self-reported sleep characteristics of middle-aged and elderly populations without neurological disease. The wording of the questions reflects the original wording as used in the primary source. Additionally, we screened for excessive daytime sleepiness with the Epworth Sleepiness Scale (ESS) [10]. The NDSQ can be completed by either patients themselves or, in the case of dementia, by their caregivers or family members. In this study, none of the PD patients were demented, and all completed the questionnaire by themselves.
2.3 Statistical Analyses
Missing data were excluded from the analyses. We used the nocturnal sleeping hours and typical bedtime/waketime questions (Questions 1, 7–8) to form a measure of sleep efficiency (total sleeping hours divided by difference between typical bedtime and typical waketime). Our primary analytical approach was to conduct a principal component analysis to determine the number of components underlying patients’ responses on the NDSQ in this population. We used Kaiser’s rule of eigenvalues greater than 1 and Cattell’s scree plot method to determine the number of components. We then rotated the solution using an oblique rotation (promax, kappa=4) to obtain simple structure. We used an oblique rotation to account for the possibility that some of our components may be related to one another.
3. Results
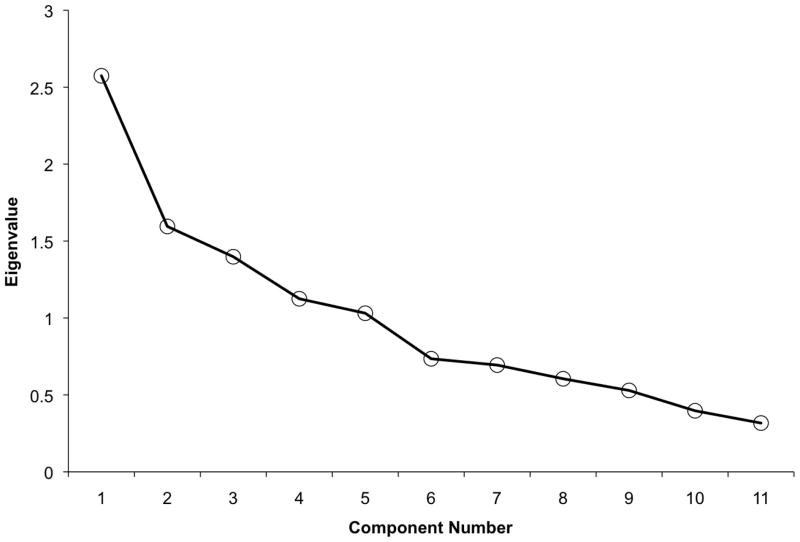
Descriptive information for the NDSQ is presented in Table 2. According to Kaiser’s rule and the Cattell’s scree plot method (Fig 1), we accepted five components to the NDSQ. The five components are illustrated in Table 3 and explained 70.20% of the total variance. Component 1 was primarily composed of sleep quality measures: the highest loadings were increased frequency of trouble falling asleep, nighttime awakenings, difficulty being able to fall back asleep, and lower sleep efficiency. No other variables loaded strongly (all ≤.15) onto Component 1. Both restless legs questions loaded strongly onto Component 2, and this component also included a moderate loading of difficulty falling asleep (cf. Question 11 regarding restlessness causing difficulty falling asleep). Component 3 included strong loadings for the vivid dreams frequency and nightmares frequency questions, with all other variables demonstrating weak to negligible loadings. The strongest loading onto Component 4 was the occurrence of nocturia. Given the definition of nocturia (waking up in the middle of the night and having to go to the bathroom), it is perhaps not surprising that there was also a moderate loading of waking in the middle of the night on Component 4. Finally, Component 5 was composed of both snoring frequency and daytime somnolence (ESS), which could possibly reflect their common association with sleep-disordered breathing (SDB). Cronbach’s alpha for the items within Component 1 (i.e., the only component with more than two items) was moderate (.53). The components did not correlate strongly with one another (rs < .23), which suggests that the underlying constructs are highly dissociable.
Table 2.
Descriptive information for the NDSQ. Sample size varied across questions due to missing data or endorsement of “don’t know” responses. The first measure is a ratio that indicates sleep efficiency, with higher scores indicating better sleep. For the remaining questions higher scores indicate greater sleep-related disturbances.
| N | Mean | Standard Deviation | |
|---|---|---|---|
| Hours Sleeping/Hours in Bed | 139 | 0.94 | 0.14 |
| Trouble Falling Asleep | 145 | 2.05 | 1.0 |
| Waking During Night | 145 | 2.84 | 1.27 |
| Waking Too Early | 145 | 2.47 | 1.11 |
| Nocturnal Bathroom Trips | 144 | 1.71 | 0.87 |
| Snoring | 127 | 2.84 | 1.19 |
| Vivid Dreams | 143 | 2.01 | 1.01 |
| Nightmares | 139 | 1.38 | 0.66 |
| RLS at bedtime | 144 | 1.48 | 0.75 |
| RLS during night | 143 | 1.64 | 0.79 |
| Daytime Sleepiness (Epworth) | 143 | 9.78 | 4.84 |
Fig. 1.
Cattell scree plot suggesting five components to the sleep questionnaire. Kaiser’s eigenvalue rule converged with the five component solution.
Table 3.
The loadings of the principal component analysis using an oblique (promax, kappa=4) rotation and eigenvalue greater than 1 cutoff. Descriptive labels for Components 1–5 are provided.
| Component 1 (Sleep Quality) | Component 2 (Restless Legs) | Component 3 (Dreams/Nightmares) | Component 4 (Nocturia) | Component 5 (Sleep-Disordered Breathing) | |
|---|---|---|---|---|---|
| Hours Sleeping/Hours in Bed | −.69 | .02 | −.12 | −.01 | .26 |
| Trouble Falling Asleep | .54 | .38 | .18 | −.13 | −.19 |
| Waking During Night | .59 | −.04 | −.01 | .53 | .11 |
| Waking Too Early | .88 | −.15 | −.20 | .04 | .20 |
| Nocturnal Bathroom Trips | .00 | .17 | .09 | .83 | −.06 |
| Snoring | .13 | .14 | .07 | −.44 | .65 |
| Vivid Dreams | −.03 | −.02 | .87 | .02 | .03 |
| Nightmares | .01 | −.13 | .82 | .11 | .15 |
| RLS at bedtime | .02 | .87 | −.09 | .00 | .11 |
| RLS during night | −.12 | .86 | −.07 | .24 | .05 |
| Daytime Sleepiness (Epworth) | −.15 | .05 | .11 | .15 | .81 |
| Eigenvalues for Unrotated Solution | 2.57 | 1.60 | 1.40 | 1.13 | 1.03 |
| Eigenvalues for Rotated Solution | 2.10 | 1.85 | 1.61 | 1.32 | 1.34 |
3.1 Additional Analyses
We conducted additional principal component analyses to determine the generalizeability and potential limitations of our findings. First, a similar 5-component structure obtained when using Varimax rotation rather than oblique rotation. Second, we conducted a principal component analysis (oblique rotation) after treating the “don’t know” responses to snoring as “sometimes” responses [11]. Again, a 5-component structure was observed and snoring continued to load the highest for Component 5. Third, we tested whether using an extraction factor method indicating six factors (rather than relying on eigenvalues > 1) improved the congruence of the results with our hypotheses [ref; Table 1]. Indicating six factors increased the cumulative variance explained (76.88%), though the sixth component only had an eigenvalue of 0.74. As illustrated in Table 4, the results were similar to our original principal component analysis, with two exceptions: sleep efficiency and trouble falling asleep only weakly loaded onto Component 1, and snoring and Epworth daytime sleepiness loaded onto separate factors (Components 4 and 6).
Table 4.
The loadings of the principal component analysis using an oblique (promax, kappa=4) rotation and forcing a 6 factor solution. Descriptive labels for Components 1–6 are provided.
| Component 1 (Sleep Maintenance) | Component 2 (Restless Legs) | Component 3 (Dreams/Nightmares) | Component 4 (Daytime Sleepiness) | Component 5 (Nocturia) | Component 6 (Snoring) | |
|---|---|---|---|---|---|---|
| Hours Sleeping/Hours in Bed | −.24 | −.11 | −.03 | .68 | −.23 | −.24 |
| Trouble Falling Asleep | .27 | .41 | .20 | −.40 | −.19 | −.02 |
| Waking During Night | .80 | .01 | .03 | .08 | .26 | −.21 |
| Waking Too Early | .96 | −.09 | −.15 | −.05 | −.17 | .05 |
| Nocturnal Bathroom Trips | −.01 | .06 | .00 | −.10 | .94 | −.02 |
| Snoring | −.10 | .01 | −.04 | .01 | −.03 | .96 |
| Vivid Dreams | −.19 | −.03 | .87 | −.09 | .04 | .05 |
| Nightmares | .04 | −.08 | .87 | .13 | −.04 | −.08 |
| RLS at bedtime | .01 | .91 | −.05 | .07 | −.09 | .02 |
| RLS during night | −.13 | .85 | −.07 | .05 | .22 | .01 |
| Daytime Sleepiness (Epworth) | .26 | .08 | .15 | .72 | .04 | .30 |
| Eigenvalues for Unrotated Solution | 2.57 | 1.60 | 1.40 | 1.13 | 1.03 | 0.74 |
| Eigenvalues for Rotated Solution | 2.08 | 1.96 | 1.74 | 1.30 | 1.28 | 1.31 |
3.2 Correlational analyses
We conducted correlational analyses between the sleep variables (i.e., those in Table 1) and demographic and disease-related factors. Gender was not significantly associated with any sleep variable, whereas age was significantly positively correlated with total sleep time, r(140)=.23, p<.01, and bathroom trips, r(142)=.19, p=.02, and negatively with waking too early in the morning, r(143)=−.20, p=02. Disease duration was negatively associated with sleep efficiency, r(137)=−.17, p<.05, and UPDRS motor score was positively correlated with Epworth scores, r(140)=.22, p=.01.
4. Discussion
4.1 Evaluation of the Component Structure
We originally conceptualized [8] the NDSQ and ESS as representing six structures, and this conceptualization was highly compatible with the five components yielded by the principal component analysis. The only discrepancy between our initial conceptualization of the NDSQ (Table 1) and the results of the principal component analysis was that daytime sleepiness and snoring measures loaded onto the same component. Snoring and daytime sleepiness are two of the primary symptoms of SDB; therefore, Component 5 may represent SDB, at least in these PD patients. However, this conclusion should be treated with some caution because snoring and daytime sleepiness loaded onto separate factors when forcing the principal component analysis to fit six factors.
One of the components revealed in the NDSQ clustered on sleep quality measures (Component 1). Poor sleep quality is frequently observed in PD patients. Relative to healthy age-matched controls, PD patients demonstrate difficulty with sleep maintenance [12] and possibly also with sleep initiation [3]. Component 1 of the NDSQ was primarily associated with sleep maintenance difficulties, but also to a (slightly) lesser extent with sleep initiation difficulties. Poor sleep quality in PD patients may be due to restricted range of mobility when in bed, tremor, increased frequency of periodic leg movements that may disrupt sleep [13], or greater dream enactment [14]. Though there was some overlap between the sleep initiation question (which loaded most strongly onto Component 1) and the RLS factor (Component 2), this was likely due to RLS being associated with difficulty falling asleep. Although early research suggested that 20.8% of PD patients also have RLS [15], subsequent research has indicated that these conditions have different underlying pathophysiology [16].
Additional dissociable components in the NDSQ were frequency of vivid dreams/nightmares and nocturia. The clinical relevance of vivid dreams/nightmares (Component 3) may relate to presence of REM sleep behavior disorder [3], pharmacological treatment [18], psychiatric co-morbidity [19], or possibly even narcolepsy [17], but these possibilities require further investigation and might constitute a reason to attempt polysomnography. Another aspect of sleep that may hold clinical value, but is not often incorporated into sleep questionnaires, is the assessment of nocturia (nighttime voiding), which can be a major issue in neurodegenerative conditions like PD [20]. The nocturia question only loaded strongly onto Component 4, and this component only received moderate contribution from one other factor (nocturnal awakenings). The omission of a nocturia assessment from typical sleep questionnaires may be important not only because it assesses a different component than other sleep questions (as shown by the present results), but also because nocturia is associated with increased risk for falls [21] and worsened health-related quality of life [22].
4.2 Relation to Existing Sleep Questionnaires
Existing sleep questionnaires for patients with neurodegenerative diseases include scales focused on PD [23–25] and scales focused on dream enactment behaviors [5]. We have found that, when combined with the ESS, the 12-items inquired about here provide a very quick overview of what issues may be problematic for PD patients and their caregivers. The recently published Mayo Sleep Questionnaire [7] represents another validated option for assessing reported aspects of sleep, however, its utility of population-based screening may have some limitations because it does not collect data on sleep duration or nocturia [26,28], which are both important phenomena particularly among older persons. Inclusion of ESS along with the NDSQ also allows for more detailed assessment of real world situations in which daytime sleepiness is likely to occur.
4.3 Conclusions and Future Directions
The present work used principal component analysis to evaluate the NDSQ’s ability to assess multiple aspects of reported sleep behaviors, namely, sleep quality, vivid dreams/nightmares, nocturia, SDB, and restless legs symptoms. Future work may involve the evaluation of test-retest reliability and the inclusion of patients with varying disease severity and different neurological conditions. However, based on the component structure that was revealed by principal component analysis, the NDSQ appears to be a valid and efficient metric for assessing sleep disturbances in patients with neurodegenerative disease, in this case those with PD.
Supplementary Material
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bliwise DL. Sleep disorders in Alzheimer’s disease and other dementias. Clinical Cornerstone. 2004;6:S16–S28. doi: 10.1016/s1098-3597(04)90014-2. [DOI] [PubMed] [Google Scholar]
- 2.Bliwise DL. Sleep in independently living and institutionalized elderly. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. 5. St. Louis: Elsevier Saunders; 2011. pp. 1551–61. [Google Scholar]
- 3.Bliwise DL, Trotti LM, Rye DB. Movement disorders specific to sleep and sleep in waking movement disorders. In: Watts RL, Standaert DG, Obeso JA, editors. Movement Disorders. 3. New York: McGraw Hill; 2011. pp. 935–74. [Google Scholar]
- 4.Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999;131:485–91. doi: 10.7326/0003-4819-131-7-199910050-00002. [DOI] [PubMed] [Google Scholar]
- 5.Lam SP, Li SX, Zhang J, Wing YK. Development of scales for assessment of REM sleep behavior disorder (RBD) Sleep Med. 2012 doi: 10.1016/j.sleep.2012.09.008. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 6.Buysse DJ, Reynolds CF, III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Research. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 7.Boeve BF, Molano JR, Ferman TJ, Smith GE, Lin SC, Bieniek K, et al. Validation of the Mayo Sleep Questionnaire to screen for REM sleep behavior disorder in an aging and dementia cohort. Sleep Med. 2011;12:445–53. doi: 10.1016/j.sleep.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scullin MK, Sollinger AB, Land J, Wood-Siverio C, Zanders L, Lee R, et al. Sleep and impulsivity in Parkinson’s disease. Parkinsonism Relat Disord. 2013 doi: 10.1016/j.parkreldis.2013.06.018. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinicopathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–4. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 11.Bliwise DL, Nekich JC, Dement WC. Relative validity of self-reported snoring as a symptom of sleep apnea in a sleep clinic population. CHEST. 1991;99:600–8. doi: 10.1378/chest.99.3.600. [DOI] [PubMed] [Google Scholar]
- 12.Factor SA, McAlarney T, Sanchez-Ramos JR, Weiner WJ. Sleep disorders and sleep effect in Parkinson’s disease. Mov Disord. 2004;5:280–5. doi: 10.1002/mds.870050404. [DOI] [PubMed] [Google Scholar]
- 13.Wetter TC, Collado-Seidel V, Yassouridis A, Trenkwalder C. Sleep and periodic leg movement patterns in drug-free patients with Parkinson’s disease and multiple system atrophy. Sleep. 2000;23:361–367. [PubMed] [Google Scholar]
- 14.Gagnon JF, Bédard MA, Fantini ML, Petit D, Panisset M, Rompre S, et al. REM sleep behavior disorder and REM sleep without atonia in Parkinson’s disease. Neurology. 2002;59:585–9. doi: 10.1212/wnl.59.4.585. [DOI] [PubMed] [Google Scholar]
- 15.Ondo WG, Vuong KD, Jankovic J. Exploring the relationship between Parkinson disease and restless legs syndrome. Arch Neurol. 2002;59:421–4. doi: 10.1001/archneur.59.3.421. [DOI] [PubMed] [Google Scholar]
- 16.Chokroverty S. Introduction: comorbid disorders and special populations. In: Hening WA, Chokroverty S, Allen R, Earley C, editors. Restless legs syndrome. Philadelphia, PA: Saunders; 2009. pp. 161–72. [Google Scholar]
- 17.Lee JH, Bliwise DL, Lebret-Bories E, Guilleminault C, Dement WC. Dream disturbed sleep in insomnia and narcolepsy. J Nerv Ment Dis. 1993;181:320–324. doi: 10.1097/00005053-199305000-00008. [DOI] [PubMed] [Google Scholar]
- 18.Thompson DF, Pierce DR. Drug-induced nightmares. Ann Pharmacother. 1999;33:93–98. doi: 10.1345/aph.18150. [DOI] [PubMed] [Google Scholar]
- 19.Levin R, Fireman G. Nightmare prevalence, nightmare distress, and self-reported psychological disturbance. Sleep. 2002;25:205–212. [PubMed] [Google Scholar]
- 20.Vaughan CP, Eisenstein R, Bliwise DL, Endeshaw YK, Nagamia ZJ, Wolf RA, Johnson TM. Self-rated sleep characteristics and bother from nocturia. Int J Clin Pract. 2012;66:369–73. doi: 10.1111/j.1742-1241.2011.02868.x. [DOI] [PubMed] [Google Scholar]
- 21.Stewart RB, Moore MT, May FE, Marks RG, Hale WE. Nocturia: a risk factor for falls in the elderly. J Am Geriatr Soc. 1992;40:1217–20. doi: 10.1111/j.1532-5415.1992.tb03645.x. [DOI] [PubMed] [Google Scholar]
- 22.Coyne KS, Zhou Z, Bhattacharyya SK, Thompson CL, Dhawan R, Versi E. The prevalence of nocturia and its effect on health-related quality of life and sleep in a community sample in the USA. BJU Int. 2003;92:948–54. doi: 10.1111/j.1464-410x.2003.04527.x. [DOI] [PubMed] [Google Scholar]
- 23.Chaudhuri KR, Pal S, DiMarco A, Whately-Smith C, Bridgman K, Mathew R, et al. The Parkinson’s disease sleep scale: a new instrument for assessing sleep and nocturnal disability in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2002;73:629–35. doi: 10.1136/jnnp.73.6.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chaudhuri KR, Martinez-Martin P, Brown RG, Sethi K, Stocchi F, Odin P, et al. The metric properties of a novel non-motor symptoms scale for Parkinson’s disease: Results from an international pilot study. Mov Disord. 2007;22:1901–11. doi: 10.1002/mds.21596. [DOI] [PubMed] [Google Scholar]
- 25.Martinez-Martin P, Visser M, Rodriguez-Blazquez C, Marinus J, Chaudhuri K, van Hilten JJ. SCOPA-sleep and PDSS: Two scales for assessment of sleep disorder in Parkinson’s disease. Mov Disord. 2008;23:1681–1688. doi: 10.1002/mds.22110. [DOI] [PubMed] [Google Scholar]
- 26.Qureshi AI, Giles WH, Croft JB, Bliwise DL. Habitual sleep patterns and risk for stroke and coronary heart disease: a 10-year follow-up from NHANES I. Neurology. 1997;48:904–11. doi: 10.1212/wnl.48.4.904. [DOI] [PubMed] [Google Scholar]
- 27.Foley DJ, Monjan AA, Brown SL, Simonsick EM. Sleep complaints among elderly persons: An epidemiologic study of three communities. Sleep. 1995;18:425–32. doi: 10.1093/sleep/18.6.425. [DOI] [PubMed] [Google Scholar]
- 28.Middelkoop HAM, Smilde-van den Doel DA, Neven AK, Kamphuisen HAC, Springer CP. Subjective sleep characteristics of 1,485 males and females aged 50–93: effects of sex and age, and factors related to self-evaluated quality of sleep. J Gerontol Med Sci. 1996;51A:M108–M115. doi: 10.1093/gerona/51a.3.m108. [DOI] [PubMed] [Google Scholar]
- 29.Bliwise DL, Tinklenberg JR, Yesavage JA. Timing of sleep and wakefulness in Alzheimer’s Disease patients residing at home. Biol Psychiatry. 1992;31:1163–5. doi: 10.1016/0006-3223(92)90162-s. [DOI] [PubMed] [Google Scholar]
- 30.Lavigne GJ, Montplaisir JY. Restless legs syndrome and sleep bruxism: prevalence and association among Canadians. Sleep. 1994;17:739–43. [PubMed] [Google Scholar]
- 31.Phillips B, Young T, Finn L, Asher K, Hening WA, Purvis C. Epidemiology of restless legs symptoms in adults. Arch Intern Med. 2000;160:2137–41. doi: 10.1001/archinte.160.14.2137. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.