Abstract
Background
It is unknown which patient will benefit most from hospital admission after transient ischemic attack (TIA).Our aim was to define predictors of a positive hospital outcome
methods
We used two cohorts of TIA patients: the University of Texas at Houston Stroke center (UTH); and Tel-Aviv Sourasky medical Center in Israel (TASMC) for external validation. We retrospectively reviewed medical records and imaging data.
We defined positive yield (PY) of the hospital admission as identification of stroke etiologies that profoundly changes clinical management.
Results
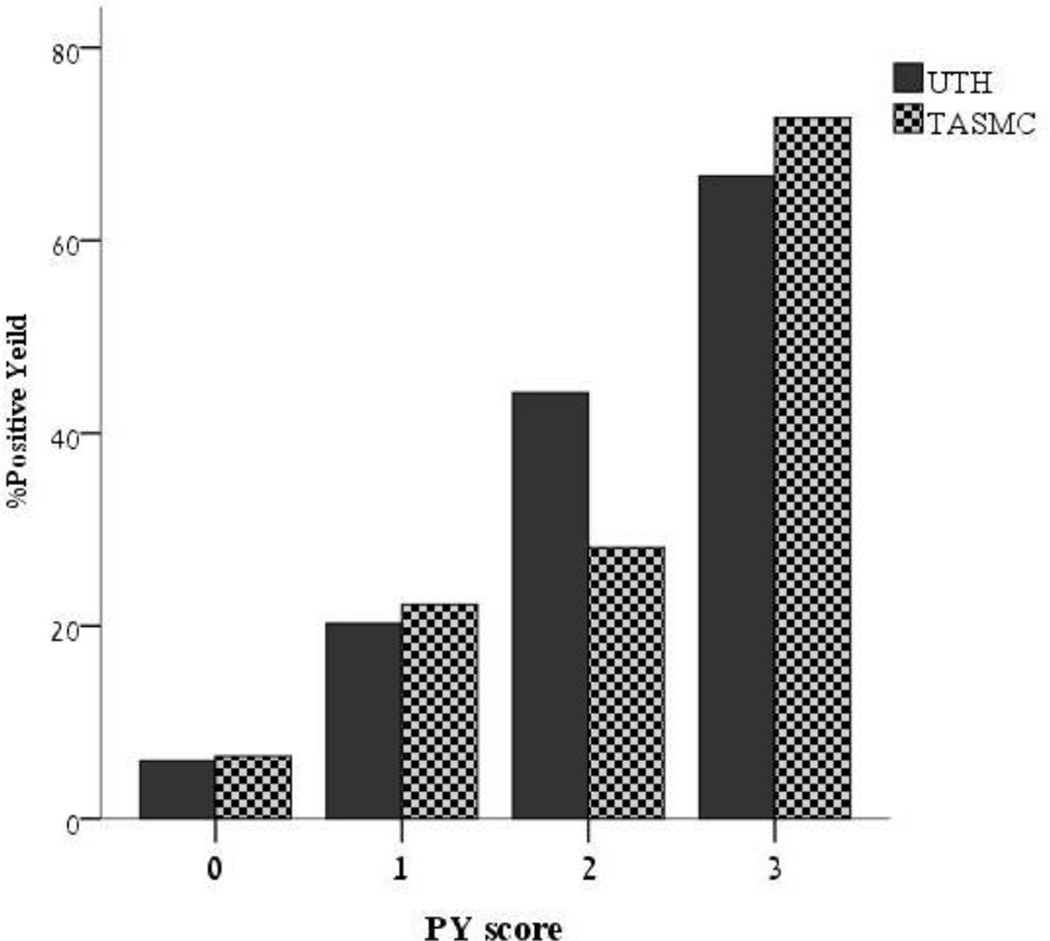
The UTH cohort included 178 patients. 24.7% had PY. In the multivariate analysis, the following were associated with PY: Coronary desease (CAD); age; acute infarct on DWI. We then derived a composite score termed the PY score to predict PY. One point is scored for: age>60, CAD, and acute infarct on DWI. The proportion of PY by PY score was as follows: 0- 6%; 1- 22%; 2- 47%; 3- 67% (p<0.001). In the validation cohort PY score was highly predictive of PY and performed in a very similar manner.
Conclusions
Our data suggest, the PY score may enable physician to make better admission decisions and result in better, safer and more economical care for TIA patients.
Keywords: TIA, hospital admission, stroke prevension
Introduction
There is consensus that some patients experiencing TIA are at high short-term risk of stroke. Several studies have identified risk factors for stroke after TIA, which may be useful in making initial management decisions, of which the ABCD2 score is currently the prediction standard[1]. While ABCD2 and other prediction scores provide valuable information on the patients' actual risk of stroke, these scores do not predict which patients to hospitalize and which patients will have findings on stroke work-up that will change medical decision making.
There are three clinical approaches to the management of TIA patients who present to the emergency department[2, 3]: Admission of all patients; Admission according to cut offs using prediction scoring such as ABCD2; and transfer to an ambulatory TIA clinic. With little concrete data to support such approaches, the optimal management of TIA patients remains poorly defined. Admitting TIA patients to the hospital permits rapid diagnostic evaluation to uncover modifiable risk factors such as carotid artery stenosis and atrial fibrillation. These may be treated immediately and drastically reduce the patients short and long-term stroke risk. Rates of adherence to secondary prevention may also improve after a hospital stay[4]. Lastly, in-hospital observation of patients with TIA enables one to treat an imminent stroke. On the other hand, hospital costs are rising and in-hospital workup exposes the patient to a variety of hospital-acquired infections and overall increases the burden on the already-stretched medical systems of industrialized countries.
The aim of our study was to estimate the additive value of hospitalization in patients after TIA. Hospitalization of a TIA patient may be valuable if it leads to immediate changes in medical management. We therefore sought to identify, on a large cohort, variables that would predict which TIA patients are found to have a positive finding on diagnostic work-up that led to a change in medical management beyond prescribing an antiplatelet agent and a statin. We then created a scoring system that predicted which patients would have a positive finding and validated the score on an independent cohort in another country.
Methods
For this study, we used two cohorts of TIA patients: One from the stroke program at the University of Texas in Houston Stroke (UTH cohort) and another from the Tel-Aviv Sourasky medical Center in Israel (TASMC cohort). The TASMC cohort is a subset of the TABASCO study[5] which is an observational study of patients with a first-ever stroke or TIA. Both centers routinely admit all TIA patients for standard stroke work-up that includes at minimum a brain CT scan, carotid Doppler, EKG monitoring, and echocardiogram. The UTH cohort was used for derivation of the prediction score and the TASMC cohort was used for external validation. The UTH cohort consisted of consecutive TIA patients from 8/07 to 6/08 hospitalized in the stroke unit with a diagnosis of TIA as per the WHO criteria. The TASMC cohort consisted of 128 consecutive patients with a first-ever TIA hospitalized between April 2006 and August 2011. We retrospectively reviewed medical records and collected demographic data, medical background, clinical characteristics, and imaging of the qualifying event. All patients underwent MRI on admission. We specifically collected the presence of acute infarcts on the DWI sequence. The primary end point of this work was positive yield (PY) of the hospital admission. We defined PY as identification of stroke etiologies that in turn led to a change in management (Table 1). The following were defined as PY: carotid stenosis ≥ 60% ipsilateral to the TIA-localized hemisphere; atrial fibrillation, left atrial thrombus; focal left ventricular wall motion abnormalities; left ventricular apical aneurysm; left ventricular thrombus; and arterial dissection. The ABCD2 score as well as the ABCD-I score[6] were calculated for all patients. In order to identify predictors of PY, we ran a univariate analysis on all clinical and radiological variables. Those variables that were positive on the univariate analysis were entered into a logistic regression model with PY as the dependent variable. The variables that were independently associated with PY were combined to create the PY score. We than applied the PY score to the TASMC cohort for external validation.
Table 1.
findings considered "positive yeald" during a hospital admission
| PY elements |
|---|
| Ipsilateral carotid stenosis ≥ 60% |
| Cervical artery dissection |
| atrial fibrillation |
| left atrial thrombus |
| Atrial septal defect |
| focal left ventricular wall motion abnormalities |
| left ventricular apical aneurysm |
| left ventricular thrombus |
| Valvular disease |
Statistical analysis
We used Chi-square and Fisher's exact test with categorical variables and Student's t-test with continuous variables with PY as the factor for the univariate analysis. For the multivariate analysis, we used bivariate logistic regression with PY as the dependent variable. All analysis were done using SPSS version 17.0 (SPSS inc. Chicago IL).
Results
The UTH cohort included 178 patients. The patient characteristics are shown in table 1. Two patients (1.1%) had a stroke during their hospital stay- both with high ABCD2, and 44 patients (24.7%) had a PY. Variables associated with PY on the univariate analysis were: age (p=0.013); acute infarct on DWI (p<0.001); history of TIA (p=0.08), CAD (p=0.07) and CHF (p=0.037). ABCD2 was not associated with a PY (p=0.33) as well as ABCD-I (p=0.18). In the multivariate analysis, the following were found to be independently associated with PY: history of CAD (OR=2.5, 95%CI 1.03–6.1, p=0.042); age (OR=1.03/year, 95% CI 1.001–1.0059, p=0.041); acute infarct on DWI (OR=7.4, 95% CI 3.1–17.6, p<0.001). Using these variables, we derived a composite score termed the PY score that predicts PY. The patient is scored one point for each of these: age>60, history of CAD, and acute infarct on DWI. The proportion of PY by PY score was as follows: 0- 6%; 1- 22%; 2- 47%; 3–67% (p<0.001 for the trend). An attempt was made to produce a more complex score by weighing each variable according to the OR received in the multivariate analysis. This score had a slightly lower C statistic then the simpler model (0.74 vs 0.754) and therefore we opted for the simple and more accurate alternative.
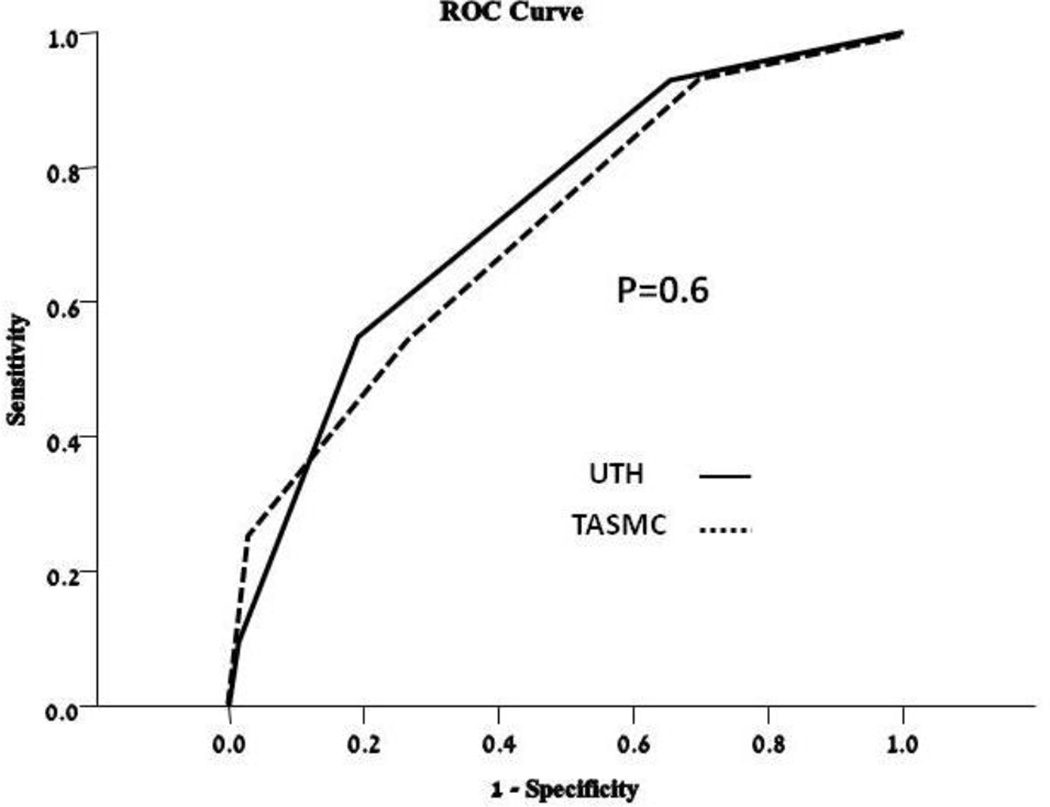
The validation TASMAC cohort included 128 TIA patients. Their baseline characteristics did not differ significantly from the derivation cohort- with the exception of stroke/TIA history (Table 1). 31 patients (24.2%) of patients had PY. The PY score was highly predictive of PY in the TASMC cohort and performed in a very similar manner (figure 1). The ROC curves (figure 2) of the UTH and TASMC cohorts yielded a C of 0.754 and 0.71 respectively (p=0.5 comparing the two ROC curves). Table 2 presents the sensitivity, specificity, negative and positive predictive values for two cut-off values of the PY score.
Figure 1.
Positive hospital yield by PY score in both cohorts
Figure 2.
ROC curves of PY score for both cohorts
Table 2.
Patients' characteristics
| UTH N=177 |
TASMC N=128 |
|
|---|---|---|
| Age (mean±SD) | 64±15 | 67±10 |
| ABCD2 (median, range) | 4 (1–7) | 4(1–7) |
| Stroke after admission(%) | 2 (1.1) | 0 (0) |
| Medical History | ||
| CAD | 42 (23) | 30 (23.4) |
| HTN | 124 (69) | 83 (65) |
| DM | 58 (33) | 32 (25) |
| AF | 20 (11) | 13 (10) |
| Stroke\TIA | 116 (65) | 0(0) |
| CHF | 12 (7) | 3 (2.3) |
| HPL | 77 (43) | 45 (35) |
| Admission Glucose mg/dL(mean±SD) | 131±46 | 120±48 |
| DWI+ (%) | 37 (21) | 28 (21) |
Of the patients with PY idenentified during their hospital stay, 77% had a major treatment change. 50% were started on anticoagulation and the remainder had carotid intervention. One patient had undergone closure of a, atrial septal defect. When no major change was introduced the common causes were contraindication to anticoagulation, patient refusal or deferral of the treatment decision to a later date.
Discussion
This study focused on the yield of a positive finding on diagnostic work-up during hospitalization of TIA patients. TIAs represent an enormous burden on the healthcare system, regularly comprising a third of stroke hospital admissions[2]. It has been argued that most patients do not benefit from hospital admission since the majority of the basic work up can be done in the emergency room or in "express" TIA clinics[7]. That may be true but the infrastructure for such clinics it not widely available and burdening busy emergency departments. It would be valuable to identify which patients who have no neurological deficits need hospitalization Risk of impending stroke is one reason[8]. The ABCD2 score can help identify patients at high risk for short-term stroke[1]. However, only a minority of TIA patients have high ABCD2 scores and few of these actually go on to have a stroke[1] In fact, in both our cohorts combined, only 2 patients had a stroke while in the hospital. Apart from treating a stroke, the firmest rationale for hospital admission is to uncover and modify risks for stroke. For instance, a patient who has paroxysmal atrial fibrillation will benefit from anticoagulation[9]; a patient with tight carotid stenosis needs to be revascularized within 48 hours of his TIA to maximize the procedural benefit[10]; a patient with left ventricular aneurysm and mural clot will benefit from anticoagulation[11]. On the other hand, patients without such risks are typically treated with antiplatelet agents and likely can be sent from the ED to the outpatient setting.
We identified several predictors of a positive hospital yield after a TIA. Not surprisingly, the predictors we identified also predict the two major contributors to high risk TIAs, namely, atrial fibrillation and carotid artery stenosis. Both atrial fibrillation and carotid atherosclerosis are more common in the aging population and in patients with coronary artery disease[10, 12]. DWI- positive TIAs are actually strokes, often embolic in origin[13], and point towards a large artery or cardiac source.
The advantage of our PY score is that it performs well across two hospitals in two different areas of the world, in two different patient populations. According to our analysis (table 2) it appears that a PY score greater or equal to 1 has an excellent sensitivity and NPV while retaining a sensible PPV. These results mean that discharged patients with PY<1 have less than a 6% chance of harboring an etiology for TIA that requires hospitalization while admitted patients with ≥1 have a reasonable chance of being helped significantly by undergoing a hospital work-up. We think that placing the cut-off at 2 seriously undermines the sensitivity and would likely defeat the purpose of the PY score. To illustrate with an example, using the Py score=1 cut-off in our combined cohorts, 84 patients (27.5%) would not be admitted and only 5 of those patients (1.6%) would be discharged home having a modifiable risk factor (cardiac source or significant carotid stenosis) that would have been detected as part of the routine hospitalized stroke work-up. On the other hand, 134 patients (43.9%) of those admitted to the hospital would then be discharged after the routine stroke work-up detected no modifiable risk factors. We believe that these figures represent a reasonable clinical cost-benefit analysis that may provide a more rational care for TIA patients. In our cohort 77% of patients with PY had a major treatment change. This change typically modified their stroke risk in a substantial way and represents the heights of preventive medicine in neurology. Identifying these patients and applying these measures in a timely manner is of the utmost importance Our study has several limitations. It is retrospective in nature and thus our findings should ideally be validated prospectively. Therefore, we used an external independent validation cohort to try and overcome this limitation. The near-perfect performance of the PY score on the validation cohort provides a strong signal that our findings are sound. Performing an MRI on all TIA patients emergently may be impractical in areas where this modality is not widely available. This can change over time as MRI technology becomes cheaper and more widespread. In the meantime, we must strive to better detect a clinically meaningful predictor that can be obtained more easily. Such a predictor has not been identified yet in our cohort. Certainly CT cannot substitute for MRI given its limited sensitivity to small acute infarcts.
Alternatively, one may argue that a patient that already has a score of 1 without the MRI needs scanning since he is going to be hospitalized even if his scan is DWI negative.
In summary, the PY score may enable physicians to make better admission decisions and result in better, safer and more economical care for TIA patients.
Table 3.
predictive values of two PY cutoffs
| PY score cut-off for hospital admission | ||
|---|---|---|
| PY ≥1 | PY ≥ 2 | |
| Sensitivity | 94% | 59% |
| Specificity | 37% | 84% |
| PPV | 39% | 61% |
| NPV | 94% | 82% |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure:
The authors have nothing to disclose
References
- 1.Johnston SC, Rothwell PM, Nguyen-Huynh MN, Giles MF, Elkins JS, Bernstein AL, et al. Validation and refinement of scores to predict very early stroke risk after transient ischaemic attack. Lancet. 2007 Jan 27;369(9558):283–292. doi: 10.1016/S0140-6736(07)60150-0. [DOI] [PubMed] [Google Scholar]
- 2.Easton JD, Saver JL, Albers GW, Alberts MJ, Chaturvedi S, Feldmann E, et al. Definition and evaluation of transient ischemic attack: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; and the Interdisciplinary Council on Peripheral Vascular Disease. The American Academy of Neurology affirms the value of this statement as an educational tool for neurologists. Stroke. 2009 Jun;40(6):2276–2293. doi: 10.1161/STROKEAHA.108.192218. [DOI] [PubMed] [Google Scholar]
- 3.Giles MF, Rothwell PM. Transient ischaemic attack: clinical relevance, risk prediction and urgency of secondary prevention. Curr Opin Neurol. 2009 Feb;22(1):46–53. doi: 10.1097/WCO.0b013e32831f1977. [DOI] [PubMed] [Google Scholar]
- 4.Ovbiagele B, Saver JL, Fredieu A, Suzuki S, Selco S, Rajajee V, et al. In-hospital initiation of secondary stroke prevention therapies yields high rates of adherence at follow-up. Stroke. 2004 Dec;35(12):2879–2883. doi: 10.1161/01.STR.0000147967.49567.d6. [DOI] [PubMed] [Google Scholar]
- 5.Ben Assayag E, Korczyn AD, Giladi N, Goldbourt U, Berliner AS, Shenhar-Tsarfaty S, et al. Predictors for poststroke outcomes: the Tel Aviv Brain Acute Stroke Cohort (TABASCO) study protocol. Int J Stroke. 2011 Nov 2; doi: 10.1111/j.1747-4949.2011.00652.x. [DOI] [PubMed] [Google Scholar]
- 6.Giles MF, Albers GW, Amarenco P, Arsava MM, Asimos A, Ay H, et al. Addition of brain infarction to the ABCD2 Score (ABCD2I): a collaborative analysis of unpublished data on 4574 patients. Stroke. 2010 Sep;41(9):1907–1913. doi: 10.1161/STROKEAHA.110.578971. [Multicenter Study Research Support, Non-U.S. Gov't] [DOI] [PubMed] [Google Scholar]
- 7.Rothwell PM, Giles MF, Chandratheva A, Marquardt L, Geraghty O, Redgrave JN, et al. Effect of urgent treatment of transient ischaemic attack and minor stroke on early recurrent stroke (EXPRESS study): a prospective population-based sequential comparison. Lancet. 2007 Oct 20;370(9596):1432–1442. doi: 10.1016/S0140-6736(07)61448-2. [Comparative Study Research Support, Non-U.S. Gov't] [DOI] [PubMed] [Google Scholar]
- 8.Johnston SC, Gress DR, Browner WS, Sidney S. Short-term prognosis after emergency department diagnosis of TIA. JAMA. 2000 Dec 13;284(22):2901–2906. doi: 10.1001/jama.284.22.2901. [DOI] [PubMed] [Google Scholar]
- 9.Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007 Jun 19;146(12):857–867. doi: 10.7326/0003-4819-146-12-200706190-00007. [DOI] [PubMed] [Google Scholar]
- 10.Rothwell PM, Eliasziw M, Gutnikov SA, Warlow CP, Barnett HJ. Endarterectomy for symptomatic carotid stenosis in relation to clinical subgroups and timing of surgery. Lancet. 2004 Mar 20;363(9413):915–924. doi: 10.1016/S0140-6736(04)15785-1. [Meta-Analysis Research Support, Non-U.S. Gov't Research Support. U.S. Gov't, P.H.S.] [DOI] [PubMed] [Google Scholar]
- 11.Mahajan N, Ganguly J, Simegn M, Bhattacharya P, Shankar L, Madhavan R, et al. Predictors of stroke in patients with severe systolic dysfunction in sinus rhythm: role of echocardiography. Int J Cardiol. [Comparative Study Letter] 2010 Nov 5;145(1):87–89. doi: 10.1016/j.ijcard.2009.05.039. [DOI] [PubMed] [Google Scholar]
- 12.Kannel WB, Benjamin EJ. Current perceptions of the epidemiology of atrial fibrillation. Cardiol Clin. 2009 Feb;27(1):13–24. doi: 10.1016/j.ccl.2008.09.015. vii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ay H, Koroshetz WJ, Benner T, Vangel MG, Wu O, Schwamm LH, et al. Transient ischemic attack with infarction: a unique syndrome? Ann Neurol. 2005 May;57(5):679–686. doi: 10.1002/ana.20465. [DOI] [PubMed] [Google Scholar]