Abstract
There is growing evidence for an interdependence of inflammation, coagulation and thrombosis in acute and chronic inflammatory diseases. Inflammatory bowel diseases (IBD) are associated with a hypercoagulable state and an increased risk of thromboembolism. While the IBD-associated prothrombogenic state has been linked to the inflammatory response, the mediators that link these two conditions remain unclear. Recent evidence suggests that interleukin-6 (IL-6) may be important in this regard. The objective of this study was to more fully define the contribution of IL-6 to the altered platelet function that occurs during experimental colitis. The number of immature and mature platelets, activated platelets, and platelet-leukocyte aggregates (PLA) were measured in wild type and IL-6−/− mice with dextran sodium sulfate (DSS) induced colonic inflammation. DSS treatment of WT mice was associated with significant increases in the number of both immature and mature platelets, activated platelets, and PLAs. These platelet responses to DSS were not observed IL-6−/− mice. Chronic IL-6 infusion (via an Alzet pump) in WT mice reproduced all of the platelet abnormalities observed in DSS colitic mice. IL-6 infused mice also exhibited an acceleration of thrombus formation in arterioles, similar to DSS. These findings implicate IL-6 in the platelet activation and enhanced PLA formation associated with experimental colitis, and support a role for this cytokine as a mediator of the enhanced thrombogenesis in IBD.
Keywords: thrombosis, inflammatory bowel disease, thrombocytosis, dextran-sodium sulfate
Introduction
Inflammatory bowel disease (IBD) is associated with hypercoagulable state and enhanced thrombosis. Clinical studies described clot formation in large arteries and veins as well as in microvasculature, with microvascular clots detected both within inflamed bowel and at distant sites.1-3 The enhanced thrombosis has been demonstrated in animal models of chronic colonic inflammation, including the DSS and T-cell transfer models.4-6 Analysis of blood samples from IBD patients and animal models of colitis suggest that the enhanced thrombosis may result from an activated coagulation system and inhibition of fibrinolysis.7,8 However, there is also a large body of evidence that implicates abnormalities in platelet function as a potential underlying cause of the enhanced thrombus development.9 Data obtained from IBD patients and more recent animal experiments suggest that these platelet abnormalities are generally manifested as thrombocytosis, the appearance of immature platelets, enhanced platelet activation, and spontaneous in vivo platelet-leukocyte aggregate formation.10-14
Efforts to better understand the potential mediators of the accelerated thrombosis and coagulation in different pathologic conditions have revealed an intimate link between coagulation and the inflammatory response. Proinflammatory cytokines are considered an important link between inflammation and the prothrombotic, hypercoagulable state observed in several pathological conditions, including sepsis.15 Several proinflammatory cytokines including tumor necrosis factor alpha (TNF-α), interleukin (IL)-1β and interleukin (IL)-6 have been implicated in the thrombogenic responses.3.15-17 While all of these cytokines are known to be prothrombotic, IL-6 appears to be the dominant cytokine that mediates this response.18-20 We have recently reported that IL-6 levels in plasma are significantly elevated in colitic mice14, and that either genetic or immunologic blockade of IL-6 effectively attenuates the accelerated microvascular thrombosis that accompanies experimental colitis.13 Similarly, we have demonstrated a role for IL-6 in the hyper-reactivity of platelets in colitic mice to thrombin stimulation.13 Whether IL-6 is also responsible for the platelet activation and platelet-leukocyte aggregate (PLA) formation that are associated with colitis remains unknown. Similarly, it is unclear whether the platelet abnormalities and accelerated thrombus development evidenced in colitic mice can be recapitulated in otherwise normal mice that receive a chronic infusion of IL-6 that mimics the cytokine level detected in experimental IBD.
The overall objective of this study was to more fully define the contribution of IL-6 to the platelet abnormalities elicited by colonic inflammation. In addition, we determined whether the altered platelet responses and enhanced thrombus formation caused by colonic inflammation can be reproduced by chronic IL-6 infusion. We evaluated the role/influence of IL-6 in mediating the appearance of activated platelets and the spontaneous generation of platelet-leukocyte aggregates that are observed in experimental IBD. Finally, the contribution of IL-6 to colitis-enhanced extra intestinal thrombosis was evaluated using chronic IL-6 infusion. Our findings support a major role for IL-6 in the enhanced thrombocytosis, platelet hyper-reactivity, PLA formation, and accelerated thrombus development in extra-intestinal tissue during colonic inflammation.
MATERIALS AND METHODS
Animals
Male C57BL/6J and IL-6 deficient mice were purchased from Jackson Laboratory (Bar Harbor, ME) were studied between 8- to 12-weeks of age. The mice were maintained under specific pathogen-free conditions and given ad libitum access to standard mouse chow and water. All animal procedures described herein were approved by Institutional Animal Care and Use Committee of Louisiana State University Health Sciences Center-Shreveport.
Induction of colitis
Mice received 3% (wt/vol) dextran-sodium sulfate (DSS; 40 kD; MP Biomedicals, Solon, OH) dissolved in filtered-purified drinking water for a period of 7 days to induce acute colonic inflammation.4 The first day of DSS feeding was defined as day-0. Control mice received filtered water only.
Assessment of disease progression
Disease activity index (DAI), ranging from 0 to 4, was used for clinical assessment of disease severity and calculated from using stool consistency, fecal blood, weight loss, and macroscopic evaluation of the anus, as previously described.14 DSS treatment resulted in clinical responses that are consistent with colitic disease activity were confirmed by daily measurements of DAI.
Plasma IL-6 concentration
IL-6 concentrations in plasma samples were measured using a cytometric bead array (CBA). The blood samples were obtained from separate groups of mice not subjected to any other manipulation, and withdrawn via a tail vein bleed into heparinized capillary tubes. A blood sample was withdrawn and collected in an Eppendorf tube, which was then centrifuged at 5000 rpm × 10 minutes to separate the plasma. IL-6 concentrations the plasma samples were measured with the CBA as per the manufacturer’s instruction (BD Biosciences, San Jose, CA).
Chronic IL-6 infusion
Murine recombinant IL-6 (BioLegend) was infused at a rate of 20 pg/g/min by an micro-osmotic Alzet pump (MODEL 1007D; 0.5 μl/hour) that was implanted subcutaneously for 7 days. Preliminary studies were performed to determine the IL-6 infusion rate (20 pg/g/min) that yielded an elevated plasma concentration that closely mimicked the level detected on day-6 in DSS treated mice. WT (no pump) and saline-pump implanted WT mice were used as control groups.
Blood sampling and cell counts
Mice were placed under a heat-lamp, and the tip of tail (~1mm) was cut with scissors, and heparinized capillary tubes were used to collect blood samples. Whole blood samples obtained from tail-vein bleed were used for measuring leukocytes (3% citric acid & 10% crystal violet) and platelet (1% buffered ammonium oxalate) counts with a hemocytometer.
Assessment of activated, immature and mature platelets
Murine blood obtained by tail-vein bleed was mixed with heparin (20U/mL). Platelets were identified by their characteristic light scattering and membrane expression of the specific platelet glycoprotein IIb (CD41) detected with rat anti-mouse CD41-APC antibody (eBiosciences, Inc). Two-color staining of JON/A-PE (Emfret Analytics, Wurzburg, Germany) and thiazole orange (TO, Sigma-Aldrich, St Louis, MO)14 was used. Platelet activation was assessed by the binding of the JON/A-PE antibody to the activation epitope of GPIIb/IIIa.21 Appropriate rat IgGs were used to determine non-specific binding. Immature platelets were identified using TO (1μg/mL dissolved in PBS). Fresh blood was diluted 1:5 and stained for 15 min at 20°C and analyzed with a LSRII flow cytometer (BD Biosciences, San Jose, CA). 20,000-50,000 events were collected, and the data were analyzed using FACSDiva software (BD Biosciences, San Jose, CA). The immature platelet population was identified by setting a TO-high gate that is 5% of the total platelet population, as previously described.22,23
Platelet-leukocyte aggregate formation
To investigate in vivo platelet-leukocyte interactions, 50 μL of heparin-anticoagulated blood was incubated with rat anti-mouse CD16/CD32 antibody to block the FcγIII/II receptors. 4-color flow cytometry assay, as described previously (Yan et al, 2013), was used to divide the platelet-leukocyte aggregates (PLA, (CD45.2+/CD41+)) into platelet-neutrophil (PNA (CD45.2+/Gr-1+/CD41+)), platelet-monocyte (PMA (CD45.2+/F4/80+/CD41+)), and platelet-lymphocyte (PLyA, estimated as PLA − (PNA + PNA)) aggregate sub-populations. Saturating concentrations of rat anti-mouse CD45.2-FITC, Gr-1-PE, F4/80-eFluor450, CD41-APC, and isotype controls (eBioscience, San Diego, CA) were used for labeling leukocytes and platelets. Red blood cells were lysed with Caltag high-yield lysing solution (Invitrogen, Camarillo, CA). When assessing the proportion of leukocytes involved in PLA formation, CD45.2 and CD41 double-positive events were recorded as a percentage of a total of 20,000 gated leukocytes. The percentage of leukocytes forming PLAs was multiplied by the corrected WBC count.
Thrombus formation in cremaster muscle microcirculation
Mice were anesthetized with 50 mg/kg sodium pentobarbital i.p., and the cremaster muscle was surgically prepared for intravital fluorescence microscopic experiments, as previously described.4, 24 Arterioles (35-45 μm in diameters) with wall shear rates of >400 s−1 were selected for study. To induce thrombus formation in cremasteric microvessels, all mice received 10 mL/kg FITC-dextran i.v., and was allowed to circulate for 10-15 min before photoactivation. Red blood cell velocities and wall shear rates were determined in arterioles before photoactivation, which was initiated by exposing a 100-μm length of microvessel to epi-illumination with a 175-W xenon lamp and a fluorescein filter cube. Daily measurements of excitation power density were recorded to maintain a value within 1% of 1.74 W/cm2, as previously described.5,24 Epi-illumination was continuously applied to the vessels, and thrombus formation was quantified by determining: 1) the time of onset of platelet deposition/aggregation within the microvessel (onset time); and 2) the time required for complete flow cessation for >60 sec (cessation time).
Experimental protocols
The first series of experiments focused on the platelet responses to DSS-induced colonic inflammation in WT and IL-6−/− mice. Blood samples were obtained throughout the DSS time-course (DSS days 2, 4, and 6) for blood cell counts and flow-cytometric measurements. During the DSS treatment, body weight change and DAI were recorded for both WT and IL-6−/− mice. After DSS day-6, the mice were killed with an overdose of anesthetic, the colon was excised for measurement of bowel length and weight and then divided for histologic processing. The spleen was also excised for weight measurement.
Preliminary experiments were performed to measure plasma IL-6 concentration in DSS-colitic mice. Blood samples were obtained from a separate group of WT and WT-DSS (day 6) mice for these measurements. Subsequently, different doses of IL-6 were infused into WT mice to determine the optimal dose of IL-6 infusion that best mimic the IL-6 concentration observed in DSS-colitic mice. Mice were infused (via an Alzet pump) with either 1μg, 2.5μg, 4μg, or 5μg of IL-6 per mouse over a 6-day period. An IL-6 dose of 5μg (20 pg/g/min) per mouse was selected for the remainder of the chronic IL-6 infusion experiments, which focused on evaluating the platelet responses to chronic IL-6 infusion. A separate group of DSS and IL-6 infused mice were used to study the time-course of changes of blood cell counts and flow-cytometric measurements of platelet function. WT mice implanted with saline-loaded Alzet pumps were used as experimental controls.
Separate experiments were performed to evaluate thrombus formation in cremaster muscle arterioles of WT, WT-DSS, and IL-6 infused mice. WT mice infused with saline were used as experimental controls.
Data analysis and statistics
Blood cell counts and the different indices of platelet dysfunction within the same experimental group were compared between treatment days using a one-way analysis of variance (ANOVA) with a Dunnett’s post-hoc test. Comparisons between different experimental groups (i.e. WT vs IL-6−/− and WT-DSS vs WT+IL-6 infusion) over the treatment time-course were performed using a two-way ANOVA with a Bonferroni post-test for multi-group comparison. Differences in the rate of thrombus development between treatment groups were compared using one-way ANOVA and Dunnett’s post-test. All values are reported as means ± SE. Statistical significance was set at P < 0.05.
RESULTS
IL-6 deficiency abolishes DSS-induced thrombocytosis and reduces the appearance of both immature and mature platelets
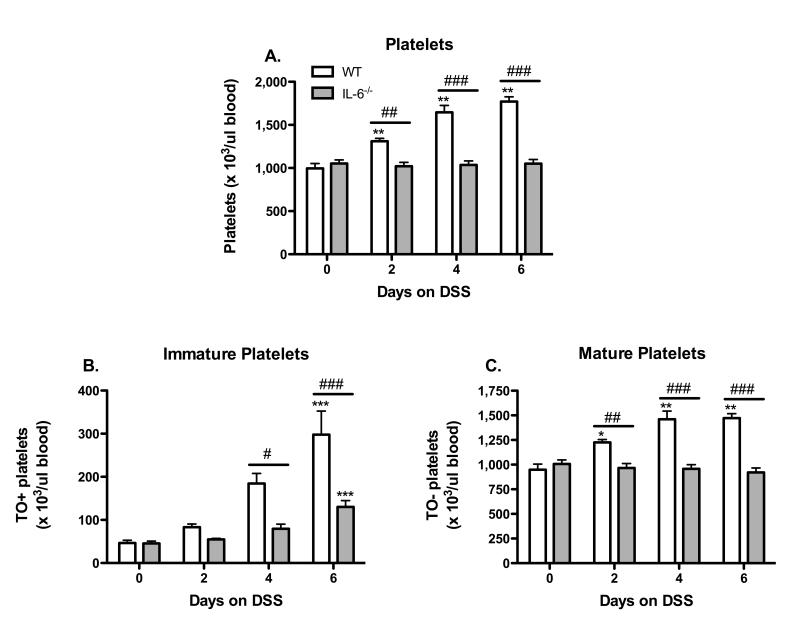
All WT mice placed on DSS showed a marked time-dependent increase in blood platelet count. The thrombocytosis observed on days 2, 4 & 6 of DSS treatment in WT mice was not detected in IL-6−/− mice (Figure 1A). Thiazole orange (TO), a fluorescent nucleic acid intercalating dye, was used to detect and quantify immature platelets. WT mice exhibited a significant increase in the number of immature platelets (compared to day-0) on days 4 & 6 of DSS treatment (Figure 1B). In IL-6 deficient mice, an increased number of immature platelets was detected only DSS day 6, however, this response was greatly reduced compared to WT DSS day 6 mice. The number of mature platelets was significantly increased on days 2 - 6 of DSS treatment in WT mice, and this increase was not evident in IL-6−/− mice (Figure 1C).
Figure 1. IL-6 deficiency prevents DSS-induced thrombocytosis.
(A) Time-course of changes in circulating platelet counts during the development of DSS-induced colonic inflammation. The appearance of (B) immature and (C) mature platelets in blood of DSS-colitic mice. Values are reported as means ± SE. For WT: n = 5 (day 0), n = 6 (day 2 & 4), and n = 9 (day 6). For IL-6−/−: n = 11 (day 0, 4, 6), and n = 8 (day 2). *, P < 0.05 versus day 0; #, P < 0.05 for WT versus IL-6−/−.
IL-6 deficiency prevents the appearance of activated platelets in mice with DSS-colitis
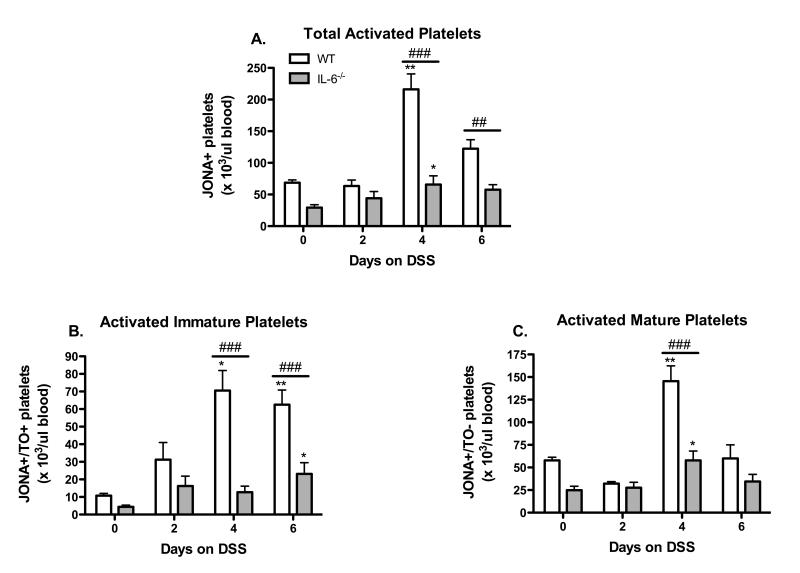
The number of circulating activated platelets was determined using an antibody (JON/A) that recognizes the activated conformation of the murine GPIIb/IIIa integrin on the platelet surface. The time-course of changes in the number of total, immature and mature platelets during the development of DSS-induced colonic inflammation is presented in Figure 2. The total number of circulating JON/A-positive platelets (Figure 2 A) was significantly increased on DSS days 4 (compared to day-0). A similar pattern was noted for activated mature platelets (Figure 2C), however, activated immature platelets were increased in number on both days 4 & 6. These changes in number of activated platelets were not detected in IL-6 deficient mice.
Figure 2. IL-6 deficiency prevents platelet activation in DSS-treated mice.
(A) Total circulating activated platelets identified as JONA+ platelets were monitored throughout the DSS time-course. (B) Activated immature and (C) activated mature platelets were also quantified. Values are reported as means ± SE. . For WT: n = 5 (day 0), n = 6 (day 2 & 4), and n = 9 (day 6). For IL-6−/−: n = 11 (day 0, 4, 6), and n = 8 (day 2). *, P < 0.05 versus day 0; #, P < 0.05 for WT versus IL-6−/−.
DSS-induced colitis results in IL-6 dependent formation of platelet-leukocyte aggregates
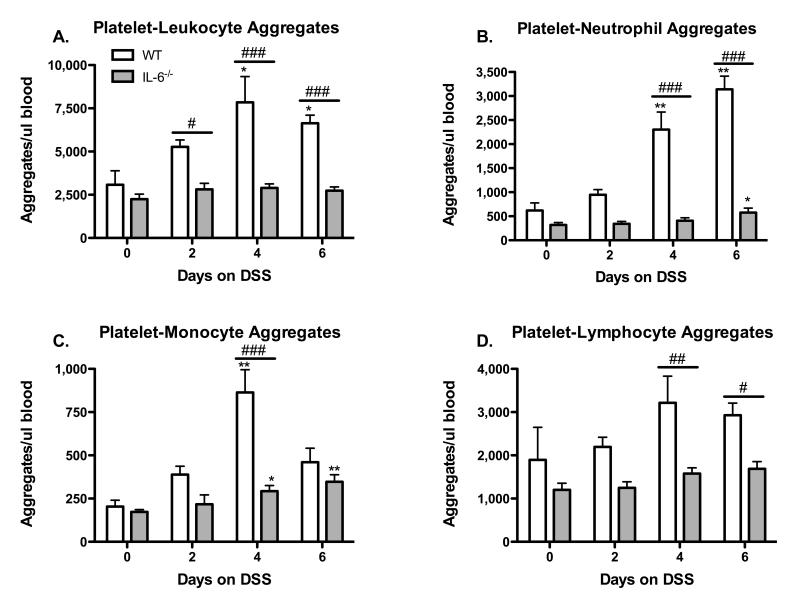
The formation of platelet aggregates with leukocytes in peripheral blood samples was assessed by 4-color flow cytometry (Figure 3). WT mice placed on DSS showed significant increases in circulating PLAs on DSS days 4 & 6, however, this response was not observed in IL-6−/− mice (Fig. 3 A). Further analysis revealed a similar pattern for platelet aggregation with either neutrophils (Panel B), monocytes (Panel C), or lymphocytes (Panel D), i.e., the increased aggregate formation observed in WT mice was not detected in IL-6−/− mice.
Figure 3. Effects of IL-6 deficiency on DSS-induced formation of platelet-leukocyte aggregates.
CD45.2+/CD41+ events were counted as platelet aggregates with total leukocytes (A). Platelet-neutrophil (B), platelet-monocyte (C), and platelet-lymphocyte aggregates (D) were identified by gating on the CD45.2+/CD41+ PLA population. Values are reported as means ± SE. For WT: n = 5 (day 0), n = 6 (day 2 & 4), and n = 9 (day 6). For IL-6−/−: n = 10 (day 0), n = 5 (day 2), n = 11 (day 4), and n = 8 (day 6). *, P < 0.05 versus day 0; #, P < 0.05 for WT versus IL-6−/−.
The elevated plasma IL-6 concentration detected in DSS colitic mice can be reproduced by chronic IL-6 infusion
IL-6 levels were significantly elevated on DSS day 6 (37.1 ± 3.4 pg/ml) compared to control (6.7 ± 0.4 pg/ml) values. Chronic (6 day) infusion of IL-6 at 20 pg/g/min resulted in a plasma concentration of 33 ± 5.8 pg/ml, which was not significantly different from the DSS day-6 values. Consequently, this dose of IL-6 was used for the remainder of the study involving chronic IL-6 infusion.
Chronic IL-6 infusion elicits a thrombocytosis response similar to DSS colitis
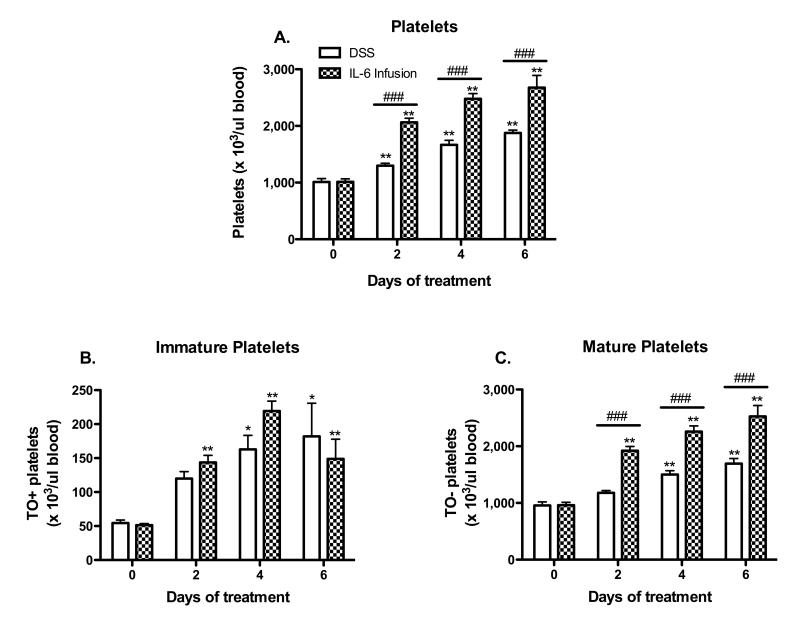
Previous studies from our lab have shown that DSS-treatment elicits significant thrombocytosis.13, 14 Figure 4 demonstrates that a 6-day infusion of IL-6 in WT mice elicits changes in blood platelet count that are qualitatively similar to those detected in DSS colitic mice. The increases in total and mature platelet counts were significantly higher in the IL-6 infused (vs DSS colitic) mice, while quantitatively similar changes in the number of immature platelets were noted in IL-6 infused and DSS colitic mice. WT mice implanted with saline-loaded Alzet pumps were used as controls, and did not exhibit significant changes over the 6-day period, when compared to WT mice without pump implantation.
Figure 4. Chronic IL-6 infusion induces thrombocytosis responses similar to DSS colitis.
(A) Time-course of changes in circulating platelet counts during the development of DSS-induced colitis and IL-6 infusion. The appearance of (B) immature and (C) mature platelets in blood was determined in both DSS-colitic and IL-6 infused mice. Values are reported as means ± SE. For DSS: n = 5 (day 0) and n = 6 (days 2, 4, 6). For IL-6 infusion: n = 5 (day 0) and n = 6 (days 2, 4, 6). *, P < 0.05 versus day 0; #, P < 0.05 for WT versus IL-6 infused mice.
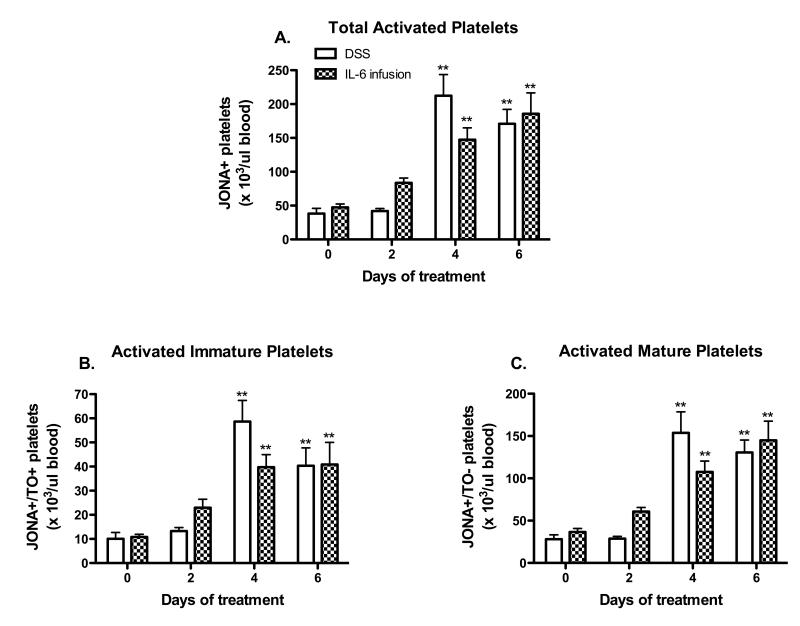
Chronic IL-6 infusion recapitulates the platelet activation observed in DSS colitic mice
Figure 5 demonstrates that WT mice infused for 6 days with IL-6 exhibit similar changes in platelet activation as observed in DSS colitic mice. Similar numbers of activated cells were noted for total (Figure 5A), immature (Figure 5B) and mature (Figure 5C) platelets.
Figure 5. IL-6 infusion elicits a platelet activation response similar to DSS-colitis.
(A) Total circulating activated platelets were monitored throughout the DSS and IL-6 infusion time-course. (B) Activated immature and (C) activated mature platelets were also quantified. Values are reported as means ± SE. For DSS: n = 5 (day 0) and n = 6 (days 2, 4, 6). For IL-6 infusion: n = 5 (day 0) and n = 6 (days 2, 4, 6). *, P < 0.05 versus day 0; #, P < 0.05 for WT versus IL-6 infused mice.
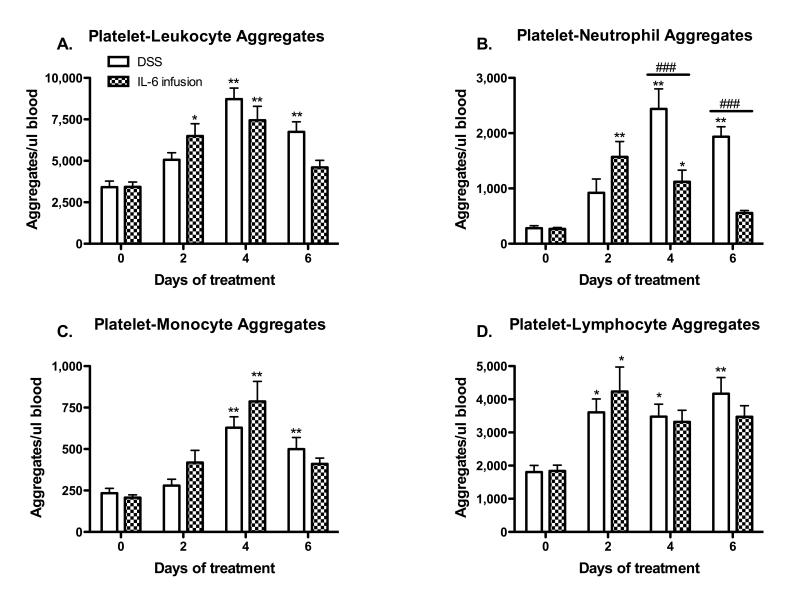
IL-6 infusion promotes platelet-leukocyte aggregate formation similar to DSS-colitis
Flow cytometric analysis of blood samples from IL-6 infused and DSS colitic mice revealed similar changes in the magnitude and time-course of platelet aggregate formation with total leukocytes (Figure 6A), monocytes (Figure 6C) and lymphocytes (Figure 6D). However, a notable difference between the IL-6 infusion and DSS colitis groups was the less intense generation of platelet-neutrophil aggregates in the IL-6 infused group (Figure 6B).
Figure 6. A comparison of platelet-leukocyte aggregate formation in IL-6 infused and DSS-colitic mice.
CD45.2+/CD41+ events were counted as platelet aggregates with total leukocytes (A). Platelet-neutrophil (B), platelet-monocyte (C), and platelet-lymphocyte aggregates (D) were identified by gating on the CD45.2+/CD41+ PLA population. Values are reported as means ± SE. For DSS: n = 5 (day 0) and n = 6 (days 2, 4, 6). For IL-6 infusion: n = 5 (day 0) and n = 6 (days 2, 4, 6). *, P < 0.05 versus day 0; #, P < 0.05 for WT versus IL-6 infused mice.
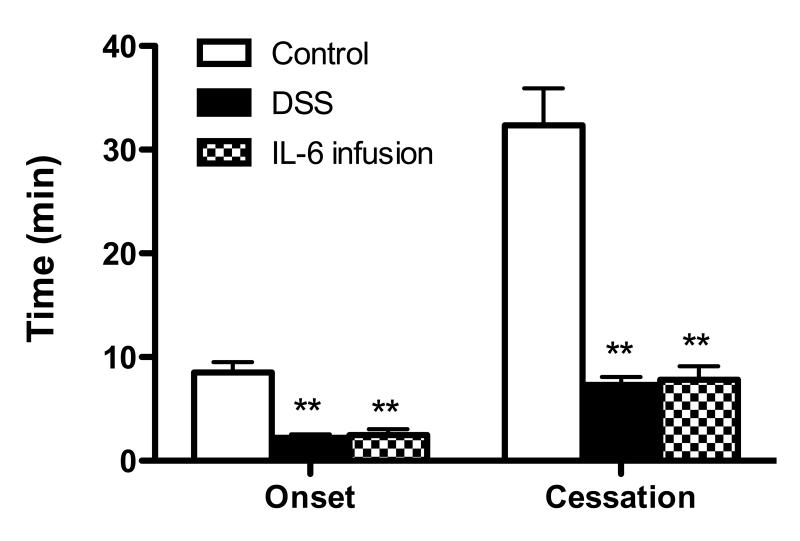
Chronic IL-6 infusion and DSS colitis accelerate microvascular thrombus formation
We have previously demonstrated that DSS colitis leads to accelerated thrombus development in extra-intestinal tissue and that the response is prevented by genetic or immunological blockade of IL-6.4, 13 In this study, we addressed the potential for chronically infused IL-6 to alter thrombus development in cremaster muscle arterioles induced by the light/dye method and compared the response to thrombus development in DSS colitic mice. As shown in Figure 7, chronic infusion of IL-6 in WT mice lead to an acceleration of thrombus formation (relative to controls), as reflected by the shortened time for onset of the thrombus as well as shorter time for complete blood flow cessation due to thrombus occlusion. The magnitude of the enhancement of thrombus development detected in IL-6 infused mice was very similar to the response noted in DSS colitic mice.
Figure 7. Thrombus development in cremaster muscle arterioles of IL-6 infused and DSS colitic mice.
Time of onset and time to blood flow cessation during thrombus formation during light/dye exposure. Values are reported as means ± SE. For each experimental group, n = 6. *, P < 0.05 versus control.
Discussion
Studies of hemostasis and coagulation in different pathologic conditions have revealed an intimate linkage between thrombosis and inflammation8, 15, however the significance of this relationship to the pathophysiology of IBD remains poorly defined. While activation of the coagulation cascade and platelet activation have both been implicated in the induction of the prothrombotic state in IBD, most attention has been focused on the contribution of pro- and anti-coagulant mechanisms in IBD-associated thrombogenesis.3, 25, 26 Less effort has been devoted to defining the potential contribution of circulating platelets to this condition despite numerous clinical reports that describe significant increases in the number and activation state of platelets in human IBD.9, 27 A recent report from our laboratory implicates IL-6 as a mediator of the thrombocytosis and enhanced microvascular thrombosis that accompanies experimental colitis.13 In the present study, an effort was made to evaluate the contribution of IL-6 to the platelet activation elicited in DSS-induced colonic inflammation and to determine whether chronic IL-6 infusion recapitulates the platelet abnormalities and enhanced thrombus development associated with experimental colitis. Our findings indicate that increased platelet activation and enhanced platelet-leukocyte interactions are important targets of IL-6 action that predispose the vasculature to thrombus development during colonic inflammation.
Reactive thrombocytosis is a frequent accompaniment to IBD, with 50 to 100% increases in platelet count observed during active IBD, when compared to control subjects.28-30 The results of both clinical11, 27 and animal studies14 suggest that platelet count may be a useful biomarker of IBD disease activity. We have previously reported that DSS and T-cell transfer-induced colitis is associated with thrombocytosis14 and that IL-6 deficient mice with DSS colitis do not exhibit this response13. Our analysis of the time-course of changes in platelet count during DSS colitis in wild type and IL-6−/− mice in the present study add further support for a role of IL-6 in mediating the thrombocytosis. Our novel findings in the IL-6 infusion experiments, which recapitulated the thrombocytosis response detected in DSS colitis, lends further support to IL-6 as a mediator of this response. Our findings are consistent with previous reports that describe the ability of IL-6 to enhance platelet production in mice and other species by stimulating megakaryocytosis.18, 31, 32 This cytokine is also capable of indirectly stimulating platelet production by enhancing hepatic output of thrombopoietin, a potent thrombopoietic agent that also targets the megakaryocyte.33
Another characteristic feature of the blood abnormalities detected in IBD patients is platelet activation.9,27 Platelets obtained from IBD patients display an increased expression of various activation markers on the platelet surface such as, GPIIb/IIIa, P-selectin, and CD40L.27 An increase in the number of circulating activated platelets has also been demonstrated in DSS and T-cell transfer models of murine colitis.14 The present study provides the first evidence to implicate IL-6 in the platelet activation response elicited in DSS colitis. Two lines of evidence is provided to support this view: 1) DSS colitis increases the number of activated platelets in blood of wild type mice, but not IL-6−/− mice, and 2) chronic infusion of IL-6 into wild type mice (to achieve IL-6 concentrations detected in DSS colitic mice) yields qualitatively and quantitatively similar increases in the numbers of circulating activated platelets, which were reflected in both the mature and immature platelet populations. While we provide evidence to support the involvement of IL-6 in the platelet activation that accompanies experimental colitis, the mechanisms that account for this action remain unclear. We have previously proposed that the platelet activation in IBD may be related to enhanced production and appearance of immature platelets in blood.13 This hypothesis was based on the known hyper-reactivity of newly produced (immature) platelets to agonist stimulation.34 However, in the present study, we observed that DSS colitis as well as chronic IL-6 infusion resulted in increased blood counts of both activated immature and mature platelets. Since both platelet populations exhibit comparable activation responses, it appears unlikely that IL-6 promotes platelet activation merely by producing more immature platelets.
The activated phenotype that is assumed by circulating platelets in human and experimental IBD predisposes these cells to binding to other circulating blood cells (e.g., leukocytes) and to vascular endothelium. These cell-cell interactions may result in an amplification of the inflammatory response and might predispose the vasculature to impaired perfusion and thrombosis.1, 3 The formation of platelet-leukocyte aggregates (PLA) in circulating blood has been demonstrated in both human 10, 12 and experimental IBD14. We have previously shown that the increased PLA formation in DSS colitic mice is selectin-dependent & likely reflects an interaction between P-selectin on activated platelets with its constitutively expressed counter-receptor on leukocyte (P-selectin glycoprotein ligand-1). In the present study, we provide the first evidence to implicate IL-6 as a mediator of the PLA formation that accompanies IBD. While DSS colitis in wild type mice was associated with an enhanced formation of platelet-neutrophil, platelet-monocyte, and platelet-lymphocyte aggregates, this was not detected in IL-6−/− mice. Furthermore, a nearly identical pattern of aggregate formation was noted in wild type mice that were subjected to chronic infusion with IL-6. These IL-6 mediated responses likely reflect the influence of the cytokine on platelet activation and the increased surface expression of adhesion molecules that sustain the heterotypic aggregation of platelets.
The pathophysiological relevance of enhanced PLA formation in IBD and other chronic inflammatory diseases remains unclear. However, it has been proposed that PLA formation may serve to intensify a focal inflammatory response and to amplify the systemic consequences of that response.2, 28, 35 For example, platelet binding to monocytes enhances RANTES accumulation on the leukocyte surface and promotes its extravasation.36 Moreover, the attachment of platelets to neutrophils greatly enhances that capacity of the leukocyte to generate superoxide.37-39 Recent studies have also shown that the interaction of platelets with neutrophils enhances the formation and stabilization of neutrophil extracellular traps (NET), which have been linked to deep vein thrombosis.12, 40, 41 The appearance of PLA in systemic blood of IBD patients and in animal models of colitis may also explain the enhanced extra-intestinal development of microscopic and macroscopic thrombi.
We have previously demonstrated that vascular beds distant from the intestine exhibit an increased vulnerability to thrombus development in arterioles.4 Furthermore, we have reported that the DSS colitis-enhanced thrombus development is significantly blunted following either genetic or immunologic blockade of IL-6.13 The results of the chronic IL-6 infusion experiments performed in the present study further support a role for IL-6 in the accelerated thrombus development in DSS colitis. Infusing IL-6 at a rate that produced an elevated plasma concentration similar to that detected in DSS colitis accelerated thrombus formation in arterioles in a manner nearly identical to that observed in DSS colitic mice.
In conclusion, our study provides evidence that implicates IL-6 as a critical mediator of the platelet activation and platelet-leukocyte aggregation that occurs during experimental colitis. In addition, we provide supportive evidence to implicate this cytokine in the thrombocytosis and enhanced extra-intestinal thrombosis that accompanies DSS colitis. The major contribution of IL-6 to these platelet-dependent responses suggests that the cytokine may be an effective therapeutic target for prevention of a potentially fatal consequence of inflammatory bowel disease.
Acknowledgments
Supported by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases (P01 DK43785-20)
References
- 1.Danese S, Papa A, Saibeni S, Repici A, Malesci A, Vecchi M. Inflammation and coagulation in inflammatory bowel disease: The clot thickens. Am J Gastroenterol. 2007;102:174–186. doi: 10.1111/j.1572-0241.2006.00943.x. [DOI] [PubMed] [Google Scholar]
- 2.Irving PM, Pasi KL, Rampton DS. Thrombosis and inflammatory bowel disease. Clin Gastroenterol Hepatol. 2005;3:617–628. doi: 10.1016/s1542-3565(05)00154-0. [DOI] [PubMed] [Google Scholar]
- 3.Yoshida H, Granger DN. Inflammatory bowel disease: a paradigm for the link between coagulation and inflammation. Inflamm Bowel Dis. 2009;15:1245–1255. doi: 10.1002/ibd.20896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anthoni C, Russell J, Wood KC, Stokes KY, Vowinkel T, Kirchhofer D, Granger DN. Tissue factor: a mediator of inflammatory cell recruitment, tissue injury, and thrombus formation in experimental colitis. J Exp Med. 2007;204:1595–1601. doi: 10.1084/jem.20062354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yoshida H, Russell J, Senchenkova EY, Almeida Paula LD, Granger DN. Interleukin-1beta mediates the extra-intestinal thrombosis associated with experimental colitis. Am J Pathol. 2010;177:2774–2781. doi: 10.2353/ajpath.2010.100205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yoshida H, Yilmaz CE, Granger DN. Role of tumor necrosis factor-alpha in the extraintestinal thrombosis associated with colonic inflammation. Inflamm Bowel Dis. 2011;17:2217–2223. doi: 10.1002/ibd.21593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Danese S, Semeraro S, Papa A, I. Roberto I, Scaldaferri F, Fedeli G, Gasbarrini G, Gasbarrini A. Extraintestinal manifestations in inflammatory bowel disease. World J Gastroenterol. 2005;11:7227–7236. doi: 10.3748/wjg.v11.i46.7227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Esmon CT. The impact of the inflammatory response on coagulation. Thromb Res. 2004;114:321–327. doi: 10.1016/j.thromres.2004.06.028. [DOI] [PubMed] [Google Scholar]
- 9.Danese S, Scaldaferri F, Papa A, Pola R, Sans M, Gasbarrini G, Pola P, Gasbarrini A. Platelets: new players in the mucosal scenario of inflammatory bowel disease. Eur Rev Med Pharmacol Sci. 2004;8:193–198. [PubMed] [Google Scholar]
- 10.Collins CE, Rampton DS, Rogers J, Williams NS. Platelet aggregation and neutrophil sequestration in the mesenteric circulation in inflammatory bowel disease. Eur J Gastroenterol Hepatol. 1977;9:1213–1217. [PubMed] [Google Scholar]
- 11.Harries AD, Fitzsimons E, Fifield R, Dew MJ, Rhoades J. Platelet count: a simple measure of activity in Crohn’s disease. Br Med J (Clin Res Ed) 1983;286:1476. doi: 10.1136/bmj.286.6376.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Irving PM, Macey MG, Shah U, Webb L, Langmead L, Rampton DS. Formation of platelet-leukocyte aggregates in inflammatory bowel disease. Inflamm Bowel Dis. 2004;10:361–372. doi: 10.1097/00054725-200407000-00007. [DOI] [PubMed] [Google Scholar]
- 13.Senchenkova EY, Komoto S, Russell J, Almeida Paula LD, Yan SL, Granger DN. Interleukin-6 mediates the platelet abnormalities and thrombogenesis associated with experimental colitis. Am J Pathol. 2013;183(1):173–81. doi: 10.1016/j.ajpath.2013.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yan SL, Russell J, Harris NR, Senchenkova EY, Yildirim A, Granger DN. Platelet Abnormalities during Colonic Inflammation. Inflamm Bowel Dis. 2013;19:1245–53. doi: 10.1097/MIB.0b013e318281f3df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levi M, van der Poll T. Two-way interactions between inflammation and coagulation. Trends Cardiovasc Med. 2005;15:254–259. doi: 10.1016/j.tcm.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 16.Danese S, Motte L, Fiocchi C. Platelets in inflammatory bowel disease: clinical, pathogenic, and therapeutic implications. Am J Gastroenterol. 2004;99:938–945. doi: 10.1111/j.1572-0241.2004.04129.x. [DOI] [PubMed] [Google Scholar]
- 17.Reinecker HC, Steffen M, Witthoeft T, Pflueger I, Schreiber S, MacDermott RP, Raedler A. Enhanced secretion of tumour necrosis factor-alpha, IL-6, and IL-1 beta by isolated lamina propria mononuclear cells from patients with ulcerative colitis and Crohn’s disease. Clin Exp Immunol. 1993;94:174–181. doi: 10.1111/j.1365-2249.1993.tb05997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ishibashi T, Kimura H, Uchida T, Kariyone S, Friese P, Burstein SA. Human interleukin 6 is a direct promoter of maturation of megakaryocytes in vitro. Proc Natl Acad Sci U S A. 1989;86:5953–5957. doi: 10.1073/pnas.86.15.5953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mitsuyama K, Matsumoto S, Masuda J, Yamasakii H, Kuwaki K, Takedatsu H, Sata M. Therapeutic strategies for targeting the IL-6/STAT3 cytokine signaling pathway in inflammatory bowel disease. Anticancer Res. 2007;27:3749–3756. [PubMed] [Google Scholar]
- 20.Mudter J, Neurath MF. Il-6 signaling in inflammatory bowel disease: pathophysiological role and clinical relevance. Inflamm Bowel Dis. 2007;13:1016–1023. doi: 10.1002/ibd.20148. [DOI] [PubMed] [Google Scholar]
- 21.Bergmeier W, Schulte V, Brockhoff G, Bier U, Zirngibl H, Nieswandt B. Flow cytometric detection of activated mouse integrin alphaIIbbeta3 with a novel monoclonal antibody. Cytometry. 2002;48:80–86. doi: 10.1002/cyto.10114. [DOI] [PubMed] [Google Scholar]
- 22.Ault KA, Knowles C. In vivo biotinylation demonstrates that reticulated platelets are the youngest platelets in circulation. Exp Hematol. 1995;23:996–1001. [PubMed] [Google Scholar]
- 23.Robinson M, Machin S, Mackie I, Harrison P. In vivo biotinylation studies: specificity of labelling of reticulated platelets by thiazole orange and mepacrine. Br J Haematol. 2000;108:859–864. doi: 10.1046/j.1365-2141.2000.01939.x. [DOI] [PubMed] [Google Scholar]
- 24.Rumbaut RE, Slaff DW, Burns AR. Microvascular thrombosis models in venules and arterioles in vivo. Microcirculation. 2005;12:259–274. doi: 10.1080/10739680590925664. [DOI] [PubMed] [Google Scholar]
- 25.Andoh A, Yoshida T, Yagi Y, Bamba S, Hata K, Tsujikawa T, Kitoh K, Sasaki M, Fujiyama Y. Increased aggregation response of platelets in patients with inflammatory bowel disease. J Gastroenterol. 2006;41:47–54. doi: 10.1007/s00535-005-1721-x. [DOI] [PubMed] [Google Scholar]
- 26.Atreya R, Neurath MF. Involvement of IL-6 in the pathogenesis of inflammatory bowel disease and colon cancer. Clin Rev Allergy Immunol. 2005;28:187–196. doi: 10.1385/CRIAI:28:3:187. [DOI] [PubMed] [Google Scholar]
- 27.Collins CE, Cahill MR, Newland AC, Rampton DS. Platelets circulate in an activated state in inflammatory bowel disease. Gastroenterology. 1994;106:840–845. doi: 10.1016/0016-5085(94)90741-2. [DOI] [PubMed] [Google Scholar]
- 28.Collins CE, Rampton DS. Review article: platelets in inflammatory bowel disease--pathogenetic role and therapeutic implications. Aliment Pharmacol Ther. 1997;11:237–247. doi: 10.1046/j.1365-2036.1997.153328000.x. [DOI] [PubMed] [Google Scholar]
- 29.Heits F, Stahl M, Ludwig D, Stange EF, Jelkmann W. Elevated serum thrombopoietin and interleukin-6 concentrations in thrombocytosis associated with inflammatory bowel disease. J Interferon Cytokine Res. 1999;19:757–760. doi: 10.1089/107999099313604. [DOI] [PubMed] [Google Scholar]
- 30.Webberley MJ, Hart MT, Melikian V. Thromboembolism in inflammatory bowel disease: role of platelets. Gut. 1993;34:247–251. doi: 10.1136/gut.34.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Asano S, Okano A, Ozawa K, Nakahata T, Ishibashi T, Koike K, Kimura H, Y. Tanioka Y, Shibuya A, Hirano T, et al. In vivo effects of recombinant human interleukin-6 in primates: stimulated production of platelets. Blood. 1990;75:1602–1605. [PubMed] [Google Scholar]
- 32.Ishibashi T, Kimura H, Shikama Y, Uchida T, Kariyone S, Hiran T, Kishimoto T, Takatsuki F, Akiyama Y. Interleukin-6 is a potent thrombopoietic factor in vivo in mice. Blood. 1989;74:1241–1244. [PubMed] [Google Scholar]
- 33.Kaser A, Brandacher G, Steurer W, Kaser S, Offner FA, Zoller H, Theurl I, Widder W, Molnar C, Ludwiczek O, Atkins MB, Mier JW, Tilg H. Interleukin-6 stimulates thrombopoiesis through thrombopoietin: role in inflammatory thrombocytosis. Blood. 2001;98:2720–2725. doi: 10.1182/blood.v98.9.2720. 2001. [DOI] [PubMed] [Google Scholar]
- 34.Thattaliyath B, Cykowski M, Jagadeeswaran P. Young thrombocytes initiate the formation of arterial in zebrafish. Blood. 2005;106:118–124. doi: 10.1182/blood-2004-10-4118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pamuk GE, Vural O, Turgut B, Demir M, Umit H, Tezel A. Increased circulating platelet-neutrophil, platelet-monocyte complexes, and platelet activation in patients with ulcerative colitis: a comparative study. Am J Hematol. 2006;81:753–759. doi: 10.1002/ajh.20655. [DOI] [PubMed] [Google Scholar]
- 36.Huo Y, Schober A, Forlow SB, Smith DF, Hyman MC, Jung S, Littman DR, Weber C, Ley K. Circulating activated platelets exacerbate atherosclerosis in mice deficient in apolipoprotein E. Nat Med. 2003;9:61–67. doi: 10.1038/nm810. [DOI] [PubMed] [Google Scholar]
- 37.Nagata K, Tsuji T, Todoroki N, Katagiri Y, Tanoue K, Yamazaki Y, Hanai N, Irimura T. Activated platelets induce superoxide anion release by monocytes and neutrophils through P-selectin (CD62) J Immunol. 1993;151:3267–3273. [PubMed] [Google Scholar]
- 38.Polanowska-Grabowska R, Wallace K, Field JJ, Chen L, Marshall MA, Figler R, Gear AR, Linden J. P-selectin-mediated platelet-neutrophil aggregate formation activates neutrophils in mouse and human sickle cell disease. Arterioscler Thromb Vasc Biol. 2010;30:2392–2399. doi: 10.1161/ATVBAHA.110.211615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zarbock A, Singbartl K, Ley K. Complete reversal of acid-induced acute lung injury by blocking of platelet-neutrophil aggregation. J Clin Invest. 2006;116:3211–3219. doi: 10.1172/JCI29499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Langer HF, E.Y. Choi EY, H. Zhou H, R. Schleicher R, K.J. Chung KJ, Z. Tang Z, K. Gobel K, K. Bdeir K, A. Chatzigeorgiou A, C. Wong C, S. Bhatia S, M.J. Kruhlak MJ, J.W. Rose JW, J.B. Burns JB, Hill KE, Qu H, Zhang Y, Lehrmann E, Becker KG, Wang Y, Simon DI, Nieswandt B, Lambris JD, Li X, Meuth SG, Kubes P, Chavakis T. Platelets contribute to the pathogenesis of experimental autoimmune encephalomyelitis. Circ Res. 2012;110:1202–1210. doi: 10.1161/CIRCRESAHA.111.256370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.von Bruhl ML, Stark K, Steinhart A, Chandraratne S, Konrad I, Lorenz M, Khandoga A, Tirniceriu A, Coletti R, Kollnberger M, Byrne RA, Laitinen I, Walch A, Brill A, Pfeiler S, Manukyan D, Braun S, Lange P, Riegger J, Ware J, Eckart A, Haidari S, Rudelius M, Schulz C, Echtler K, Brinkmann V, Schwaiger M, Preissner KT, Wagner DD, Mackman N, Engelmann B, Massberg S. Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo. J Exp Med. 2012;209:819–835. doi: 10.1084/jem.20112322. [DOI] [PMC free article] [PubMed] [Google Scholar]