Abstract
Background
Restricted interests are a class of repetitive behavior in autism spectrum disorders (ASD) whose intensity and narrow focus often contribute to significant interference with daily functioning. While numerous neuroimaging studies have investigated executive circuits as putative neural substrates of repetitive behavior, recent work implicates affective neural circuits in restricted interests. We sought to explore the role of affective neural circuits and determine how restricted interests are distinguished from hobbies or interests in typical development.
Methods
We compared a group of children with ASD to a typically developing (TD) group of children with strong interests or hobbies, employing parent report, an operant behavioral task, and functional imaging with personalized stimuli based on individual interests.
Results
While performance on the operant task was similar between the two groups, parent report of intensity and interference of interests was significantly higher in the ASD group. Both the ASD and TD groups showed increased BOLD response in widespread affective neural regions to pictures of their own interest. When viewing pictures of other children's interests, the TD group showed a similar pattern, whereas BOLD response in the ASD group was much more limited. Increased BOLD response in the insula and anterior cingulate cortex distinguished the ASD from the TD group, and parent report of the intensity and interference with daily life of the child's restricted interest predicted insula response.
Conclusions
While affective neural network response and operant behavior are comparable in typical and restricted interests, the narrowness of focus that clinically distinguishes restricted interests in ASD is reflected in more interference in daily life and aberrantly enhanced insula and anterior cingulate response to individuals’ own interests in the ASD group. These results further support the involvement of affective neural networks in repetitive behaviors in ASD.
Keywords: Autism, restricted interests, reward, repetitive behavior, fMRI, insula, salience
Introduction
Individuals with autism spectrum disorders (ASD) face social, communication, and behavioral challenges that profoundly impact their lives, as well as those of their families and communities. Among these core symptoms is the presence of restricted or repetitive behaviors (RRB). RRB includes a broad range of behaviors from motor mannerisms to more complex, cognitively-mediated behaviors including rituals, rigid insistence on continuity in environment or routine, and restricted interests occupying the majority of leisure time (Bodfish, Symons, Parker, & Lewis, 2000; Lam & Aman, 2007). These behaviors may contribute to social isolation and interfere with daily life (South, Ozonoff, & McMahon, 2005; Turner-Brown, Lam, Holtzclaw, Dichter, & Bodfish, 2011).
Although all RRBs share the basic characteristic of behavioral inflexibility, they are dissociable into multiple factors that reflect the division between motor and cognitive-based behavior (Bishop et al., 2012; Cuccaro et al., 2003; Lam, Bodfish, & Piven, 2008; Szatmari et al., 2006; Lam & Aman, 2007). Some factor analyses further suggest that restricted interests constitute a unique factor (Lam & Aman, 2007; Lam et al., 2008). Restricted interests are intense, sometimes all-consuming, hobbies, interests, or areas of expertise. They can manifest as common, age-appropriate hobbies (e.g., a particular video game in adolescence) engaged to an extreme degree that limits a child's involvement in other activities and interferes with social relationships and family routines (Mercier, Mottron, & Belleville, 2008; South et al., 2005; Turner-Brown et al., 2011). In other cases, the subject of restricted interests is one not commonly shared with peers, such as accumulating extensive knowledge about different models of vacuum cleaners.
It is clear from educational and behavioral studies that restricted interests are rewarding for individuals with ASD. Access to restricted interests is an effective classroom reinforcer for children with ASD (Turner-Brown et al., 2011; South et al., 2005; Mercier et al., 2008; Charlop-Christy & Haymes, 1998; Boyd, Conroy, Mancil, Nakao, & Alter, 2007). Autobiographical narratives from high functioning individuals with ASD describe restricted interests using language that suggests intense positive emotion (Mercier et al., 2008). Furthermore, parental reports indicate that restricted interests are volitionally and habitually engaged, are often difficult to interrupt, and interfere with family life, daily functioning, and in some cases, personal health and wellness (Turner-Brown et al., 2011; South et al., 2005; Charlop-Christy & Haymes, 1998). These data suggest that restricted interests provide a source of pleasure and reward for individuals with ASD, but there is little empirical evidence for whether or how that may differ from typical hobbies and interests, or for the role of affective neural circuitry in sustaining restricted interests.
Recent studies have focused on neural motivational systems in ASD, reporting diminished behavioral (Lin, Rangel, & Adolphs, 2013) and neural response to social rewards (Scott-Van Zeeland, Dapretto, Ghahremani, Poldrack, & Bookheimer, 2010; Delmonte et al., 2012; Dichter, Richey, Rittenberg, Sabatino, & Bodfish, 2012; Kohls et al., 2012; Lin, Rangel, & Adolphs, 2013; Richey et al., 2013), in the striatum and ventromedial prefrontal regions. This evidence has advanced the “social motivation hypothesis” of ASD (Chevallier, Kohls, Troiani, Brodkin, & Schultz, 2012; Kohls, Chevallier, Troiani, & Schultz, 2012), but it is unclear whether reward system dysfunction is limited to social reward in ASD, or represents a more widespread deficit. Altered neural response to monetary reward has also been reported (Kohls et al., 2012b; Delmonte et al., 2012; Scott-Van Zeeland et al., 2010; Dichter et al., 2012b), but the specific neural loci and direction of difference varies among studies. A study of primary reward by our lab demonstrated aberrantly enhanced response of insula and anterior cingulate cortex to food reward cues in ASD (Cascio et al., 2012). These two regions are heavily connected to the limbic system, and are believed to constitute a salience network (Seeley, Menon, Schatzberg, Keller, Glover et al., 2007; Menon & Uddin, 2010), integrating reward and arousal information.
While individuals with ASD show diminished ventral striatal response to monetary rewards, response to objects that are commonly the focus of restricted interests (e.g., trains, computers) is intact (Dichter et al., 2012b). This result suggested the intriguing possibility that ASD is associated with a sparing of reward system function in response to objects that are the focus of repetitive behaviors. However, while this study highlighted a possible role of neural reward systems in restricted interests, it used a standardized stimulus set, and therefore did not test restricted interests directly. It is unknown to what degree the individual participants’ actual interests were represented by the standardized stimulus set, which depicted a range of common interests. In the current study, we sought to build upon this study and further explore the role of affective neural systems in restricted interests by presenting individually tailored stimulus sets targeted to the restricted interest of each participant with ASD, focusing on children rather than adults. Using a combination of parent report, operant behavioral measurement, and fMRI, we explored the affective basis of restricted interests in a sample of children with ASD, comparing operant and neural response to images of each individual's interest to a parallel set of images gathered from other participants’ stimulus sets. Using this novel approach, the current study is the first to directly investigate the affective neural response to restricted interests in children with ASD.
Materials and methods
Participants
21 children and adolescents with a diagnosis of ASD and 23 children and adolescents with typical development (TD) were recruited through the Vanderbilt Kennedy Center Treatment and Research Institute for Autism Spectrum Disorders (TRIAD) and community advertisements. Cognitive ability was measured for all participants using the Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999); a full-scale IQ score of at least 70 was required for inclusion. To verify diagnosis of ASD, individuals in the ASD group were administered the Autism Diagnostic Observation Schedule (Lord, Rutter, DiLavore, & Risi, 1999), and caregivers were interviewed with the Autism Diagnostic Interview-Revised (LeCouteur, Lord, & Rutter, 2003); both assessments were administered by a research-reliable assessor and interpreted by a licensed clinical psychologist, who determined that all children in the ASD group met DSM-IV-TR criteria for ASD. Exclusion criteria included: use of psychotropic medications (children taking stimulants were included but abstained from medication for 24 hours before their scan (Kimko, Cross, & Abernethy, 1999)), history of medical conditions associated with autism such as Fragile X, tuberous sclerosis, and epilepsy, recent history of psychiatric or neurologic diagnoses other than ASD, MRI contraindications, and, for the control group, presence of a first-degree relative with an ASD. All parents gave informed consent and participants gave informed assent prior to beginning the study. Following additional exclusion for attention and compliance in the MRI (detailed below), the final sample consisted of 19 children in the ASD group and 18 in the TD group; participant characteristics are summarized in Table 1. All procedures were reviewed and approved by the Human Research Protection Program at Vanderbilt University.
Table 1. Participant Characteristics.
Demographic characteristics of participants. Values given are mean (standard deviation) for age, full-scale IQ, and number of runs included for the autism spectrum disorder (ASD) and typically developing comparison (TD) groups.
| Group | ASD | TD | T (p) |
|---|---|---|---|
| N | 19 | 18 | |
| Age | 12.58 (2.48) | 13.11 (3.44) | −.542 (.59) |
| Full Scale IQ | 109.52 (13.96) | 104.22 (12.45) | 1.22 (.23) |
| Included Runs | 4.58 (0.77) | 4.50 (0.71) | .325 (.75) |
Interest assessment
Parent report
During phone screening, parents were interviewed informally about their child's interests, hobbies, and free-time activities. Children were considered eligible if: a) engagement with their primary interest occupied a minimum of one hour per day on average, and b) the content of their interest could be represented pictorially (e.g., old movies, whereas listening to music would not be an eligible interest because of difficulty conveying the salient feature(s) visually). To gain further information about each child's interest, parents of children in both groups were administered the Yale Special Interests Interview (YSII, (South, Klin, & Ozonoff, 1999)), about the primary interest chosen based on the phone screen. The YSII determines the presence or absence of a restricted interest based on the intensity, duration and degree of specialized knowledge about the subject of interest. If a restricted interest is present, the YSII provides a measure of severity based on the degree to which it interferes with functioning in several contexts. The interests presented pictorially to children in the experimental tasks were those confirmed with the YSII to be most salient at the time of their participation in the study. The YSII severity score was analyzed with a one-way ANOVA.
Stimuli and operant task
Following the general methodology of Aharon et al. (2001), we designed a task to quantify the reward value of statically presented images of the child's primary interest. Implemented with Matlab ® 2007a and Psychophysics Toolbox (Brainard, 1997), 38 pictures related to each child's interest (“Own” condition) and 38 non-overlapping pictures collected from other children's stimulus sets (“Other” condition) were sequentially displayed in random order for a default duration of five seconds. Images were obtained through internet search engines and were all in jpeg format, landscape layout and had final dimensions of 800×600 pixels per inch. Each picture contained no more than 6 words of text (e.g., movie titles were allowed, but not more extensive text that the child might spend time reading). Images in the “Other” condition were screened carefully to avoid overlap with the participant's own interest.
For each picture displayed, participants could press separate keys to either increase or decrease the display time for that image (see supplemental materials for more detail). The “reward” value of each image was defined by its total display time. For each participant, mean display time was calculated separately for the sets of “Own” and “Other” images. Keypress task data were analyzed using a repeated measures ANOVA with picture type (Own, Other) as the within-subjects factor and group (ASD, TD) as the between-subjects factor.
fMRI task
Block design
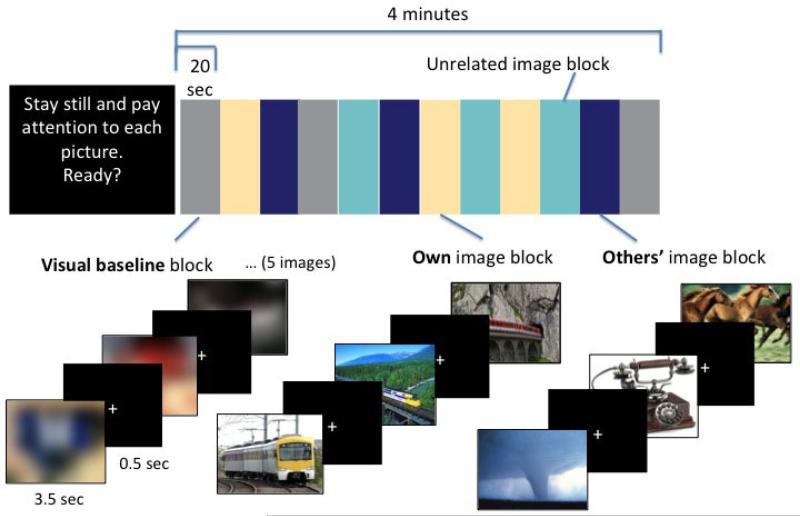
The fMRI task consisted of five functional runs, each lasting four minutes and consisting of twelve 20-second blocks presented in a pseudo-randomized order that was fixed across participants. Within each run, there were 3 blocks in each of four conditions: 1) own interest, 2) others’ interests, 3) primary reward (not included in the current analysis, see Cascio et al., 2012), and 4) visual baseline. Each 20-second block consisted of 5 images from the condition, each presented for 3.5 seconds and followed by a white fixation cross on a black background for 500 msec before the next image appeared. Images comprising the stimulus sets for conditions 1) and 2) were the same stimuli used in the keypress task. The visual baseline condition consisted of randomly selected images from the other three conditions that were rotated 180 degrees and blurred with a Gaussian filter using Photoshop ® (Adobe, San Jose, CA). Stimuli were presented using Eprime 2.0 (Psychology Software Tools, Inc., Sharpsburg, PA), and projected onto a screen viewed using an angled mirror. An example of stimuli for a representative participant (with an interest in trains) and the block design are displayed in Figure 1.
Figure 1.
fMRI task design for a single sample run, and example stimuli for a participant whose restricted interest is trains (Own image block). Within each of the 5 runs, blocks of each condition were arranged in a pseudorandom sequence (random except that each began and ended with a visual baseline block) that was fixed across participants.
Image acquisition
All images were acquired using a 3.0 Tesla Philips Achieva MRI scanner with an eight-channel SENSE head coil. Whole-brain functional images were acquired using axial oblique slices (tilted 15° anterior higher than posterior relative to the AC-PC line) with an isotropic 2.5 mm3 voxel size (TR=2 s, TE=25 msec, flip angle=90°, acquisition matrix=96 × 96, no gap). High-resolution anatomical images were acquired in the sagittal plane using a T1-weighted volumetric 3D SPGR sequence (TR=7.9 msec, TE=3.7 msec, flip angle = 7°, acquisition matrix: 256×256, 1 mm3 isotropic resolution). Participants were lying comfortably on the scanner bed with foam cushioning between the head and the birdcage coil. During the structural, scout, and reference scans, participants watched a favorite video (unrelated to the targeted interest). When the functional scans began, instructions were simply to pay attention to each picture, with a reminder of the recognition memory test that would follow the scan.
Post-scan memory test
For the “Own” and “Other” conditions, participants were tested after the scanning session to confirm that they were attending during the passive viewing paradigm. For each condition, the 38 previously-viewed images were combined with 19 novel images; all images were presented in randomized order using Eprime 2.0. Participants were instructed to press “1” if they had seen the image in the scanner, and “2” if they had not. On each trial, the child was given feedback regarding response accuracy. Hit and false alarm rates were calculated individually by condition to verify attention to the stimuli across all conditions. fMRI data for children whose hit rate was less than 75% for old images in either condition or false alarm rate was more than 25% for new images in either condition (d’ of at least 1.32) were excluded from the final analysis. Using this criterion, 3 children with TD were excluded from imaging analyses.
Image processing and analysis
Images were analyzed using SPM5 running in Matlab 7.13.0 (R2011b) (http://www.fil.ion.ucl.ac.uk/spm/). Functional images in each run were realigned to the first volume and resliced. Next, all realigned functional volumes were warped to the standard MNI template brain for group comparison. Functional images were smoothed with a Gaussian kernel of 6 mm FWHM.
First level analysis was specified for each participant using the general linear model design, modeled using the canonical hemodynamic response function (HRF). The robust weighted least squares toolbox was used to inversely weight volumes according to their variance due to noise, thereby minimizing the contribution of volumes with motion spikes. Each model was estimated with the classical restricted maximum likelihood approach for spatially smoothed images. Realignment parameters were used to identify runs that had > 3mm translational and/or 3° rotational motion in any direction for exclusion in first-level contrast specification. Inclusion of individual participant data in second level analyses required that three or more functional runs met inclusion criteria for motion. Based on this criterion, 2 participants from each group were excluded from second-level analyses. Thus, the final sample was 19 children in the ASD group and 18 children in the TD group; for both groups, all children in the final sample were male. Independent samples t tests confirmed that the mean number of included runs did not differ by group (t(35)=0.325, p=.75), nor did the groups in the final sample differ by age (t(35)=−0.542, p=.59) or IQ (t(35)=1.22, p=.23). Characteristics of the final sample are detailed in Table 1.
Second level (group) analysis was completed in two stages: 1) using one-sample t tests to create contrasts between conditions within groups, and 2) using two-sample t tests to compare contrasts between the two groups. In keeping with our a priori hypotheses, a mask comprising regions responsive to reward was created using a combination of AAL regions (Maldijan, Laurienti, Kraft, & Burdette, 2003) for amygdala, anterior cingulate cortex, medial prefrontal cortex, orbitofrontal cortex, and insula, and the Harvard-Oxford atlas (http://www.fmrib.ox.ac.uk/fsl/data/atlas-descriptions.html), for the nucleus accumbens.
Within-groups contrasts were created first to compare activation in each condition (i.e., Own Interest, Others’ Interests) to the visual baseline condition, as well as to each other (i.e., Own vs. Other), in the ASD and TD groups separately. Between-group contrasts were then created to examine the group differences in the same contrasts. For an initial exploratory analysis, a threshold of Z > 2.58 (uncorrected p-value of 0.005), and a cluster size of at least 10 voxels was used to identify clusters with statistically significant BOLD response within the mask. We then subjected significant clusters to correction for family-wise error using AlphaSim (http://afni.nimh.nih.gov/pub/dist/doc/manual/AlphaSim.pdf), a Monte Carlo simulation procedure that determines the probability of a false positive from the frequency count of cluster sizes. Masks for each region of interest were registered to functional image space and used with a corrected p-value of .05 and smoothing kernel of 6mm in 5000 iterations of the simulation procedure.
Extraction of percent signal change and correlation with behavioral variables
BOLD signal was extracted from a functionally-defined ROI (see below) from the between groups contrast for Own-Other and correlated with both the YSII severity score measuring the interference and intensity of the interest in daily life, and the operant keypress task score (ratio of time spent viewing Own vs. Other pictures) in the ASD group. Because the BOLD signal and YSII scores were not normally distributed, Spearman's correlation coefficients were computed.
Results
Operant task
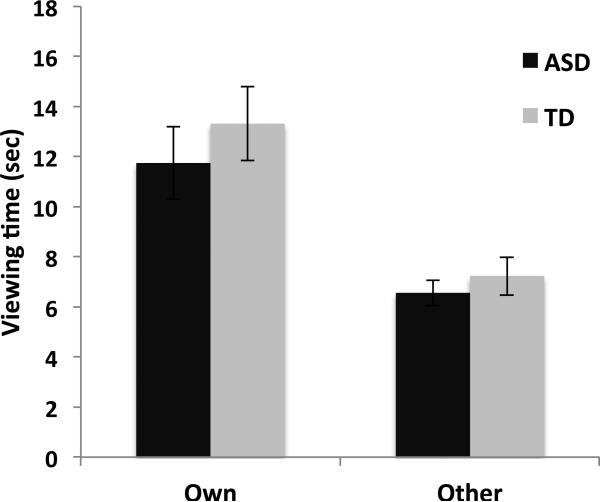
Across all participants, mean display time was 12.52 seconds for “Own” pictures versus 6.89 seconds for “Other” images, suggesting that children across groups showed a preference for pictures related to their own interest. There was a significant effect of picture type (Own vs. Other, F(1,34) = 45.61, p < 0.0001), but no significant main effect of group (F(1,34) = 0.687, p = 0.41) and no interaction between group and picture type (F(1,34) = 0.002, p = 0.97). The results of this task measuring the reward value of pictures related to children's interests are depicted in Figure 2.
Figure 2.
Behavioral keypress results. Viewing time for the ASD (dark bars) and TD (light bars) groups, for their own personalized stimuli (left) versus other children's stimuli (right). Viewing time results directly from participant effort expenditure to increase or decrease viewing duration from the default value of 5 sec.
Yale Special Interests Interview (YSII)
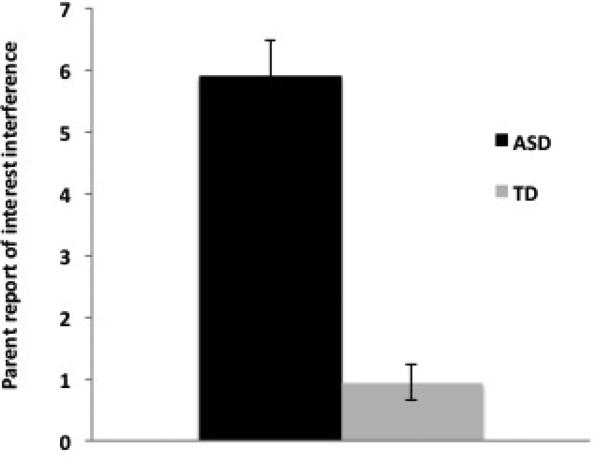
In contrast to the non-significant effect of group for picture viewing, the two groups differed significantly in parent report of the degree to which interests or hobbies interfered with other aspects of daily life. The average YSII severity rating for the ASD group was 5.89 (of a possible 12 points), while the average for the TD group was 0.94 (F(1,34) = 55.41, p < 0.0001). The results of the parent interview measuring everyday interest intensity and interference are summarized in Figure 3.
Figure 3.
Parent-report data from the YSII for the ASD (dark bars) and TD (light bars) groups. The Y-axis represents the severity score from the YSII measuring the intensity and interference of the interest in multiple domains of daily life.
fMRI: Within-group contrasts
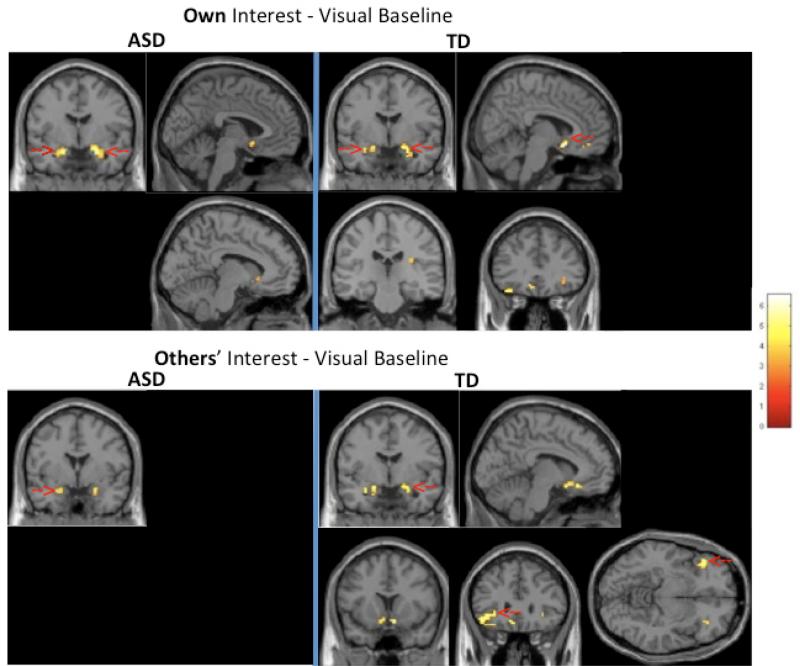
In both groups, viewing one's own interest elicited significantly higher BOLD signal relative to viewing the blurred baseline image (Own-Baseline) in bilateral amygdala when corrected for multiple comparisons. In the TD group, there was also a significant activation in the medial frontal gyrus (MFG) for this contrast. Exploratory uncorrected (p<.005, cluster size ≥10) results revealed additional regions associated with the processing of emotion and reward (bilateral subgenual anterior cingulate in both groups; posterior insula and inferior frontal gyrus (IFG) in the TD group, and the head of the caudate in the ASD group).
In the Other-Baseline contrast, both groups showed unilateral amygdala activation, and the TD group showed a large activation with a peak voxel in the orbitofrontal cortex (OFC), extending into inferior frontal gyrus (IFG) and anterior insula. In the exploratory analysis without correction for multiple comparisons (p<.005, cluster size ≥10), this contrast also elicited bilateral nucleus accumbens, in the TD group. In contrast, the ASD group's only significant clusters in the Other-Baseline contrast were in bilateral amygdala, even at the more lenient uncorrected threshold. Within-group results for these contrasts are summarized in Table 2 and Figure 4.
Table 2. Within-group contrasts.
Within-groups significant clusters (p (uncorrected) < 0.005, minimum cluster = 10 voxels), in each of the three contrasts examined: own interest – visual baseline, others’ interests – visual baseline, and own interest – others’ interests. Clusters that survived correction using 5000 iterations of a Monte Carlo simulation are shown in BOLD and denoted with an
| Own-Baseline |
|||||||
|---|---|---|---|---|---|---|---|
| Group | Region | x | y | z | K | Zmax | p(uncorr) |
| ASD | R amygdala | 23 | −3 | −20 | 93* | 4.63 | .000 |
| L amygdala | −18 | −3 | −20 | 63* | 4.15 | .000 | |
| R subgenual ACC | 8 | 15 | −15 | 19 | 3.42 | .000 | |
| L subgenual ACC | −3 | 10 | −10 | 11 | 3.19 | .001 | |
| L caudate (head) | −13 | 25 | −8 | 16 | 3.15 | .001 | |
| TD | R amygdala | 23 | −5 | −18 | 48* | 4.26 | .000 |
| L amygdala | −20 | −5 | −18 | 36* | 3.64 | .000 | |
| R subgenual ACC | 5 | 13 | −13 | 28 | 4.45 | .000 | |
| L subgenual ACC | −5 | 8 | −13 | 25 | 4.47 | .000 | |
| R IFG | 33 | 30 | −8 | 16 | 3.27 | .001 | |
| L IFG | −38 | 33 | −20 | 20 | 3.71 | .000 | |
| R posterior insula | 33 | 23 | 20 | 11 | 3.04 | .001 | |
| L MFG | −8 | 30 | −15 | 55* | 4.05 | .000 | |
| Other-Baseline |
|||||||
|---|---|---|---|---|---|---|---|
| Group | Region | x | y | z | K | Zmax | p(uncorr) |
| ASD | R amygdala | −25 | −3 | −23 | 50* | 4.44 | .000 |
| L amygdala | 23 | −3 | −20 | 22 | 3.73 | .000 | |
| TD | R amygdala | 25 | −5 | 18 | 28 | 3.63 | .000 |
| L amygdala | −20 | −5 | −18 | 36* | 4.00 | .000 | |
| L subgenual ACC | −8 | 25 | −15 | 10 | 3.41 | .000 | |
| L OFC (extending through IFG and anterior insula) | −40 | 33 | −20 | 115* | 3.99 | .000 | |
| R IFG | 35 | 33 | −5 | 12 | 3.36 | .000 | |
| R Nac | 8 | 13 | −15 | 11 | 4.19 | .000 | |
| L Nac | −8 | 10 | −15 | 21 | 3.13 | .001 | |
| Own-Other |
|||||||
|---|---|---|---|---|---|---|---|
| Group | Region | x | y | z | K | Zmax | p(uncorr) |
| ASD | R IFG | 23 | 28 | −15 | 13 | 3.14 | .001 |
| L IFG | −35 | 30 | −5 | 11 | 3.78 | .000 | |
| TD | ACC | −5 | 3 | 30 | 16 | 3.24 | .001 |
ACC: anterior cingulate cortex; IFG: inferior frontal gyrus; MFG: medial frontal gyrus; OFC: orbitofrontal cortex; Nac: nucleus accumbens.
Figure 4.
One sample maps depicting significant clusters (uncorrected p<.005; cluster size ≥ 10) in the “Own” interest (top panel) and “Other” interest (bottom panel) conditions, relative to the visual baseline condition, for the ASD (left) and TD (right) groups. Clusters that remained after correction for multiple comparisons are highlighted with red arrows.
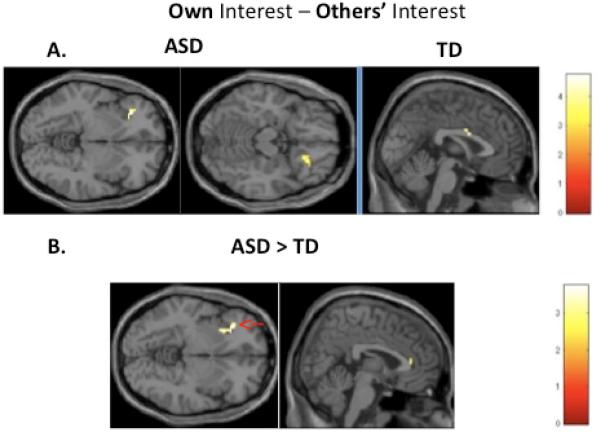
The more stringent contrast (Own-Other) was also examined for each group. No clusters in these contrasts survived the correction for multiple comparisons. In the uncorrected (p<.005, cluster size ≥10) exploratory analysis, for the ASD group, viewing images of one's own interest, relative to viewing images of others’ interests, generated significantly increased BOLD response in bilateral IFG. In the TD group, this contrast yielded a cluster in the middle anterior cingulate cortex (ACC). Within-group results for this contrast are summarized in Table 2 and Figure 5a.
Figure 5.
Maps of the most stringent contrast (Own-Other). A. One sample map showing significantly active clusters in the ASD (left) and TD (right) groups for this contrast. None of these clustered remained after correction for multiple comparisons. B. Two sample maps showing clusters that were significantly more active in the ASD than the TD group. Clusters that remained after correction for multiple comparisons are highlighted with red arrows. There were no clusters that were significantly more active in the TD than ASD group in this contrast.
fMRI: Between-group contrasts
When directly comparing the ASD and TD groups, the “Own-Other” contrast elicited a significantly greater response in the ASD than the control group in left anterior insula, which survived correction for multiple comparisons with AlphaSim. With the more lenient uncorrected threshold, we also observed a cluster in the anterior cingulate cortex with greater BOLD response in ASD than TD for the “Own-Other” contrast. The reverse contrast (i.e., “Other-Own”) yielded no clusters that were significantly more active in the control group compared to the ASD group, nor did direct group comparisons of Own and Other conditions relative to baseline. Between-group comparisons are summarized in Table 3 and Figure 5b.
Table 3. Between-group contrasts.
Between-groups significant clusters (p (uncorrected) < 0.005, minimum cluster size = 10 voxels) in the own interest – others’ interests contrast. Clusters that survived correction using 5000 iterations of a Monte Carlo simulation are shown in BOLD and denoted with an
| Own-Other |
|||||||
|---|---|---|---|---|---|---|---|
| Group | Region | x | y | z | k | Zmax | p(uncorr) |
| ASD>TD | L insula | −30 | 28 | −5 | 35* | 3.41 | .000 |
| ACC | 0 | 35 | 20 | 10 | 2.81 | .002 | |
| TD>ASD | [none] | ||||||
No significant clusters were observed for either of the other two contrasts’ two sample t tests, in either direction. ACC: anterior cingulate cortex.
Correlation of signal change with behavioral measures
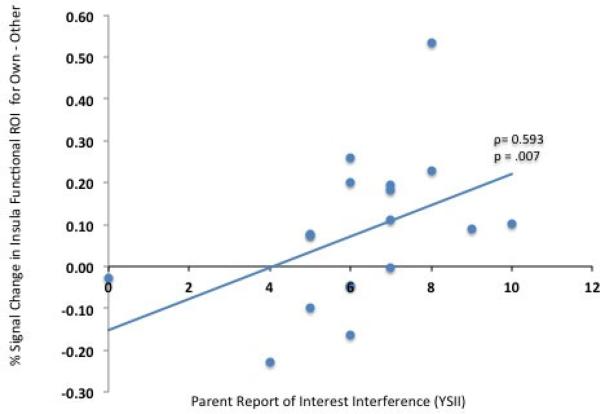
The average percent signal change was extracted from the cluster that distinguished the two groups (left anterior insula) and correlated with both the overall severity score from the YSII and the Own vs. Other viewing time ratio from the behavioral keypress task in the ASD group. Percent signal change in this cluster was significantly positively correlated with degree of interest intensity and interference reported in the YSII (ρ= 0.593, p = .007), and positively, but not significantly, correlated with the relative duration of viewing time for the child's own interest images versus those of other children in the keypress task (ρ = .362, p = .14). The relation between the YSII score and percent signal change in this functional ROI in the ASD group is depicted in Figure 6.
Figure 6.
Correlation (for the ASD group only) between parent report of interest severity from the YSII and percent signal change in the left insula that was significantly more active in the ASD group for the Own-Other contrast.
Discussion
Our results support the hypothesis that intense hobbies and interests elicit behavioral and neural reward responses in both children with ASD and typically developing children with a strong hobby or interest, adding to and extending to children previous findings in adults with ASD demonstrating intact object reward. Both groups exhibited similar viewing preference for images related to their own interest compared to those of other children in the operant task. Both groups also showed significant BOLD response increases in bilateral amygdala when viewing pictures of their own interest relative to a blurred baseline visual image, suggesting some overlap in the behavioral and neural foundations of rewarding activities for all children. Although the amygdala is a complex structure with a variety of functions, these include processing of emotionally relevant events, and, in combination with prefrontal and ventral striatal regions, signaling the current reward value of a stimulus (Adolphs, 2010). Equivalent amygdala response to images of one's own interest in ASD and TD groups suggests that the behavioral and neural response to highly salient objects is intact in ASD, which was further supported by the absence of clusters with significant group differences for this condition.
The narrowness of focus that parents report as causing interference in daily life may be better understood by considering the neural response to pictures of other children's interests. For the TD group, these pictures relative to baseline elicited not only amygdala response, but also a widespread activation in left OFC that extended into other frontal regions and, in the exploratory analysis, bilateral nucleus accumbens. In contrast, only the amygdala was active in the ASD group when looking at images of other children's interests, for both the corrected threshold and the more lenient exploratory one. Related to its role in signaling salient stimuli, the amygdala responds generally to novelty (Blackford, Buckholtz, Avery, & Zald, 2010). Thus, it is possible that the novelty of unfamiliar pictures was rewarding to the TD group, but not the ASD group. This interpretation, however, must be considered with caution in light of the absence of group differences for this condition.
More support for the idea that one's own interests are uniquely rewarding in ASD comes from the direct comparison of response to “own” interests versus “others’” interests between the groups. Greater BOLD response in the ASD than the TD group was evident in the left anterior insula for this contrast. Further, the response in the insula was significantly and positively correlated with the degree of interference and intensity of the interest reported by parents of the ASD group. In addition to insula, the anterior cingulate cortex was significantly more active in children with ASD than TD in the exploratory analysis. These two regions together constitute a “salience network” that evaluates the affective significance of external stimuli (Seeley et al., 2007; Menon & Uddin, 2010). Heightened response to one's own (versus others’) interests that is specific to the ASD group supports the notion that restricted interests have affective significance for individuals with ASD that goes beyond the reward response, and may impact the interface of reward, autonomic, and attentional mechanisms that determine engagement with environmental stimuli. This interpretation is consistent with the ASD group showing similar operant behavior to the TD group in a controlled laboratory environment where little else was vying for attention, but higher parent-reported levels of interference in daily life where the salience of restricted interests may compete with many other possible stimuli.
Although it had the strength of a unique design using personalized stimuli and combining behavioral, neural, and parent report data, our study also had several limitations. The use of a passive viewing design was intended to allow free visual exploration without heavy cognitive demand, but did not allow for separation of reward anticipation (‘wanting”) from hedonic experience (“liking”). In spite of this, we see patterns of activation that are similar to those seen in previous studies of the reward system in ASD (Kohls et al., 2012; Dichter et al., 2012). The use of individualized stimulus sets based on restricted interests was a unique attribute of our study that optimized the emotional salience of the reward stimuli, allowing us to confirm intact affective neural response to objects of interest in ASD in comparison to baseline, and aberrantly enhanced response in comparison to other children's objects of interest. However, an inherent drawback to this approach was the heterogeneity of stimuli across individuals. We endeavored to address this by using a standard size and resolution for each image and utilizing a stringent control condition. Regardless, this heterogeneity likely introduced additional noise into our data and thus limited our power to detect differences, as suggested by several regions that showed response differences at an uncorrected threshold but did not survive correction for multiple comparisons. Finally, we excluded children taking psychotropic medications, which was necessary to prevent confounding effects on the BOLD response, but may have limited the generalizability of our findings to the broader population of children with ASD.
Conclusion
The results reported here add to a picture of intact/aberrantly enhanced response of neural reward systems in ASD to nonsocial stimuli, particularly those related to restricted interests, despite previously demonstrated deficits in social reward. It is plausible that the enhanced response of the insula, the critical node of the salience network, seen here to objects of interest takes the place of responses to social rewards in ASD, though we did not test social reward directly in this study. Thus, the implications of these findings, including their role in the etiology of both social deficits and repetitive behaviors in ASD, will require much further work to elucidate.
Key points.
Repetitive behaviors such as restricted interests implicate positive affect and reward, but the role of neural affective systems have not been directly tested using functional neuroimaging (fMRI).
fMRI studies in ASD suggest impaired response to social reward, which may extend to other kinds of reward, such as money.
Using personalized pictures of individual interests and comparing children with ASD to children with typical development (TD) who also had strong interests, we found intact and enhanced reward response in ASD using an operant task and fMRI.
The groups differed in parent-reported interference of the interest, and in the response of the ‘salience network’, both of which were higher in ASD, suggesting enhanced affective response for stimuli related to restricted interests.
Acknowledgments
The authors wish to thank Mikle South for providing helpful input on the use of the YSII, Matthew Hoscheit, and Akua Cosby for assistance with data collection and management, and David Royal for programming a pilot version of the picture interest task. CJC is supported by K01MH090232 from NIMH; this research was also supported in part by 1 UL1 RR024975 from NCRR/NIH and 5P30EY008126-23.
Footnotes
Conflict of interest statement: No conflict of interests declared.
The authors have declared that they have no competing or potential conflicts of interest.
References
- Adolphs R. What does the amygdala contribute to social cognition? Annals of the New York Academy of Sciences. 2010;1191:42–61. doi: 10.1111/j.1749-6632.2010.05445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aharon I, Etcoff N, Ariely D, Chabris CF, O'Connor E, Breiter HC. Beautiful faces have variable reward value: fMRI and behavioral evidence. Neuron. 2001;32:537–551. doi: 10.1016/s0896-6273(01)00491-3. [DOI] [PubMed] [Google Scholar]
- Bishop SL, Hus V, Duncan A, Huerta M, Gotham K, Pickles A, Kreiger A, Buja A, Lund S, Lord C. Subcategories of Restricted and Repetitive Behaviors in Children with Autism Spectrum Disorders. Journal of Autism and Developmental Disorders. 2012 Oct. doi: 10.1007/s10803-012-1671-0. [Epub ahead of print] PMID: 23065116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackford JU, Buckholtz JW, Avery SN, Zald DH. A unique role for the human amygdala in novelty detection. NeuroImage. 2010;50(3):1188–93. doi: 10.1016/j.neuroimage.2009.12.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodfish JW, Symons FJ, Parker DE, Lewis MH. Varieties of repetitive behavior in autism: comparisons to mental retardation. Journal of Autism and Developmental Disorders. 2000;30:237–243. doi: 10.1023/a:1005596502855. [DOI] [PubMed] [Google Scholar]
- Boyd BA, Conroy MA, Mancil GR, Nakao T, Alter PJ. Effects of circumscribed interests on the social behaviors of children with autism spectrum disorders. Journal of Autism and Developmental Disorders. 2007;37:1550–1561. doi: 10.1007/s10803-006-0286-8. [DOI] [PubMed] [Google Scholar]
- Brainard D. The Psychophysics Toolbox. Spatial Vision. 1997;10:433–436. [PubMed] [Google Scholar]
- Cascio CJ, Foss-Feig JH, Heacock JL, Newsom CR, Cowan RL, Benningfield MM, et al. Response of neural reward regions to food cues in autism spectrum disorders. Journal of Neurodevelopmental Disorders. 2012;4:9. doi: 10.1186/1866-1955-4-9. doi: 10.1186/1866-1955-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlop-Christy MH, Haymes LK. Using objects of obsession as token reinforcers for children with autism. Journal of Autism and Developmental Disorders. 1998;28:189–198. doi: 10.1023/a:1026061220171. [DOI] [PubMed] [Google Scholar]
- Chevallier C, Kohls G, Troiani V, Brodkin ES, Schultz RT. The social motivation theory of autism. Trends in Cognitive Sciences. 2012;16:231–239. doi: 10.1016/j.tics.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuccaro ML, Shao Y, Grubber J, Slifer M, Wolpert CM, Donnelly SL, Abramson RK, Ravan SA, Wright HH, DeLong GR, Pericak-Vance MA. Factor analysis of restricted and repetitive behaviors in autism using the Autism Diagnostic Interview-R. Child Psychiatry and Human Development. 2003;34:3–17. doi: 10.1023/a:1025321707947. [DOI] [PubMed] [Google Scholar]
- Delmonte S, Balsters J, McGrath J, Fitzgerald J, Brennan S, Fagan A, Gallagher L. Social and monetary reward processing in autism spectrum disorders. Molecular Autism. 2012;3(1):7. doi: 10.1186/2040-2392-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter GS, Richey JA, Rittenberg AM, Sabatino A, Bodfish JW. Reward circuitry function in autims during face anticipation and outcomes. Journal of Autism and Developmental Disorders. 2012a;42:147–60. doi: 10.1007/s10803-011-1221-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter GS, Felder JN, Green SR, Rittenberg AM, Sasson NJ, Bodfish JW. Reward circuitry function in autism spectrum disorders. Social Cognitive and Affective Neuroscience. 2012b;7:160–172. doi: 10.1093/scan/nsq095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter GS, Richey JA, Rittenberg AM, Sabatino A, Bodfish JW. Reward circuitry function in autism during face anticipation and outcomes. Journal of Autism and Developmental Disorders. 2012;42:147–160. doi: 10.1007/s10803-011-1221-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimko H, Cross J, Abernethy D. Pharmacokinetics and clinical effectiveness of methylphenidate. Clinical Pharmacokinetics. 1999;37:457–470. doi: 10.2165/00003088-199937060-00002. [DOI] [PubMed] [Google Scholar]
- Kohls G, Chevallier C, Troiani V, Schultz RT. Social ‘wanting’ dysfunction in autism: neurobiological underpinnings and treatment implications. Journal of Neurodevelopmental Disorders. 2012;4(1):10. doi: 10.1186/1866-1955-4-10. doi: 10.1186/1866-1955-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohls G, Schulte-Ruther M, Nehrkorn B, Muller K, Fink GR, Kamp-Becker I, Herpertz-Dahlmann B, Schultz RK, Konrad K. Reward system dysfunction in autism spectrum disorders. Soc.Cogn Affect.Neurosci. 2012 Apr 11; [Epub ahead of print]. PMID: 22419119. [Google Scholar]
- Lam KS, Aman MG. The Repetitive Behavior Scale-Revised: independent validation in individuals with autism spectrum disorders. Journal of Autism and Developmental Disorders. 2007;37:855–866. doi: 10.1007/s10803-006-0213-z. [DOI] [PubMed] [Google Scholar]
- Lam K, Bodfish J, Piven J. Evidence for three subtypes of repetitive behavior in autism that differ in familiality and association with other symptoms. Journal of Child Psychology and Psychiatry. 2008;49:1193–1200. doi: 10.1111/j.1469-7610.2008.01944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeCouteur A, Lord C, Rutter M. The Autism Diagnostic Interview-Revised (ADI-R) Western Psychological Corporation; Los Angeles: 2003. [Google Scholar]
- Lin A, Rangel A, Adolphs R. Impaired learning of social compared to monetary rewards in autism. Frontiers in Neuroscience. 2013;6:143. doi: 10.3389/fnins.2012.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore P, Risi S. The Autism Diagnostic Observation Schedule (ADOS) Western Psychological Corporation; Los Angeles: 1999. [Google Scholar]
- Maldijan JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Structure and Function. 2010;214(5-6):655–67. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercier C, Mottron L, Belleville S. A Psychosocial Study on Restricted Interests in High Functioning Persons with Pervasive Developmental Disorders. Autism. 2008;4:406–425. [Google Scholar]
- Richey J, Rittenberg A, Hughes L, Damiano C, Sabatino A, Miller S, Hanna E, Bodfish JW, Dichter GS. Common and distinct neural features of social and non-social reward processing in autism and social anxiety disorder. Social Cognitive and Affective Neuroscience. 2013 Jan 17; doi: 10.1093/scan/nss146. [Epub ahead of print] PMID: 23223206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott-Van Zeeland AA, Dapretto M, Ghahremani DG, Poldrack RA, Bookheimer SY. Reward processing in autism. Autism Research. 2010;3:53–67. doi: 10.1002/aur.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Grecius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. Journal of Neuroscience. 2007;27(9):2349–56. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- South M, Klin A, Ozonoff S. The Yale Special Interests Interview. Unpublished Work. 1999.
- South M, Ozonoff S, McMahon WM. Repetitive behavior profiles in Asperger syndrome and high-functioning autism. Journal of Autism and Developmental Disorders. 2005;35:145–158. doi: 10.1007/s10803-004-1992-8. [DOI] [PubMed] [Google Scholar]
- Szatmari P, Georgiades S, Bryson S, Zwaigenbaum L, Roberts W, Mahoney W, et al. Investigating the structure of the restricted, repetitive behaviours and interests domain of autism. Journal of Child Psychology and Psychiatry. 2006;47:582–590. doi: 10.1111/j.1469-7610.2005.01537.x. [DOI] [PubMed] [Google Scholar]
- Turner-Brown LM, Lam KS, Holtzclaw TN, Dichter GS, Bodfish JW. Phenomenology and measurement of circumscribed interests in autism spectrum disorders. Autism. 2011;15:437–456. doi: 10.1177/1362361310386507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence (WASI) Harcourt Assessment; San Antonio, TX: 1999. [Google Scholar]