Abstract
Background
The US Food and Drug Administration’s meta-analyses of placebo-controlled antidepressant trials found approximately twice the rate of suicidal behaviors among children and adults 24 years of age and younger who were randomized to receive antidepressant medication, compared with those who were randomized to placebo. Rates of suicidal behavior were similar for subjects 25 to 64 years of age whether they received antidepressants or placebo, and subjects 65 years of age or older randomized to antidepressants were found to have lower rates of suicidal behavior. Age stratified FDA meta-analyses did not have adequate power to investigate rates of suicidal behaviors by antidepressant drug class.
Objective
To assess the risk of deliberate self-harm associated with the two most commonly prescribed classes of antidepressant agents.
Design
Propensity score matched cohort study of incident users of antidepressant agents.
Setting
Population-based health care utilization data of US residents.
Patients
US residents 10 to 64 years of age with a recorded diagnosis of depression who initiated use of selective serotonin reuptake inhibitors (SSRIs) or serotonin norepinephrine reuptake inhibitors (SNRIs) between January 1, 1998 and December 31, 2010.
Main Outcome Measures
ICD-9 external cause of injury codes E950.x-E958.x (deliberate self-harm).
Results
102,647 patients between 10 and 24 years of age and 338,021 patients between 25 and 64 years of age initiated therapy with antidepressants. Among 10–24 year olds, prior to propensity score matching, 75,675 patients initiated therapy with SSRIs and 5,344 initiated SNRIs. After matching there were 5,344 SNRI users and 10,688 SSRI users. Among the older cohort, 36,037 SNRI users were match to 72,028 SSRI users (from an unmatched cohort of 225,952 SSRI initiators). Regardless of age cohort, patients initiating SSRIs and patients initiating SNRIs had similar rates of deliberate self-harm. Restriction to patients with no antidepressant use in the past 3 years did not alter our findings.
Conclusions
Our findings of similar rates of deliberate self-harm for depressed patients who initiate treatment with either an SSRI or an SNRI suggests that physicians who have decided that their patients would benefit from initiating antidepressant therapy need not weigh differential suicide risk when deciding which class of antidepressant to prescribe.
1. Introduction
The first suggestion from placebo-controlled trials that some antidepressants might increase the risk of suicide came from a 2003 report to the Food and Drug Administration (FDA) by GlaxoSmithKline, the manufacturer of the drug paroxetine (a selective serotonin reuptake inhibitor [SSRI]). [1] That report documented an increased risk of possible suicide-related adverse events (SREs) in paroxetine-treated pediatric patients with major depressive disorder. The FDA subsequently requested that manufacturers of 8 other widely used antidepressants search for similar evidence in their antidepressant databases of pediatric trials. The FDA investigation culminated in two of the largest meta-analyses of placebo-controlled trials of antidepressants ever undertaken, which, taken together found that children and adults 24 years of age and younger who were randomized to receive antidepressant medication, compared with those who were randomized to placebo, appear to have approximately twice the rate of suicidal behaviors. [2] [3] Suicidal behavior event rates were similar for subjects 25 to 64 years of age whether they received antidepressants or placebo, and subjects 65 years of age or older randomized to antidepressants were found to have lower rates of suicidal behavior. [3]
The FDA meta-analyses are the largest efforts ever undertaken to use randomized data to assess clinically relevant questions about possible suicide risk associated with antidepressant therapy. Nevertheless, suicide-related outcomes are rare events, even among high-risk subjects, and the FDA analyses lacked the power to determine whether some antidepressant classes or agents may be safer than others with respect to suicide risk. Consequently, FDA advisories warn of an increased risk of suicide after starting any antidepressant, regardless of class or formulation. There are, however, reasons to believe that antidepressants might differ in their associated suicide risks, perhaps most markedly across classes of antidepressants where different mechanisms of action can lead to differences in other adverse pathophysiological effects linked to suicide risk such as anxiety, difficulty falling asleep, akathisia, and adverse discontinuation effects, [4–15] the latter being a more likely consequence of commonly prescribed serotonin-norepinephrine reuptake inhibitor (SNRI) agents, which have much shorter half-lives, compared with commonly used SSRI agents. [14]
Consistent with the possibility of differential risk across classes of antidepressants, the 2006 FDA meta-analysis of placebo-controlled pediatric trials reported a stronger effect estimate for SREs with venlafaxine (the predominant SNRI prescribed today) than for other antidepressants (most of which were SSRIs), albeit with wide confidence intervals. A secondary analysis of FDA findings by Smith found that antidepressants with longer half-lives tended to be associated with lower risk of suicidality. [14] These considerations notwithstanding, non-randomized studies that have compared suicide risks across antidepressant agents [16–23] have reported somewhat mixed results, with most, but not all [19, 24] finding no or limited differences in suicide risk by antidepressant class. The two studies that found differential risk by antidepressant class did not agree, however, on the antidepressant class or agent that was associated with higher suicide risk, with one identifying substantially higher risk among older male patients on SSRIs, compared with older males on non-SSRI antidepressants (mostly tricyclic agents), [24] and the other identifying venlafaxine as consistently associated with higher risk of suicide and suicide attempts, compared with SSRIs and tricyclic antidepressants. [19]
Since the clinical efficacy of antidepressants does not clearly favor one class of antidepressants over another [25, 26], identifying whether suicide-related risk varies across antidepressants could be of immediate clinical relevance. The current study, the largest US cohort study to include children and adults to investigate this question, examines the risk of Deliberate self-harm among new initiators of SSRIs and SNRIs. The size of our population of new users of antidepressants (over 300,000 of whom initiate use of SSRIs or SNRIs over the study period) and the inclusion of more recent data than prior work has the advantage of conferring greater power to detect differences, if they exist, between the two most widely prescribed classes of antidepressants today: the SSRIs and the SNRIs. The power to discriminate between these two classes of antidepressants is not a trivial advantage as type II error is more likely with smaller studies and is especially important in guiding clinical judgment based on null findings. [27]
2. Methods
2.1 Patients and data source
The current cohort study involves US patients 10 to 64 years of age with a recorded diagnosis of depression who initiated use of selective serotonin reuptake inhibitors (SSRIs) or serotonin norepinephrine reuptake inhibitors (SNRIs) between January 1, 1998 and December 31, 2010. Initiation was defined as filling an SSRI or SNRI antidepressant prescription without evidence of having filled a prescription for any class of antidepressants in the preceding 12 months. Such initiators are referred to throughout as “new users”. Primary analyses focused on the first treatment episode initiated during the study period. Eligibility required evidence of depression as indicated by a diagnosis of depression (International Classification of Diseases, Ninth Revision (ICD-9) codes 296.2x, 296.3x, 298.0x, 300.4x, 309.0x, 309.1x, 311.xx, 293.83, 296.90, 309.28) recorded during the year prior to antidepressant initiation. Subjects were required to be actively enrolled in a health plan with prescription benefits that contributed data to our claims database (see below) during the 15 months prior to initiation (i.e., 12 months for baseline covariate assessment and an additional 3 months to allow uniform assessment of all patients based on a 60 day grace period and a usual antidepressant supply of 30 days).
The PharMetrics Claims Database used in this study was purchased from IMS Health and is comprised of commercial health plan information obtained from managed care plans throughout the United States. The database includes medical and pharmaceutical claims for over 61 million unique patients from over 98 health plans (approximately 16 million covered lives per year). The database includes inpatient and outpatient diagnoses (in ICD-9-CM format) and procedures (in Current Procedural Terminology (CPT) and Healthcare Common Procedure Coding System (HCPCS) formats), as well as both retail and mail order records of all reimbursed dispensed prescriptions. Available data on prescriptions include the National Drug Code (NDC) as well as the quantity, number of days supplied, and the date of dispensing. Additional data elements include demographic variables (age, gender, geographic region), provider specialty, and start and stop dates of health-plan enrollment. Only health plans that submit data for all members are included in the database. Records in the PharMetrics database are generally representative of the national, commercially insured (non-Medicaid/Medicare) population in terms of age and gender. Our analyses focus on persons 64 years of age and younger, the age-group for which our data are nationally representative and for which we had sufficient sample size to examine the relation between antidepressant drug class and deliberate self-harm.
2.2 Antidepressant medication exposure
Antidepressant medications were classified as SSRIs or SNRIs. SSRIs included citalopram hydrobromide, fluoxetine hydrochloride, fluvoxamine maleate, paroxetine hydrochloride, and sertraline hydrochloride; SNRIs included venlafaxine and Duloxetine hydrochloride. We did not examine tricyclic antidepressants or monoamine oxidase inhibitors given their infrequent use. Bupropion (Wellbutrin) was excluded from analysis due to its frequent use for smoking cessation. Patients initiating more than one antidepressant agent on the same day were excluded.
2.3 Follow-up and study end point
Exposure status was assigned based on the initiated medication. Study follow-up began on the day after initiation of the first antidepressant therapy. For each patient we created a record of drug coverage by listing consecutive prescription fills, based on dispensing dates and reported days supply. When a dispensing occurred before the previous prescription should have run out, use of the new prescription is assumed to begin the day after the end of the old prescription. Since users of any prescription medicine, especially chronic users, may experience relatively brief episodes without a supply of medicine or may skip taking the medicine some days, our primary analyses allowed for up to 60 extra days to elapse beyond the provided days supply before censoring (i.e., we use a 60 day grace period, twice the most common days supply).
Patients were also censored at the date they switched agents (including when switching occurred within antidepressant class), added other antidepressant agents to the initiated regimen (i.e., treatment augmentation), 360 days after the index date, ended enrollment in their health insurance plan, or the end of the study period, whichever came first. New users were not allowed to become new users again; patients who were prevalent users at the start of their enrollment were allowed to become new users later during the study period.
The occurrence of deliberate self-harm (DSH) at least one day after initiating antidepressant therapy was our outcome of interest. Deliberate self-harm was defined as a medical claim with an International Classification of Diseases, Ninth Revision (ICD-9) external cause of injury code (E-code) of deliberate self-harm (E950.x-E958.x).
2.4 Patient characteristics
Patient characteristics assessed included age, sex, and several indicators of past year medical comorbidity including the number of acute hospitalizations for non-psychiatric reasons, constituents of the Charlson Comorbidity Index score, number of distinct generic drugs filled, and number of outpatient visits. Psychiatric risk factors included the number of acute psychiatric hospitalizations, the number of acute hospitalizations for substance abuse, psychiatric comorbidity, and prior DSH. All patients had at least one depression diagnosis in the year prior to their index date. A hierarchy of depression severity was constructed for each patient as a function of the proximity of the most recent depression diagnosis to antidepressant initiation (i.e., within 30 days of antidepressant initiation vs. within 31–360 days of initiation) and whether the diagnosis was an inpatient or outpatient diagnosis. For inpatient diagnoses, depression diagnosis was further characterized as to whether the diagnosis was the primary or secondary diagnosis of record. For outpatient diagnoses, persons with a single depression diagnosis were distinguished from those with multiple depression diagnoses in the year prior to initiation of antidepressant therapy. Other psychiatric disorders were defined as the presence of at least one inpatient or outpatient diagnosis. These included anxiety or sleep disorders, substance abuse, psychotic disorder, ADHD, cognitive impairment or dementia, and personality disorder. In addition to psychiatric comorbidities, we measured a number of general medical comorbidities, including malignant neoplasms, opiate use, stroke and transient ischemic attack, Parkinson disease, seizure disorders, urinary incontinence, cardiovascular disease, and chronic lung disease. Patients with past year mania, bipolar disorder, or schizophrenic disorder were excluded from analyses.
2.5 Statistical analyses
We first stratified patients into two age groups, those 10–24 years of age and those 25–64 years of age, guided by the age-related risk of suicidal behavior identified in the FDA’s meta-analyses of placebo-controlled antidepressant trials. [3] We then estimated propensity scores for treatment initation of SSRIs vs. SNRIs based on the patient characteristics described above. Propensity scores were estimated separately for our younger and older age groups. Up to two patients initiated on SSRI therapy were matched to every patient initiated on SNRI therapy based on the propensity score using an adaptation of a published algorithm [Parsons]. [28]. As we selected SSRI patients who most closely matched the less numerous SNRI patients, the research question we address is what would have happened, with respect to future Deliberate self-harm, if people who were treated with SNRIs had instead been treated with SSRI therapy (i.e., effect in the treated rather than in everybody). Since the only antidepressants that have been approved for treatment of depression in children are an SSRIs (fluoxetine, escitalopram), our matching algorithm has the added benefit of asking a more germane clinical question than had our reference population been SSRI users.
Crude DSH rates were calculated for patients exposed to SSRI vs. SNRI antidepressants over the entire exposure period. After examination of Kaplan-Meier (KM) plots of the matched cohorts, crude rates were also reported for three time periods: the first 30, 31–90, and 91–360 days after initiating therapy. Exact methods were used to calculate 95% confidence intervals. Poisson regression was used to estimate the 90-day risk difference and Cox proportional hazards regression was used to estimate the 1-year hazard ratio of antidepressant class on DSH.
Sensitivity analyses examined how robust our findings were to a range of grace periods (7, 14, 30, 90, 180 and 360 days) and to analyses that focused on the first treatment carried forward (FTCF), the latter meaning that changes in antidepressant exposure do not result in censoring and, as such, are analogous to “as randomized” analyses in randomized controlled trials. Additional subgroup analyses restricted subjects to those without a prior DSH, and to those who had not received antidepressants in the 3 years prior to their index date. We also plotted Kaplan-Meier curves for DSH–free survival as a function of the duration of continuous use of the index antidepressant for up to one year.
3. Results
Between January 1, 1998 and December 31, 2010, 102,647 patients between 10 and 24 years of age with baseline depression and 338,021 patients between 25 and 64 years of age with baseline depression initiated therapy with antidepressants. Of these, 70% initiated SSRIs (76.4% of the younger age group; 68.1% of the older), 10% SNRIs (5.5% of younger, 11.0% of older), 3% tri-or tetracyclic antidepressants (2.2% younger; 3.1% older), and 17% with other antidepressants (15.9% younger, 17.7% older). Sertraline constituted 25% of all SSRI initiation, followed by 22% Citalopram, 21% Escitalopram, 20% Fluoxetine, 12% Paroxetine and few Fluvoxamine initiators. The breakdown of SSRI initiation in the younger (older) age group was: 28% (25%) Sertraline, 27% (17%) Fluoxetine, 19% (23%) Citalopram, 16% (22%) Escitalopram, and 9.5% (12%) Paroxetine. Among those initiating SNRIs, 66% (73% of younger; 65% of older) initiated with Venlafaxine; the remaining initiated with Duloxetine.
As shown in Table 1, age-group stratified propensity score matched cohorts of SSRI vs. SNRI users were balanced across most baseline covariates, including measures of psychiatric burden. Medical comorbidity was very infrequent among the younger cohorts; among the older cohorts the frequency of medical disorders was evenly distributed across drug class categories.
Table 1a.
Baseline characteristics of patients with depression, ages 10–24 years, initiating antidepressant therapy before and after propensity score matching on the probability of receiving SNRI therapy
| Pre-Matched Cohort | Propensity Score Matched Cohort | |||
|---|---|---|---|---|
| Characteristic | SSRI N=75,675 |
SNRI N=5,344 |
SSRI N=10,688 |
SNRI N=5,344 |
| Age | ||||
| 10–15 | 18,151 (24.0%) | 557 (10.4%) | 1,214 (11.4%) | 557 (10.4%) |
| 16–19 | 33,385 (44.1%) | 2,083 (39.0%) | 4,240 (39.7%) | 2,083 (39.0%) |
| 20–24 | 24,139 (31.9%) | 2,704 (50.6%) | 5,234 (49.0%) | 2,704 (50.6%) |
| Sex, M | 26,393 (34.9%) | 1,883 (35.2%) | 3,830 (35.8%) | 1,883 (35.2%) |
| Severity Level of Depression Diagnosis | ||||
| T1: Primary Inpatient diagnosis <=30 Days Pre-Index Date | 3,043 (4.0%) | 246 (4.6%) | 545 (5.1%) | 246 (4.6%) |
| T2: Primary Inpatient diagnosis 31–360 Days Pre-Index Date | 520 (0.7%) | 48 (0.9%) | 108 (1.0%) | 48 (0.9%) |
| T3: Non-Primary Inpatient diagnosis <=360 Days Pre-Index Date | 1,575 (2.1%) | 128 (2.4%) | 234 (2.2%) | 128 (2.4%) |
| T4: 2+ Outpatient diagnosis <=360 Days Pre-Index Date | 43,060 (56.9%) | 3,233 (60.5%) | 5,908 (55.3%) | 3,233 (60.5%) |
| T5: 1 Outpatient diagnosis <=360 Days Pre-Index Date | 27,477 (36.3%) | 1,689 (31.6%) | 3,893 (36.4%) | 1,689 (31.6%) |
| Anxiety Disorders | 18,003 (23.8%) | 1,444 (27.0%) | 3,106 (29.1%) | 1,444 (27.0%) |
| Baseline DSH | 1,005 (1.3%) | 63 (1.2%) | 146 (1.4%) | 63 (1.2%) |
| Primary Inpatient Depression Diagnosis | 3,563 (4.7%) | 294 (5.5%) | 653 (6.1%) | 294 (5.5%) |
| No Depression diagnosis w/in 30 Days of Index Date | 10,891 (14.4%) | 1,200 (22.5%) | 2,606 (24.4%) | 1,200 (22.5%) |
| Baseline Suicidal Ideation (2006–2010 only) | 1,609 (3.5%) | 91 (2.9%) | 253 (3.8%) | 91 (2.9%) |
| Cognitive Impairment/Dementia | 27 (0.0%) | 0 (0.0%) | 0 | 0 |
| Personality Disorder | 730 (1.0%) | 77 (1.4%) | 158 (1.5%) | 77 (1.4%) |
| Substance Abuse | 6,211 (8.2%) | 548 (10.3%) | 1,148 (10.7%) | 548 (10.3%) |
| Use of Any Opiate | 15,880 (21.0%) | 1,374 (25.7%) | 2,679 (25.1%) | 1,374 (25.7%) |
| Number of Distinct Drug Prescriptions Filled | ||||
| 1 (AD Only) | 12,202 (16.1%) | 807 (15.1%) | 1,449 (13.6%) | 807 (15.1%) |
| 2–3 | 23,189 (30.6%) | 1,480 (27.7%) | 2,912 (27.2%) | 1,480 (27.7%) |
| 4–5 | 16,994 (22.5%) | 1,110 (20.8%) | 2,317 (21.7%) | 1,110 (20.8%) |
| 6–9 | 16,489 (21.8%) | 1,250 (23.4%) | 2,615 (24.5%) | 1,250 (23.4%) |
| 10+ | 6,801 (9.0%) | 697 (13.0%) | 1,395 (13.1%) | 697 (13.0%) |
| Number of Psychiatric Hospitalizations, 1+ | 3,715 (4.9%) | 309 (5.8%) | 682 (6.4%) | 309 (5.8%) |
| Number of Outpatient Visits | ||||
| <5 | 15,774 (20.8%) | 1,068 (20.0%) | 1,952 (18.3%) | 1,068 (20.0%) |
| 5–9 | 21,931 (29.0%) | 1,437 (26.9%) | 3,022 (28.3%) | 1,437 (26.9%) |
| 10–19 | 23,506 (31.1%) | 1,612 (30.2%) | 3,180 (29.8%) | 1,612 (30.2%) |
| 20–39 | 11,851 (15.7%) | 967 (18.1%) | 1,981 (18.5%) | 967 (18.1%) |
| 40+ | 2,613 (3.5%) | 260 (4.9%) | 553 (5.2%) | 260 (4.9%) |
| Number of Hospitalizations for Substance Abuse, 1+ | 419 (0.6%) | 55 (1.0%) | 114 (1.1%) | 55 (1.0%) |
| Number of Hospitalizations for Other Reasons, 1+ | 4,753 (6.3%) | 358 (6.7%) | 743 (7.0%) | 358 (6.7%) |
| Colorectal Cancer | 10 (0.0%) | 2 (0.0%) | 3 (0.0%) | 2 (0.0%) |
| Prostate Cancer | 1 (0.0%) | 0 (0.0%) | ||
| Other Malignant Neoplasm Cancer | 396 (0.5%) | 43 (0.8%) | 81 (0.8%) | 43 (0.8%) |
| Congestive Heart Failure | 63 (0.1%) | 7 (0.1%) | 13 (0.1%) | 7 (0.1%) |
| Arthritis | 166 (0.2%) | 17 (0.3%) | 27 (0.3%) | 17 (0.3%) |
| Cerebrovascular Disease | 190 (0.3%) | 27 (0.5%) | 55 (0.5%) | 27 (0.5%) |
| Cluster Headaches/Migraines | 2,684 (3.5%) | 256 (4.8%) | 520 (4.9%) | 256 (4.8%) |
| Diabetes | 897 (1.2%) | 75 (1.4%) | 166 (1.6%) | 75 (1.4%) |
| Disorders of the Eye | 43 (0.1%) | 4 (0.1%) | 10 (0.1%) | 4 (0.1%) |
| Gait or Balance Disorder | 286 (0.4%) | 27 (0.5%) | 55 (0.5%) | 27 (0.5%) |
| Hyperparathyroidism | 8 (0.0%) | 1 (0.0%) | 0 (0.0%) | 1 (0.0%) |
| Osteoarthritis | 284 (0.4%) | 32 (0.6%) | 66 (0.6%) | 32 (0.6%) |
| Osteoporosis | 39 (0.1%) | 8 (0.1%) | 14 (0.1%) | 8 (0.1%) |
| Parkinsons Disease | 4 (0.0%) | 1 (0.0%) | 1 (0.0%) | 1 (0.0%) |
| Seizures | 558 (0.7%) | 43 (0.8%) | 110 (1.0%) | 43 (0.8%) |
| Urinary Incontinence | 415 (0.5%) | 22 (0.4%) | 58 (0.5%) | 22 (0.4%) |
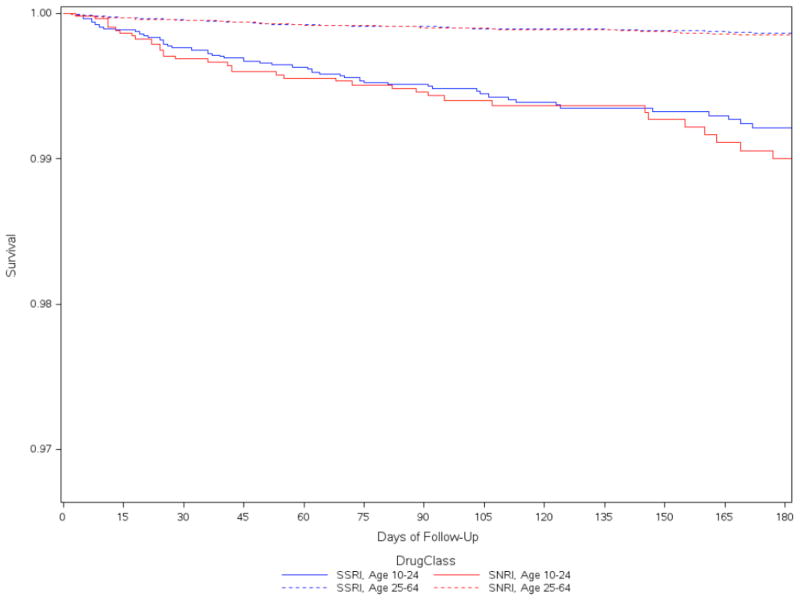
Among our younger cohort, deliberate self-harm events were identified within one year of initiating antidepressant therapy for 66 patients on SSRIs and 39 patients on SNRIs. Among our 25–64 year old age group, 81 subjects initiating an SSRI and 49 initiating SNRI subsequently had a DSH event over the first year of follow-up. Corresponding DSH rates for 10–24 year olds over the first year were 15.3 DSH events per 1000 person-years (95% confidence interval [CI]: 12.0–19.4) for SSRI users and 17.6 DSH events per 1000 person years for SNRI users (95% confidence interval [CI]: 12.7–23.8) (Table 2). Rates were highest over the first 30 days after initiation and declined further with time from the index date. Rates of DSH among adults 25–64 years of age were similar by class of antidepressant, but considerably lower than among our younger cohort (with event rates of, respectively, 2.5 and 2.8 per 1000 person-years for the older SSRI and SNRI cohorts (Table 2). For all cohorts, although the hazards were proportional throughout the 1-year follow-up period, approximately two-thirds of the DSH events that occurred over the first year of therapy occurred within the first 3 months (Table 2, Fig 1).
Table 2.
Rate of deliberate self-harm events per 1000 person-years, SNRI vs SSRI antidepressants, by age-group and time since initiating therapy
| DSH events | Person-yearsx1000 | DSH rate (per 1000 person years) | DSH rate (95% CI) by time since index date (Number of events) | |||
|---|---|---|---|---|---|---|
| 0–30 days | 31–90 days | 91–360 days | ||||
| SNRI age 10–24 | 39 | 2,212 | 17.6 (12.7–23.8) | 38.1 (22.7–60.4) (n=16) | 13.9 (7.1–24.7) (n=10) | 12.1 (6.8–20.1) (n=13) |
| SSRI age 10–24 | 66 | 4,305 | 15.3 (12.0–19.4) | 28.5 (18.7–41.7) (n=24) | 15.9 (10.4–23.5) (n=23) | 9.4 (5.9–14.4) (n=19) |
| SNRI age 25–64 | 49 | 17273 | 2.8 (2.1–3.7) | 5.6 (3.3–8.9) (n=16) | 3.2 (1.9–5.1) (n=16) | 1.8 (1.1–2.8) (n=17) |
| SSRI age 25–64 | 81 | 33050 | 2.5 (2.0–3.0) | 5.8 (4.1–8.0) (n=33) | 2.7 (1.8–3.9) (n=27) | 1.2 (0.8–1.8) (n=21) |
Figure 1.
Probability of remaining free of Deliberate Self-Harm and time since initiating SSRI vs. SNRI antidepressant therapy, by age group (primary analysis, 60 day grace period)
DSH hazard ratios were similar across antidepressant classes, both in younger patents and older patients (Table 3). Indeed, hazard ratios were very close to one in all sensitivity analyses. Hazards ratios also differed little when we varied the grace period from 7 days to 360 days and in first dose carried forward analyses.
Table 3.
Deliberate self-harm hazard ratios (HR) during 1-year follow-up comparing propensity score matched subjects initiating SNRI vs. SSRI* antidepressant therapy, by age group
| Age 10–24 | Age 25–64 | |
|---|---|---|
| Primary analyses (Grace Period=60days) | 1.17 (0.79, 1.73) | 1.18 (0.83, 1.68) |
| Grace period 7 days | 1.30 (0.79, 2.13) | 1.04 (0.67, 1.60) |
| Grace period 14 days | 1.25 (0.79, 1.99) | 1.24 (0.83, 1.86) |
| Grace period 30 days | 1.27 (0.84, 1.94) | 1.16 (0.80, 1.68) |
| Grace period 90 days | 1.17 (0.80, 1.71) | 1.19 (0.84, 1.68) |
| Grace period 180 days | 1.11 (0.77, 1.61) | 1.15 (0.82, 1.61) |
| Grace period 360 days | 1.07 (0.74, 1.55) | 1.17 (0.84, 1.62) |
| First dose carried forward | 1.07 (0.78, 1.46) | 1.04 (0.78, 1.37) |
| Treatment naïve x 3years | 0.86 (0.43, 1.74) | 1.30 (0.58, 2.89) |
The age-group specific SSRI cohort serves as the reference group for the age-group analyses.
4. Discussion
We observed little variation in the risk of DSH between classes of antidepressants among patients aged 10 to 64 years who initiated SNRI or SSRI therapy. Most events occurred in the first 3 months after treatment initiation. Our results are consistent with the findings of most other recent observational studies that reported either no evidence of differential risk across antidepressant classes or agents, or small and inconsistent differences [16–23], as well as with albeit underpowered findings from meta-analyses of randomized controlled trials. [29–31]
In contrast to a case control study of older Ontario residents (aged 66 years and older) [24] we did not observe an increased risk of suicidal acts among SSRI initiators relative to initiators of other antidepressants in our two cohorts, either in the first month of new use or thereafter, nor did we find any effect measure modification by age. The Canadian study reported that SSRIs were associated with a nearly 5-fold increased risk of suicide, compared with other antidepressants, but the risk appeared to be restricted to males and to the first month of treatment only. Our findings are also at odds with those from a large cohort study from the United Kingdom, which found higher risk of suicide and DSH among users of venlafaxine, compared with users of SSRIs and tricyclics. [19] In this UK study, venlafaxine users had a markedly higher burden of suicide risk factors at baseline than did users of SSRIs or tricyclics, including prior use of antidepressants (i.e. the UK study included prevalent users), and adjustment for measured confounders resulted in a substantial reduction in the risk estimates, suggesting, as noted by the authors, that residual confounding may have explained all or much of the observed risk.
Our decision to restrict analyses to new initiators of antidepressants with documented diagnoses of depression confers several advantages over designs that include prevalent users, chief among which are the ability to detect adverse events that follow soon after a drug is started, assess risks over time, and control for selection bias with baseline patient characteristics that are not in uenced by effects of antidepressant treatment. In addition incident user designs also mitigate potential selection bias due to past drug-related history that might affect current treatment assignment. Because we were interested in adverse outcomes, our primary analytic strategy was to censor people at the time of treatment changes. Our decision to censor data for subjects at treatment discontinuation and to use a proportional-hazards analysis adjusts for differences in treatment persistence. By censoring patient follow-up as soon as a patient switched drugs, augmented therapy with a different agent, or changed dose we avoided the problematic comparison of patients who escalate dose or otherwise change treatment in response to adverse effects, refractory depression, or worsening symptoms, any of which might be indications of elevated suicide risk, with patients who do not.
The current study is subject to several potential limitations. First, because we used administrative data our ability to adjust for the severity of psychiatric illness, as well as other potential confounders was more limited than had we used more detailed clinical data. We do, however, adjust for documented psychiatric comorbidity and co-medication, and for a construct that proxies depression severity by specifying whether a patient’s depression diagnosis occurred during an inpatient admission for depression, whether the diagnosis was a primary or secondary diagnosis, and whether the diagnosis occurred within the month prior to their index date or more remotely. Second, we had no direct measure of antidepressant adherence, only of whether and when patients filled their prescriptions. Using prescription data may, however, more accurately measure use than previous studies that relied on data from self-report surveys. Automated pharmacy records are a good source of medication data because these records are not subject to information bias [32–34] and they cover the complete history of drug use, rather than a few time intervals, as is often the case in self-report literature. In addition, automated drug use records have generally been found to have concordance of better than 90% with patient self-reports of medication use. [34] In the case of our null findings, we cannot, however, rule out a small class-related effect that was obscured by such residual misclassification.
Third, we define drug exposure in our primary analysis in a way that seeks to capture how patients fill their medications (i.e. analyses are “as treated”), but in so doing admit possible selection bias due to censoring. [35] That we observed no material differences in DSH risk across antidepressant class both immediately after initiating therapy and in the latter part of the follow-up period suggests that the null finding we observed is unlikely to be entirely attributable to such a censoring-related bias to the null. Fourth, our findings most directly apply to patients 10–64 years of age initiating therapy with either SSRIs or SNRIs. The applicability of our findings to patients 65 years of age and older or to other classes of antidepressant medication is not known. And fifth, although propensity score matching appears to have balanced measured confounders well, it does not guarantee that the SSRI and SNRI cohorts are matched on unmeasured confounders.
5. Conclusions
Our study examines whether the risk of DSH differs across the two most commonly prescribed antidepressant drug classes, not whether prescribing antidepressants for persons with depression is advisable or not. Our findings of similar DSH rates for depressed patients who initiate treatment with either an SSRI or an SNRI suggests that physicians who have decided that their patients would benefit from initiating antidepressant therapy need not weigh differential suicide risk when deciding which to class of antidepressant to prescribe.
Table 1b.
Baseline characteristics of patients with depression, ages 25–64 years, initiating antidepressant therapy before and after propensity score matching on the probability of receiving SNRI therapy
| Pre-Matched Cohort | Propensity Score Matched Cohort | |||
|---|---|---|---|---|
| Characteristic | SSRI N=225,952 |
SNRI N=36,037 |
SSRI N=72,028 |
SNRI N=36,037 |
| Age | ||||
| 25–40 | 93,806 (41.5%) | 13,227 (36.7%) | 26,992 (37.5%) | 13,227 (36.7%) |
| 41–55 | 96,389 (42.7%) | 16,712 (46.4%) | 32,307 (44.9%) | 16,712 (46.4%) |
| 56+ | 35,757 (15.8%) | 6,098 (16.9%) | 12,729 (17.7%) | 6,098 (16.9%) |
| Sex, M | 74,212 (32.8%) | 11,202 (31.1%) | 23,001 (31.9%) | 11,202 (31.1%) |
| Severity Level of Depression Diagnosis | ||||
| T1: Primary Inpatient diagnosis <=30 Days Pre-Index Date | 2,150 (1.0%) | 450 (1.2%) | 928 (1.3%) | 450 (1.2%) |
| T2: Primary Inpatient diagnosis 31–360 Days Pre-Index Date | 406 (0.2%) | 142 (0.4%) | 235 (0.3%) | 142 (0.4%) |
| T3: Non-Primary Inpatient diagnosis <=360 Days Pre-Index Date | 5,518 (2.4%) | 1,064 (3.0%) | 2,016 (2.8%) | 1,064 (3.0%) |
| T4: 2+ Outpatient Diagnosis <=360 Days Pre-Index Date | 116,171 (51.4%) | 20,737 (57.5%) | 37,969 (52.7%) | 20,737 (57.5%) |
| T5: 1 Outpatient Diagnosis <=360 Days Pre-Index Date | 101,707 (45.0%) | 13,644 (37.9%) | 30,880 (42.9%) | 13,644 (37.9%) |
| Anxiety Disorders | 54,993 (24.3%) | 9,402 (26.1%) | 19,517 (27.1%) | 9,402 (26.1%) |
| Baseline DSH | 519 (0.2%) | 93 (0.3%) | 185 (0.3%) | 93 (0.3%) |
| Primary Inpatient Depression Diagnosis | 2,556 (1.1%) | 592 (1.6%) | 1,163 (1.6%) | 592 (1.6%) |
| No Depression Diagnosis w/in 3 0 Days of Index Date | 45,707 (20.2%) | 10,522 (29.2%) | 21,068 (29.2%) | 10,522 (29.2%) |
| Baseline Suicidal Ideation (2006–2010 only) | 1,103 (0.8%) | 218 (0.9%) | 463 (1.0%) | 218 (0.9%) |
| Cognitive Impairment/Dementia | 242 (0.1%) | 47 (0.1%) | 95 (0.1%) | 47 (0.1%) |
| Personality Disorder | 1,269 (0.6%) | 257 (0.7%) | 554 (0.8%) | 257 (0.7%) |
| Substance Abuse | 20,334 (9.0%) | 3,630 (10.1%) | 7,853 (10.9%) | 3,630 (10.1%) |
| Use of Any Opiate | 72,807 (32.2%) | 13,668 (37.9%) | 27,456 (38.1%) | 13,668 (37.9%) |
| Number of Distinct Drug Prescriptions Filled | ||||
| 1 (Antidepressant Only) | 21,005 (9.3%) | 3,229 (9.0%) | 6,213 (8.6%) | 3,229 (9.0%) |
| 2–3 | 51,943 (23.0%) | 7,321 (20.3%) | 14,564 (20.2%) | 7,321 (20.3%) |
| 4–5 | 46,441 (20.6%) | 6,688 (18.6%) | 12,834 (17.8%) | 6,688 (18.6%) |
| 6–9 | 60,524 (26.8%) | 9,556 (26.5%) | 19,454 (27.0%) | 9,556 (26.5%) |
| 10+ | 46,039 (20.4%) | 9,243 (25.6%) | 18,963 (26.3%) | 9,243 (25.6%) |
| Number of Psychiatric Hospitalizations, 1+ | 2,694 (1.2%) | 608 (1.7%) | 1,197 (1.7%) | 608 (1.7%) |
| Number of Outpatient Visits | ||||
| <5 | 39,311 (17.4%) | 4,966 (13.8%) | 9,338 (13.0%) | 4,966 (13.8%) |
| 5–9 | 54,399 (24.1%) | 7,671 (21.3%) | 15,001 (20.8%) | 7,671 (21.3%) |
| 10–19 | 67,688 (30.0%) | 10,581 (29.4%) | 21,639 (30.0%) | 10,581 (29.4%) |
| 20–39 | 46,533 (20.6%) | 8,436 (23.4%) | 17,357 (24.1%) | 8,436 (23.4%) |
| 40+ | 18,021 (8.0%) | 4,383 (12.2%) | 8,693 (12.1%) | 4,383 (12.2%) |
| Number of Hospitalizations for Substance Abuse, 1+ | 1,192 (0.5%) | 241 (0.7%) | 524 (0.7%) | 241 (0.7%) |
| Number of Hospitalizations for Other Reasons, 1+ | 25,038 (11.1%) | 4,055 (11.3%) | 8,511 (11.8%) | 4,055 (11.3%) |
| Colorectal Cancer | 563 (0.2%) | 90 (0.2%) | 213 (0.3%) | 90 (0.2%) |
| Prostate Cancer | 636 (0.3%) | 101 (0.3%) | 224 (0.3%) | 101 (0.3%) |
| Other Malignant Neoplasm Cancer | 7,842 (3.5%) | 1,555 (4.3%) | 3,325 (4.6%) | 1,555 (4.3%) |
| Congestive Heart Failure | 2,477 (1.1%) | 449 (1.2%) | 938 (1.3%) | 449 (1.2%) |
| Arthritis | 2,110 (0.9%) | 576 (1.6%) | 1,143 (1.6%) | 576 (1.6%) |
| Cerebrovascular Disease | 4,777 (2.1%) | 875 (2.4%) | 1,838 (2.6%) | 875 (2.4%) |
| Cluster Headaches/Migraines | 10,188 (4.5%) | 2,256 (6.3%) | 4,642 (6.4%) | 2,256 (6.3%) |
| Diabetes | 17,361 (7.7%) | 3,235 (9.0%) | 6,737 (9.4%) | 3,235 (9.0%) |
| Disorders of the Eye | 285 (0.1%) | 49 (0.1%) | 105 (0.1%) | 49 (0.1%) |
| Gait or Balance Disorder | 2,426 (1.1%) | 522 (1.4%) | 1,065 (1.5%) | 522 (1.4%) |
| Hyperparathyroidism | 325 (0.1%) | 80 (0.2%) | 159 (0.2%) | 80 (0.2%) |
| Osteoarthritis | 14,325 (6.3%) | 3,217 (8.9%) | 6,528 (9.1%) | 3,217 (8.9%) |
| Osteoporosis | 3,776 (1.7%) | 863 (2.4%) | 1,758 (2.4%) | 863 (2.4%) |
| Parkinson’s Disease | 231 (0.1%) | 36 (0.1%) | 72 (0.1%) | 36 (0.1%) |
| Seizures | 1,641 (0.7%) | 302 (0.8%) | 686 (1.0%) | 302 (0.8%) |
| Urinary Incontinence | 2,421 (1.1%) | 547 (1.5%) | 1,152 (1.6%) | 547 (1.5%) |
Acknowledgments
Drs Miller, Azrael, Pate, White, Stürmer and Ms Swanson have no conflicts of interest to report. Drs Miller, Azrael, Stürmer and Pate received support for this work from an investigator initiated research grant from the National Institute of Mental Health (PI Miller; RO1MH085021). Dr. Til Stürmer receives investigator-initiated research funding and support as Principal Investigator (R01 AG023178) from the National Institute on Aging at the National Institutes of Health. He also receives research funding as Principal Investigator of the UNC-DEcIDE center from the Agency for Healthcare Research and Quality. Dr. Stürmer does not accept personal compensation of any kind from any pharmaceutical company, though he receives salary support from the Center for Pharmacoepidemiology and from unrestricted research grants from pharmaceutical companies (GlaxoSmithKline, Merck, Sanofi) to the department of epidemiology, University of North Carolina at Chapel Hill. Dr White owns stock in GlaxoSmithKline. The statements, findings, conclusions, views, and opinions contained and expressed in this article are based in part on data obtained under license from the following IMS Health Incorporated information service(s): LifeLink® Information Assets-Health Plan Claims Database (1997–2010), IMS Health Incorporated. All Rights Reserved. The statements, findings, conclusions, views, and opinions contained and expressed herein are not necessarily those of IMS Health Incorporated or any of its affiliated or subsidiary entities.
References
- 1.Andy Mosholder briefing document. [Accessed 11/11/12.];Food and Drug Administration Psychopharmacologic Drugs Advisory Committee Web site. Available at: http://www.fda.gov/ohrms/dockets/ac/04/transcripts/4006T1.pdf.
- 2.Hammad TA, Laughren T, Racoosin J. Suicidality in pediatric patients treated with antidepressant drugs. Arch Gen Psychiatry. 2006;63(3):332–9. doi: 10.1001/archpsyc.63.3.332. [DOI] [PubMed] [Google Scholar]
- 3.Stone M, et al. Risk of suicidality in clinical trials of antidepressants in adults: analysis of proprietary data submitted to US Food and Drug Administration. BMJ. 2009;339:b2880. doi: 10.1136/bmj.b2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baldassano CF, et al. Akathisia: a review and case report following paroxetine treatment. Compr Psychiatry. 1996;37(2):122–4. doi: 10.1016/s0010-440x(96)90572-6. [DOI] [PubMed] [Google Scholar]
- 5.Hansen L, Wilkinson DG. Drug induced akathisia, suicidal ideation and its treatment in the elderly. Int J Geriatr Psychiatry. 2001;16(2):231–3. doi: 10.1002/1099-1166(200102)16:2<231::aid-gps303>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 6.Healy D, Whitaker C. Antidepressants and suicide: risk-benefit conundrums. J Psychiatry Neurosci. 2003;28(5):331–7. [PMC free article] [PubMed] [Google Scholar]
- 7.Kasantikul D. Drug-induced akathisia and suicidal tendencies in psychotic patients. J Med Assoc Thai. 1998;81(7):551–4. [PubMed] [Google Scholar]
- 8.Lipinski JF, Jr, et al. Fluoxetine-induced akathisia: clinical and theoretical implications. J Clin Psychiatry. 1989;50(9):339–42. [PubMed] [Google Scholar]
- 9.Power AC, Cowen PJ. Fluoxetine and suicidal behaviour. Some clinical and theoretical aspects of a controversy. Br J Psychiatry. 1992;161:735–41. doi: 10.1192/bjp.161.6.735. [DOI] [PubMed] [Google Scholar]
- 10.Teicher MH, Glod CA, Cole JO. Antidepressant drugs and the emergence of suicidal tendencies. Drug Saf. 1993;8(3):186–212. doi: 10.2165/00002018-199308030-00002. [DOI] [PubMed] [Google Scholar]
- 11.Hamilton MS, Opler LA. Akathisia, suicidality, and fluoxetine. J Clin Psychiatry. 1992;53(11):401–6. [PubMed] [Google Scholar]
- 12.Pfeiffer PN, et al. Comorbid anxiety as a suicide risk factor among depressed veterans. Depress Anxiety. 2009;26(8):752–7. doi: 10.1002/da.20583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wojnar M, et al. Sleep problems and suicidality in the National Comorbidity Survey Replication. J Psychiatr Res. 2009;43(5):526–31. doi: 10.1016/j.jpsychires.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith EG. Association between antidepressant half-life and the risk of suicidal ideation or behavior among children and adolescents: confirmatory analysis and research implications. J Affect Disord. 2009;114(1–3):143–8. doi: 10.1016/j.jad.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 15.Weiss JJ, Gorman JM. Antidepressant adherence and suicide risk in depressed youth. Am J Psychiatry. 2005;162(9):1756–7. doi: 10.1176/appi.ajp.162.9.1756-a. [DOI] [PubMed] [Google Scholar]
- 16.Jick H, Kaye JA, Jick SS. Antidepressants and the risk of suicidal behaviors. JAMA. 2004;292(3):338–43. doi: 10.1001/jama.292.3.338. [DOI] [PubMed] [Google Scholar]
- 17.Martinez C, et al. Use of venlafaxine compared with other antidepressants and the risk of sudden cardiac death or near death: a nested case-control study. BMJ. 2010;340:c249. doi: 10.1136/bmj.c249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martinez C, et al. Antidepressant treatment and the risk of fatal and non-fatal self harm in first episode depression: nested case-control study. BMJ. 2005;330(7488):389. doi: 10.1136/bmj.330.7488.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rubino A, et al. Risk of suicide during treatment with venlafaxine, citalopram, fluoxetine, and dothiepin: retrospective cohort study. BMJ. 2007;334(7587):242. doi: 10.1136/bmj.39041.445104.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schneeweiss S, et al. Comparative safety of antidepressant agents for children and adolescents regarding suicidal acts. Pediatrics. 2010;125(5):876–88. doi: 10.1542/peds.2009-2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schneeweiss S, et al. Variation in the risk of suicide attempts and completed suicides by antidepressant agent in adults: a propensity score-adjusted analysis of 9 years’ data. Arch Gen Psychiatry. 2010;67(5):497–506. doi: 10.1001/archgenpsychiatry.2010.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Valenstein M, et al. Antidepressant agents and suicide death among US Department of Veterans Affairs patients in depression treatment. J Clin Psychopharmacol. 2012;32(3):346–53. doi: 10.1097/JCP.0b013e3182539f11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Valuck RJ, et al. Antidepressant treatment and risk of suicide attempt by adolescents with major depressive disorder: a propensity-adjusted retrospective cohort study. CNS Drugs. 2004;18(15):1119–32. doi: 10.2165/00023210-200418150-00006. [DOI] [PubMed] [Google Scholar]
- 24.Juurlink DN, et al. The risk of suicide with selective serotonin reuptake inhibitors in the elderly. Am J Psychiatry. 2006;163(5):813–21. doi: 10.1176/ajp.2006.163.5.813. [DOI] [PubMed] [Google Scholar]
- 25.Cipriani A, et al. Comparative efficacy and acceptability of 12 new-generation antidepressants: a multiple-treatments meta-analysis. Lancet. 2009;373(9665):746–58. doi: 10.1016/S0140-6736(09)60046-5. [DOI] [PubMed] [Google Scholar]
- 26.Cipriani A, et al. Metareview on short-term effectiveness and safety of antidepressants for depression: an evidence-based approach to inform clinical practice. Can J Psychiatry. 2007;52(9):553–62. doi: 10.1177/070674370705200903. [DOI] [PubMed] [Google Scholar]
- 27.Freiman JA, et al. The importance of beta, the type II error and sample size in the design and interpretation of the randomized control trial. Survey of 71 “negative” trials. N Engl J Med. 1978;299(13):690–4. doi: 10.1056/NEJM197809282991304. [DOI] [PubMed] [Google Scholar]
- 28.Parsons L. [Accessed October 1, 2012.];Reducing bias in a propensity score matched-pair sample using greedy matching techniques. 2001 http://www2.sas.com/proceedings/sugi26/p214-26.pdf.
- 29.Mosholder AD, Willy M. Suicidal adverse events in pediatric randomized, controlled clinical trials of antidepressant drugs are associated with active drug treatment: a meta-analysis. J Child Adolesc Psychopharmacol. 2006;16(1–2):25–32. doi: 10.1089/cap.2006.16.25. [DOI] [PubMed] [Google Scholar]
- 30.Dubicka B, Hadley S, Roberts C. Suicidal behaviour in youths with depression treated with new-generation antidepressants: meta-analysis. Br J Psychiatry. 2006;189:393–8. doi: 10.1192/bjp.bp.105.011833. [DOI] [PubMed] [Google Scholar]
- 31.Hetrick S, et al. Selective serotonin reuptake inhibitors (SSRIs) for depressive disorders in children and adolescents. Cochrane Database Syst Rev. 2007;(3):CD004851. doi: 10.1002/14651858.CD004851.pub2. [DOI] [PubMed] [Google Scholar]
- 32.Piper JM, Ray WA, Griffin MR. Effects of Medicaid eligibility expansion on prenatal care and pregnancy outcome in Tennessee. JAMA. 1990;264(17):2219–23. [PubMed] [Google Scholar]
- 33.Strom BL, Carson JL. Use of automated databases for pharmacoepidemiology research. Epidemiol Rev. 1990;12:87–107. doi: 10.1093/oxfordjournals.epirev.a036064. [DOI] [PubMed] [Google Scholar]
- 34.West SL, et al. Recall accuracy for prescription medications: self-report compared with database information. Am J Epidemiol. 1995;142(10):1103–12. doi: 10.1093/oxfordjournals.aje.a117563. [DOI] [PubMed] [Google Scholar]
- 35.Suissa S. Immeasurable time bias in observational studies of drug effects on mortality. Am J Epidemiol. 2008;168(3):329–35. doi: 10.1093/aje/kwn135. [DOI] [PubMed] [Google Scholar]