Abstract
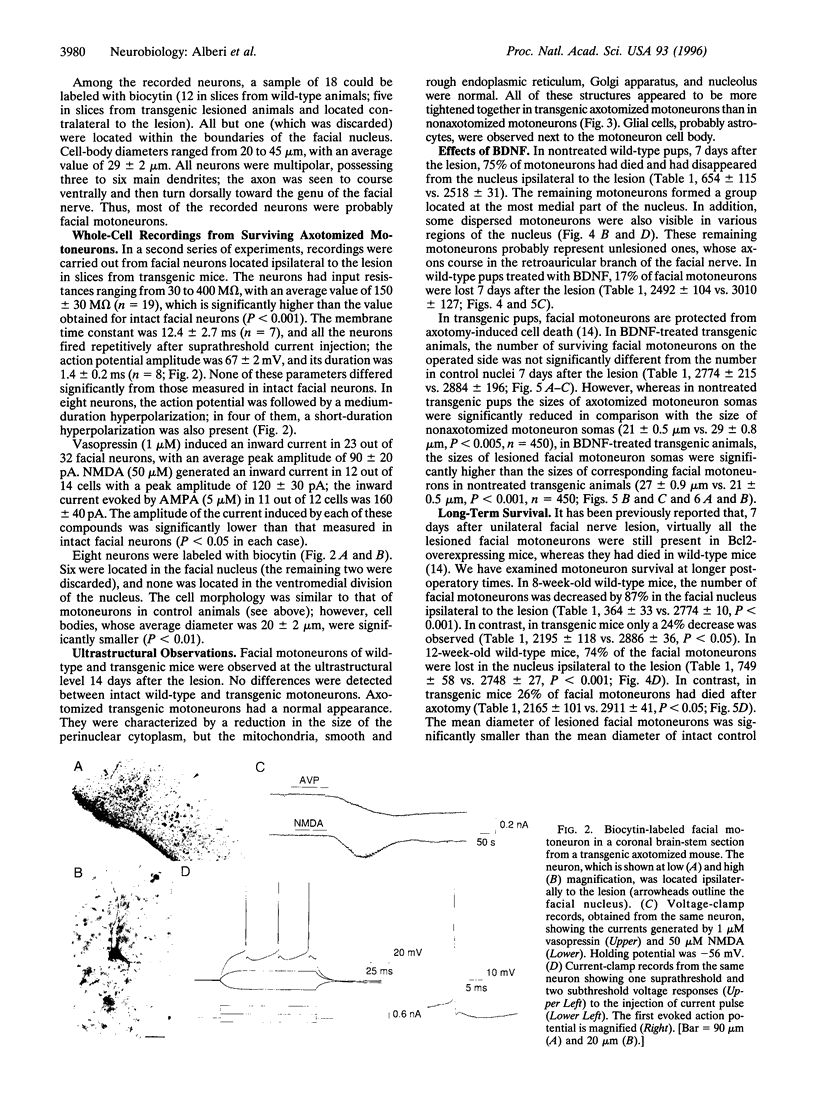
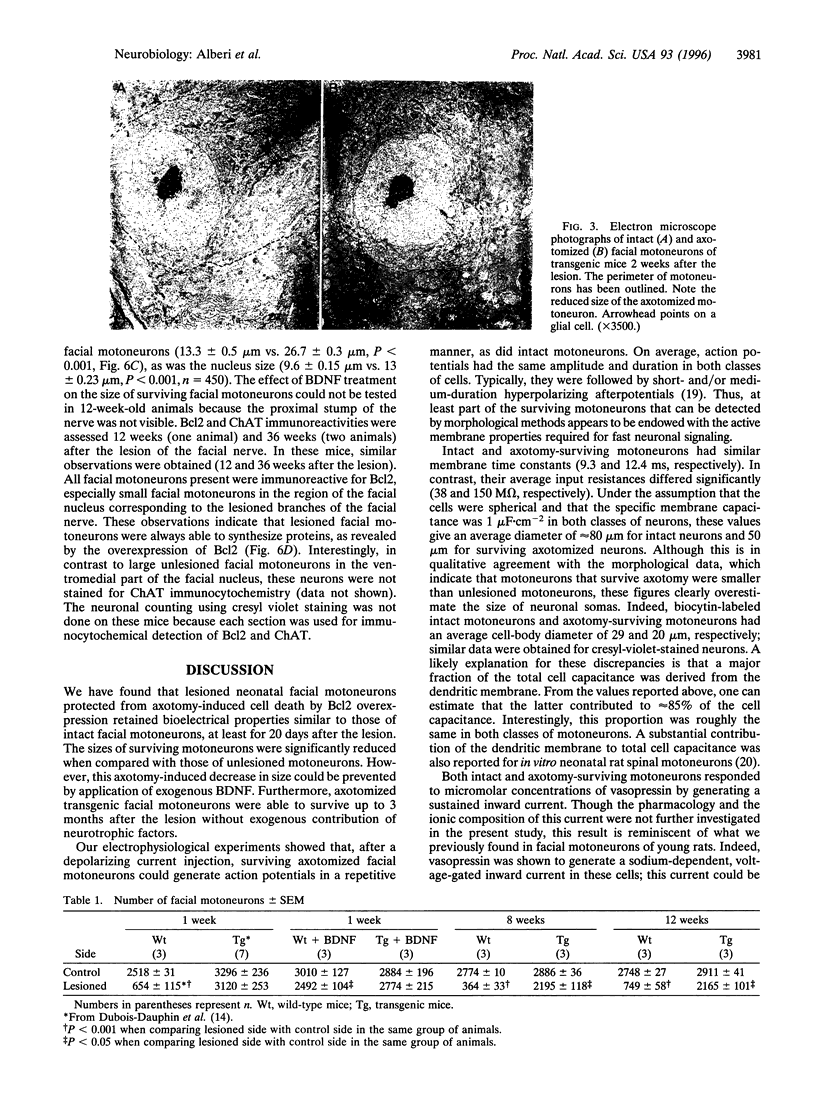
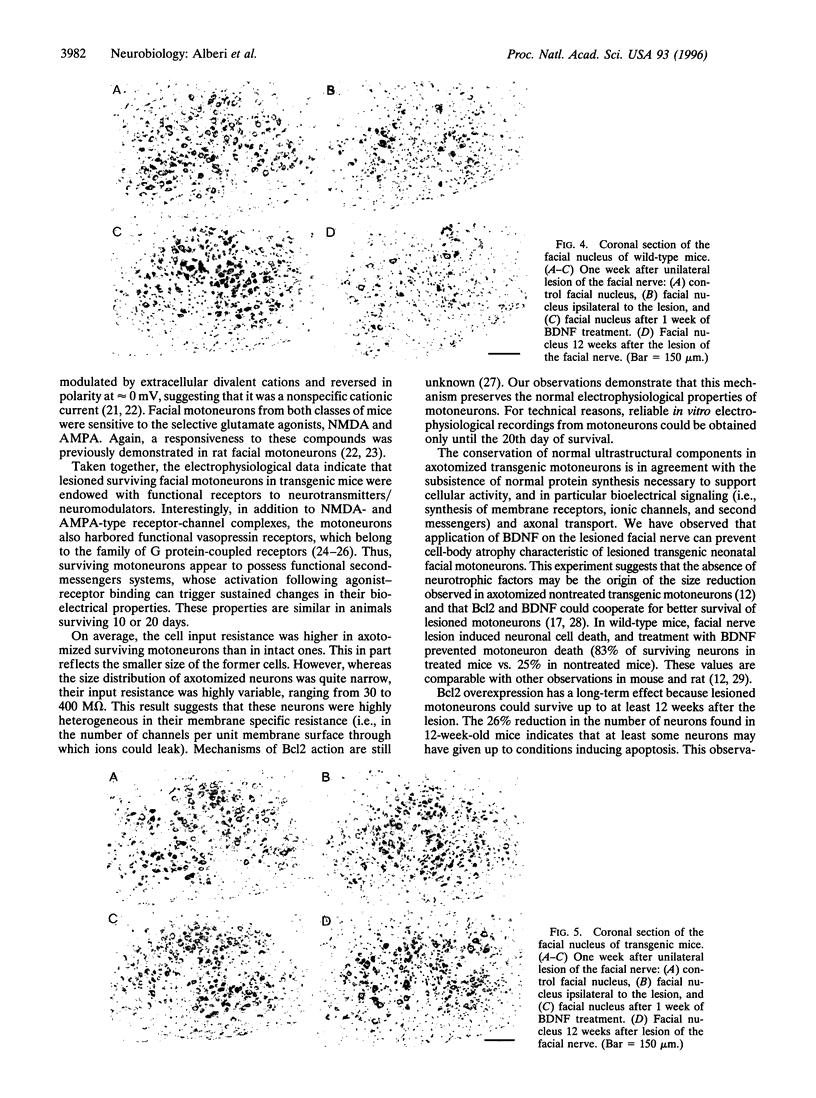
Bcl2 overexpression prevents axotomy-induced neuronal death of neonatal facial motoneurons, as defined by morphological criteria. However, the functional properties of these surviving lesioned transgenic neurons are unknown. Using transgenic mice overexpressing the protein Bcl2, we have investigated the bioelectrical properties of transgenic facial motoneurons from 7 to 20 days after neonatal unilateral axotomy using brain-stem slices and whole cell patch-clamp recording. Nonaxotomized facial motoneurons from wild-type and transgenic mice had similar properties; they had an input resistance of 38 +/- 6 M omega and fired repetitively after injection of positive current pulses. When cells were voltage-clamped at or near their resting membrane potential, alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA), N-methyl-D-aspartic acid (NMDA), or vasopressin generated sustained inward currents. In transgenic axotomized mice, facial motoneurons could be found located ipsilaterally to the lesion; they had an input resistance of 150 +/- 30 M omega, indicating that they were smaller in size, fired repetitively, and were also responsive to AMPA, NMDA, and vasopressin. Morphological measurements achieved 1 week after the lesion have shown that application of brain-derived neurotrophic factor prevented the reduction in size of axotomized transgenic motoneurons. These data indicate that Bcl2 not only prevents morphological apoptotic death of axotomized neonatal transgenic motoneurons but also permits motoneurons to conserve functional electrophysiological properties.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberi S., Dubois-Dauphin M., Dreifuss J. J., Raggenbass M. Modulation by divalent cations of the current generated by vasopressin in facial motoneurons. Brain Res. 1993 Oct 8;624(1-2):326–330. doi: 10.1016/0006-8993(93)90097-7. [DOI] [PubMed] [Google Scholar]
- Allsopp T. E., Kiselev S., Wyatt S., Davies A. M. Role of Bcl-2 in the brain-derived neurotrophic factor survival response. Eur J Neurosci. 1995 Jun 1;7(6):1266–1272. doi: 10.1111/j.1460-9568.1995.tb01116.x. [DOI] [PubMed] [Google Scholar]
- Allsopp T. E., Wyatt S., Paterson H. F., Davies A. M. The proto-oncogene bcl-2 can selectively rescue neurotrophic factor-dependent neurons from apoptosis. Cell. 1993 Apr 23;73(2):295–307. doi: 10.1016/0092-8674(93)90230-n. [DOI] [PubMed] [Google Scholar]
- Blanton M. G., Lo Turco J. J., Kriegstein A. R. Whole cell recording from neurons in slices of reptilian and mammalian cerebral cortex. J Neurosci Methods. 1989 Dec;30(3):203–210. doi: 10.1016/0165-0270(89)90131-3. [DOI] [PubMed] [Google Scholar]
- Clatterbuck R. E., Price D. L., Koliatsos V. E. Further characterization of the effects of brain-derived neurotrophic factor and ciliary neurotrophic factor on axotomized neonatal and adult mammalian motor neurons. J Comp Neurol. 1994 Apr 1;342(1):45–56. doi: 10.1002/cne.903420106. [DOI] [PubMed] [Google Scholar]
- Craig R. W. The bcl-2 gene family. Semin Cancer Biol. 1995 Feb;6(1):35–43. doi: 10.1006/scbi.1995.0005. [DOI] [PubMed] [Google Scholar]
- Davies A. M. The Bcl-2 family of proteins, and the regulation of neuronal survival. Trends Neurosci. 1995 Aug;18(8):355–358. doi: 10.1016/0166-2236(95)93928-q. [DOI] [PubMed] [Google Scholar]
- Dubois-Dauphin M., Frankowski H., Tsujimoto Y., Huarte J., Martinou J. C. Neonatal motoneurons overexpressing the bcl-2 protooncogene in transgenic mice are protected from axotomy-induced cell death. Proc Natl Acad Sci U S A. 1994 Apr 12;91(8):3309–3313. doi: 10.1073/pnas.91.8.3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois-Dauphin M., Raggenbass M., Widmer H., Tribollet E., Dreifuss J. J. Morphological and electrophysiological evidence for postsynaptic localization of functional oxytocin receptors in the rat dorsal motor nucleus of the vagus nerve. Brain Res. 1992 Mar 13;575(1):124–131. doi: 10.1016/0006-8993(92)90431-8. [DOI] [PubMed] [Google Scholar]
- Farlie P. G., Dringen R., Rees S. M., Kannourakis G., Bernard O. bcl-2 transgene expression can protect neurons against developmental and induced cell death. Proc Natl Acad Sci U S A. 1995 May 9;92(10):4397–4401. doi: 10.1073/pnas.92.10.4397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-García M., García I., Ding L., O'Shea S., Boise L. H., Thompson C. B., Núez G. bcl-x is expressed in embryonic and postnatal neural tissues and functions to prevent neuronal cell death. Proc Natl Acad Sci U S A. 1995 May 9;92(10):4304–4308. doi: 10.1073/pnas.92.10.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield S. A. Cell death in Parkinson's disease. Essays Biochem. 1992;27:103–118. [PubMed] [Google Scholar]
- Hirasawa A., Shibata K., Kotosai K., Tsujimoto G. Cloning, functional expression and tissue distribution of human cDNA for the vascular-type vasopressin receptor. Biochem Biophys Res Commun. 1994 Aug 30;203(1):72–79. doi: 10.1006/bbrc.1994.2150. [DOI] [PubMed] [Google Scholar]
- Javoy-Agid F., Ruberg M., Taquet H., Bokobza B., Agid Y., Gaspar P., Berger B., N'Guyen-Legros J., Alvarez C., Gray F. Biochemical neuropathology of Parkinson's disease. Adv Neurol. 1984;40:189–198. [PubMed] [Google Scholar]
- Li L., Oppenheim R. W., Lei M., Houenou L. J. Neurotrophic agents prevent motoneuron death following sciatic nerve section in the neonatal mouse. J Neurobiol. 1994 Jul;25(7):759–766. doi: 10.1002/neu.480250702. [DOI] [PubMed] [Google Scholar]
- Lo A. C., Houenou L. J., Oppenheim R. W. Apoptosis in the nervous system: morphological features, methods, pathology, and prevention. Arch Histol Cytol. 1995 Jun;58(2):139–149. doi: 10.1679/aohc.58.139. [DOI] [PubMed] [Google Scholar]
- Mah S. P., Zhong L. T., Liu Y., Roghani A., Edwards R. H., Bredesen D. E. The protooncogene bcl-2 inhibits apoptosis in PC12 cells. J Neurochem. 1993 Mar;60(3):1183–1186. doi: 10.1111/j.1471-4159.1993.tb03275.x. [DOI] [PubMed] [Google Scholar]
- Meyer M., Matsuoka I., Wetmore C., Olson L., Thoenen H. Enhanced synthesis of brain-derived neurotrophic factor in the lesioned peripheral nerve: different mechanisms are responsible for the regulation of BDNF and NGF mRNA. J Cell Biol. 1992 Oct;119(1):45–54. doi: 10.1083/jcb.119.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel A., O'Carroll A. M., Brownstein M. J., Lolait S. J. Molecular cloning and expression of a rat V1a arginine vasopressin receptor. Nature. 1992 Apr 9;356(6369):523–526. doi: 10.1038/356523a0. [DOI] [PubMed] [Google Scholar]
- Nishimura Y., Schwindt P. C., Crill W. E. Electrical properties of facial motoneurons in brainstem slices from guinea pig. Brain Res. 1989 Nov 13;502(1):127–142. doi: 10.1016/0006-8993(89)90468-x. [DOI] [PubMed] [Google Scholar]
- Oppenheim R. W. Cell death during development of the nervous system. Annu Rev Neurosci. 1991;14:453–501. doi: 10.1146/annurev.ne.14.030191.002321. [DOI] [PubMed] [Google Scholar]
- Pollin M. M., McHanwell S., Slater C. R. The effect of age on motor neurone death following axotomy in the mouse. Development. 1991 May;112(1):83–89. doi: 10.1242/dev.112.1.83. [DOI] [PubMed] [Google Scholar]
- Price D. L. New perspectives on Alzheimer's disease. Annu Rev Neurosci. 1986;9:489–512. doi: 10.1146/annurev.ne.09.030186.002421. [DOI] [PubMed] [Google Scholar]
- Raggenbass M., Goumaz M., Sermasi E., Tribollet E., Dreifuss J. J. Vasopressin generates a persistent voltage-dependent sodium current in a mammalian motoneuron. J Neurosci. 1991 Jun;11(6):1609–1616. doi: 10.1523/JNEUROSCI.11-06-01609.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sendtner M., Dittrich F., Hughes R. A., Thoenen H. Actions of CNTF and neurotrophins on degenerating motoneurons: preclinical studies and clinical implications. J Neurol Sci. 1994 Jul;124 (Suppl):77–83. doi: 10.1016/0022-510x(94)90187-2. [DOI] [PubMed] [Google Scholar]
- Sendtner M., Holtmann B., Kolbeck R., Thoenen H., Barde Y. A. Brain-derived neurotrophic factor prevents the death of motoneurons in newborn rats after nerve section. Nature. 1992 Dec 24;360(6406):757–759. doi: 10.1038/360757a0. [DOI] [PubMed] [Google Scholar]
- Smith R. G., Appel S. H. Molecular approaches to amyotrophic lateral sclerosis. Annu Rev Med. 1995;46:133–145. doi: 10.1146/annurev.med.46.1.133. [DOI] [PubMed] [Google Scholar]
- Snider W. D., Elliott J. L., Yan Q. Axotomy-induced neuronal death during development. J Neurobiol. 1992 Nov;23(9):1231–1246. doi: 10.1002/neu.480230913. [DOI] [PubMed] [Google Scholar]
- Takahashi T. Membrane currents in visually identified motoneurones of neonatal rat spinal cord. J Physiol. 1990 Apr;423:27–46. doi: 10.1113/jphysiol.1990.sp018009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibonnier M., Auzan C., Madhun Z., Wilkins P., Berti-Mattera L., Clauser E. Molecular cloning, sequencing, and functional expression of a cDNA encoding the human V1a vasopressin receptor. J Biol Chem. 1994 Feb 4;269(5):3304–3310. [PubMed] [Google Scholar]
- Widmer H., Dreifuss J. J., Raggenbass M. N-methyl-D-aspartate and vasopressin activate distinct voltage-dependent inward currents in facial motoneurones. Brain Res. 1992 Oct 16;593(2):215–220. doi: 10.1016/0006-8993(92)91310-b. [DOI] [PubMed] [Google Scholar]
- Zhong L. T., Sarafian T., Kane D. J., Charles A. C., Mah S. P., Edwards R. H., Bredesen D. E. bcl-2 inhibits death of central neural cells induced by multiple agents. Proc Natl Acad Sci U S A. 1993 May 15;90(10):4533–4537. doi: 10.1073/pnas.90.10.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]