Abstract
Background
We examined maternal depressive symptoms (MDS) as longitudinal predictors of actigraphy-measured sleep; children's respiratory sinus arrhythmia (RSA) was tested as a moderator of these relations.
Method
271 children (145 boys and 126 girls) participated in a three-wave study (M age at T1 = 9.38 years), with a one-year lag between waves. Children wore actigraphs to derive sleep parameters. RSA reactivity was assessed during a social stress test.
Results
Contrary to hypotheses, MDS were related to less sleep over time for children exhibiting greater RSA withdrawal. Consistent with hypotheses, MDS were related longitudinally to decreased sleep activity for children exhibiting less RSA withdrawal.
Conclusions
Findings illustrate the importance of maternal influences and physiological regulation as predictors of children's sleep.
Keywords: Maternal Depression, Sleep, Autonomic, Parasympathetic, Children
INTRODUCTION
Maternal depressive symptoms (MDS) may promote child sleep problems (El-Sheikh, Kelly, Bagley, & Wetter, 2012; Seifer, 2011). Insufficient and poor quality sleep are prevalent in children and are associated with mental health problems (Astill, Van der Heijden, Ijzendoorn, & Van Someren, 2012). Identification of family variables that can impact this important bioregulatory system is warranted (El-Sheikh, 2011). This investigation examines longitudinal relations between MDS and objective measures of child sleep and considers parasympathetic nervous system (PNS) reactivity indexed by respiratory sinus arrhythmia (RSA) as a moderator of associations. Herein, the term sleep problems refers to shorter duration and worse quality sleep relative to children in the sample.
MDS are associated with parent reports of sleep problems in infants and preschoolers (Seifer, 2011; Warren, Howe, Simmens, & Dahl, 2006), but there has been little research on older children or using objective sleep measures. In one of the few exceptions, El-Sheikh and colleagues (2012) examined actigraphically measured sleep and found that family conflict mediated the association between MDS and children's (M age = 9.44 years) sleep duration and quality. That study used the T1 sample of the current study, which uses a larger data set now available from three waves. The use of actigraphy in this investigation avoids potential biases for negative perceptions by depressed mothers (Grills & Ollendick, 2002).
Individual differences in PNS activity modulate the degree of internalizing and externalizing problems among children exposed to family adversity (El-Sheikh & Erath, 2011). The vulnerability or protective functions of PNS activity may extend to children's sleep outcomes. Activation of the PNS—the vagal brake—reduces heart rate and supports emotion regulation and social engagement (Porges, 2007). Flexible withdrawal of PNS influence (vagal withdrawal) in the context of stress results in a rapid yet moderate increase in heart rate, enabling engaged and well-regulated responses to environmental demands. Greater vagal withdrawal has been linked with fewer child-reported sleep problems and higher sleep duration and sleep quality (El-Sheikh & Buckhalt, 2005; Elmore-Staton, El-Sheikh, Vaughn, & Arsiwalla, 2012). Greater vagal withdrawal, indexed by respiratory sinus arrhythmia reactivity (RSA-R), is also related to fewer externalizing, internalizing, and cognitive problems in childhood (Graziano & Derefinko, 2013).
Respiratory sinus arrhythmia (RSA) refers to variability in heart rate across the breathing cycle and serves as a valid marker of vagal output to the heart (Berntson, Cacioppo, & Grossman, 2007). Reduced RSA in response to stress or challenge is an index of vagal withdrawal. In the present study, lower (i.e., more negative) RSA-R scores indicate greater vagal withdrawal (i.e., greater RSA withdrawal). Children with lower RSA withdrawal may have poorer sleep, particularly in the context of MDS due to their less adaptive responses to stress; in contrast, children with greater RSA withdrawal may exhibit relatively good sleep even in the context of MDS.
We examined longitudinal associations between MDS and actigraphy-based sleep parameters (duration and quality). Sleep duration and quality are differentially related to developmental outcomes (Dewald, Meijer, Oort, Kerkhof, & Bogels, 2010) and thus we examined these sleep parameters separately. We examined associations between MDS and sleep in older children and young adolescents since the influence of family stress on child sleep may increase across child development (Weinraub et al., 2012). Bidirectional associations were also examined because children's sleep may affect MDS (Teti & Crosby, 2012). We expected that MDS would predict children's increased sleep problems over time; expectations regarding the converse direction were tentative given the paucity of research with older children. Finally, we expected that greater RSA withdrawal would ameliorate the negative effects of MDS on child sleep.
METHOD
Participants
Drawn from the Auburn University Sleep Study (AUSS; 339 families recruited from public schools in the United States), 282 families participated at T1 and an additional 57 families were recruited at T2. Four AUSS families were excluded due to outliers and 64 did not have sleep or maternal depression data. The final analytic sample size was 271 at T1, 264 at T2, and 259 at T3.
Children (8-10 years old) at T1 (2009-2010) with no diagnosed learning disability or sleep disorder were eligible for participation. Data were collected from 2010-2011 at T2 and from 2011-2012 at T3. Children who participated for the first time at T1 vs. T2 did not differ in sex, race, body mass index (BMI), asthma status, sleep, or RSA, but children who participated for the first time at T2 were older, p < .05, and MDS were higher, p < .001.
At T1, the mean age of children was 9.38 years (SD = 8.03 months); 46.5% were female; 37% were African American and the others were European American. Socioeconomic status (SES) was assessed with income-to-needs ratio (U.S. Department of Commerce; www.commerce.gov) (M = 1.61; SD = .97). Children's mean age was 10.39 years (SD = 7.81 months) at T2 and 11.32 (SD = 7.72) at T3.
For the 282 families participating initially at T1, 55 (19.5%) were lost to attrition at any subsequent time point. Of those participating initially at T2 (n = 46 in the analytic sample), 3 (6.5%) were lost to attrition at T3. There were no significant differences between retained and attrited families on any study variable.
Procedure
This study was approved by the institution's review board; informed consent and assent were obtained. Procedures were identical across waves. Actigraphy data were collected during the regular school year, excluding holidays. Children wore the actigraphs on their non-dominant wrists for seven consecutive nights; sleep diaries were used to cross-validate actigraphic assessments (Acebo & Carskadon, 2001). Nights with medication used for an acute illness were excluded.
Children visited the laboratory for physiological assessment after completion of actigraphy (M = 1.67 to 4.03 days across waves). After a three-minute adaptation, RSA was assessed during a three-minute baseline while children were asked to sit quietly. Later, children completed a modified version of the Trier Social Stress Task (Kudielka, Hellhammer, & Kirschbaum, 2007).Children were given three minutes to prepare a speech about something interesting that happened to them in the past year, and were asked to deliver the three-minute speech; RSA during the speech was examined. Children were told that several researchers would view the speech and evaluate it through a one-way mirror. Mothers completed questionnaires. Families were compensated for their participation.
Measures
Maternal depressive symptoms (T1-T3)
Mothers completed the Center for Epidemiologic Studies Depression Scale (CESD; Radloff, 1977), which consists of 20 items rated on a 4-point scale. Reliability in the current sample was excellent (α = .89–.91 across waves). Based on the cut-off score of ≥ 16, 27.1% at T1, 27.5% at T2, and 28.6% at T3 of mothers had potentially clinical levels of depression.
Child depressive symptoms (T2-T3)
Children completed the Children's Depression Inventory (CDI; Kovacs, 1992); α = .81 to .87 across waves. One item assessing suicidal ideation was not administered and two items assessing sleep were removed. Because depressed children may experience sleep problems and maternal depression may confer a greater genetic risk for depression (Goodman et al., 2011), concurrent levels of child depression in relation to sleep problems were controlled at T2 and T3. Based on a cut-off score of 12, 6.8% and 5.5% of children reported potentially clinical levels of depression at T2 and T3, respectively.
RSA (T1-T3)
Data were collected with the MW1000A acquisition system (Mindware Technologies LTD., Gahanna, OH). Electrodes were placed on the child's chest in a modified lead-II configuration. Cardiovascular activity was recorded with an ECG activity amplifier module and disposable pediatric snap ECG electrodes. Spectral analysis of thoracic impedance was used to derive respiration. Data were scored using Mindware analysis software (HRV 3.0.17). Data were reviewed for artifacts and missing or misplaced R-peaks and were edited manually. The natural log of the high-frequency power (.15–.40 Hz) was used to derive RSA (Berntson et al., 1997). Data during the 3-min baseline and during the speech task were averaged to form composite baseline (RSA-B) and stress task scores, respectively. RSA reactivity (RSA-R) was computed by subtracting RSA-B from the stress task RSA, such that lower (i.e., more negative) RSAR scores indicate greater vagal withdrawal. RSA-B was included as a control variable in all models. The percentages of children exhibiting RSA withdrawal at T1, T2, and T3 were 56.9%, 51.5%, and 51.2%, respectively; there were no significant differences in RSA-R across time.
Sleep (T1-T3)
Motion during sleep was monitored in one-minute epochs using zero crossing mode with an Octagonal Basic Motionlogger (Ambulatory Monitoring, Inc., Ardsley, NY). Data were scored using the Sadeh algorithm (Sadeh, Sharkey, & Carskadon, 1994) and ActME software (Action W2, 2002). We examined: (1) Sleep Minutes—from sleep onset to wake time; (2) Sleep Activity—% of epochs with physical activity. Scores are the average across available nights. Children had valid data for most nights (M = 5.62 to 6.13 nights across waves).
Control variables
Child age, race, and gender were controlled. Child height and weight were recorded in the laboratory and used to compute BMI (Must, Dallal, & Dietz, 1991). Mothers reported on child diagnosis of chronic illness and pubertal development (Pubertal Development Scale; Petersen et al., 1988).
Analysis Plan
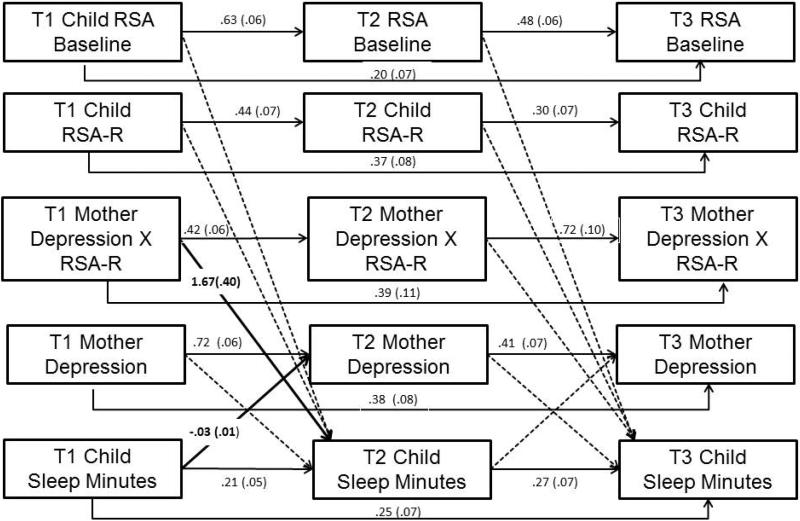
Full information maximum likelihood was used and an autoregressive cross-lag model was fit using AMOS v. 19 in which MDS predicted children's sleep at the next time point and, at the same time, children's sleep predicted MDS. Variables within the same time point were allowed to correlate (Figure 1). Analyses controlled for concurrent child depressive symptoms, providing a conservative test of the relation between MDS and children's sleep problems. Additionally, time-invariant covariates were child age, sex, race, and whether the child had a severe chronic illness (e.g., sickle cell; n = 18) or was diagnosed with asthma (n = 41), and the time-varying covariates were child BMI and puberty status.
Figure 1.
Auto-regression cross-lag model of mothers’ depression symptoms and children's sleep minutes as moderated by children's RSA-R. Note. Covariances among variables within the same time point were estimated, but not shown here. Model included the following (correlated) covariates as predictors of children's sleep at T2 and T3: child age, sex, race, chronic illness, asthma, and time-varying covariates of child depression symptoms, BMI, and puberty. Solid lines denote significant paths (all p < .01), dotted lines denote non-significant paths.
Children's RSA-R to the Trier task, RSA-B, and the interaction between MDS and RSA-R were also included as predictors of children's sleep problems. All predictors were mean-centered before creating the two-way interaction term and significant interactions were probed using an online interaction calculator (Preacher, Curran, & Bauer, 2006).
RESULTS
Table 1 shows descriptive statistics and correlations. Two models tested prediction of children's (a) sleep minutes, and (b) sleep activity.
Table 1.
Means, Standard Deviations, and Inter-correlations among Study Variables
| M | SD | 1. | 2. | 3. | 4. | 5. | 6. | 7. | 8. | 9. | 10. | 11. | 12. | 13. | 14. | 15. | 16. | 17. | 18. | 19. | 20. | 21. | 22. | 23. | 24. | 25. | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. T1 Mother CESD | 10.54 | 8.51 | — | ||||||||||||||||||||||||
| 2. T1 RSA Baseline | 6.94 | 1.05 | .02 | — | |||||||||||||||||||||||
| 3. T1 RSA-R | −.12 | .69 | .01 | −.39** | — | ||||||||||||||||||||||
| 4. T1 Sleep Minutes | 457.29 | 57.90 | −.11 | .11 | −.05 | — | |||||||||||||||||||||
| 5. T1 Sleep Activity | 41.42 | 12.77 | .04 | −.12† | .05 | −.56** | — | ||||||||||||||||||||
| 6. T2 Mother CESD | 12.02 | 9.65 | .59** | .07 | −.03 | −.23** | .12† | — | |||||||||||||||||||
| 7. T2 RSA Baseline | 7.08 | 1.16 | −.10 | .53** | −.07 | .10 | .02 | −.01 | — | ||||||||||||||||||
| 8. T2 RSA-R | −.07 | .81 | .15* | −.01 | .28** | .04 | −.12 | .04 | −.41** | — | |||||||||||||||||
| 9. T2 Sleep Minutes | 444.09 | 50.82 | −.15* | .19** | −.17* | .33** | −.18** | −.09 | .11† | −.08 | — | ||||||||||||||||
| 10. T2 Sleep Activity | 40.60 | 13.52 | .02 | −.11 | .04 | −.11 | .56** | −.07 | −.02 | −.07 | −.48** | — | |||||||||||||||
| 11. T3 Mother CESD | 11.91 | 10.09 | .54** | .02 | .03 | −.08 | .03 | .56** | −.01 | .06 | −.07 | −.004 | — | ||||||||||||||
| 12. T3 RSA Baseline | 6.96 | 1.21 | .08 | .45** | −.10 | .11 | .05 | −.02 | .61** | −.09 | .09 | .06 | .03 | — | |||||||||||||
| 13. T3 RSA-R | −.03 | .88 | −.03 | −.12 | .31** | −.06 | −.10 | .14† | −.24** | .33** | −.06 | −.05 | −.02 | −.49* | — | ||||||||||||
| 14. T3 Sleep Minutes | 435.78 | 59.53 | −.04 | .07 | −.04 | .36** | −.24** | −.05 | .02 | −.07 | .34** | −.16* | −.10 | .12† | −.08 | — | |||||||||||
| 15. T3 Sleep Activity | 39.17 | 13.93 | −.02 | −.07 | −.01 | −.17* | .52** | −.09 | .001 | −.01 | −.15* | .57** | −.04 | −.04 | −.01 | −.57** | — | ||||||||||
| Covariates | |||||||||||||||||||||||||||
| 16. Age (T2; in months) | 124.67 | 7.84 | −.01 | .05 | .01 | −.20** | .08 | −.00 | −.02 | −.06 | −.17** | .02 | .06 | −.10 | .03 | −.09 | −.02 | — | |||||||||
| 17. Child Sex | -- | -- | .01 | .02 | .11 | −.13† | .13† | −.08 | .10 | −.05 | −.10 | .15* | .00 | .14* | .08 | −.12† | .13* | .04 | — | ||||||||
| 18. Race | -- | -- | .09 | .09 | .12† | −.19** | −.12† | .12† | .08 | .14* | −.08 | −.24** | −.07 | .05 | .10 | −.20 | −.11† | −.06 | −.03 | — | |||||||
| 19. Chronic Illness | -- | -- | −.06 | −.09 | .02 | −.01 | −.10 | .01 | −.05 | −.02 | .08 | −.11 | −.06 | −.09 | .14† | .00 | .05 | −.02 | .07 | .18** | — | ||||||
| 20. Asthma Status | -- | -- | −.01 | −.03 | .13† | −.21** | .25** | .04 | −.00 | .17* | .01 | .09 | −.01 | .12† | −.05 | .01 | .03 | .07 | .07 | .10 | .05 | — | |||||
| 21. T2 BMI | 20.22 | 5.26 | .08 | −.20** | .07 | −.24** | .02 | .08 | −.13† | .11 | −.27** | .04 | .13† | −.17* | .03 | −.28** | .12† | .09 | −.01 | .12† | −.02 | .05 | — | ||||
| 22. T2 Puberty Status | 1.74 | .54 | −.02 | −.09 | −.04 | −.15* | .02 | .02 | −.05 | .06 | −.04 | −.08 | −.02 | −.09 | −.07 | −.09 | −.03 | .25** | −.42** | .20** | −.00 | .07 | .26** | — | |||
| 23. T2 CDI | 4.10 | 4.49 | .15* | .03 | −.03 | .10 | −.04 | .02 | .01 | .02 | .03 | .07 | .29* | −.02 | .01 | .04 | .06 | .00 | .00 | −.04 | .03 | −.05 | −.05 | −.05 | — | ||
| 24. T3 BMI | 21.66 | 5.56 | .03 | −.15* | .05 | −.19** | .00 | .08 | −.09 | .08 | −.23** | .01 | .14* | −.15* | .01 | −.25** | .09 | .11† | −.02 | .08 | −.03 | .01 | .95** | .29** | −.06 | — | |
| 25. T3 Puberty Status | 2.06 | .63 | .00 | −.04 | −.00 | −.17* | .04 | .06 | −.12† | .12† | −.09 | .00 | .03 | −.17* | .01 | −.09 | −.01 | .28** | −.44** | .13* | .00 | .06 | .24** | .76** | −.02 | .24** | — |
| 26. T3 CDI | 3.92 | 4.28 | −.02 | −.01 | −.05 | .09 | −.01 | −.06 | .01 | .01 | .11 | .03 | .07 | −.00 | −.02 | .02 | .05 | −.02 | −.06 | .02 | .01 | −.11 | −.01 | −.02 | .55** | −.02 | −.01 |
Note. CESD = Center for Epidemiological Studies on Depression; RSA = respiratory sinus arrhythmia; RSA-R = respiratory sinus arrhythmia reactivity to social stress test [change in RSA from baseline]; BMI = body mass index; CDI = Children's Depression Inventory.
p < .10
p < .05
p < .01
***p < .001.
Sleep Minutes
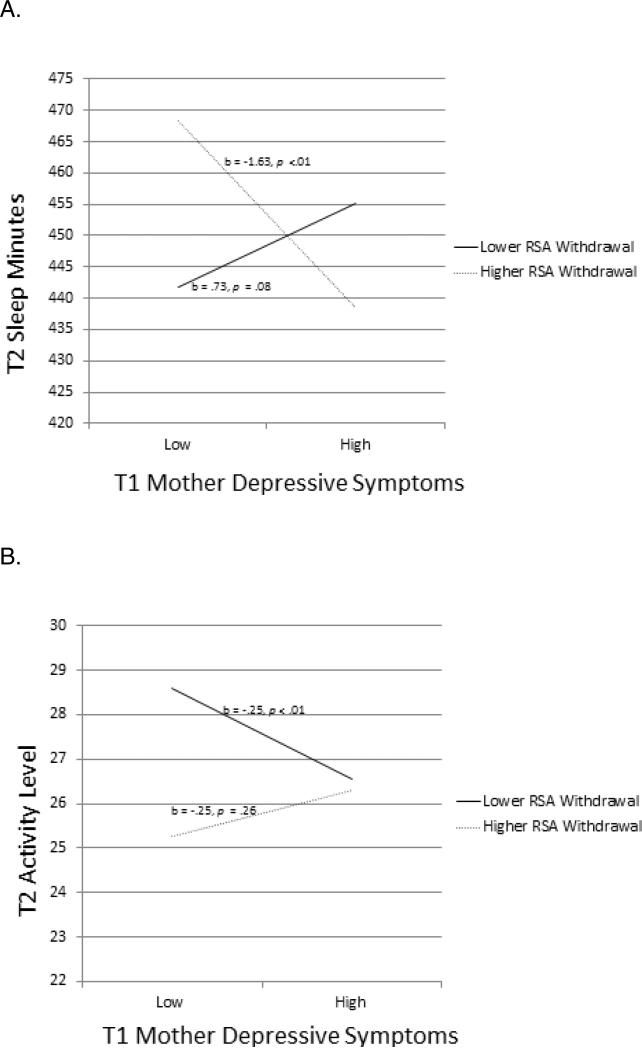
This model (Figure 1) was a good fit to the sample data, χ2(193) = 313.04, p <.001, χ2/df = 1.62, CFI = .93, RMSEA = .05 (90% Confidence Interval [CI]: .042-.06). All autoregressive effects were significant. MDS interacted with T1 RSA-R in predicting T2 sleep minutes (Figure 2A; Simple slopes significant for values of RSA-R lower than −.10 and greater than .76). For children with lower RSA-R, there was no association between T1 MDS and T2 sleep minutes. For children with higher RSA-R, there was a negative association between MDS and sleep minutes. In the context of lower MDS, children exhibiting high levels of RSA-R slept longer (predicted M = 7 hrs and 48 min) over time than those with lower RSA-R (7 hrs and 18 min). Reflective of reciprocal relations, greater sleep minutes at T1 were associated with decreased MDS at T2 (Figure 1).
Figure 2.
Two-way interaction between mothers’ depressive symptoms and child RSA-R at T1 predicting children's (a) sleep minutes and (b) sleep activity at T2. .
Sleep Activity
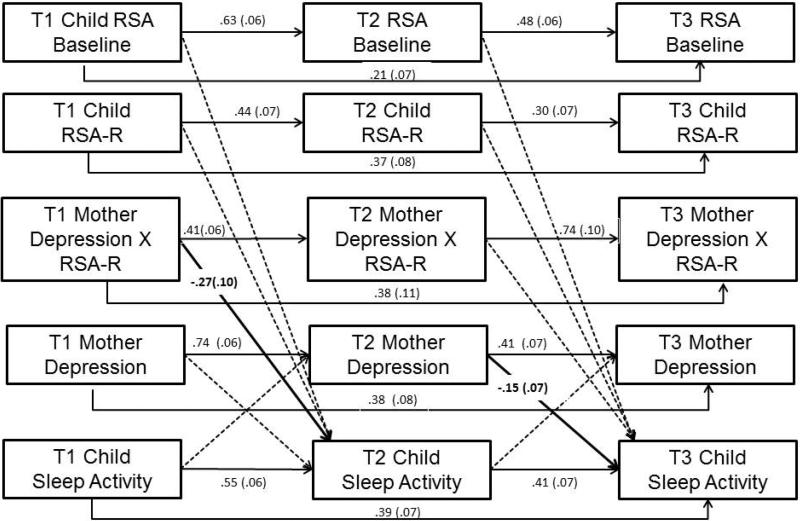
This model (Figure 3) provided a good fit to the data, χ2(193) = 309.11, p < .00, χ2/df = 1.60, CFI = .94, RMSEA = .05 (90% CI: .04-.06). All autoregressive paths were significant. There was a significant interaction between MDS and children's T1 RSA-R predicting T2 sleep activity (Figure 2B; simple slopes were significant for values of RSAR lower than −1.68 and greater than .48). For children with lower RSA-R, T1 MDS predicted decreases in sleep activity over time; no association was found for children with higher RSA-R. However, sleep activity was consistently lower for children with higher RSA-R compared to those with lower RSA-R. Further, MDS at T2 predicted decreases in sleep activity at T3; this relation was not moderated by RSA-R (Figure 3). Sleep activity did not predict MDS.
Figure 3.
Auto-regression cross-lag model of mothers’ depression symptoms and children's sleep minutes as moderated by children's RSA-R. Note. Covariances among variables within the same time point were estimated, but not shown here. Model included the following (correlated) covariates as predictors of children's sleep at T2 and T3: child age, sex, race, chronic illness, asthma, and time-varying covariates of child depression symptoms, BMI, and puberty. Solid lines denote significant paths (all p < .01), dotted lines denote non-significant paths.
Post-hoc Analyses
Relations between MDS, RSA-R, and sleep were significant between the first two time points but not between T2 and T3. This raises the question of whether associations are stronger earlier compared to later in development. A chi-square difference test examined whether the strength of association between T1 MDS and T2 child sleep was the same as compared to this relation one year later (i.e., T2 MDS to T3 sleep). A model where the T1-T2 and T2-T3 paths were freely estimated did not provide a better fit to the data than a model where these two paths were constrained to be equal for either the model with sleep minutes, Δχ2 (1) = 1.45, or the model with sleep activity, Δχ2 (1) = 0.37. Moreover, the moderation effects did not differ for either T1-T2 vs. T2-T3 sleep minutes, Δχ2 (3) = 4.66, or sleep activity, Δχ2 (3) = 4.69.
DISCUSSION
We examined longitudinal associations between MDS and children's sleep problems, with child RSA-R as a moderator of associations. MDS interacted with RSA-R to predict sleep over time, even with conservative models controlling for many potential confounds. Consistent with expectations, there were more sleep minutes among children with higher RSA withdrawal and non-depressed mothers. These results are consistent with an arousal regulation model of sleep (Dahl, 1996), as children with well-regulated responses to normal social stress and without the stress of MDS experienced longer sleep.
However, for children with higher RSA withdrawal, MDS were related to fewer Sleep Minutes, such that children with more depressed mothers and higher RSA withdrawal slept for fewer minutes. Research suggests that normally adaptive physiological responses to stress, such as vagal withdrawal, may predict negative bioregulatory outcomes in the context of chronic high stress. El-Sheikh and Hinnant (2011) cited allostatic load (McEwen & Stellar, 1993) to explain reductions in baseline RSA over time among boys who exhibited RSA withdrawal to stress and were exposed to high or increasing marital conflict. The adaptive role of vagal withdrawal may be diminished when it is repeatedly required in the context of high stress, resulting in maladaptive biological regulation of stress and ultimately sleep problems. MDS is directly linked with lower baseline RSA (Gentzler, Rottenberg, Kovacs, George, & Morey, 2012); this lower baseline RSA combined with further RSA withdrawal may result in hyperarousal (Boyce et al., 2001) and interfere with sleep. Indeed, children's lower baseline RSA in conjunction with greater RSA withdrawal is associated with poorer sleep quality (El-Sheikh, Erath, & Bagley, 2013). Another possibility is that children with higher vagal withdrawal, potentially reflecting greater sensitivity to stress, may be more susceptible to adverse consequences of maternal depression, consistent with the biological sensitivity to context framework (Boyce & Ellis, 2005; Ellis & Boyce, 2008).
Analyses predicting sleep activity also yielded mixed support for hypotheses. Consistent with expectations, children with greater RSA withdrawal demonstrated lower sleep activity than children with lower RSA withdrawal across the range of MDS. Moreover, MDS were not associated with more sleep activity among children with greater RSA withdrawal. In contrast to expectations, MDS predicted lower sleep activity for children with lower RSA withdrawal. MDS may be particularly stressful for children with less adaptive stress responses (i.e., lower RSA withdrawal); lower sleep activity may reflect a biological compensatory mechanism among these children (even if these children sleep for a relatively low or normal number of minutes).
The different patterns of interaction for sleep minutes and sleep activity highlight the need to examine multiple indices of sleep-wake regulation. Sleep minutes indexes sleep amount, while sleep activity measures sleep quality or fragmentation. These different aspects of sleep are not necessarily predicted by the same variables in the same way. Additional longitudinal research will illuminate these effects, including physiological responses that provide protection or increase vulnerability to sleep disruptions. Of course, there are genetic determinants of sleep and biological vulnerabilities (Armitage et al., 2009) and given the frequent association between depressive symptoms and sleep, mothers who are depressed may also have sleep problems and children's sleep problems may be related to shared genes or prenatal influences. Nevertheless, controlling for children's depressive symptoms in analyses strengthens some of the conclusions.
The longitudinal study design permitted examination of bidirectional associations, which indicated that longer child sleep at age 9 predicted decreased MDS when children were age 10. This result is consistent with the literature conducted with infants and younger children (Countermine & Teti, 2010). Even though older children and young adolescents are not as likely to signal their awakening to parents as infants are, it is plausible that such signaling may disrupt mothers’ sleep, which in turn may underlie the observed associations. There are many mechanisms of effects, however, that can connect children's sleep with maternal depression that warrant assessment (e.g., increased agitation and stress, dysfunctional cognitions).
These findings should be interpreted in light of study limitations. Although this is a longitudinal study examining potential bidirectional effects, the study design cannot be used to conclusively determine causality. We note that there were several nonsignificant findings, and that interactions were not consistently significant across time points. Further, the magnitude of relations between study variables may have been attenuated in this community sample and larger effect sizes may be observed in clinical samples of depressed mothers or children with clinically significant sleep problems. It is also possible that the role of RSA-R differs for children in clinical samples. Assessment of paternal depression and children's sleep is also warranted. Children were in late childhood and early adolescence, and most associations were observed in the first part of the study.
Despite these limitations, the present study builds on prior research showing relations between maternal depression and child sleep through examination of objective sleep measures, a longitudinal design, and physiological reactivity as a moderator. Findings consistently show evidence for differential susceptibility to poor sleep based on PNS activity and MDS in late childhood and early adolescence. Sleep plays an important role in brain function, supporting adaptive connectivity and reactivity of brain regions devoted to emotion and emotion regulation (Gujar, McDonald, Nishida, & Walker, 2010) and therefore has important implications for the development of psychiatric problems (Astill et al., 2012).
KEY POINTS.
Longitudinal relations between maternal depression and child sleep duration and quality were examined. Physiological regulation indexed by children's RSA reactivity to a lab stressor was assessed as a moderator of relations.
Maternal depressive symptoms were associated with less sleep minutes for children exhibiting higher RSA withdrawal, and lower sleep activity for children exhibiting lower RSA withdrawal.
Children with less depressed mothers and greater RSA withdrawal exhibited relatively high sleep minutes and relatively low sleep activity.
Sleep problems can be cause, consequence, or symptom of mental health problems and understanding of the development of sleep problems in childhood is critical.
ACKNOWLEDGEMENTS
The project was supported by Grant Number R01HL093246 from the National Heart, Lung, and Blood Institute awarded to Mona El-Sheikh. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. We thank study participants, school personnel, and lab staff.
Footnotes
Conflicts of interest statement: No conflicts declared.
REFERENCES
- Acebo C, Carskadon M. Providence. Brown University; RI: Bradley Sleep Center: 2001. Scoring actigraphs data using ACTION-W2. [Google Scholar]
- Armitage R, Flynn H, Hoffmann R, Vazquez D, Lopez J, Marcus S. Early developmental changes in sleep in infants: The impact of maternal depression. Sleep. 2009;32(5):693–696. doi: 10.1093/sleep/32.5.693. doi: http://www.ncbi.nlm.nih.gov/pubmed/19480236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astill RG, Van der Heijden KB, Van Ijzendoorn MH, Van Someren EJ. Sleep, cognition, and behavioral problems in school-age children: A century of research meta-analyzed. Psychological Bulletin. 2012;138:1109–1138. doi: 10.1037/a0028204. [DOI] [PubMed] [Google Scholar]
- Beauchaine TP. Vagal tone, development, and Gray's motivational theory: Toward an integrated model of autonomic nervous system functioning in psychopathology. Development and Psychopathology. 2001;13:183–214. doi: 10.1017/s0954579401002012. [DOI] [PubMed] [Google Scholar]
- Beauchaine TP. Physiological markers of emotion and behavior dysregulation in externalizing psychopathology. Monographs of the Society for Research in Child Development. 2012;77:79–86. doi: 10.1111/j.1540-5834.2011.00665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berntson GG, Bigger JT, Eckberg DL, Grossman P, Kaufmann PG, Malik M, Nagaraja HN, Porges SW, Saul JP, Stone PH, Van der Molen MW. Heart rate variability: Origins, methods, and interpretive caveats. Psychophysiology. 1997;34:623–648. doi: 10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Cacioppo JT, Grossman P. Whither vagal tone. Biological Psychology. 2007;74:295–300. doi: 10.1016/j.biopsycho.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Boyce WT, Ellis BJ. Biological sensitivity to context: I. An evolutionary-developmental theory of the origins and functions of stress reactivity. Development and Psychopathology. 2005;17:271–301. doi: 10.1017/s0954579405050145. [DOI] [PubMed] [Google Scholar]
- Boyce WT, Quas J, Alkon A, Smider NA, Essex MJ, Kupfer DJ. Autonomic reactivity and psychopathology in middle childhood. The British Journal of Psychiatry. 2001;179:144–150. doi: 10.1192/bjp.179.2.144. [DOI] [PubMed] [Google Scholar]
- Calkins SD, Leerkes EM, Marcovitch S, O'Brien M. Moderate vagal withdrawal in 3.5 year-old children is assocated with optimal performance on executive function tasks. Developmental Psychobiology. 2010;52:603–608. doi: 10.1002/dev.20462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chorney DB, Detweiler MF, Morris TL, Kuhn BR. The interplay of sleep disturbance, anxiety, and depression in children. Journal of Pediatric Psychology. 2008;33:339–348. doi: 10.1093/jpepsy/jsm105. [DOI] [PubMed] [Google Scholar]
- Countermine MS, Teti DM. Sleep arrangements and maternal adaptation in infancy. Infant Mental Health Journal. 2010;31:647–663. doi: 10.1002/imhj.20276. [DOI] [PubMed] [Google Scholar]
- Dahl RE. The regulation of sleep and arousal: Development and psychopathology. Development and Psychopathology. 1996;8:3–27. [Google Scholar]
- Dahl RE, Lewin DS. Pathways to adolescent health sleep regulation and behavior. Journal of Adolescent Health. 2002;31(6 Suppl):175–184. doi: 10.1016/s1054-139x(02)00506-2. doi: http://www.ncbi.nlm.nih.gov/pubmed/12470913. [DOI] [PubMed] [Google Scholar]
- Dewald JF, Meijer AM, Oort FJ, Kerkhof GA, Bogels SM. The influence of sleep quality, sleep duration and sleepiness on school performance in children and adolescents: A meta-analytic review. Sleep Medicine Reviews. 2010;14(3):179–189. doi: 10.1016/j.smrv.2009.10.004. doi:10.1016/j.smrv.2009.10.004. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M. Introduction and overview: Salient issues in the consideration of sleep in context. In: El-Sheikh M, editor. Sleep and Development: Familial and Socio-Cultural Considerations. Oxford University Press; New York: 2011. pp. xi–xvi. [Google Scholar]
- El-Sheikh M, Buckhalt JA. Vagal regulation and emotional intensity predict children's sleep problems. Developmental Psychobiology. 2005;46:307–317. doi: 10.1002/dev.20066. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M, Erath SA. Family conflict, autonomic nervous system functioning, and child adaptation: Sate of the science and future directions. Development and Psychopathology. 2011;23:703–721. doi: 10.1017/S0954579411000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sheikh M, Erath SA, Keller PS. Children's sleep and adjustment: the moderating role of vagal regulation. Journal of Sleep Research. 2007;16:396–405. doi: 10.1111/j.1365-2869.2007.00618.x. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M, Hinnant JB. Marital conflict, respiratory sinus arrhythmia, and allostatic load: Interrelations and associations with the development of children's externalizing behavior. Development and Psychopathology. 2011;23:815–829. doi: 10.1017/S0954579411000320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sheikh M, Kelly RJ, Bagley EJ, Wetter EK. Parental depressive symptoms and children's sleep: The role of family conflict. Journal of Child Psychology and Psychiatry. 2012;53:806–814. doi: 10.1111/j.1469-7610.2012.02530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis BJ, Boyce WT. Biological sensitivity to context. Current Directions in Psychological Science. 2008;17:183–187. [Google Scholar]
- Elmore-Staton L, El-Sheikh M, Vaughn B, Arsiwalla DD. Preschoolers’ daytime respiratory sinus arrhythmia and nighttime sleep. Physiology & Behavior. 2012;107:414–417. doi: 10.1016/j.physbeh.2012.07.005. [DOI] [PubMed] [Google Scholar]
- England MJ, Sim LJ. Depression in parents, parenting, and children: Opportunities to improve identification, treatment, and prevention. The National Academies Press; Washington, DC: 2009. [PubMed] [Google Scholar]
- Gentzler AL, Rottenberg J, Kovacs M, George CJ, Morey JN. Atypical development of resting respiratory sinus arrhythmia in children at high risk for depression. Developmental Psychobiology. 2012;54:556–567. doi: 10.1002/dev.20614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman SH, Rouse MH, Connell AM, Robbins Broth M, Hall CM, Heyward D. Maternal depression and child psychopathology: A meta-analytic review. Clinical Child and Family Psychology Review. 2011;14:1–27. doi: 10.1007/s10567-010-0080-1. [DOI] [PubMed] [Google Scholar]
- Goodman SH, Tully EC. Children of depressed mothers: Implications for the etiology, treatment, and prevention of depression in children and adolescents. In: Abela JRZ, Hankin BL, editors. Handbook of depression in children and adolescents. Guilford; New York: 2008. pp. 415–440. [Google Scholar]
- Grills AE, Ollendick TH. Issues in parent-child agreement: The case of structured diagnostic interviews. Clinical Child and Family Psychology Review. 2002;5:57–83. doi: 10.1023/a:1014573708569. [DOI] [PubMed] [Google Scholar]
- Gujar N, McDonald SA, Nishida M, Walker MP. A role for REM sleep in recalibrating the sensitivity of the human brain to specific emotions. Life Sciences & Medicine, Cerebral Cortex. 2010;21(1) doi: 10.1093/cercor/bhq064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs M. Children's depression inventory. Multi-Health Systems; New York: 1992. [Google Scholar]
- Kudielka BM, Hellhammer DH, Kirschbaum C. Ten years of research with the Trier Social Stress Test&Revisited. In: Harmon Jones E, Winkielman P, editors. Social Neuroscience: Integrating Biological and Psychological Explanations of Social Behavior. Guilford Press; New York: 2007. pp. 56–83. [Google Scholar]
- Lovejoy MC, Graczyk PA, O'Hare E, Neuman G. Maternal depression and parenting behavior: A meta-analytic review. Clinical Psychology Review. 2000;20:561–592. doi: 10.1016/s0272-7358(98)00100-7. [DOI] [PubMed] [Google Scholar]
- Lustberg L, Reynolds CF. Depression and insomnia: Questions of cause and effect. Sleep Medicine Reviews. 2000;4:253–262. doi: 10.1053/smrv.1999.0075. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Stellar E. Stress and the individual: Mechanisms leading to disease. Archives of Internal Medicine. 1993;153:2093–2101. [PubMed] [Google Scholar]
- Must A, Dallal GE, Dietz WH. Reference data for obesity: 85th and 95th percentiles of body mass index(wt/ht^2) and triceps skinfold thickness. American Journal of Clinical Nutrition. 1991;53:839–846. doi: 10.1093/ajcn/53.4.839. [DOI] [PubMed] [Google Scholar]
- Owens JA. The ADHD and sleep conundrum: A review. Journal of Developmental & Behavioral Pediatrics. 2005;26:312–322. doi: 10.1097/00004703-200508000-00011. [DOI] [PubMed] [Google Scholar]
- Petersen A, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: Reliability, validity, and initial norms. Journal of Youth and Adolescence. 1988;17(2):117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- Porges SW. The polyvagal perspective. Biological Psychology. 2007;74:116–143. doi: 10.1016/j.biopsycho.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preacher KJ, Curran P, Bauer DJ. Computational tools for probing interaction effects in multiple linear regression, multilevel modeling, and latent curve analysis. Journal of Educational and Behavioral Statistics. 2006;31:437–448. [Google Scholar]
- Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Sadeh A, Sharkey KM, Carskadon MA. Activity-based sleep-wake identification: An empirical test of methodological issues. Sleep. 1994;17:201–207. doi: 10.1093/sleep/17.3.201. [DOI] [PubMed] [Google Scholar]
- Seifer R. Parental psychopathology and children's sleep. In: El-Sheikh M, editor. Sleep and development: Familial and socio-cultural considerations. Oxford University Press; New York, NY: 2011. pp. 79–98. [Google Scholar]
- Teti DM, Crosby B. Maternal depressive symptoms, dysfunctional cognitions, and infant night waking: The role of maternal nighttime behavior. Child Development. 2012;83:939–953. doi: 10.1111/j.1467-8624.2012.01760.x. doi:10.1111/j.1467-8624.2012.01760.x. [DOI] [PubMed] [Google Scholar]
- Warren SL, Howe G, Simmens SJ, Dahl RE. Maternal depressive symptoms and child sleep: Models of mutual influence over time. Development and Psychopathology. 2006;18:1–16. doi: 10.1017/S0954579406060019. [DOI] [PubMed] [Google Scholar]
- Weinraub M, Bender RH, Friedman SL, Susman EJ, Knoke B, Bradley R, Houts R, Williams J. Patterns of developmental change in infants’ nighttime sleep awakenings from 6 through 36 months of age. Developmental Psychology. 2012;48:1511–1528. doi: 10.1037/a0027680. [DOI] [PubMed] [Google Scholar]