Abstract
Cancer cachexia is the syndrome of weight loss, loss of appetite, and wasting of skeletal muscle and adipose tissue experienced by many individuals with cancer. Currently, few effective treatment and prevention strategies are available for these patients, due in part to a poor understanding of the mechanisms contributing to cachexia. Insulin resistance has been associated with cancer cachexia in epidemiological, human, and animal research. The present experiment was designed to examine the ability of Exendin-4, a GLP-1 agonist and insulin sensitizing agent, to prevent the development of cachexia symptoms in male Sprague Dawley rats bearing the Yoshida sarcoma. Following tumor implantation or sham surgery, rats were treated daily with saline or Exendin-4 (3 μg/kg body weight/day) and were monitored for tumor growth and cachexia symptoms for 21–23 days. As a result of large variability in treatment effects, data were analyzed separately for animals with large and small tumors. Exendin-4 treatment reduced tumor growth and prevented the development of cancer cachexia symptoms in animals with small, but not large, tumors. In addition, insulin levels were preserved in Exendin-4-treated tumor-bearing animals. The results of this experiment demonstrate a novel preventative therapy for cancer cachexia and a novel use of Exendin-4. Further research is necessary to determine the mechanisms through which Exendin-4 exerts these potent effects.
Keywords: Skeletal Muscle Mass, Plasma Insulin Level, Cancer Cachexia, Yoshida Sarcoma, Final Tumor Weight
Introduction
Cancer cachexia, a syndrome of weight loss, loss of appetite, and wasting of body tissues, contributes significantly to both morbidity and mortality in individuals with cancer [10]. Those who experience cachexia have reduced survival time, as well as diminished psychological and physical health [3, 49]. In addition, cachectic cancer patients often display more negative side effects and a decreased therapeutic response during chemotherapy [15, 19, 42]. The majority of individuals with cancer will experience some degree of cachexia during the course of their disease [49, 50], making cancer cachexia a clinically relevant syndrome for which there are currently no effective methods of treatment or prevention.
One potential avenue of cachexia prevention involves a potent mediator of energy balance. Insulin function is often impaired in individuals with and animal models of cancer cachexia [1, 5, 6, 9, 11, 13, 14, 16, 20, 21, 24, 26, 29, 33, 36, 41, 45–48]. Given the ability of insulin dysfunction to result in altered body composition in other animal models of disease [31], there is interest in determining the role that insulin resistance may play in cachexia development [23]. Insulin sensitizing agents have been examined previously, with varying success, in the context of cancer cachexia treatment [11, 12, 30, 34, 37, 38], but few have been examined with the goal of prevention [1].
The majority of insulin release is dependent upon the action of incretin hormones, including GLP-1 [2, 22]. GLP-1 receptors are found throughout the central nervous system and the periphery, including skeletal and smooth muscle, and adipose tissue, and utilize receptor signaling pathways that include INSR, IRS-1, and IRS-2 [2, 17]. GLP-1 agonists, such as Exendin-4, have become attractive therapeutic options for improving insulin sensitivity. Exendin-4 lowers blood glucose levels, increases insulin secretion, improves beta-cell function, and promotes peripheral insulin sensitivity [8, 52]. Exendin-4 increases insulin sensitivity in isolated myotubules and adipocytes [25]. Additionally, this peptide has potent anti-inflammatory effects [4] and has been shown to reduce cell proliferation in a number of cancer cell lines [27, 28, 32]. Exendin-4 has not previously been investigated for the treatment or prevention of cancer cachexia.
Currently, no therapies are effective in the treatment or prevention of cancer cachexia. Given the ability of Exendin-4 to exert insulin sensitizing effects, the present experiment investigated the effects of chronic Exendin-4 treatment on the development of cancer cachexia in rats implanted with the Yoshida sarcoma. It was hypothesized that Exendin-4 treatment would improve insulin sensitivity and prevent the development of cachexia symptoms, while reducing tumor growth.
Materials and Methods
Experimental Animals
Sixty-eight male Sprague Dawley rats (65–69 days old; Harlan Sprague Dawley, Indianapolis, IN, USA) served as experimental subjects. Rats were individually housed in hanging wire mesh cages in a temperature- and humidity-controlled room, with a 12:12 h light/dark cycle (lights on at 04:00). Following arrival in the laboratory, rats were allowed to acclimate to the laboratory environment for 1 week prior to the start of the experiment and received ad libitum access to purified rodent chow (AIN-76A; Dyets Inc., Bethlehem, PA, USA). Throughout the experiment, all rats received ad libitum access to tap water and purified rodent chow. All procedures were approved by the Purdue University Animal Care and Use Committee.
After acclimation to the laboratory environment, rats were then weight-matched (starting body weights 291–355 g) and divided into two experimental groups, which differed in tumor status. The control group (n = 20) received a sham surgery. The tumor-bearing group (TB, n = 48) was subcutaneously implanted with the Yoshida sarcoma. Surgery was performed on experimental day 0.
Tumors and Tumor Implantation
Yoshida sarcoma ascites tumor cells were purchased from the Development Therapeutics Program at the National Cancer Institute (Bethesda, MD, USA). A detailed description of the tumor implantation process has been described previously [24]. Briefly, donor animals were used to perpetuate the tumor line in the laboratory. Following an overdose of sodium pentobarbital, the tumor tissue was quickly dissected from the donor animal, divided into fragments, and placed on ice. The animal receiving the tumor was anesthetized under isoflurane anesthesia, and a tumor fragment (approximately 6 mm3) was subcutaneously implanted on the right flank between the upper and lower limbs. All TB animals received tumor tissue from the same donor animal. Sham surgeries were performed in control animals. Surgeries were performed on experimental day 0.
Tumor volume was determined through measurement of tumor size by external digital caliper, with the longest longitudinal diameter (length, L), greatest transverse diameter (width, W), and the great vertical diameter (height, H) recorded daily. Tumor volume was calculated using a standard ellipsoid formula [V = π/6 × (L)(W)(H)] [51].
Chronic Exendin-4 Treatment
On day 1, animals were further divided into two treatment groups (n = 10, 24 for control saline and TB saline; n = 10, 24 for control Exendin-4 and TB Exendin-4). One group received a daily i.p. injection of Exendin-4 (3 μg/kg body weight; American Peptide Company, Sunnyvale, CA, USA), while the other received a similar volume of sterile, physiological saline. In a pilot experiment, this dose of Exendin-4 was found to increase insulin sensitivity in non-tumor-bearing animals after 14 days of treatment, with minimal decreases in body weight or food intake. Daily injections were performed 3 h prior to the start of the dark cycle.
Analysis of Body Composition
Body composition was analyzed on day 20 in conscious rats using the EchoMRI system (Echo Medical Systems, Houston, TX, USA). The EchoMRI system has been validated in mice and rats, and its results are well correlated with chemical carcass analysis [40]. Data are presented as a percentage of body fat. Hind leg diameter was utilized as a measure of skeletal muscle mass. The contralateral hind leg, relative to the tumor, was extended and the diameter of the upper leg was measured via external digital caliper and recorded.
Sacrifice
After 21–23 days, rats were sacrificed under ether inhalation anesthesia followed by rapid decapitation approximately 4 h prior to the start of the dark cycle. Trunk blood was collected for analysis of non-fasting blood glucose and plasma insulin levels. Blood glucose levels were measured using the Precision Xtra Glucose Monitoring System (Abbott Laboratories, Abbott, IL, USA). The remaining blood samples were centrifuged at 2,000 rpm for 15 min at 4 °C, and plasma was removed and stored at −80 °C for subsequent analysis of plasma insulin levels by radioimmunoassay. Exendin-4 or saline injections were not administered on the day of sacrifice.
The epididymal fat pads were removed from each animal and weighed, as a measure of terminal fat mass. Additionally, hind leg diameter, both contralateral and ipsilateral to the tumor, was recorded, as a measure of terminal skeletal muscle mass. In TB rats, the tumor was also removed and weighed. Small samples of epididymal fat and quadriceps muscle were collected and placed in RNAlater for analysis of mRNA expression by QPCR.
Radioimmunoassay (RIA)
Plasma insulin levels were determined using a commercial Rat Insulin RIA kit (Millipore, Billerica, MA, USA), with upper and lower detection limits of 0.1 and 10 ng/mL, respectively. All samples were run in duplicate and per manufacturer’s instructions. Unknown concentrations of insulin were calculated based on a standard curve generated for each kit.
Quantitative Real-Time Polymerase Chain Reaction (QPCR)
RNA was isolated from tissue samples for determination of mRNA expression. Tissue samples (100 mg) were homogenized in 2 mL of Trizol Reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. cDNA was synthesized from 0.5 to 3 μg of RNA using the SuperScript III First-Strand Synthesis System (Invitrogen) and diluted in nuclease-free water for storage at −80 °C. Primers for housekeeping genes and genes of interest are listed in Table 1. QPCR was performed in duplicate using a BioRad iCycler and Maxima Sybr Green solution (Thermo Scientific, Barrington, IL, USA) with two-step (L32 and Atrogin-1) or three-step (β-actin and HSL) amplification for 40 cycles. L32 was amplified from each sample for use as an endogenous control for Atrogin-1, while β-actin served as an endogenous control for HSL. The method of Pfaffl was used to determine expression of the genes of interest [43]. Expression was calculated based on normalization to the housekeeping gene, relative to the efficiency of the housekeeping and interest genes in the target tissue.
Table 1.
Primers sequences for QPCR analysis of mRNA expression
| Gene | Primer sequence |
|---|---|
| L32 | Forward: 5′-CAG ACG CAC CAT CGA AGT TA-3′ |
| Reverse: 5′-AGC CAC AAA GGA CGT GTT TC-3′ | |
| β-Actin | Forward: 5′-CGT GGG CCG CCC TAG GCA CCA-3′ |
| Reverse: 5′-CTC TTT GAT GTC ACG CAC GAT TTC-3′ | |
| Atrogin-1 | Forward: 5′-GTC CAG AGA GTC GGC AAG TC-3′ |
| Reverse: 5′-GTC GGT GAT CGT GAG ACC TT-3′ | |
| Hormone-sensitive lipase (HSL) | Forward: 5′-GAA TAT CAC GGA GAT CGA GG-3′ |
| Reverse: 5′-CCG AAG GGA CAC GGT GAT GC-3′ |
Statistical Analyses
Data are presented as mean ± standard error of the mean. The variance in tumor growth observed in saline- and Exendin-4-treated animals was significantly different during this experiment, with Exendin-4-treated rats having significantly greater variance than saline-treated rats. This led to the hypothesis that Exendin-4 treatment may have been more or less effective in some animals based on tumor size and growth rate. To better quantify the effects of Exendin-4 treatment on cancer cachexia symptoms, tumor-bearing rats in both treatment groups were divided into tertiles based on final tumor volume on the day of sacrifice (days 21–23). All data from TB animals from the upper (TBU) and lower (TBL) tertiles were analyzed, while data from TB animals falling in the middle tertile were excluded from the present analysis. Final groups sample sizes were (1) n = 9 TBU saline, (2) n = 10 for TBU Exendin-4, (3) n = 7 for TBL saline, and (4) n = 8 for TBL Exendin-4. Saline- and Exendin-4-treated control groups had group sizes of 10 animals each.
Tumor volume was analyzed in TBU and TBL rats in separate two-way ANOVAs, with treatment (saline or Exendin-4) and time serving as independent variables. Planned Bonferroni comparisons were performed when appropriate. Final tumor weight was analyzed via two-way ANOVA with tertile (upper or lower) and treatment (saline or Exendin-4) as independent variables, with planned Bonferroni comparisons as appropriate.
Terminal change in body weight, minus tumor weight in the TB group, was analyzed via two-way ANOVA, with treatment (saline or Exendin-4) and tumor status (control, TBL, or TBU) serving as independent variables. Planned Bonferroni comparisons were performed when appropriate. Average daily food intake data, presented as a mean of daily food intake throughout the 21–23 days of the study, as well as body adiposity, hind leg diameter, blood glucose levels, and plasma insulin levels, were similarly analyzed by two-way ANOVA, with planned Bonferroni comparisons when appropriate.
Results
Change in Body Weight and Food Intake
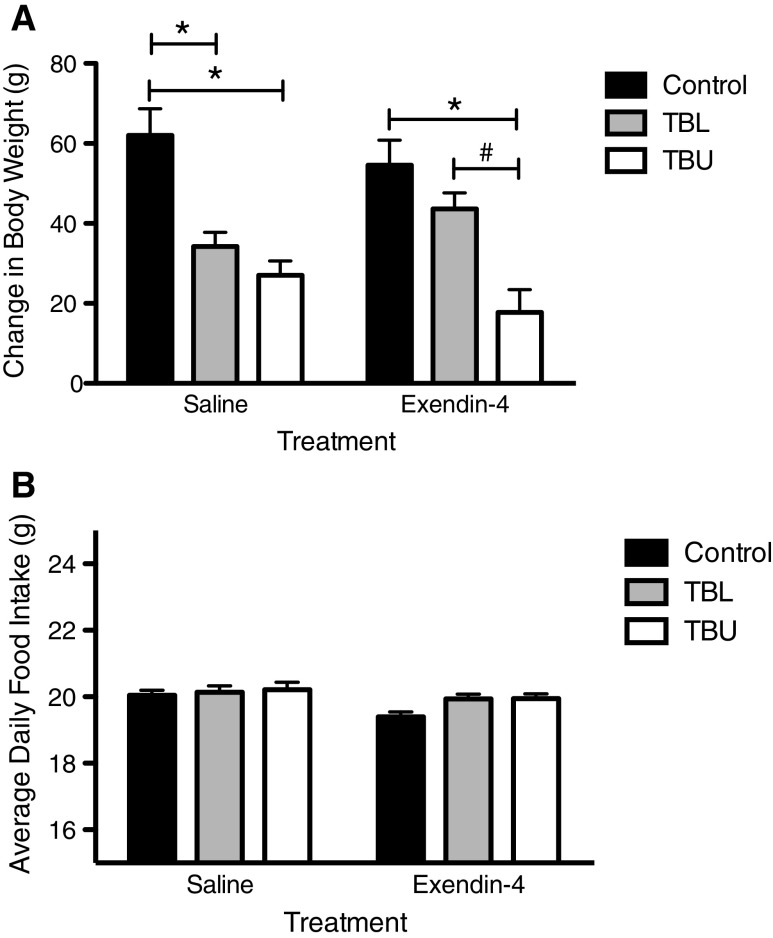
Terminal change in body weight was calculated by subtracting the weight of the tumor at the time of sacrifice from the total weight gained during the experiment for each rat (Fig. 1a). In the saline-treated groups, TB rats gained significantly less body weight as compared to controls (p < 0.01 for TBL, p < 0.001 for TBU). However, in rats treated with Exendin-4, only rats in the upper tertile of tumor volume experienced less weight gain as compared to controls and TBL rats (p < 0.05 for TBU). In contrast, TBL rats gained a comparable amount of weight to control rats (p > 0.05).
Fig. 1.
Change in body weight and average daily food intake in control and tumor-bearing rats (TBL, lower tertile; TBU, upper tertile) following chronic saline or Exendin-4 treatment. a Terminal change in body weight, minus tumor weight, was significantly lower in saline-treated TBL and TBU rats, as compared to controls (p < 0.05). In contrast, terminal change in body weight was significantly lower in Exendin-4 treated TBU rats, as compared to both control and TBL rats. No significant differences were found between control and TBL Exendin-4-treated rats. b Food intake was significantly lower in Exendin-4-treated rats, as compared to saline-treated rats, but no differences were observed based on tumor status. *p < 0.05 compared to control, #p < 0.05 compared to TBL
No differences in food intake were observed between groups based on tumor status (Fig. 1b).
Tumor Volume and Final Tumor Weight
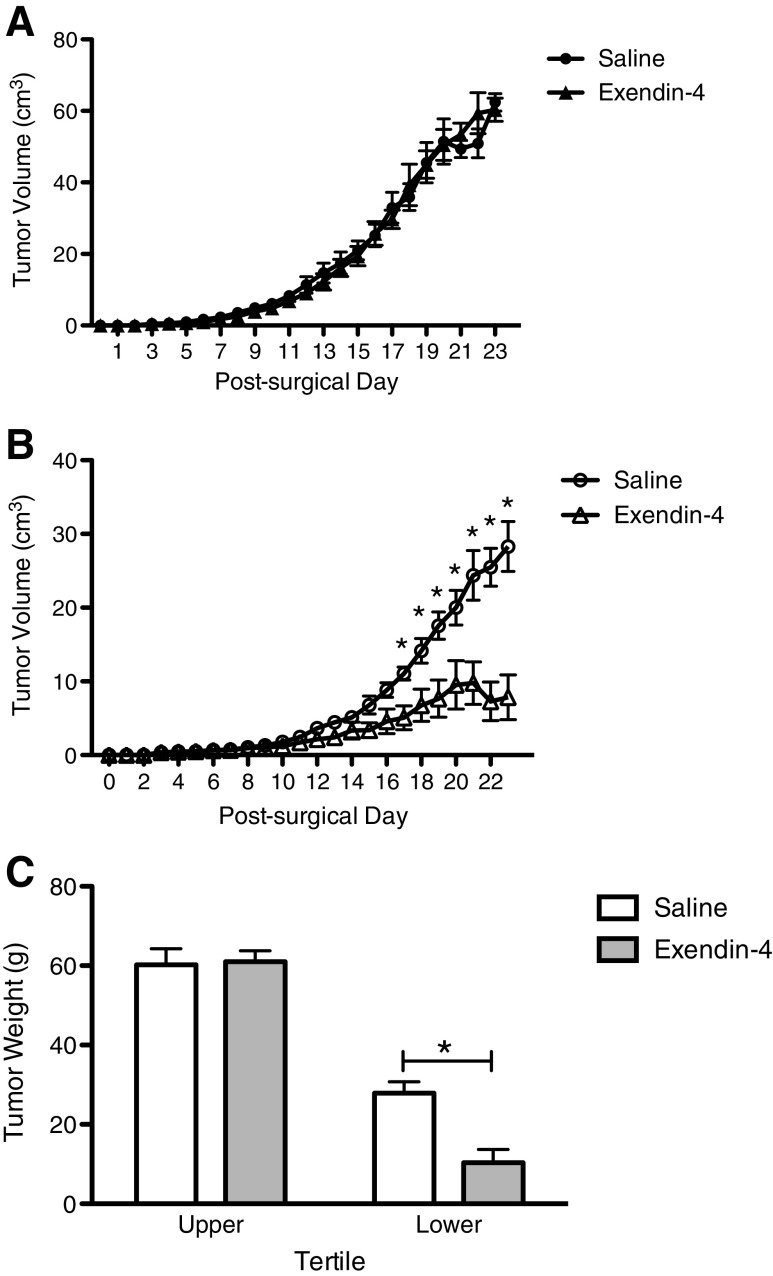
During the experiment, no significant difference in tumor volume was observed between saline and Exendin-4 treated TBU rats (Fig. 2a). In contrast, among TBL rats, significant differences between treatment groups were observed. While tumor volume in both groups increased over time, Exendin-4-treated TBL rats exhibited a smaller tumor volume starting at day 17 and continuing to the end of the experiment (day 17–23, p < 0.05 for all) (Fig. 2b).
Fig. 2.
Tumor volume and final tumor weight in saline- and Exendin-4-treated rats bearing the Yoshida sarcoma. a In TBU rats, tumor volume was comparable between saline- and Exendin-4-treated groups. b In TBL rats, tumor volume was significantly lower in Exendin-4-treated animals from days 17 to 23, as compared to saline-treated animals (p < 0.05). c On the day of sacrifice, tumors were removed and weighed. While no differences were observed between saline- and Exendin-4-treated TBU rats, tumors isolated from Exendin-4-treated TBL rats weighed significantly less than those isolated from saline-treated TBL rats. *p < 0.05
Final tumor weight followed a similar pattern. Exendin-4 treatment reduced final tumor weight in TBL rats as compared to saline treatment, but there was no difference in tumor weight between saline-treated and Exendin-4-treated TBU rats (Fig. 2c, p < 0.05).
Body Composition
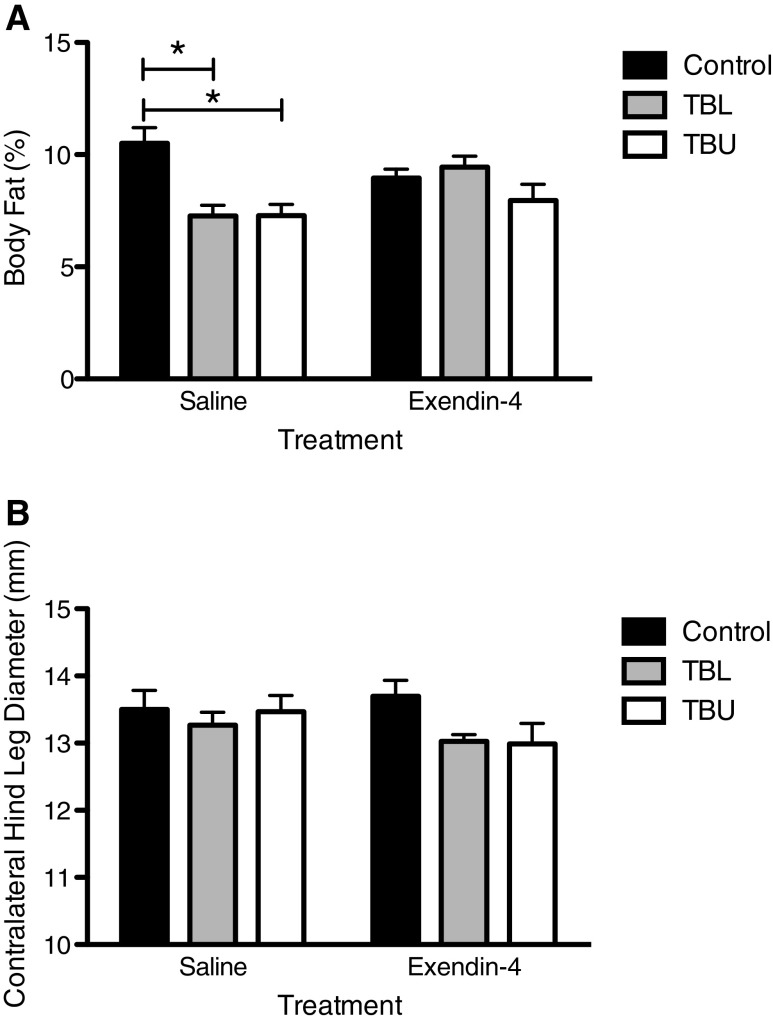
On day 20, saline-treated tumor-bearing animals exhibited a lower percentage of body fat, as compared to saline-treated controls (p < 0.01 for both TBU and TBL) (Fig. 3a). In contrast, this effect was not observed in Exendin-4-treated rats. Analysis of contralateral hind leg diameter revealed no significant differences based on tumor status or treatment on day 20 (Fig. 3b).
Fig. 3.
Body composition on day 20. a Percent body fat, as measured by EchoMRI, was reduced in saline-treated TBU and TBL rats, as compared to controls (p < 0.05). In contrast, no differences were observed among Exendin-4-treated rats. b No significant differences in contralateral hind leg diameter were observed between groups
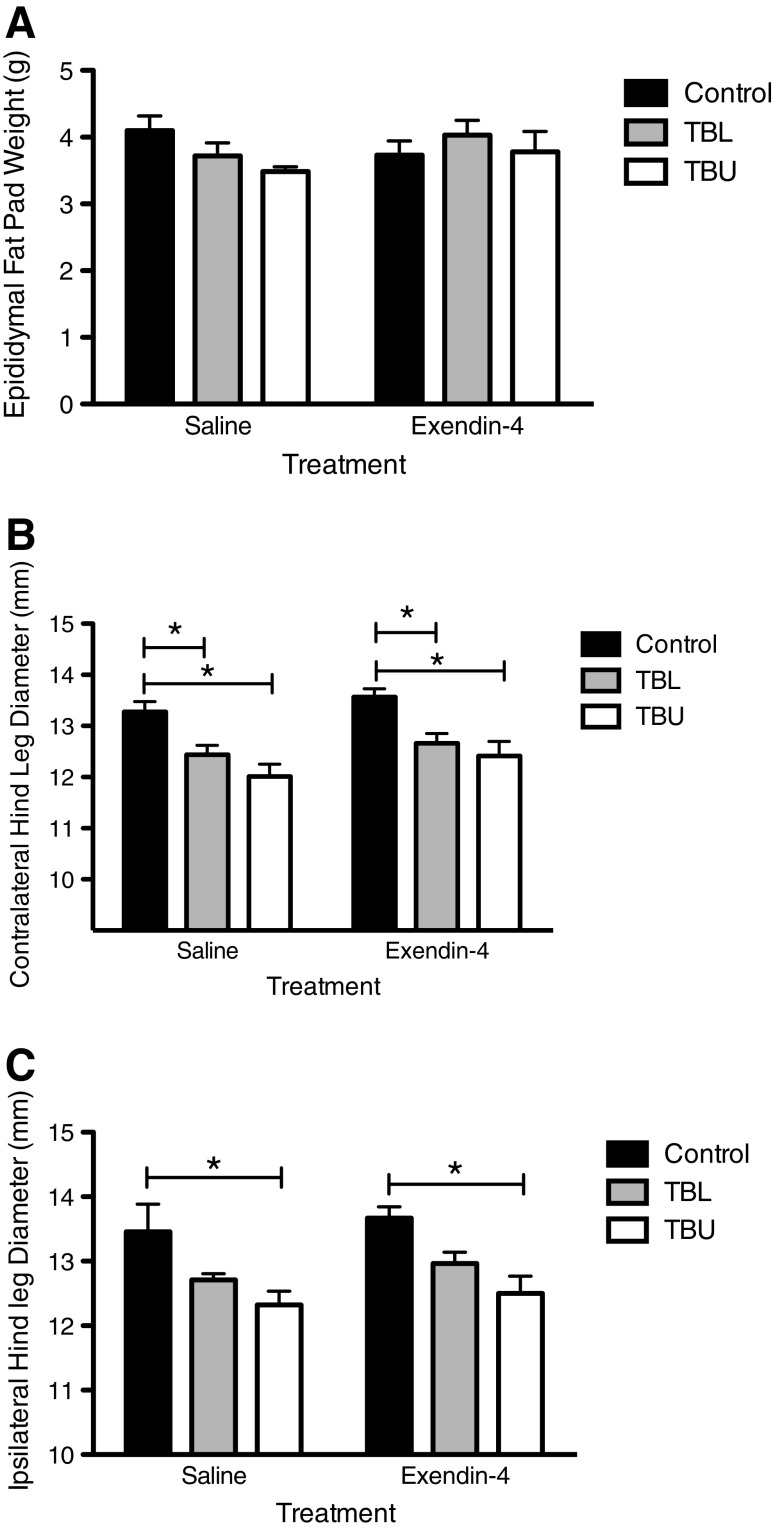
Terminal analyses were performed to further quantify body composition. Analysis of epididymal fat pad weight by two-way ANOVA revealed no significant effect of tumor status or treatment (Fig. 4a). On the day of sacrifice, lower contralateral hind leg diameter was observed in TBU and TBL animals, as compared to control animals (Fig. 4b). This effect was observed in both saline-treated and Exendin-4-treated animals. Similar results were observed with ipsilateral hind leg diameter; however, this effect was only observed in TBU, but not TBL, rats. Again, this effect was observed in both treatment groups.
Fig. 4.
Terminal body composition measurements. a No differences in epididymal fat pad weight were observed. b Contralateral hind leg diameter was lower in TBL and TBU animals in both treatment groups, compared to controls. c Ipsilateral hind leg diameter was lower in TBU rats in both treatment groups, as compared to controls. *p < 0.05
Plasma Insulin and Blood Glucose Levels
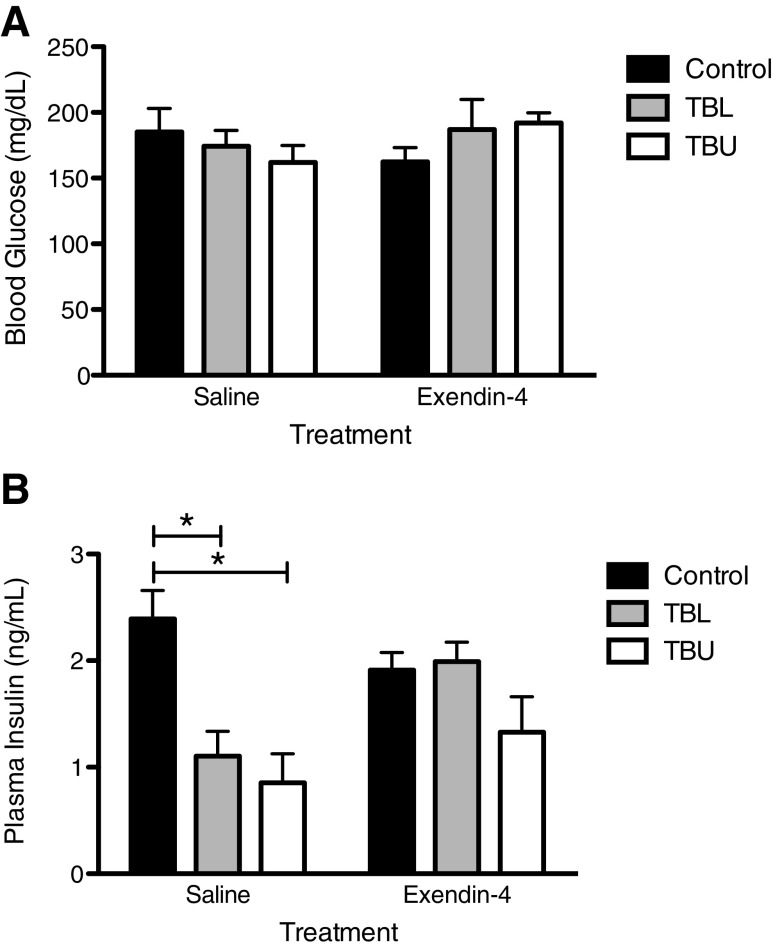
Blood glucose and plasma insulin levels were measured at the time of sacrifice, in a non-fasted state. No significant differences in blood glucose levels were observed (Fig. 5a). In contrast, plasma insulin levels exhibited a different pattern (Fig. 5b). In saline-treated rats, both TBU and TBL had lower insulin levels, as compared to non-tumor-bearing controls. However, in Exendin-4-treated rats, insulin levels were comparable in control, TBU, and TBL rats (p < 0.05 for all).
Fig. 5.
Terminal blood glucose and plasma insulin levels. a No significant differences in blood glucose levels were observed. b Plasma insulin levels were reduced in saline-treated TBL and TBU rats, compared to controls. However, Exendin-4 treatment ameliorated this effect. *p < 0.05
QPCR
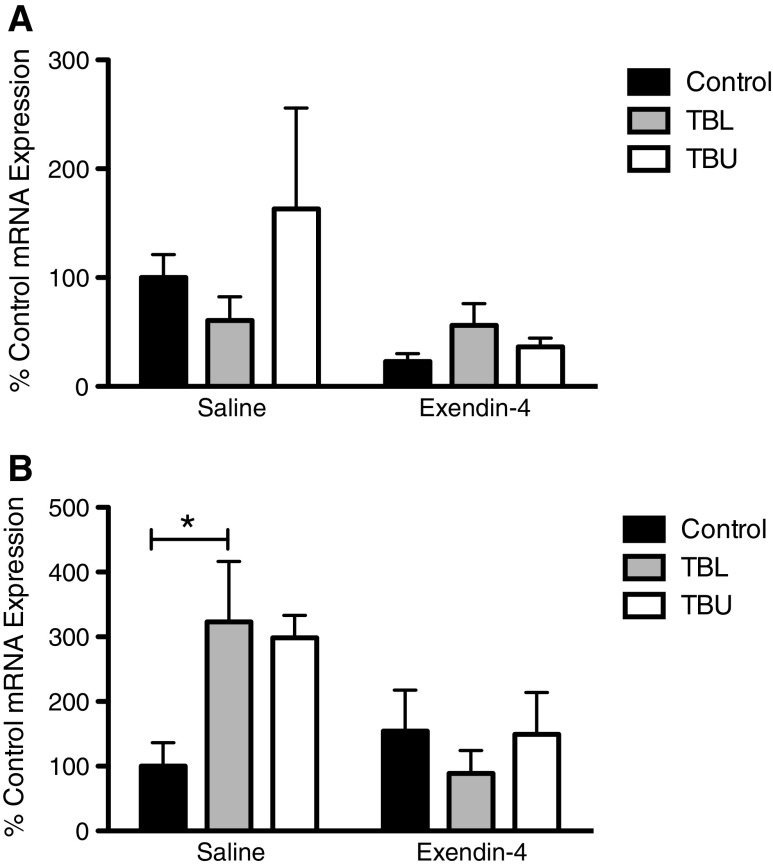
Additional analyses examined expression of wasting-related genes. In epididymal fat samples, HSL expression was not significantly different between groups (Fig. 6a). In quadriceps muscle samples, Atrogin-1 expression was increased in TBL rats, as compared to controls, in the saline-treated condition (p < 0.05) (Fig. 6b). This effect was not present in Exendin-4-treated animals.
Fig. 6.
Hormone-sensitive lipase (HSL) and Atrogin-1 mRNA expression in epididymal fat and quadriceps muscle, respectively. a No significant differences were observed in HSL expression. b Atrogin-1 expression was higher in saline-treated tumor-bearing rats, though this difference only reached statistical significance in TBL rats. Exendin-4 treatment resulted in Atrogin-1 expression that was comparable to controls in TBL and TBU rats. *p < 0.05
Discussion
Currently, no effective treatments are available for patients with cancer cachexia and no prevention strategies exist. As a result, a large proportion of individuals with cancer will experience some degree of cancer cachexia [49, 50]. Cancer cachexia contributes significantly to both morbidity and mortality in individuals with cancer, making the prevention and treatment of cancer cachexia an important research focus. The present experiment was undertaken to determine the effects of chronic treatment with Exendin-4 on the development of cancer cachexia symptoms in rats bearing the Yoshida sarcoma.
Saline-treated animals bearing the Yoshida sarcoma experienced significant symptoms of cancer cachexia. TBL and TBU rats had lower levels of body weight gain, as well as lower levels of both body fat and skeletal muscle mass. A reduction in food intake was not observed in this experiment. While minimal (approximately 5 %) differences have been observed previously in this model, differences of this magnitude are considered minimal [24]. Plasma insulin levels were significantly lower in TBL and TBU animals, as compared to controls. As further evidence of cachexia symptoms, expression of Atrogin-1 mRNA was higher in saline-treated TBL rats (non-significant elevation in TBU rats), as compared to control rats, suggesting an increase in skeletal muscle degradation via the ubiquitin–proteasome pathway. While TB rats were divided into upper and lower tertiles based on tumor growth, both TBL and TBU groups treated with saline developed the characteristic cachexia symptoms. This indicates that tumor burden itself may not be an important factor in determining the onset of cancer cachexia symptoms. These findings are consistent with the human literature [20, 26, 33, 41], and with previous experiments in the Yoshida sarcoma and other animal models [1, 9, 13, 16, 24, 29, 36, 46, 48].
Chronic treatment with Exendin-4 prevented the development of some cachexia symptoms in tumor-bearing animals, particularly in the lower tertile of tumor growth. Within the TBL group, Exendin-4-treated rats experienced weight gain and had fat mass comparable to their non-tumor-bearing controls. In addition, plasma insulin levels were comparable to those of controls. Reduced lean mass, as measured by hind leg diameter, was observed in both Exendin-4-treated and saline-treated TBL and TBU rats, indicating that Exendin-4 treatment did not ameliorate muscle mass loss. Despite this, QPCR analysis indicated Exendin-4 treatment prevented the increase in Atrogin-1 expression associated with the tumor-bearing state, such that Atrogin-1 expression was not elevated above control levels in TBL or TBU groups. These results suggest that the ubiquitin–proteasome pathway may be less active, despite a decrease in lean mass. It is possible that the wasting associated with the cachexia syndrome has stopped, but muscle rebuilding has not yet occurred. Further experiments must investigate the potential effects of Exendin-4 in this avenue, possibly in conjunction with a high-protein diet or pharmacological treatment to increase muscle protein synthesis and rebuild lost muscle mass.
In addition to altering the development of cachexia symptoms in tumor-bearing animals, Exendin-4 treatment resulted in reduced tumor growth in animals in the TBL group. While the upper tertiles of tumor growth for each treatment were similar, tumors were significantly smaller in the lower tertile of Exendin-4-treated rats, as compared to the lower tertile of saline-treated rats. It is possible that treatment with Exendin-4 altered tumor growth through direct and/or indirect mechanisms.
Previous studies have demonstrated that application of Exendin-4 to certain cancer cell lines decreases cell proliferation. GLP-1 has been observed to exert independent effects on cell growth and proliferation, making this peptide and its receptor novel targets for cancer therapeutics. Exendin-4 treatment decreases cell proliferation in isolated breast [32] and colon cancer cells [28]. However, similar effects were not seen in pancreatic cancer cells [27], or primary mammary or liver cells [32]. GLP-1 receptors are expressed on a number of tumor cells [18, 28, 32], though GLP-1 receptor expression has not been measured in the Yoshida sarcoma. In addition, substantial overlap is presented between GLP-1 receptor signaling pathways and those known to affect tumor growth. For example, the Wnt/beta-catenin pathway controls cell growth and regulation in cancer cells and normal cells, and interacts with the GLP-1 receptor [18]. The nature of these interactions has not yet been well characterized. In future experiments, levels of circulating GLP-1, as well as expression of GLP-1 receptors on Yoshida sarcoma tumors, should be investigated to determine the involvement of GLP-1 and GLP-1 receptors in cancer and cancer cachexia.
Indirectly, Exendin-4 may improve the elements of insulin sensitivity, which may lead to an environment that is less conducive to tumor growth in general. While glucose tolerance and insulin sensitivity were not directly measured following Exendin-4 treatment, plasma insulin levels were comparable in control and TB rats treated with Exendin-4, while insulin levels were lower in saline-treated TB rats. Previous research has linked insulin dysregulation to increased tumor growth and cancer incidence in both humans and animals [44]. A preservation of proper insulin levels may indicate a preservation of insulin sensitivity in these animals and could lead to reduced tumor growth. Additionally, Exendin-4 is known to produce potent anti-inflammatory effects, which could indirectly result in a reduction in tumor growth in these animals. TNF-α, IL-1, and IL-6 are significant mediators of tumor growth and inflammation [39], as well as cachexia symptoms [35], and expression of these peptides is often increased in individuals with diabetes [7]. Daily treatment with a synthetic Exendin-4 analog reduced mRNA expression of TNF-α and IL-1, and reduced circulating levels of IL-6 in insulin resistant individuals with type II diabetes [4]. The influence of Exendin-4 on inflammation in the present model has yet to be investigated.
The present experiment demonstrates the potential of Exendin-4 treatment to act as a preventative agent in the development of Yoshida sarcoma-induced cancer cachexia. These data represent a novel use of Exendin-4, one of few compounds to prevent the development of cancer cachexia symptoms in any animal model. These results could have important implications for cachexia treatment in individuals with cancer, and the effects of Exendin-4 should be investigated in other models, including human xenograft models, to further explore these implications. Future experiments should attempt to determine if these effects are the result of a direct effect on tumor growth or indirect effects on insulin sensitivity and other parameters. In addition, the differential effects of treatment in animals which develop large or small tumors should be explored, as some inherent tumor-related factors, such as pro-inflammatory cytokines, may alter the effectiveness of Exendin-4 treatment. Such mechanistic knowledge would be helpful in determining the utility of this treatment in a clinical setting.
Acknowledgments
The authors would like to acknowledge Meredith Cobb and Melissa McCurley (for technical assistance), and Dr. Terry Powley, Dr. Terry Davidson, and Dr. Jim Fleet (for editorial comments and suggestions). This work was supported by National Institutes of Health DK078654 (KPK) and by the National Institutes of Health, National Cancer Institute R25CA128770 (D. Teegarden) Cancer Prevention Internship Program administered by the Oncological Sciences Center and the Discovery Learning Research Center at Purdue University (MAH).
Conflict of Interest Statement
The authors have no conflicts of interest to disclose.
References
- 1.Asp ML, Tian M, Wendel AA, Belury MA (2010) Evidence for the contribution of insulin resistance to the development of cachexia in tumor-bearing mice. Int J Cancer 126 [DOI] [PubMed]
- 2.Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132:27. doi: 10.1053/j.gastro.2007.03.054. [DOI] [PubMed] [Google Scholar]
- 3.Baracos VE. Hypercatabolism and hypermetabolism in wasting states. Curr Opin Clin Nutr Metab Care. 2002;5:3. doi: 10.1097/00075197-200205000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Chaudhuri A, Ghanim H, Vora N, Sia CL, Korzeniewski K, Dhindsa S, Makdissi A, Dandona P. Exenatide exerts a potent anti-inflammatory effect. J Clin Endocrinol Metab. 2012;97(1):10. doi: 10.1210/jc.2011-1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Copeland GP, Al-Sumidaie AM, Leinster SJ, Davis JC, Hipkin LH. Glucose metabolism in patients with gastrointestinal malignancy but without excessive weight loss. Eur J Surg Oncol. 1987;13(1):6. [PubMed] [Google Scholar]
- 6.Copeland GP, Leinster SJ, Davis JC, Hipkin LJ. Insulin resistance in patients with colorectal cancer. Br J Surg. 1987;74(11):5. doi: 10.1002/bjs.1800741124. [DOI] [PubMed] [Google Scholar]
- 7.Dandona P, Aljada A, Bandyopadhyay A. Inflammation: the link between insulin resistance, obesity, and diabetes. Trends Immunol. 2004;25(1):4–8. doi: 10.1016/j.it.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 8.Davidson JA. Advances in therapy for type 2 diabetes: GLP-1 receptor agonists and DPP-4 inhibitors. Cleve Clin J Med. 2009;76:11. doi: 10.3949/ccjm.76.s5.05. [DOI] [PubMed] [Google Scholar]
- 9.el Razi Neto S, Zorn TMT, Curi R, Carpinelli AR. Impairment of insulin secretion in pancreatic islets isolated from Walker 256 tumor-bearing rats. Am J Physiol Cell Physiol. 1996;271:5. doi: 10.1152/ajpcell.1996.271.3.C804. [DOI] [PubMed] [Google Scholar]
- 10.Evans WJ, Morley JE, Argiles J, Bales C, Baracos V, Guttridge D, Jatoi A, et al. Cachexia: a new definition. Clin Nutr. 2008;27:793–799. doi: 10.1016/j.clnu.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 11.Fernandes LC, Carpinelli AR, Hell NS, Curi R (1991) Improvement of cancer cachexia and decrease of Walker 256 tumor growth by insulin administration in rats. Cancer Ther Control 1(10)
- 12.Fernandes LC, Curi R. Reversion of Walker 256 tumor cachexia by insulin treatment: possible mechanisms involved and perspectives for future research. Endocr Relat Cancer. 1997;4:465–474. doi: 10.1677/erc.0.0040465. [DOI] [Google Scholar]
- 13.Fernandes LC, Machado UF, Nogueira CR, Carpinelli AR, Curi R (1990) Insulin secretion in Walker 256 tumor cachexia. Am J Physiol Endocrinol Metab 258 [DOI] [PubMed]
- 14.Glickman AS, Rawson RW (1956) Diabetes and altered carbohydrate metabolism in patients with cancer. Cancer 9 [DOI] [PubMed]
- 15.Gordon JN, Green SR, Goggin PM. Cancer cachexia. Q J Med. 2005;98:10. doi: 10.1093/qjmed/hci127. [DOI] [PubMed] [Google Scholar]
- 16.Guaitani A, Recchia M, Carli M, Rocchetti M, Bartosek I, Garattini S. Walker carcinoma 256: a model for studies on tumor-induced anorexia and cachexia. Oncology. 1982;39:6. doi: 10.1159/000225631. [DOI] [PubMed] [Google Scholar]
- 17.Han VK, Hynes MA, Jin C, Towle AC, Lauder JM, Lund PK. Cellular localization of proglucagon/glucagon-like peptide messenger RNAs in the rat brain. J Neurosci Res. 1986;16:11. doi: 10.1002/jnr.490160110. [DOI] [PubMed] [Google Scholar]
- 18.Heller C, Kuhn MC, Mulders-Opgenoorth B, Schott M, Willenberg HS, Scherbaum WA, Schinner S. Exendin-4 upregulates the expression of Wnt-4, a novel regulator of pancreatic beta-cell proliferation. Am J Physiol Endocrinol Metab. 2011;301:9. doi: 10.1152/ajpendo.00144.2011. [DOI] [PubMed] [Google Scholar]
- 19.Hofbauer KG, Anker SD, Inui A, Nicholson JR. Pharmacotherapy of cachexia. Boca Raton: CRC; 2006. [Google Scholar]
- 20.Holroyde CP, Reichard GA. Carbohydrate metabolism in cancer cachexia. Cancer Treat Rep. 1981;65:5. [PubMed] [Google Scholar]
- 21.Holroyde CP, Gabuzda TG, Putnam RC, Paul P, Reichard GA. Altered glucose metabolism in metastatic cancer. Cancer Res. 1975;35:5. [PubMed] [Google Scholar]
- 22.Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87:31. doi: 10.1152/physrev.00034.2006. [DOI] [PubMed] [Google Scholar]
- 23.Honors MA, Kinzig KP. The role of insulin resistance in the development of muscle wasting during cancer cachexia. J Cachex Sarcopenia Muscle. 2012;3:7. doi: 10.1007/s13539-011-0051-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Honors MA, Kinzig KP. Characterization of the Yoshida sarcoma: a model of cancer cachexia. Support Care Cancer. 2013 doi: 10.1007/s00520-013-1839-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Idris I, Patiag D, Gray S, Donnelly R. Exendin-4 increases insulin sensitivty via a PI-3-kinase-dependent mechanism: contrasting effects of GLP-1. Biochem Pharmacol. 2002;63:4. doi: 10.1016/S0006-2952(01)00924-8. [DOI] [PubMed] [Google Scholar]
- 26.Jasani B, Donaldson LJ, Ratcliffe JG, Sokhi GS. Mechanism of impaired glucose tolerance in patients with neoplasia. Br J Cancer. 1978;38:6. doi: 10.1038/bjc.1978.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koehler JA, Drucker DJ. Activation of glucagon-like peptide-1 receptor signaling does not modify growth or apoptosis of human pancreatic cancer cells. Diabetes. 2006;55:11. doi: 10.2337/db05-1145. [DOI] [PubMed] [Google Scholar]
- 28.Koehler JA, Kain T, Drucker DJ. Glucagon-like peptide-1 receptor activation inhibits growth and augments apoptosis in murine CT26 colon cancer cells. Endocrinology. 2011;152(9):11. doi: 10.1210/en.2011-1201. [DOI] [PubMed] [Google Scholar]
- 29.Lazarus DD, Destree AT, Mazzola LM, McCormack TA, Dick LR, Xu B, Huang JQ, Pierce JW (1999) A new model of cancer cachexia: contribution of the ubiquitin-proteasome pathway. Am J Physiol Endocrinol Metab 277 [DOI] [PubMed]
- 30.Lazarus DD, Kambayashi T, Lowry SF, Strassmann G. The lack of an effect by insulin or insulin-like growth factor-1 in attenuating colon-26-mediated cancer cachexia. Cancer Lett. 1996;103:7. doi: 10.1016/0304-3835(96)04197-3. [DOI] [PubMed] [Google Scholar]
- 31.Lee SW, Dai G, Hu Z, Wang X, Du J, Mitch WE (2004) Regulation of muscle protein degradation: coordinated control of apoptotic and ubiquitin–proteasome systems by phosphatidylinositol 3 kinase. J Am Soc Nephrol 15 [DOI] [PubMed]
- 32.Ligumsky H, Wolf I, Israeli S, Haimsohn M, Ferber S, Karasik A, Kaufman B, Rubinek T. The peptide-hormone glucagon-like peptide-1 activates cAMP and inhibits growth of breast cancer cells. Breast Cancer Res Treat. 2012;132:449–461. doi: 10.1007/s10549-011-1585-0. [DOI] [PubMed] [Google Scholar]
- 33.Lundholm K, Holm G, Schersten Y. Insulin resistance in patients with cancer. Cancer Res. 1978;38:6. [PubMed] [Google Scholar]
- 34.Lundholm K, Korner U, Gunnebo L, Sixt-Ammilon P, Fouladiun M, Daneryd P, Bosaeus I. Insulin treatment in cancer cachexia: effects on survival, metabolism, and physical functioning. Clin Cancer Res. 2007;13(9):8. doi: 10.1158/1078-0432.CCR-06-2720. [DOI] [PubMed] [Google Scholar]
- 35.Matthys P, Billiau A. Cytokines and cachexia. Nutrition. 1997;13(9):763–770. doi: 10.1016/S0899-9007(97)00185-8. [DOI] [PubMed] [Google Scholar]
- 36.McCarthy HD, McKibbin PE, Perkins AV, Linton EA, Williams G. Alterations in hypothalamic NPY and CRF in anorexic tumor-bearing rats. Am J Physiol Endocrinol Metab. 1993;264:6. doi: 10.1152/ajpendo.1993.264.4.E638. [DOI] [PubMed] [Google Scholar]
- 37.Moley JF, Morisson SD, Norton JA. Insulin reversal of cancer cachexia. Cancer Res. 1985;45:7. [PubMed] [Google Scholar]
- 38.Moley JF, Morrison SD, Gorschboth CM, Norton JA. Body composition changes in rats with experimental cancer cachexia: improvement with exogenous insulin. Cancer Res. 1988;48:4. [PubMed] [Google Scholar]
- 39.Nelson D, Ganss R. Tumor growth or regression: powered by inflammation. J Leukoc Biol. 2006;80:6. doi: 10.1189/jlb.1105646. [DOI] [PubMed] [Google Scholar]
- 40.Nixon JP, Zhang M, Wang C, Kuskowski M, Novak CM, Levine JA, Billington CJ, Kotz CM. Evaluation of a quantitative magnetic resonance imaging system for whole body composition analysis in rodents. Obesity. 2010;18(8):1652–1659. doi: 10.1038/oby.2009.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Norton JA, Maher M, Wesley R, White D, Brennan MF. Glucose intolerance in sarcoma patients. Cancer. 1984;54:6. doi: 10.1002/1097-0142(19841215)54:12<3022::AID-CNCR2820541234>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 42.Ovesen L, Allingstrup L, Hannibal J, Mortensen EL, Hansen OP. Effect of dietary counseling on food intake, body weight, response rate, survival, and quality of life in cancer patients undergoing chemotherapy: a prospective, randomized study. J Clin Oncol. 1993;11:8. doi: 10.1200/JCO.1993.11.10.2043. [DOI] [PubMed] [Google Scholar]
- 43.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29(9):6. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pollak M. Insulin and insulin-like growth factor signaling in neoplasia. Nat Rev Cancer. 2008;8:14. doi: 10.1038/nrc2536. [DOI] [PubMed] [Google Scholar]
- 45.Schein PS, Kisner D, Haller D, Blecher M, Hamosh M. Cachexia of malignancy: potential role of insulin in nutritional management. Cancer. 1979;43:8. doi: 10.1002/1097-0142(197905)43:5+<2070::AID-CNCR2820430715>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 46.Tanaka Y, Eda H, Tanaka T, Udagawa T, Ishikawa T, Horii I, Ishitsuka H, Kataoka T. Experimental cancer cachexia induced by transplantable colon 26 adenocarcinoma in mice. Cancer Res. 1990;50:6. [PubMed] [Google Scholar]
- 47.Tayek JA. A review of cancer cachexia and abnormal glucose metabolism in humans with cancer. J Am Coll Nutr. 1992;11:12. doi: 10.1080/07315724.1992.10718249. [DOI] [PubMed] [Google Scholar]
- 48.Temparis S, Asensi M, Taillandier D, Aurousseau E, Larbaud D, Obled A, Bechet D, Ferrara M, Estrela JM, Attaix D. Increased ATP-ubiquitin-dependent proteolysis in skeletal muscle of tumor-bearing rats. Cancer Res. 1994;54:6. [PubMed] [Google Scholar]
- 49.Teunissen CC, Wesker W, Kruitwagen C, de Haes HC, Voest EE, de Graeff A. Symptom prevalence in patients with incurable cancer: a systematic review. J Pain Symptom Manag. 2007;34(1):11. doi: 10.1016/j.jpainsymman.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 50.Tisdale MJ. Cachexia in cancer patients. Nat Rev Cancer. 2002;2:10. doi: 10.1038/nrc927. [DOI] [PubMed] [Google Scholar]
- 51.Tomayko MM, Patrick Reynolds C. Determination of subcutaneous tumor size in athymic (nude) mice. Cancer Chemother Pharmacol. 1989;24:7. doi: 10.1007/BF00300234. [DOI] [PubMed] [Google Scholar]
- 52.Wang A, Hu Z, Hu J, Du J, Mitch WE. Insulin resistance accelerates muscle protein degradation: activation of the ubiquitin–proteasome pathway by defects in muscle cell signaling. Endocrinology. 2006;147:9. doi: 10.1210/en.2005-1305. [DOI] [PubMed] [Google Scholar]