Abstract
Aims
To determine if methamphetamine-dependent (MD) individuals exhibit behavioral or neural processing differences in risk-taking relative to healthy comparison participants (CTL).
Design
This was a cross-sectional study comparing two groups’ behavior on a risk-taking task and neural processing as assessed using functional magnetic resonance imaging (fMRI).
Settings
The study was conducted in an inpatient treatment center and a research fMRI facility in the United States.
Participants
Sixty-eight recently abstinent MD individuals recruited from a treatment program and forty CTL recruited from the community completed the study.
Measurements
The study assessed risk-taking behavior (overall and post-loss) using the Risky Gains Task (RGT), sensation-seeking, impulsivity and blood-oxygenation level dependent activation in the brain during the decision phase of the RGT.
Findings
Relative to CTL, MD displayed decreased activation in the bilateral rostral anterior cingulate cortex (ACC) and greater activation in the left insula across risky and safe decisions (p<.05). Right mid insula activation among CTL did not vary between risky and safe decisions, but among MD it was higher during risky relative to safe decisions (p<.05). Among MD, lower activation in the right rostral ACC (r=−.39, p<.01) and higher activation in the right mid insula (r=.35, p<.01) during risky decisions were linked to a higher likelihood of choosing a risky option following a loss.
Conclusions
Methamphetamine-dependent individuals show disrupted risk-related processing in both anterior cingulate and insula, brain areas that have been implicated in cognitive control and interoceptive processing. Attenuated neural processing of risky options may lead to risk-taking despite experiencing negative consequences.
Keywords: Decision-making, neuroimaging, relapse, treatmen
Introduction
Amphetamine-type stimulants, including methamphetamine, are the second most widely-used class of illicit drugs worldwide [1]. Behavioral studies have shown that methamphetamine users select risky options more often than healthy participants [2], alcohol abusers [3] or opiate dependent participants [4]. Risk-taking can be defined as selecting an option that has a variably distributed outcome [5] and often involves foregoing an option with a small, sure benefit in favor of one that offers the potential for either big gains or losses [6]. Considering the potential negative outcomes associated with methamphetamine use, it is important to understand why methamphetamine users exhibit greater levels of risk-taking.
Neuroimaging studies of healthy volunteers suggest that the insula [7, 8] and anterior cingulate cortex (ACC) [9, 10] contribute to the evaluation of risky decisions. In particular, attenuated ACC activation has been associated with higher levels of risk-taking among polydrug abusers (i.e. heroin, marijuana, cocaine or amphetamine) relative to comparison groups [4, 11, 12]. Moreover, studies examining methamphetamine-dependent (MD) individuals during simple decision-making tasks revealed decreased activation of the insula and ACC [13-16] relative to comparison groups. However, no neuroimaging studies have been conducted to examine whether primary methamphetamine dependent individuals show altered ACC or insula activation during risk-taking in particular. Examining neural activation during risk-taking among MD individuals may help explain whether misrepresentation of risk contributed to their heightened risk-taking propensity, which might ultimately explain continued methamphetamine use despite adverse consequences.
The present study used the Risky Gains Task [2, 17] during functional magnetic resonance imaging (fMRI) to examine behavioral and neural differences in risky decision making between MD and healthy comparison (CTL) participants. We hypothesized that MD would exhibit decreased ACC and insula activation relative to CTL during risky decisions. We also predicted that MD relative to CTL would take more risks overall and be more likely to continue taking risks following a loss.
Methods
Participants
Sixty-eight MD participants (15 female) were recruited through 28-day inpatient Alcohol and Drug Treatment Programs (ADTP) at the San Diego Veterans Affairs Medical Center and Scripps Green Hospital (La Jolla, CA). All participants had ceased using methamphetamine an average of 34.0 ± 3.4 days prior to participation (range of 15 to 207) and were randomly screened for the presence of drugs as part of the treatment program. Semi-structured clinical interviews at time of testing revealed that no subjects were experiencing symptoms of withdrawal during neuroimaging sessions.
Forty healthy, age-matched CTL participants (14 female) were recruited through internet ads, fliers, and local newspapers in the San Diego area. Eligible CTL participants endorsed (1) no lifetime history of DSM-IV Axis I disorders; (2) no lifetime history of DSM-IV substance dependence; and (3) no current drug or alcohol related problems or intoxication as confirmed by toxicology screen. Subjects were informed that the study was examining behavior and brain characteristics related to stimulant dependence. Written consent was obtained from all participants after study procedures had been fully explained.
Lifetime DSM-IV Axis I diagnoses (including substance abuse and dependence) and Axis II antisocial personality disorder (ASPD) were assessed by experienced interviewers using the Semi Structured Assessment for the Genetics of Alcoholism (SSAGA), a validated [18], semi-structured interview that allows for quantification of lifetime drug use. Diagnoses were based on consensus between a clinician specialized in substance use disorders (MPP) and trained study personnel. The following were exclusion criteria for all groups: (1) anti-social personality disorder; (2) current (past 6 months) Axis I panic disorder, social phobia, post-traumatic stress disorder, major depressive disorder; (3) lifetime bipolar disorder, schizophrenia, and obsessive compulsive disorder; (4) current severe medical disorders requiring inpatient treatment or frequent medical visits; (5) use of medications that affect the hemodynamic response (e.g. antihypertensives, insulin or thyroid medication) within the past 30 days; (6) current positive urine toxicology test; and (7) history of head injuries with loss of consciousness for longer than 5 minutes. During evaluation, 102 participants (68 MD, 34 CTL) performed the North American Adult Reading Test [NAART; 19] and 3 CTL performed the Wechsler Test of Adult Reading [WTAR; 20] to provide a measure of verbal intelligence (VIQ). Scores from both tests were transformed to create a standardized VIQ index. VIQ was not obtained for three CTL. Characteristics are summarized in Table 1.
Table 1. Subject Characteristics by Group.
Distributions of substance use means and standard deviations are highly skewed between and within groups. The strength of the relationship between these variables and brain activation and behavior among MD is more important than group differences in central tendency.
| Characteristic | Comparison (CTL) | Methamphetamine-Dependent (MD) | ||
|---|---|---|---|---|
| N | % | N | % | |
| Participants | 40 | 68 | ||
| Female | 14 | 35 | 15 | 22 |
| White | 24 | 60 | 41 | 60 |
| Black | 5 | 13 | 8 | 12 |
| Hispanic | 2 | 5 | 13 | 19 |
| Asian | 4 | 10 | 4 | 6 |
| Pacific islander | 1 | 3 | 2 | 3 |
| Unspecified ethnicity | 4 | 10 | 0 | 0 |
| Alcohol dependencea | 0 | 0 | 10 | 15 |
| Marijuana dependencea | 0 | 0 | 7 | 10 |
| Cocaine dependencea | 0 | 0 | 11 | 16 |
| Opiate dependencea | 0 | 0 | 2 | 3 |
| Mean | SD | Mean | SD | |
| Age (years) | 35.6 | 11.5 | 38.2 | 10.5 |
| Education (years)b | 14.9 | 1.7 | 13.0 | 1.6 |
| Verbal IQc | 112.0 | 9.4 | 108.9 | 9.4 |
| Sensation Seekingb,d | 17.6 | 5.7 | 22.8 | 6.2 |
| Impulsivityb,e | 33.8 | 6.4 | 46.8 | 7.6 |
| Alcohol (drinks/week)b,f | 3.4 | 4.4 | 22.0 | 38.4 |
| Nicotine (cigarettes/day)b,f | 1.2 | 3.7 | 13.3 | 8.7 |
| Methamphetamineb,g | 0.2 | 1.0 | 13144 | 28023.6 |
| Marijuanab,g | 38.4 | 158.3 | 8180.0 | 25007.5 |
| Cocaineb,g | 1.1 | 4.3 | 2007.2 | 5518.8 |
| Opiatesb,g | 0.1 | 0.8 | 64.7 | 366.1 |
| Prescription stimulantsb,g | 0.0 | 0.2 | 49.5 | 390.2 |
Met criteria for comorbid dependence with methamphetamine dependence.
CTL significantly different from MD, two-sample t test, p<0.001.
Assessed by either the North American Adult Reading Test (NAART; Uttl, 2002) or the Wechsler Test of Adult Reading (WTAR; Wechsler, 2001).
Based on data from 37 CTL and 58 MD
Based on data from 37 CTL and 60 MD
Recent patterns of use.
Number of uses across the lifespan.
Risky Gains Task
The Risky Gains Task (RGT) has been used in a number of risk-taking studies [17, 21]. Subjects were informed that the goal was to earn as many points as possible; points were exchanged for money at the end of the task. Participants started the task with zero points and were told they could not end with a negative point total. They earned points by selecting, with a button press, one of three options—20, 40 or 80—on each trial. Options appeared in ascending order for 1 second each. Participants were told 20 was the safe option and 40 and 80 were risky options. If a participant responded when 20 appeared, the trial ended and she earned 20 points. If the participant bypassed the 20 option, the 40 option appeared. On some trials, the participant immediately lost 40 points and the trial ended. Otherwise, she could respond and earn 40 points. If the 40 option was bypassed, the 80 option appeared. Like the 40 option, sometimes the participant immediately lost 80 points. Otherwise, she could respond and receive 80 points. A pleasant beep sounded after wins (+20, +40, +80) but a buzz sounded after losses (−40, −80). The task consisted of 96 trials, each lasting 3.5 seconds regardless of the participant's response. The three trial types were presented in a predetermined, randomized order: 54 unpunished (+20, +40, +80), 24 punished (−40) and 18 punished (−80). If a participant selected 20 on a −40 trial they received 20 points. Similarly, if they selected 20 or 40 on a −80 trial, they received 20 or 40 points, respectively. Unbeknownst to participants, the number of negative trials for 40 and 80 options (−40 and −80) was set so that choosing the same option on each trial would produce the same final points total, i.e. choosing risky or safe provided no advantage.
Personality measures
Two questionnaires assessing personality traits related to risk-taking were used to complement behavioral risk-taking measures. Fifty-eight MD and thirty-seven CTL participants completed the Sensation-Seeking Scale [22], a forty-item two-choice questionnaire that assesses preference for sensation-seeking, including risky behaviors. Sixty MD and thirty-seven CTL participants completed the Barratt Impulsiveness Scale [23], a thirty-item, likert-scaled questionnaire that assesses impulsivity.
Image Acquisition
A fMRI run sensitive to blood-oxygenation level dependent (BOLD) contrast was collected using a Signa EXCITE 3T scanner (GE Healthcare, Milwaukee, Wisconsin T2*-weighted echoplanar imaging; TR=2000 ms, TE=32 ms, FoV=230×230 mm2, 64×64 matrix, 30 2.6 mm axial slices with 1.4 mm gap, flip angle=90°, total duration: 8 min, 32 sec, 3.59×3.59×2.6 mm3 voxels). For anatomical reference, a high-resolution, T-1 weighted image (TR=8 ms, TE=3 msec, FoV=250×250 mm2, 192×256 matrix interpolated to a 256×256 matrix, flip angle=12°, 172 sagitally acquired slices, .97×.97×1 mm3 voxels) was collected during the same session.
Behavioral analysis
The frequencies of safe (20) and risky (40, 80) choices were compared overall and following a loss. 40 and 80 choices were combined as a single metric of risk-taking. Thus, the frequency of safe choices, p, was the complement of the frequency of risky choices, 1-p. A two-way repeated measures ANOVA examined frequency of risky choices to test for a main effect of group (i.e. MD versus CTL), condition (i.e. risk overall versus risk post-loss), and a group by condition interaction.
fMRI preprocessing
Data were preprocessed using Analysis of Functional NeuroImages (AFNI) software. Echoplanar images were aligned to anatomical images. Outlier voxels were identified in the aligned images based on whether a given time point greatly exceeded the mean number of voxel outliers for the time series; time points with high numbers of outlier voxels were excluded from subsequent analyses. Images were spatially smoothed using a 4mm Gaussian filter. Functional echo-planar images were co-registered to anatomical images and then visually inspected. Preprocessed time-series data were analyzed using a multiple regression model based on a BOLD response function with a 4-6 second peak. A general linear model fit was performed using AFNI's 3dDeconvolve function, wherein regressors for safe (+20) and risky (+40, +80) decisions were defined as starting at trial onset and concluding when a) the subject made a response or b) punishment (−40, −80) was delivered. Baseline activation was calculated from the BOLD signal during intertrial intervals and null trials (i.e. fixation on crosshairs without responding). Three motion regressors (roll, pitch and yaw) and a regressor for slow linear drift were included in the linear model fit as regressors of non-interest. Percent signal change (PSC) was calculated by dividing the regressor of interest by the baseline regressor.
fMRI group analysis
A linear mixed-effects (LME) analysis was conducted using R statistical software (www.cran.org). Group (MD, CTL) and decision (safe, risky) were fixed effects in the model and individual participants were treated as random effects. LME analysis examined main effect of group and group by decision (i.e. risky versus safe) interaction. Analyses were performed voxel-wise but restricted to a limbic mask that included the insula and ACC based on a priori hypotheses. Threshold adjustment based on Monte Carlo simulations (AFNI's Alpha Sim) was applied to guard against identifying false-positive activations. A-priori voxel-wise probability of p<.05 in a cluster of 384 μL for the ACC and 320 μL for the insula (6 and 5 voxels, respectively) resulted in an a-posteriori probability of p<.05. Center-of-mass coordinates are reported in Talairach space (x, y, z) and labeled using Talairach Daemon software [24]. Whole brain patterns of activation for task, along with group differences in activation and group by decision interactions are presented in Supplemental Figures 1, 2 and 3.
Follow-up exploratory analysis
fMRI robust regressions. To explore the implications of brain activation identified by LME analysis among MD, voxel-wise Huber [25] robust regressions were performed to identify clusters of activation related to predictor variables. Using PSC (restricted to the mask including ACC and insula) during risky decisions as the dependent variable, this analysis explored the relationship between MD's substance use history or behavioral performance and brain activation at each voxel. Seven Huber regressions were conducted, examining 1) overall frequency of risky choice, 2) frequency of risky choice following a loss, 3) lifetime methamphetamine use (log transformed) controlling for lifetime marijuana and cigarettes/day, 4) lifetime methamphetamine use controlling for lifetime cocaine and alcoholic drinks/week, 5) years of methamphetamine use controlling for age, 6) days since last use of methamphetamine and 7) impulsivity and sensation-seeking (see Supplemental Figures 4 and 5). Using Alpha-Sim to correct for multiple comparisons, a-priori voxel-wise probability of p<.05 in a cluster of 384 μL for the ACC and 320 μL for the insula (6 and 5 voxels, respectively) resulted in an a-posteriori probability of p<.05.
Results
Demographics
MD and CTL were of similar age (t=1.2, p=.23; see Table 1). Although CTL had an average of two more years of education compared to MD (t=5.8, p<.001), verbal IQs were similar between groups (t=1.6, p=.10). MD had used significantly more substances (e.g., methamphetamine, marijuana, prescription stimulants, cocaine) across their lifespan than CTL (p<.001). MD also smoked more cigarettes per day and drank more alcohol per week than CTL (p<.001).
Behavioral Data and Personality Measures
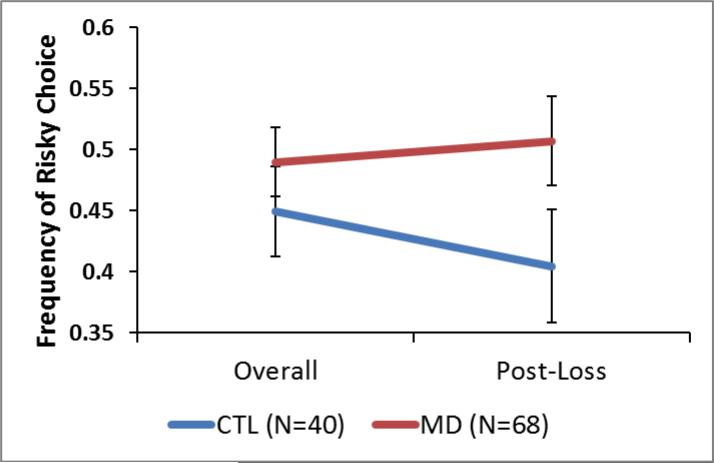
To determine whether MD showed heightened risk-taking behavior, a two-way, repeated-measures ANOVA was conducted with prior condition (overall or post-loss) as a within-subject factor. As seen in Figure 1, there was no main effect of group (F1,106=2.6, p=.11) or condition (F1,106=1.5, p=.23) on risk-taking. There was a trend for a group-by-condition interaction effect on risk-taking (F1,106=2.7, p=.10). Mean risk-taking frequency was lower among CTL following a loss relative to overall (overall mean=.45, SEM=.04; post-loss mean=.38, SEM=.05) whereas MD continued to take risks at the same frequency following a loss (overall mean=.49, SEM=.03; post-loss mean=.50, SEM=.04). MD scored significantly higher on measures of sensation-seeking (t=4.1, p<.001; group difference estimate: 5.3 points, 95%CI 4.2, 6.3) and impulsivity (t=8.7, p<.001; group difference estimate: 13.0 points, 95%CI 11.9, 14.1) than CTL (see Table 1). History of drug use (e.g. cocaine, methamphetamine) was unrelated to risk-taking behavior (see Supplemental Table 1).
Figure 1.
Behavioral Results. Two-way repeated ANOVA showed no main effect of condition (post-loss or overall; p=.23), group (p=.11) or group by condition interaction (p=.10). Error bars represent standard error from the mean.
Imaging Data
Group main effect
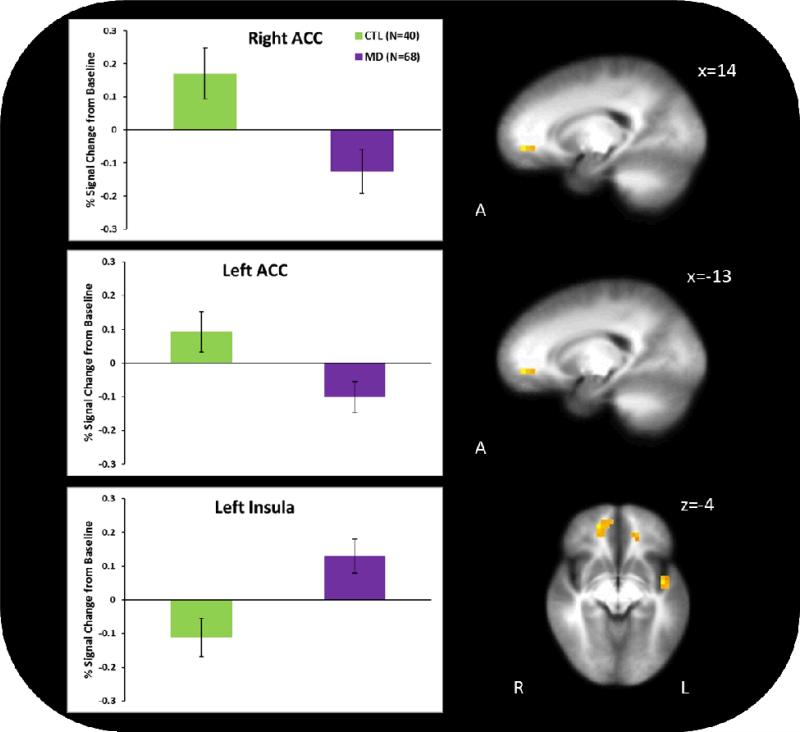
During the decision-making phase of the RGT (see Figure 2), across risky and safe choices, MD exhibited lower activation in bilateral rostral ACC (BA 32; right cluster: x=14, y=45, z=−3, Vol.=768 μL; left cluster: x=−13, y=40, z=−2, Vol.=448 μL) but greater activation in left posterior insula (BA 13; x=−44, y=-5, z=−4; Vol.=448μL).
Figure 2.
Group differences in activation during decision-making. Methamphetamine-Dependent (MD) participants showed significantly less activation than comparison participants (CTL) in the bilateral rostral anterior cingulate cortex (ACC) and significantly more activation in the left posterior insula during the decision making phase of the Risky Gains Task. Error bars represent standard error from the mean.
Group by decision interaction
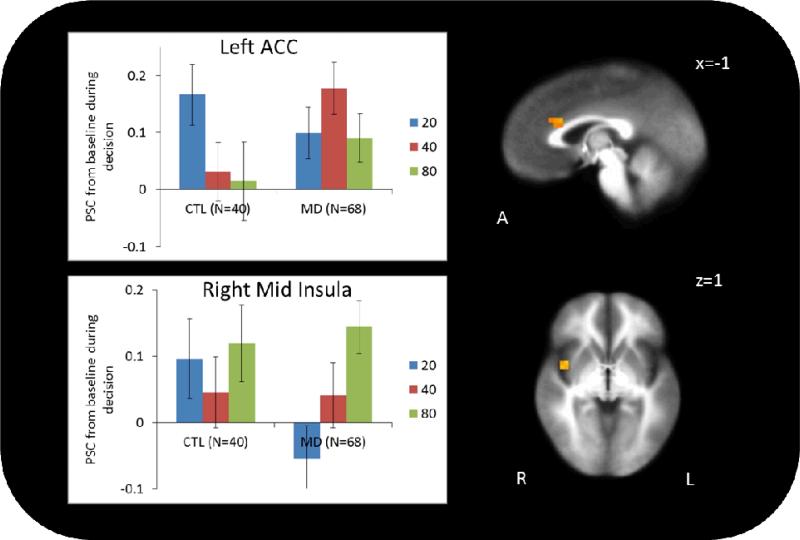
As seen in Figure 3, LME indicated significant group by decision interactions in left dorsal ACC (BA 32/33; x=−1, y=21, z=22, Vol.=448 μL) and right mid insula (BA 13; x=40, y=1, z=1, Vol.=320 μL). Specifically, whereas CTL showed diminished left dorsal ACC activation during risky relative to safe choices, MD showed no change. In the right mid insula, CTL showed no difference in activation between safe and risky choices, but MD showed greater activation during risky relative to safe choices, with greater activation during 80 than 40 decisions.
Figure 3.
Group by decision (risky versus safe) interaction. In the left dorsal ACC, CTL showed significantly less activation during risky relative to safe decisions. This is consistent with prior evidence in healthy volunteers that ACC activation is greatest during risk-aversion. MD showed no difference in activation between safe and risky decisions. In the right mid insula, CTL did not have different activation between safe and risky decisions, but MD showed significantly more activation during risky relative to safe decisions, with the highest activation during 80-point decisions. Error bars represent standard error from the mean.
Brain – Behavior Relationships
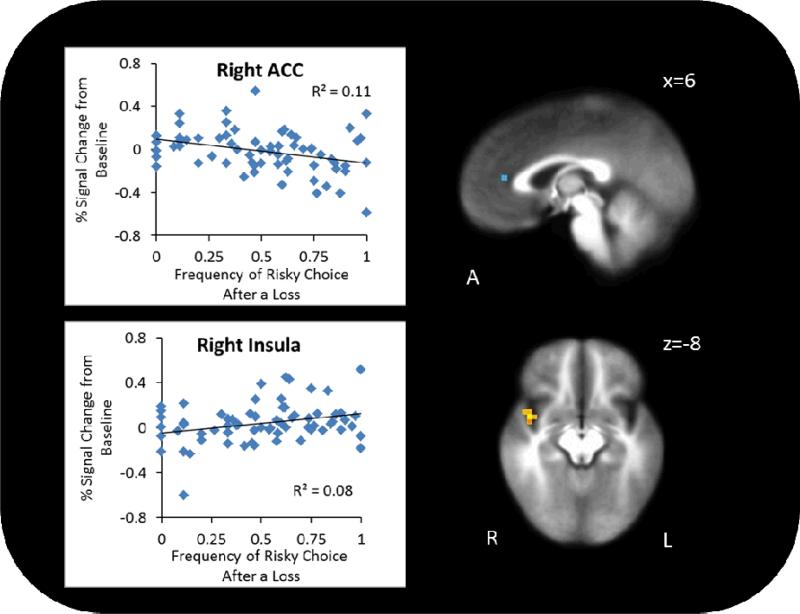
To determine if brain activation during risky decisions among MD was related to RGT performance, robust regressions were conducted examining the relationship between brain activation and risky choices a) overall and b) post-loss. Robust regression revealed no clusters with activity that correlated with overall risk-taking. However, as seen in Figure 4, frequency of risky choices post-loss significantly predicted lower right rostral ACC activation (x=−2, y=−35, z=12, Vol.=448 μL, r=−.39, p<.01) and higher right mid insula activation (x=42, y=4, z=−8, Vol.=320 μL, r=.35, p<.01) during risky decisions. There was a positive relationship between sensation-seeking and right posterior insula activation when controlling for impulsivity (r=.30, p=.01; see Supplemental Figure 4) and a negative relationship between impulsivity and bilateral posterior insula activation when controlling for sensation-seeking (r=−.031, p=.01; see Supplemental Figure 5).
Figure 4.
In the rostral right ACC, less activation was linked to greater likelihood of choosing a risky option following a loss. In the right mid insula, greater activation was linked to greater likelihood of choosing a risky option following a loss.
Brain – Clinical Characteristics Relationships
The relationship between brain activation during risky decisions and substance use was also examined within MD. After controlling for age, MD with a longer use history showed greater activation in bilateral mid insula (left cluster: x=−43, y=−25, z=18, Vol.=3136 μL r= 0.51, p<.01; right cluster: x=47, y=−26, z=19, Vol.=960 μL, r=.48, p<.01). After controlling for cocaine, cigarette, marijuana and alcohol use, lifetime number of methamphetamine uses was positively related to right rostral ACC activation (x=−2, y=−43, z=8, Vol.=2240 μL, r= 0.36, p<.01). No clusters in the ACC or insula showed a relationship with time since last use of methamphetamine.
Discussion
This study examined whether MD individuals exhibit altered behavioral and neural processing characteristics during risk-related decision-making and yielded three main results. First, relative to CTL, MD participants showed decreased activation in the bilateral rostral ACC, but increased activation in the left posterior insula during decision-making. Second, MD, but not CTL, individuals showed increased activation during risky relative to safe decisions in right mid insula, with the highest activation during 80-point decisions. Third, behavioral data provide some evidence that MD were more likely to continue engaging in risky behavior following a loss, and this tendency was associated with attenuated rostral ACC activity, which further supports the ACC's role in moderating risk-related behaviors. Together, these results support the notion that MD individuals show disrupted risk-related processing in both ACC and insula, brain areas that have been implicated in cognitive control and interoceptive processing.
Previous work in healthy volunteers showed that dorsal ACC activation during risk assessment was greater among risk-averse, rather than risk tolerant, individuals [9]. Consistently, in the present study CTL showed higher left dorsal ACC activation when they chose to avoid risk. MD, on the contrary, had equivalent activation regardless of choice, suggesting they may fail to distinguish between risky and safe options. Similarly, another study showed that MD showed comparable activation in the left dorsal ACC during easy and difficult options in a delay discounting task, whereas controls had differential activation [14].
MD participants also showed decreased bilateral rostral ACC activation during decision-making relative to CTL. Alternatively, the relatively attenuated activation may not reflect the individual's appreciation of the risk but rather the relative lack of this appreciation to significantly influence their selection of choices. MD participants with less rostral ACC activation during risky decisions were more likely to continue taking risks following a loss. The negative relationship between ACC activation and risk-taking following a loss may explain the evidence for a behavioral interaction effect, where MD continued taking risks following a loss while CTL shifted to a risk-averse strategy. Studies in rats have shown that chronic administration of amphetamine led to diminished firing of ACC neurons in response to excitatory inputs and impaired learning to avoid a lever that produced a shock [26]. Moreover, disruptions in subcortical-to-prefrontal circuits containing the ACC led rats to choose risky options more frequently, even after rewards for the risky option were reduced [27]. Diminished ACC activation in MD could relate to an inability to integrate newly obtained information about probability (e.g. a loss) into assessment of risky options.
Contrary to our hypothesis, MD, but not CTL, participants showed an increase in right mid insula activation during risky relative to safe decisions, with the highest activation during 80-point decisions. Mid insula activation was highest in MD participants who had used methamphetamine for the greatest number of years. The insula has been implicated as a key structure for generating representations of how a decision will make a person feel [28, 29]. For instance, drug related cues and craving produce heightened insula activity in cocaine-dependent individuals [30]. In conjunction with evidence that cocaine dependent individuals show greater insula activation after winning money relative to a comparison group [31], stimulant use may be associated with enhanced anticipation of reward in the insula. MD had higher activity in the left posterior insula across all decisions. Heightened insula activation among MD participants may signal oversight of possible losses, since insula activation during risk was positively correlated with the likelihood of continuing to choose a risky option following a loss. Moreover, as posterior insula has been shown to be part of the decision-making neural network [32, 33], increased insula activation among MD patients may reflect increased processing demands to fulfill the decision task. Hypersensitivity to reward in the insula may explain why MD individuals continue using drugs in the face of negative consequences.
The present study provided some evidence for a behavioral interaction effect, where MD participants were more likely than CTL to continue taking risks following a loss. This is consistent with previous studies showing that MD participants take more risks relative to comparison groups [3]. Risk-taking in the present task was a state-dependent measure, but trait-measurements of risk may produce a larger effect size. For example, adolescents with a history of substance abuse scored significantly higher than adolescents without such a history on a trait index of risk-taking [34]. In the present study MD participants scored significantly higher on trait measures of sensation seeking and impulsivity, personality measures that overlap with risk-taking. Future studies should employ state and trait measures of risk-taking to capture more of the total variance in risk-behavior profiles.
Although methamphetamine was the primary substance use disorder in these individuals, we cannot rule out that some of the observed behavioral and neural processing differences are a result of a combination of drugs impacting the brain's ability to appropriately process benefits and risks of an option. Nearly a third of MD participants met criteria for comorbid diagnosis of past dependence on another substance (e.g. alcohol, cocaine, marijuana). Also, greater cigarette and marijuana use was linked to increased insula activation, complicating interpretation of methamphetamine-specific effects. Nonetheless, the relationship between lifetime methamphetamine use and ACC activation remained significant even after controlling for cigarette, cocaine, alcohol and marijuana use, indicating the presence of methamphetamine-specific effects. Further, since comorbid substance use is prevalent among methamphetamine users [35], comorbid substance use in the present sample may make our results more generalizable.
Since our data are cross-sectional, it is possible that these neural differences pre-dated onset of MD. However, the correlations between years of methamphetamine use and insula activation suggest that altered activity is related to substance use. Rodent work suggests that repeated amphetamine administration leads to diminished ACC response to excitatory inputs [26]. Taken together, these results suggest that MD is associated with altered risk-related processing and that this alteration may be a consequence of years of drug use.
This study examines a particular stage of MD, early remission, and may reflect multiple adjustment processes. Previous studies suggest that risk-taking behavior decreases after a period of abstinence [36], but it remains unknown whether neural processing dysfunctions associated with methamphetamine dependence persist into remission or whether they undergo regenerative changes. For example, heightened insula activation during risk-taking could reflect increased insular sensitivity during the early remission state. Since past research has indicated that insula activation during decision-making offers predictive value for future methamphetamine relapse [37], future investigations should examine the extent to which brain activation during risk-taking offers prognostic information.
The present results are the first to examine neural processing of risk among a group primarily dependent on methamphetamine and suggest risk processing in the ACC and insula may be disrupted in MD individuals. ACC disruption may relate to a diminished impact of losses toward future decisions. Insular processing among MD individuals may focus on potential rewards resulting from decisions at the expense of losses. This may explain why MD individuals continue risky behavior even after experiencing negative outcomes. As altered risk-related processing and behavior was observed soon after MD participants entered treatment, it may contribute to relapse likelihood. Treatment providers should take this deficiency into consideration.
Supplementary Material
Acknowledgements
We would like to thank T. Flagan, H. Donovan, D. Leland, M. Mortezaei and B. Friedrich for assistance and support during data acquisition. We are grateful to Dr. Fred Berger from the Scripps McDonald Center, San Diego, for the recruitment of patients. This work was supported by grants from the National Institute on Drug Abuse (R01-DA016663, P20-DA027834, R01-DA027797, and R01-DA018307 to Martin Paulus).
Footnotes
Conflict of interest declaration: None
References
- 1.United Nations Office on Drugs and Crime . World Drug Report 2012. United Nations Office on Drugs and Crime; Vienna, Austria: 2012. [Google Scholar]
- 2.Leland DS, Paulus MP. Increased risk-taking decision-making but not altered response to punishment in stimulant-using young adults. Drug Alcohol Depend. 2005;78(1):83–90. doi: 10.1016/j.drugalcdep.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 3.Gonzalez R, Bechara A, Martin EM. Executive functions among individuals with methamphetamine or alcohol as drugs of choice: preliminary observations. J Clin Exp Neuropsychol. 2007;29(2):155–9. doi: 10.1080/13803390600582446. [DOI] [PubMed] [Google Scholar]
- 4.Ersche KD, et al. Abnormal frontal activations related to decision-making in current and former amphetamine and opiate dependent individuals. Psychopharmacology (Berl) 2005;180(4):612–23. doi: 10.1007/s00213-005-2205-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lane SD, Cherek DR. Analysis of risk taking in adults with a history of high risk behavior. Drug Alcohol Depend. 2000;60(2):179–87. doi: 10.1016/s0376-8716(99)00155-6. [DOI] [PubMed] [Google Scholar]
- 6.Platt ML, Huettel SA. Risky business: the neuroeconomics of decision making under uncertainty. Nat Neurosci. 2008;11(4):398–403. doi: 10.1038/nn2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Preuschoff K, Quartz SR, Bossaerts P. Human insula activation reflects risk prediction errors as well as risk. J Neurosci. 2008;28(11):2745–52. doi: 10.1523/JNEUROSCI.4286-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rudorf S, Preuschoff K, Weber B. Neural correlates of anticipation risk reflect risk preferences. J Neurosci. 2012;32(47):16683–92. doi: 10.1523/JNEUROSCI.4235-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown JW, Braver TS. Risk prediction and aversion by anterior cingulate cortex. Cogn Affect Behav Neurosci. 2007;7(4):266–77. doi: 10.3758/cabn.7.4.266. [DOI] [PubMed] [Google Scholar]
- 10.Paulus MP, Frank LR. Anterior cingulate activity modulates nonlinear decision weight function of uncertain prospects. Neuroimage. 2006;30(2):668–77. doi: 10.1016/j.neuroimage.2005.09.061. [DOI] [PubMed] [Google Scholar]
- 11.Gowin JL, Mackey S, Paulus MP. Altered risk-related processing in substance users: Imbalance of pain and gain. Drug Alcohol Depend. 2013 doi: 10.1016/j.drugalcdep.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fishbein DH, et al. Risky decision making and the anterior cingulate cortex in abstinent drug abusers and nonusers. Brain Res Cogn Brain Res. 2005;23(1):119–36. doi: 10.1016/j.cogbrainres.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 13.London ED, et al. Cerebral metabolic dysfunction and impaired vigilance in recently abstinent methamphetamine abusers. Biological Psychiatry. 2005;58(10):770–8. doi: 10.1016/j.biopsych.2005.04.039. [DOI] [PubMed] [Google Scholar]
- 14.Hoffman WF, et al. Cortical activation during delay discounting in abstinent methamphetamine dependent individuals. Psychopharmacology. 2008;201(2):183–93. doi: 10.1007/s00213-008-1261-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nestor LJ, et al. Prefrontal hypoactivation during cognitive control in early abstinent methamphetamine-dependent subjects. Psychiatry research. 2011;194(3):287–95. doi: 10.1016/j.pscychresns.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paulus MP, et al. Decision making by methamphetamine-dependent subjects is associated with error-rate-independent decrease in prefrontal and parietal activation. Biol Psychiatry. 2003;53(1):65–74. doi: 10.1016/s0006-3223(02)01442-7. [DOI] [PubMed] [Google Scholar]
- 17.Paulus MP, et al. Increased activation in the right insula during risk-taking decision making is related to harm avoidance and neuroticism. Neuroimage. 2003;19(4):1439–48. doi: 10.1016/s1053-8119(03)00251-9. [DOI] [PubMed] [Google Scholar]
- 18.Hesselbrock M, et al. A validity study of the SSAGA--a comparison with the SCAN. Addiction. 1999;94(9):1361–70. doi: 10.1046/j.1360-0443.1999.94913618.x. [DOI] [PubMed] [Google Scholar]
- 19.Uttl B. North American Adult Reading Test: age norms, reliability, and validity. J Clin Exp Neuropsychol. 2002;24(8):1123–37. doi: 10.1076/jcen.24.8.1123.8375. [DOI] [PubMed] [Google Scholar]
- 20.Wechsler D. Wechsler test of adult reading. Pearson Psychological Corporation; San Antonio, TX: 2001. [Google Scholar]
- 21.Kruschwitz JD, et al. Nothing to lose: processing blindness to potential losses drives thrill and adventure seekers. Neuroimage. 2012;59(3):2850–9. doi: 10.1016/j.neuroimage.2011.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zuckerman M. Item revisions in the Sensation Seeking Scale Form V (SSS-V). Personality and Individual Differences. 1996;20(4):515–515. [Google Scholar]
- 23.Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. J Clin Psychol. 1995;51(6):768–74. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 24.Lancaster JL, et al. Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp. 2000;10(3):120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huber PJ. Robust Estimation of a Location Parameter. The Annals of Mathematical Statistics. 1964;35(1):73–101. [Google Scholar]
- 26.Tse MT, Cantor A, Floresco SB. Repeated amphetamine exposure disrupts dopaminergic modulation of amygdala-prefrontal circuitry and cognitive/emotional functioning. J Neurosci. 2011;31(31):11282–94. doi: 10.1523/JNEUROSCI.1810-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.St Onge JR, et al. Separate prefrontal-subcortical circuits mediate different components of risk-based decision making. J Neurosci. 2012;32(8):2886–99. doi: 10.1523/JNEUROSCI.5625-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ernst M, Paulus MP. Neurobiology of decision making: a selective review from a neurocognitive and clinical perspective. Biological Psychiatry. 2005;58(8):597–604. doi: 10.1016/j.biopsych.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 29.Lovero KL, et al. Anterior insular cortex anticipates impending stimulus significance. Neuroimage. 2009;45(3):976–83. doi: 10.1016/j.neuroimage.2008.12.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kilts CD, et al. Neural activity related to drug craving in cocaine addiction. Archives of general psychiatry. 2001;58(4):334–41. doi: 10.1001/archpsyc.58.4.334. [DOI] [PubMed] [Google Scholar]
- 31.Jia Z, et al. An initial study of neural responses to monetary incentives as related to treatment outcome in cocaine dependence. Biol Psychiatry. 2011;70(6):553–60. doi: 10.1016/j.biopsych.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wittmann M, Leland DS, Paulus MP. Time and decision making: differential contribution of the posterior insular cortex and the striatum during a delay discounting task. Exp Brain Res. 2007;179(4):643–53. doi: 10.1007/s00221-006-0822-y. [DOI] [PubMed] [Google Scholar]
- 33.Craig AD. How do you feel--now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10(1):59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- 34.Lane SD, Cherek DR. Risk taking by adolescents with maladaptive behavior histories. Exp Clin Psychopharmacol. 2001;9(1):74–82. doi: 10.1037/1064-1297.9.1.74. [DOI] [PubMed] [Google Scholar]
- 35.Substance Abuse and Mental Health Services Administration . Results from the 2011 National Survey on Drug Use and Health: Summary of National Findings. Substance Abuse and Mental Health Services Administration; Rockville, MD: 2012. NSDUH Series H-44, HHS Publication No. (SMA) 12-4713. [Google Scholar]
- 36.Aklin WM, et al. Risk-Taking Propensity Changes Throughout the Course of Residential Substance Abuse Treatment. Pers Individ Dif. 2009;46(4):454–459. doi: 10.1016/j.paid.2008.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paulus MP, Tapert SF, Schuckit MA. Neural activation patterns of methamphetamine-dependent subjects during decision making predict relapse. Arch Gen Psychiatry. 2005;62(7):761–8. doi: 10.1001/archpsyc.62.7.761. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.