Abstract
Recombinant immunotoxins (RITs) are agents being developed for cancer treatment. They are composed of an Fv that binds to a cancer cell, fused to a 38-kDa fragment of Pseudomonas exotoxin A. SS1P is a RIT that targets mesothelin, a protein expressed on mesothelioma as well as pancreatic, ovarian, lung and other cancers. Because the protein tyrosine kinase (TK) family regulates a variety of cellular processes and pathways, we hypothesized that TKs might regulate susceptibility to immunotoxin killing. To investigate their role we used siRNAs to lower the level of expression of the 88 known TKs. We identified 5 TKs, INSR, HCK, SRC, PDGFRβ, and BMX that enhance the activity of SS1P when their level of expression is lowered by siRNAs. We further investigated the Src family member HCK in this study. Knocking down of SRC slightly increased SS1P killing in A431/H9 cells, but knocking down HCK substantially enhanced killing by SS1P. We investigated the mechanism of enhancement and found that HCK knock down enhanced SS1P cleavage by furin and lowered levels of Mcl-1 and raised Bax. We then found that Src inhibitors mimic the stimulatory effect of HCK knock down, both SU6656 and SKI-606 (Bosutinib) enhanced immunotoxin killing of mesothelin expressing cells by SS1P and CD22 expressing cells by HA22 (Moxetumomab pasudotox). SU6656 also enhanced the antitumor effects of SS1P and HA22 in mouse xenograft tumor models. Our data suggest that the combination of immunotoxin with TK inhibitors may be an effective way to treat some cancers.
Keywords: Cancer treatment, Bosutinib, SU6656, Moxetumomab pasudotox, SS1P
Introduction
Recombinant immunotoxins (RITs) are chimeric proteins being developed to treat cancer. They are composed of the Fv portion of an antibody linked to a bacterial or plant toxin (1). We produce RITs by linking a 38-kDa portion of Pseudomonas exotoxin A (PE) to Fvs reacting with either CD22 present on the surface of B cell leukemias and lymphomas or with mesothelin present on mesotheliomas and several other epithelial malignancies (2, 3). HA22, also known as Moxetumomab pasudotox, is a RIT that kills CD22 expressing cells. It has been shown to be very active in drug resistant hairy cell leukemia (HCL); in a phase 1 trial it had a 90% response rate with 50% of subjects obtaining a complete remission (4). HA22 is also being tested in children with drug resistant acute lymphoblastic leukemia (ALL) and has produced several complete remissions in that disease, although the response rate is lower than in HCL (5). SS1P is a RIT targeting mesothelin-expressing tumors. When tested by itself, it had low antitumor activity in patients with mesothelioma and ovarian cancer (6, 7), but appears to have more activity when combined with cis platinium and permetrexed to treat mesothelioma (8, 9).
Our current efforts are directed at increasing immunotoxin activity in patients by determining how the steps in the pathway by which immunotoxins kill cells are regulated and using this information to identify drugs that will modify these steps and enhance cell killing (10, 11). The mechanism by which RITs kill cells is complex and although much is known it is not fully understood (12–14). Following binding to the receptor on the cell surface, the RIT is internalized by receptor-mediated endocytosis and undergoes processing by furin, which separates the Fv from the toxin. The toxin fragment, which contains a REDL sequence at its C terminus, can then bind to the KDEL receptor and be transported through the Golgi to the endoplasmic reticulum, where it escapes into the cytosol. In the cytosol it catalyzes the ADP-ribosylation and inactivation of elongation factor 2 (EF2) leading to the arrest of protein synthesis. This event initiates the apoptotic cascade by lowering Mcl-1 levels and unleashing Bak to promote apoptosis (11).
Because protein phosphorylation is a major mechanism of protein regulation, and TKs are often activated in cancer cells, we have begun to examine the role of protein phosphorylation in the killing of cells by immunotoxins SS1P and HA22. We have used siRNAs to lower the level of TKs and assess the response of cancer cells to SS1P or HA22. We recently reported that lowering expression of the insulin receptor (INSR) enhanced immunotoxin action (15). We chose to examine members of the Src family because Src kinases contribute to important cellular signal pathways, including cell growth, differentiation, cell shape and migration (16, 17). Many Src family kinases are identified as oncogenes and play important roles in tumor development (18).
We report here that knock down of HCK, and to a lesser extent, SRC, enhance immunotoxin action by a different mechanism than that regulated by the INSR. We also report that two Src family inhibitors, SU6656 and Bosutininb, enhanced immunotoxin killing of cultured cells and that SU6656 synergized with immunotoxins HA22 or SS1P to cause tumor regression in tumor bearing mice.
Material and Methods
Reagents
Immunotoxins SS1P and HA22 were purified in our laboratory as described in Pastan et al. (19). Anti-β-tubulin, 2-hydroxypropyl-beta-cyclodextran, and SU6656 were purchased from Sigma (St. Louis, MO). Bosutinib (SKI-606) was purchased from LC Laboratories (Woburn, MA). Anti-Bcl2, anti-PARP, anti-cleaved caspase-3, anti-Bax, anti-Bcl-xL, and anti-Mcl-1 were purchased from Cell Signaling (Danvers, MA). Anti-actins were purchased from Abcam (Cambridge, MA). All the siRNAs were purchased from Dharmacon (Lafayette, CO) and are listed in Supplemental Table S1. CellTilter-Glo kit was purchased from Promega (Madison, WI).
Cell culture, transfection and cytotoxicity assay
Human cell lines A431/H9, A1847, KLM-1 and CA46 were cultured in DMEM supplemented with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin in a humidified atmosphere of 5% CO2 at 37°C. The cell lines A431/H9 and A1847 were described previously (15). Pancreatic cell line KLM1 was from Dr. Udo Rudloff (NCI, NIH, Bethesda, MD). CA46 was purchased from ATCC. Their identities were confirmed by short tandem repeat analysis within a year.
Cells were transfected at 5000 cells per well in 96-well plates by the addition of 0.3 μl of 20 μM siRNA and 0.35 μl of DharmaFECT Transfection Reagent 3 in a final volume of 125 μl. After 48 hours, the cells were treated with SS1P for 72 hours. In some experiments, inhibitors were added 1 hour before SS1P. Cell viability was measured with a Cell Tilter-Glo kit. Cell viability is expressed as the percentage of luminescence with SS1P compared to control without SS1P treatment.
Western blot analysis
Cells were washed in phosphate buffered saline (PBS) and lysed by the addition of lysis buffer (50 mM Tris HCl, 150 mM NaCl, 5 mM EDTA, 1% NP40, 5 μg/ml leupeptin, 5 μg/ml aprotinin, 10 μM PMSF) on ice for 30 minutes. After high-speed centrifugation, supernatants were analyzed by SDS-PAGE, transferred to PVDF membranes and subjected to western blot analysis.
Internalization and FACS analysis
Immunotoxin SS1P was conjugated with alex-647 as described previously (15). After SS1P binds to mesothelin on the cell surface, it is internalized into the cells, and the fluorescently labeled cells are detected by FACS analysis. A431/H9 cells were transfected with siRNA for 48 hours in 6-well plates, 1 μg/ml of SS1P-Alexa-647 was added and incubated at 37°C for indicated time. The control cells are the same cells as the experimental group except there was no addition of SS1P-Alexa-647. After labeling, the cells were washed with PBS and stripped with glycine buffer containing 0.2 mol/L glycine (pH2.5) and 1 mg/ml of bovine serum albumin to remove surface bound SS1P. Cells were then trypsinized and washed with FACS buffer (PBS with 5% FBS and 0.1% NaN3) and analyzed by flow cytometry using FACS Calibur.
SS1P cleavage
A431/H9 cells were transfected with siRNA for 48 hours in 6-well plates. One μg/ml of SS1P was added and cells incubated on ice for 30 minutes to saturate SS1P binding. Cells were changed to fresh media and incubated at 37°C for the indicated time before making a total cell lysate and western blot analysis.
Real Time PCR
RNAs were isolated using Trizole reagent (Invitrogen). Reverse transcription and cDNA synthesis were performed with Quantitect Reverse transcription kit following the manufacturer’s instructions (Qiagen, Valencia, CA). Human actin primers were used as an internal control. The primer sequences are listed in Supplemental Table S1. The PCR reaction was performed using Quantifast SYBR green PCR master kit (Qiagen).
Xenograft tumor model
1.8 Million A431/H9 cells or 10 million CA46 cells with Matrigel (800 μg in 200 μl per mouse) were implanted subcutaneously into a rear leg of athymic nude mice. When tumors reached 100 mm3 (day 6 or 7), 5 or 8 mice in each group were injected intraperitoneally with 300 μg of SU6656 suspended in 20% hydroxydextran. Thirty minutes later, 8 μg SS1P or 6 μg of HA22 was injected intravenously. A total of three doses were injected every other day. Tumor volumes were calculated and measured as previously described (20). The animal protocol was approved by the National Cancer Institute Animal Care and Use Committee. All animal experiments were stopped when tumors reached 1000 mm3.
Statistics
Statistical analysis of synergy was performed by David Venzon (Biostatistics and Data Management Section, Center for Cancer Research, National Cancer Institute, Bethesda, MD). Repeated measures ANOVA was applied to the changes in successive tumor spherical diameters. Synergy was defined as an interaction effect significantly greater than the sum of the drug and SS1P effect (21).
Results
TK siRNA library screen
To identify which TKs regulate immunotoxin killing, we treated A431/H9 cells, which express high levels of mesothelin, with siRNAs targeting all of the 88 known TKs. After 48 hours we added the immunotoxin (SS1P) and incubated the cells 72 hours to allow apoptosis to occur. We assessed cell death using an assay that measures the cellular level of ATP. Nonspecific siRNA and luciferase siRNA (GL2) served as negative controls (Con), and siMSLN, targeting mesothelin served as positive control. As expected, siMSLN prevents SS1P killing (Supplemental Figure S1). We identified 12 siRNAs (BMX, FES, INSR. KDR, KIT, MUSK, PDGFR2, TNK1, HCK, SRC, YES1 and LYN) that enhanced cell killing and 3 siRNA (MATK, IGF-1R and EPHA5) that blocked cell killing. Because the siRNA pools consisted of 4 different oligonucleotides and because the pools could have off target effects we then confirmed the results using specific oligos. We found that knock down of 5 genes, SRC, HCK, PDGFRβ, BMX and INSR, reproducibly enhanced killing of cells by SS1P. Single oligos targeting FES, KDR, KIT, MUSK, TNK1, YES1 and LYN, respectively, did not reproducibly enhance SS1P toxicity. The results with INSR were described previously (15). Knock down of Src slightly enhanced SS1P killing in both A431/H9 and KB cells (IC50 value decreased 25% in both cell lines, Supplemental Fig. S2). However, knock down of HCK gene greatly enhanced SS1P toxicity, with the IC50 decreasing 3-fold as described below.
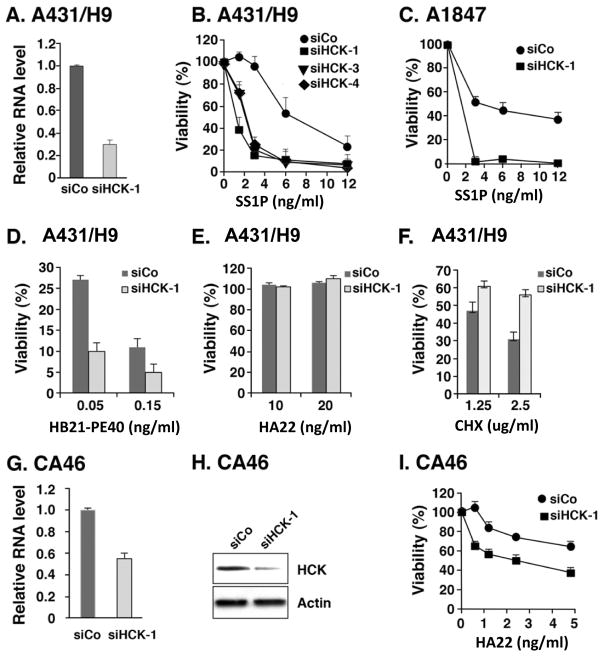
To demonstrate that the siRNA lowered HCK expression we analyzed HCK RNA by RT-PCR and found HCK RNA was decreased by 70% (Fig. 1A). The levels of HCK protein are very low in A431/H9 cells and could not be detected by antibody on western blots. To assess specificity further we used siRNAs that target other regions of HCK RNA and found that siHCK-3 and siHCK-4 also enhanced the cytotoxic action of SS1P (Fig. 1B). Since many ovarian cancers express mesothelin, we assessed the ability of siHCK-1 to enhance killing of the ovarian cancer line A1847 by SS1P, and found a marked enhancement in cytotoxicity in that cell line (Fig. 1C).
Figure 1. HCK knock down specifically enhanced immunotoxin toxicity.
A. siRNA siHCK-1 and control siRNA (GL2, luciferase siRNA) were transfected into A431/H9 cells; after 48 hours RNAs were analyzed by real time PCR. B. siHCK1, siHCK3, siHCK4 or control siRNAs were transfected into A431/H9 cells for 48 hours. Cell viability was measured by ATP assay after SS1P treated for 72 hours, and the viability (%) expressed as the percentage of luminescence with SS1P compared to control. C. siHCK or control siRNAs were transfected into A1847 cells and cell viability measured as in Figure 1B. D–F. A431/H9 cells were transfected with siCo or siHCK-1; 48 hours later, cells were treated with HB21-PE40 (D), HA22 (E) or CHX (F) for 72 hours, and cell viability was measured by ATP assay. G–I. CA46 cells were transfected with siHCK-1 or control siRNA (siCo) for 48 hours, real time PCR (G) or western blot (H) were analyzed for expression of HCK RNA (G), protein (H), or cells were treated with HA22 and cell viability was determined after 72 hours of treatment by an ATP assay (I).
To determine if the HCK knock down effect would enhance killing of A431/H9 cells by an immunotoxin targeting another receptor on A431/H9 cells, we treated the cells with immunotoxin HB21-PE40 (anti-TFR-PE40) that targets the human transferrin receptor and found that its cytotoxic activity was greatly increased upon knock down of HCK (Fig. 1D). We also tested the effects of HCK knock down using immunotoxin HA22, which targets CD22, not expressed on A431/H9 cells, and found that cytotoxicity was not induced by HCK knock down (Fig. 1E). We also studied the effects of HCK knock down using cycloheximide (CHX), which inhibits total protein synthesis. As shown in Figure 1F, knock down of HCK did not stimulate CHX-induced cell killing. These results demonstrate that knock down of HCK enhances the activity of immunotoxins that have specific receptors on target cells and does not provoke nonspecific internalization or cell killing of a non-targeted immunotoxin.
To determine if siHCK knock down would enhance killing of a lymphoma cell line, we used CA46 cells, which express CD22 and the immunotoxin HA22 that targets CD22 expressing cells. We transfected CA46 cells with siHCK-1 and found it decreased HCK RNA and protein levels by about 50% (Fig. 1G and 1H). Since transfection efficiencies are often low using non-adherent cells we ascribe this moderate degree of level of knock down to poor transfection efficiency. Nevertheless it was sufficient to allow cytotoxicity studies and as shown in Figure 1I, the cytotoxic activity of HA22 was significantly stimulated by HCK knock down; the IC50 value shifted from above 5 ng/ml to 2.4 ng/ml. We conclude that HCK knock down can enhance immunotoxin action on several different cell types.
HCK knock down stimulates SS1P processing
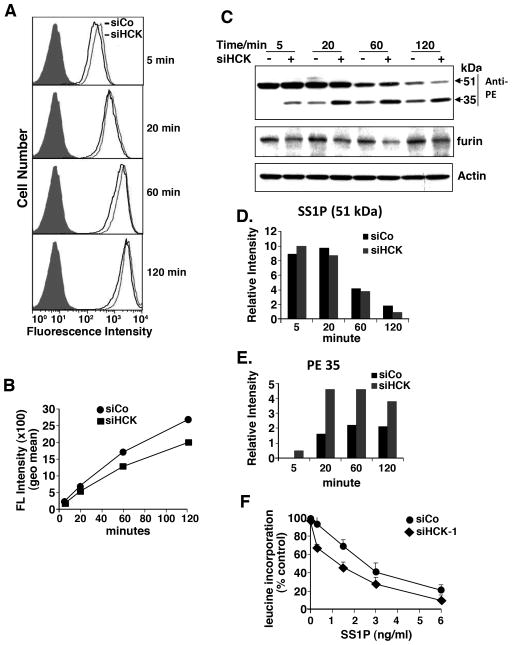
To investigate where in the intoxication pathway the stimulatory effect of HCK knock down was occurring, we examined several different steps in the intoxication pathway. We began by examining the uptake of SS1P using SS1P labeled with a fluorescent dye as previously described (15). We found that the uptake of SS1P was not increased and was actually slightly decreased upon HCK knock down (Fig. 2A–B). These data show that enhanced entry of SS1P does not explain the increase in cytotoxic activity.
Figure 2.
HCK knock down did not affect SS1P internalization, but affected SS1P cleavage. A. SS1P internalization after knock down of HCK. A431/H9 cells were transfected with siHCK-1 for 48 hours and SS1P-Alexa-647 was added and incubated at 37°C for indicated time before FACS analysis. B. SS1P fluorescence intensity (Geometric mean) analyzed by FACS (shown in A) plotted over time. C. SS1P cleavage after knock down of HCK. A431/H9 cells were transfected with siHCK-1 for 48 hours, 1 μg/ml of SS1P was added and incubated on ice for 30 minutes then chased at 37°C for indicated time. Total cell lysates were analyzed with polyclonal antibody anti-PE by western blot. PE35 is the cleaved form of SS1P. D–E. Representative western blot was scanned and analyzed by NIH image J, the relative intensity of heavy chain-toxin SS1P (D) or PE35 (E) was plotted. F. Protein synthesis inhibition assay: A431/H9 cells were transfected with siHCK-1 and control GL2 for 48 hours, SS1P was added for 20 hours; protein synthesis was measured by 3H-leucine incorporation.
The next step in the pathway is the processing of SS1P by furin, which cleaves the toxin between residues 279 and 280 of PE and generates an Fv fragment and a 35-kDa fragment of PE containing the ADP-ribosylating activity (14). While furin protein levels did not increase after knock down of HCK, the amount of the 35-kDa fragment increased about 2–3 fold at the 5, 20 and 60 minute time points when HCK is knocked down (Fig. 2C, 2D and 2E).
An increased amount of processed immunotoxin should lead to more of the toxin fragment delivered to the endoplasmic reticulum and the cytosol, a more rapid inactivation of EF2 and a decrease in protein synthesis. Protein synthesis in SS1P treated cells was measured by 3H-leucine incorporation (11). Figure 2F shows that in cells treated with increasing amounts of SS1P, there is a greater decrease in leucine incorporation in cells treated with siRNAs for HCK compared to a control siRNA. IC50 decreased from 2.5 to 1.2 ng/ml. This indicates that the fragment of PE produced by increased processing can reach the cytosol and inhibit protein synthesis to a greater extent.
Knock down of HCK decreases Mcl-1 levels and increases Bax levels
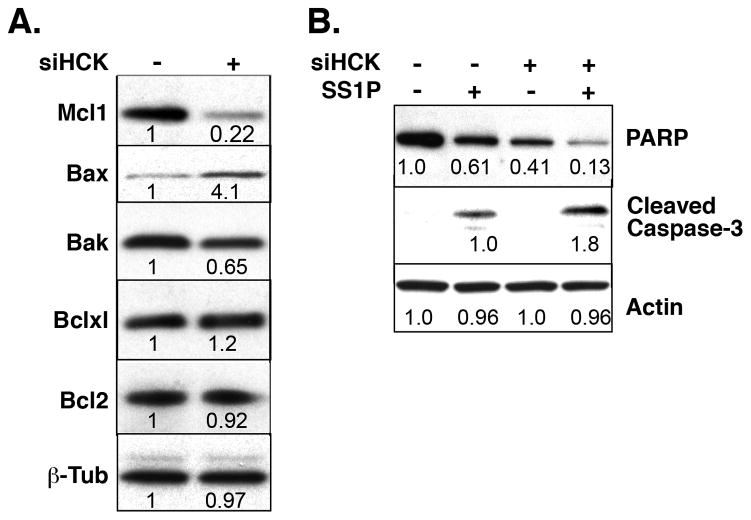
To investigate if knock down of HCK also affected the levels of proteins involved in apoptosis and previously shown to control immunotoxin killing (11), we examined the levels of Bak, Bax, Mcl-1, Bcl2 and BclxL in A431/H9 cells by western blot. Figure 3A shows that the level of the anti-apoptotic protein Mcl-1 decreased about 5-fold and the level of the pro-apoptotic protein Bax was increased about 4-fold after knock down HCK. The changes in Bclxl, Bcl2 and Bak were much smaller. We examined the markers of apoptosis PARP and cleaved caspase-3 (Fig. 3B) and found that HCK knock down by itself substantially decreased full-length PARP levels. When knock down was combined with SS1P treatment, PARP levels were further decreased and cleaved caspase-3 levels increased, as would be expected in cells undergoing apoptosis.
Figure 3. Knock down of HCK decreased Mcl-1 level and stimulated apoptosis by SS1P.
A. A431/H9 cells were transfected with siHCK-1 or control siRNA for 48 hours and cell lysates were analyzed by western blot. B. Cells were transfected for 48 hours and further treated with 10 ng/ml of SS1P for 24 hours. The relative intensities were determined from scans analyzed with NIH image J.
Src family kinase inhibitors
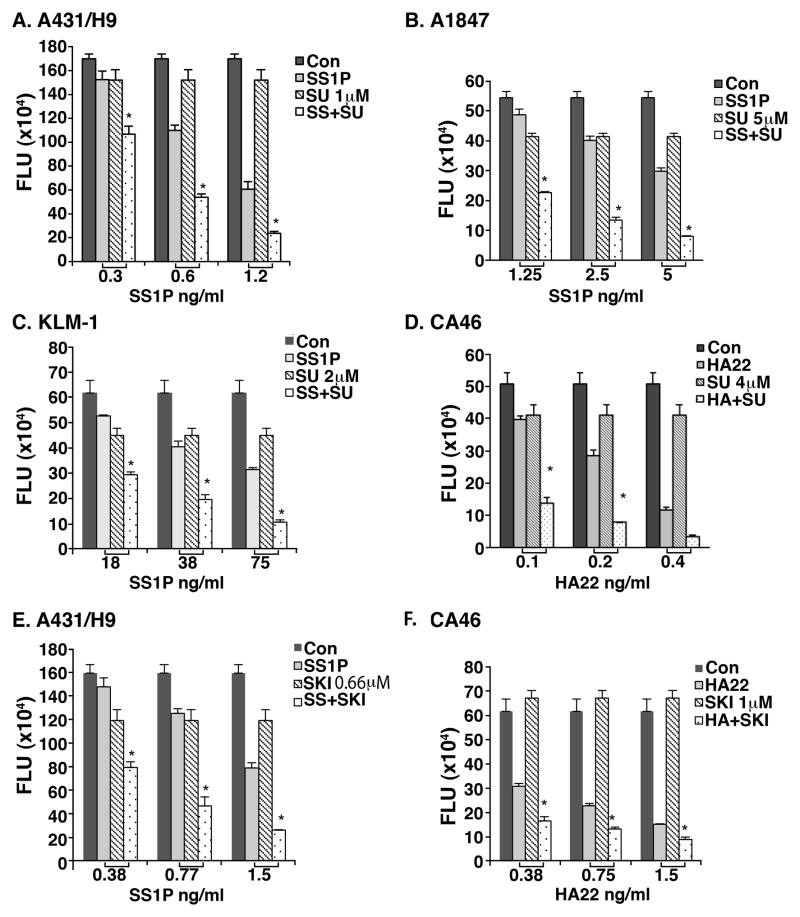
To investigate if we could enhance SS1P or HA22 activity with a TK inhibitor, we tested SU6656 and SKI-606 (Bosutinib), known to inhibit members of the Src family (22, 23). We found that combining the Src kinase inhibitor (SU6656) with SS1P gave a synergistic inhibition of cell growth compared with the addition of either agent alone, on three different epithelial cancer lines A431/H9, A1847 and KLM-1, a pancreatic cancer line (Fig. 4A–C). SU6656 also enhanced the cytotoxic activity of HA22 on the CA46 lymphoma cell line (Fig. 4D). Bosutinib/SKI606 synergistically enhanced killing of A431/H9 cells by SS1P and CA46 cells by HA22 (Fig. 4E and 4F, respectively).
Figure 4. SU6656 and SKI-606 stimulated SS1P and HA22 toxicity in cell lines.
5000 cells (A431/H9, A1847 and KLM-1) or 10, 000 cells of CA46 were plated in 96-well plates overnight. SU6656 (A–D) or SKI-606 (E–F) at indicated concentrations was added to cultures 1 hour before SS1P or HA22 addition. After 72 hours, cell viability was measured by an ATP assay. Stars (*) indicate synergistic effects of immunotoxin and SU6656 or SKI-606. SS = SS1P, Su = SU6656, and SKI = SKI606.
SU6656 enhanced immunotoxin activity in mice xenografts
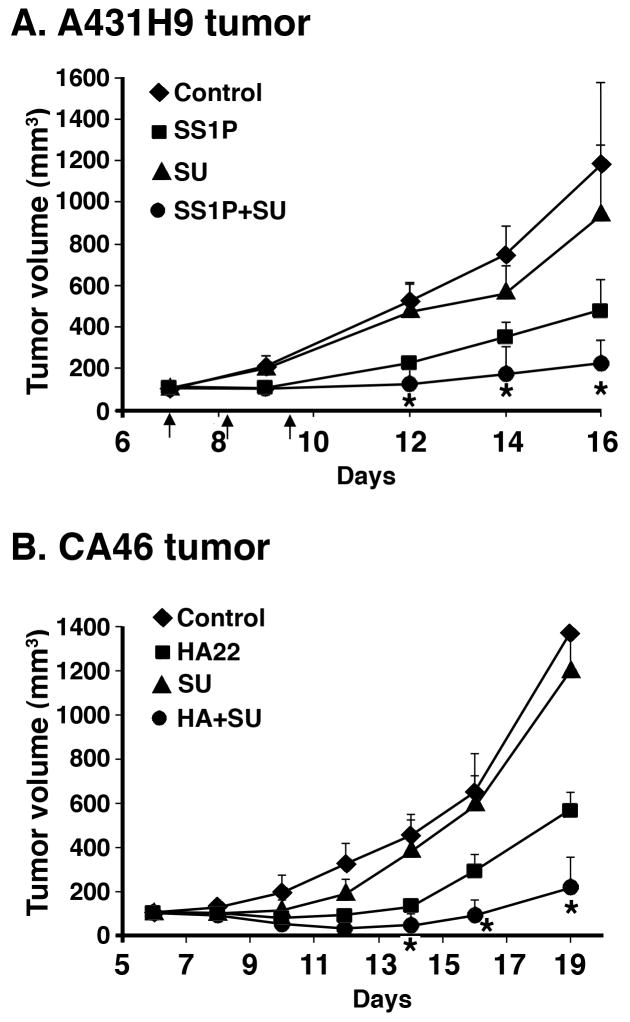
To determine if SU6656 could enhance the antitumor activity of SS1P, mice were implanted with A431/H9 cells and treatment was initiated on day 6. Mice received either SS1P or SU6656 or SS1P and Su6656 in combination. As shown in Figure 5A, when mice were treated with SU6656, there was a minimal change in tumor growth compared with control. SS1P treatment significantly reduced tumor growth, and tumor growth was retarded further when the two agents were combined. Statistical analysis indicated that the drugs showed a synergistic effect on days 12, 14 and 16 (p <0.01).
Figure 5. SU6656 enhanced SS1P and HA22 toxicity in mouse xenograft model.
A431/H9 cells or CA46 cells were implanted subcutaneously into athymic nude mice. When tumors reached 100 mm3, 3 doses of SU6656 and 3 doses of SS1P (A) or HA22 (B) were injected. The mean tumor volume of each group of five mice (A) or eight mice (B) was presented as a function of time after injection of cells. Arrows indicate the injection times. Stars (*) indicate synergistic effects of SU6656 with SS1P or HA22.
We tested the effect of SU6656 in mice bearing CA46 lymphomas and found that SU6656 alone had very little effect on the growth of the tumors but greatly enhanced the antitumor activity of HA22 (Fig. 5B). The asterisks indicate that on days 14, 16 and 19 the combination had a synergistic effect on inhibiting tumor growth (P=0.011, P<0.0001, and P=0.001, respectively).
Discussion
We have used siRNAs targeting members of the 88 TKs to identify genes that can regulate immunotoxin killing, and found that knock down of several TK genes enhanced killing of A431/H9 cells. These include HCK, which produces a large enhancement and SRC whose effect is less. There are nine members of the Src family (Src, Yes, Lyn, Fyn, Blk, Fgr, Lck, Hck and Frk); the other seven members were not confirmed to be active in our study. Because the substrate specificities of the various family members overlap, we were surprised that we did not find similar results with knock down of other family members. It is possible that Hck is specifically associated with proteins that regulate toxin action or that Hck has a substrate specificity that explains its selective effect. It is also possible that in cells other than A431/H9, other members of the Src family can regulate immunotoxin action. This can be investigated by performing knock down experiments with the other Src family members in different types of cells. However, the finding that HCK knock down also enhances killing of A1847 ovarian cancer cells and CA46 lymphoma cells indicates that Hck has an important regulatory role in several types of cancer.
We have previously shown that knock down of the INSR can enhance immunotoxin action by increasing the processing of the immunotoxin by furin (15). Knock down of HCK similarly increased the furin cleavage and increased the amount of 35-kDa PE fragment, indicating that Hck also modulates immunotoxin processing. Hck has additional effects not seen with INSR knock down. It has major effects on the levels of two important proteins that regulate apoptosis: lowering HCK decreases the anti-apoptotic Mcl-1 protein and elevates the pro-apoptotic Bax protein. This is the first report that HCK can regulate pro/anti-apoptotic protein levels. There are studies on other Src family kinases; for example, Src and Fyn play a role in the anti-apoptotic response in fibroblasts (24) and knock down of Lyn induced caspase-8 activation in a mesothelioma cell line (25). The mechanism by which HCK regulates the levels of apoptotic proteins will be studied in the future.
The protein encoded by the HCK gene was originally identified in hematopoietic cells (26), and its function is being studied in lymphoid and myeloid cells (27, 28). It is now known to be expressed in many different tissues and cell types (http://www.genecards.org/cgi-bin/carddisp.pl?gene=HCK), although its role in non-hematopoietic cells has not been extensively studied. Our studies have identified an important role for Hck in regulating the levels of Mcl-1 and Bax in an epithelial cancer cell line and the mechanism by which this occurs needs further study.
The major goal of our experiments was to identify therapeutic agents that could be used in patients to enhance immunotoxin action. Recently, Src family kinases were found to be hyper-activated in malignant mesothelioma specimens and cell lines compared with normal mesothelial cells (29), and Src activation in mesothelioma samples was found to correlate with a more advanced pathologic stage and the presence of metastasis (30). Also Lck expression was found to be correlated with resistance to dexamethasone in chronic lymphocytic leukemia (31). Moreover, Src kinase activity was found to be constitutively high in many human B lymphoma cell lines and primary lymphoma samples. The inhibitors of SFKs, PP1 and PP2 inhibited the proliferation of human B lymphomas in a dose-dependent manner (32). We identified two Src kinase inhibitors Bosutinib (SKI-606) and SU6656 that show promise. We found that both of these inhibitors would replicate the effect of HCK knock down and enhance immunotoxin killing of both epithelial and lymphoma cells. We performed antitumor experiments in mice with SU6656 and found that using a dose of SU6656, which by itself had no antitumor activity, produced synergistic antitumor effects when combined with an immunotoxin targeting mesothelin on an epithelial cancer or an immunotoxin that targets CD22 on B cell malignancies (Fig. 5). Both of these agents are in clinical trials and HA22 as a single agent has produced complete regression in several children with ALL (5). The combination of HA22 and Bosutinib, which is approved for chronic myelocytic leukemia (http://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm318203.html), might be useful for treating children with ALL who have a poor response to HA22.
Supplementary Material
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research
Footnotes
The authors declare no conflict of interest
References
- 1.Wu AM, Senter PD. Arming antibodies: prospects and changes for immunoconjugates. Nat Biotechnol. 2005;23:1137–46. doi: 10.1038/nbt1141. [DOI] [PubMed] [Google Scholar]
- 2.Pastan I, Hassan R, Fitzgerald DJ, Kreitman RJ. Immunotoxin therapy of cancer. Nat Rev Cancer. 2006;6:559–65. doi: 10.1038/nrc1891. [DOI] [PubMed] [Google Scholar]
- 3.FitzGerald DJ, Wayne AS, Kreitman RJ, Pastan I. Treatment of hematologic malignancies with immunotoxins and antibody-drug conjugates. Cancer Res. 2011;71:6300–9. doi: 10.1158/0008-5472.CAN-11-1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kreitman RJ, Tallman MS, Robak T, Coutre S, Wilson WH, Stetler-Stevenson M, et al. Phase I trial of anti-CD22 recombinant immunotoxin moxetumomab pasudotox (CAT-8015 or HA22) in patients with hairy cell leukemia. J Clin Oncol. 2012;30:1822–8. doi: 10.1200/JCO.2011.38.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wayne AS, Bhojwani D, Silverman LB, Richards K, Stetler-Stevenson M, Shah N, et al. A novel anti-CD22 immunotoxin, moxetumomab pasudotox: Phase I study in pediatric acute lymphoblastic leukemia. Blood. 2011;118:1317a. doi: 10.1182/blood-2017-02-749101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hassan R, Bera T, Pastan I. Mesothelin: a new target for immunotherapy. Clin Cancer Res. 2004;10:3937–42. doi: 10.1158/1078-0432.CCR-03-0801. [DOI] [PubMed] [Google Scholar]
- 7.Hassan R, Bullock S, Premkumar A, Kreitman RJ, Kinderler H, Willingham MC, et al. Phase I study of SS1P, a recombinant anti-mesothelin immunotoxin given as a bolus I.V. infusion to patients with mesothelin-expressing mesothelioma, ovarian, and pancreatic cancers. Clin Cancer Res. 2007;13:5144–9. doi: 10.1158/1078-0432.CCR-07-0869. [DOI] [PubMed] [Google Scholar]
- 8.Hassan R, Sharon E, Schuler B, Mallory Y, Zhang J, Ling A, et al. Antitumor activity of SS1P with pemetrexed and cisplatin for front-line treatment of pleural mesothelioma and utility of serum mesothelin as a marker of tumor response. J Clin Oncol. 2011;29 (suppl; abstr 7026) [Google Scholar]
- 9.Zhang Y, Xiang X, Hassan R, Pastan I. Immunotoxin and Taxol synergy results from a decrease in shed mesothelin levels in the extracellular space of tumors. Proc Natl Acad Sci. 2007;104:17099–104. doi: 10.1073/pnas.0708101104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Traini R, Ben-Josef G, Pastrana DV, Moskatel E, Sharma AK, Antignani A, et al. ABT-737 overcomes resistance to immunotoxin-mediated apoptosis and enhances the delivery of Pseudomonas exotoxin-based proteins to the cell cytosol. Mol Cancer Ther. 2010;9:2007–15. doi: 10.1158/1535-7163.MCT-10-0257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Du X, Youle RJ, Fitzgerald DJ, Pastan I. Pseudomonas exotoxin A-mediated apoptosis is Bak dependent and preceded by the degradation of Mcl-1. Mol Cell Biol. 2010;30:3444–52. doi: 10.1128/MCB.00813-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chiron MF, Fryling CM, Fitzgerald DJ. Furin-mediated cleavage of Pseudomonas exotoxin-derived chimeric toxins. J Biol Chem. 1997;272:3107–11. doi: 10.1074/jbc.272.50.31707. [DOI] [PubMed] [Google Scholar]
- 13.Spooner RA, Watson P. Drug targeting: learning from toxin entry and trafficking in mammalian cells. Curr Opin Drug Discov Devel. 2010;13:86–95. [PubMed] [Google Scholar]
- 14.Weldon JE, Pastan I. Guide to taming a toxin – recombinant immunotoxins constructed from Pseudomonas exotoxin A for the treatment of cancer. FEBS J. 2011;278:4683–700. doi: 10.1111/j.1742-4658.2011.08182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu XF, FitzGerald DJ, Pastan I. The insulin receptor negatively regulates the action of Pseudomonas toxin-based immunotoxins and native Pseudomonas toxin. Cancer Res. 2013;73:2281–8. doi: 10.1158/0008-5472.CAN-12-3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thomas SM, Brugge JS. Cellular functions regulated by SRC family kinases. Annu Rev Cell Dev Biol. 1997;13:513–609. doi: 10.1146/annurev.cellbio.13.1.513. [DOI] [PubMed] [Google Scholar]
- 17.Parsons SJ, Parsons JT. Src family kinases, key regulators of signal transduction. Oncogene. 2004;23:7906–9. doi: 10.1038/sj.onc.1208160. [DOI] [PubMed] [Google Scholar]
- 18.Sen B, Johnson FM. Regulation of Src family kinases in human cancers. J Signal Transduct. 2011;2011:865819. doi: 10.1155/2011/865819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pastan I, Beers R, Bera TK. Recombinant immunotoxins in the treatment of cancer. Methods Mol Biol. 2004;248:503–18. doi: 10.1385/1-59259-666-5:503. [DOI] [PubMed] [Google Scholar]
- 20.Liu XF, Xiang L, Zhang Y, Becker KG, Bera TK, Pastan I. CAPC negatively regulates NF-κB activation and suppresses tumor growth and metastasis. Oncogene. 2012;31:1673–82. doi: 10.1038/onc.2011.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tan M, Fang HB, Tian GL, Houghton PJ. Repeated-measures models with constrained parameters for incomplete data in tumour xenograft experiments. Stat Med. 2004;24:109–19. doi: 10.1002/sim.1775. [DOI] [PubMed] [Google Scholar]
- 22.Blake RA, Broome MA, Liu X, Wu J, Gishizky M, Sun L, et al. SU6656, a selective Src family kinase inhibitor, used to probe growth factor signaling. Mol Cell Biol. 2000;20:9018–27. doi: 10.1128/mcb.20.23.9018-9027.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keller G, Schafhausen P, Brummendorf TH. Bosutinib: a dual SRC/ABL kinase inhibitor for the treatment of chronic myeloid leukemia. Exp Rev Hematol. 2009;2:489–97. doi: 10.1586/ehm.09.42. [DOI] [PubMed] [Google Scholar]
- 24.Laplante P, Raymond MA, Labelle A, Abe J, Iozzo RV, Hébert MJ. Perlecan proteolysis induces an α2β1 integrin- and Src family kinase-dependent anti-apoptotic pathway in fibroblasts in the absence of focal adhesion kinase activation. J Biol Chem. 2006;281:30383–92. doi: 10.1074/jbc.M606412200. [DOI] [PubMed] [Google Scholar]
- 25.Eguchi R, Kubo S, Takeda H, Ohta T, Tabara C, Ogawa H, et al. Deficiency of Fyn protein is prerequisite for apoptosis induced by Src family kinase inhibitors in human mesothelioma cells. Carcinogenesis. 2012;33:969–75. doi: 10.1093/carcin/bgs109. [DOI] [PubMed] [Google Scholar]
- 26.Quintrell N, Lebo R, Varmus H, Bishop JM, Pettenati MJ, Le Beau MM, et al. Identification of a human gene (HCK) that encodes a protein-tyrosine kinase and is expressed in hemopoietic cells. Mol Cell Biol. 1987;7:2267–75. doi: 10.1128/mcb.7.6.2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lowell CA, Fumagalli L, Berton G. Deficiency of Src family kinases p59/61hck and p58c-fgr results in defective adhesion-dependent neutrophil functions. J Cell Biol. 1996;133:895–910. doi: 10.1083/jcb.133.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guiet R, Poincloux R, Castandet J, Marois L, Labrousse A, Le Cabec V, et al. Hematopoietic cell kinase (Hck) isoforms and phagocyte duties - from signaling and actin reorganization to migration and phagocytosis. Eur J Cell Biol. 2008;87:527–42. doi: 10.1016/j.ejcb.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 29.Menges CW, Chen Y, Mossman BT, Chernoff J, Yeung AT, Testa JR. A phosphotyrosine proteomic screen identifies multiple tyrosine kinase signaling pathways aberrantly activated in malignant mesothelioma. Genes Cancer. 1:496–505. doi: 10.1177/1947601910375273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsao AS, He D, Saigal B, Liu S, Lee JJ, Bakkannagari S, et al. Inhibition of c-Src expression and activation in malignant pleural mesothelioma tissues leads to apoptosis, cell cycle arrest, and decreased migration and invasion. Mol Cancer Ther. 2007;6:1962–72. doi: 10.1158/1535-7163.MCT-07-0052. [DOI] [PubMed] [Google Scholar]
- 31.Harr MW, Caimi PF, McColl KS, Zhong F, Patel SN, Barr PM, et al. Inhibition of Lck enhances glucocorticoid sensitivity and apoptosis in lymphoid cell lines and in chronic lymphocytic leukemia. Cell Death Differ. 2010;17:1381–91. doi: 10.1038/cdd.2010.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ke J, Chelvarajan RL, Sindhava V, Robertson DA, Lekakis L, Jennings CD, et al. Anomalous constitutive Src kinase activity promotes B lymphoma survival and growth. Mol Cancer. 2009;8:132. doi: 10.1186/1476-4598-8-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.