Abstract
To investigate whether obesity induces a leptin–Notch signaling axis in breast cancer (BC), leptin-induced Notch was determined in human MCF-7 and MDA-MB231 and mouse E0771 cells and, in E0771-BC hosted by syngeneic lean and diet-induced-obesity (DIO) C57BL/6J female mice. Lean and DIO-mice were treated for three weeks with leptin inhibitor (PEG-LPrA2) one week after the inoculation of E0771 cells. Leptin induced Notch1, 3 and 4 in BC cells, but Notch2 expression showed opposite pattern in MCF-7 compared to MDA-MB231 cells. Notch loss-of-function [DAPT and dominant negative (R218H) RBP-Jk (CSL/CBF1)] showed that a functional leptin-Notch signaling axis was involved in the proliferation and migration of E0771 cells. E0771-BC onset was affected by obesity [lean mice: 7/10 (70%) vs DIO-mice: 11/12 (92%); Pearson Chi2: P=0.06]. PEG-LPrA2 significantly reduced BC growth [untreated: 19/42; (45%) vs treated: 8/42 (19%); Pearson Chi2: p=0.008]. PEG-LPrA2 did not influence the caloric intake of mice, but increased carcass and/or body weights of lean and DIO-mice inoculated with E0771 cells, which could be related to the improvement of health conditions (less aggressive disease). Importantly, BC from obese mice had higher levels of Notch3, JAG-1 and survivin than lean mice. Inhibition of leptin signaling reduced protein levels of Notch (NICD1, NICD4, Notch3, JAG1 and survivin) and significantly decreased mRNA expression of Notch receptors, ligands, and targets. PEG-LPrA's effects were more prominent in DIO-mice. Present data suggest that leptin induces Notch, which could be involved in the reported higher incidence and aggressiveness and, poor prognosis of BC in obese patients.
Keywords: Breast cancer, Obesity, Leptin, Notch, high-fat diet, E0771 cells, PEG-LPrA2, dominant negative RBP-Jk
Introduction
The American Cancer Society estimates that about 296,980 new cases of breast cancer (BC, invasive and carcinoma in situ) and 39,620 deaths among women suffering from BC will be detected in the US in 2013. Earlier detection, increased awareness and improved treatment have resulted in a significant decrease in mortality and consequently an increase in morbidity from BC during the last decade. Currently, the chance that a woman will die from BC is about 1 in 36 (about 3%)1. Several factors such as genetic predisposition and modifiable causes, for example obesity, are thought to contribute to the incidence and development of BC2.
Several studies have shown that obesity is linked to increased rate of various cancers3, 4. Obesity, characterized by increase of adipose tissue, is associated with poorer prognosis in the majority of the studies that have examined this relationship5. Many studies have hypothesized that diet composition may play an important role in the increased rate of BC6. It has been observed that BC rates are much higher in countries consuming high-fat diets than in less developed countries where fat intake is much lower. This also leads to the notion that obesity is connected to BC incidence3, 7.
The mechanisms underlying obesity-induced risk of cancer is currently not well understood. Several factors have been proposed to contribute to the lethal relationships between obesity and development of cancer, poor prognosis, and relapse3. Obesity is considered a mild inflammatory state that generates several changes in adipocyte and glandular epithelial cell biology, and in the non-adipose cellular components of the stroma-vascular fraction. These changes in turn could promote malignant transformation of cells and positively affect cancer cell survival3, 8. Obesity negatively impacts the survival of BC patients regardless of menopausal status, as it has been associated with increased risk of recurrence and increased proportion of BC6, 9. Obese BC patients show high levels of estrogen, which increases the growth of endocrine responsive tumors. However, not all BC respond to estrogens. Additionally, estrogen levels are unrelated to BC in premenopausal women, suggesting that obesity must affect BC through other mechanisms. Correlations between BC, obesity, and markers of the metabolic syndrome (insulin, free/bioavailable IGF-1 and leptin) have been shown10. However, the individual contributions of these factors to obesity-related cancers are often contradictory and not well understood in diverse scenarios.
Leptin has been the most studied adipokine since this protein was first cloned in 199411. Leptin levels increase proportionally to the size of adipose tissue. Higher levels of leptin are found in female, postmenopausal women and obese individuals12. Leptin is secreted by adipose tissue and BC cells2. Leptin signaling and its crosstalk to several oncogenic signals regulate angiogenesis, stimulate survival, proliferation, and migration of BC cells. Leptin can also induce pro-oncogenic actions in the stroma-vessel components2, 13. Compelling evidence for leptin's oncogenic role in BC was found in obese ob/ob and db/db mice, which are leptin–deficient and unable to develop mammary tumors. This was true even when they were crossed with MTTV-TGF-α mice (prone to develop BC)14, 15. Remarkably, these leptin-deficient and obese mice that are unable to develop BC were found to have high levels of insulin/IGF-1. Furthermore, we have also provided solid evidence sustaining an oncogenic role for leptin signaling in BC16-18. Inhibition of leptin signaling via pegylated-leptin receptor antagonist 2 (PEG-LPrA2) decreased BC growth and reduced the levels of several oncogenic molecules in several mouse models. Additionally, other studies have shown that high levels of leptin in obese women can impact transformed BC cells to induce an alteration to a more aggressive phenotype 19.
Notch is a hallmark of BC. Notch expression is associated to angiogenesis, proliferation, differentiation, apoptosis, and a more aggressive BC and poor prognosis2. Notch receptors are mammalian transmembrane proteins that bind membrane-bound ligands expressed by adjacent cells. The Notch family consists of four receptors Notch1-Notch4 and five ligands: Jagged 1(JAG1), JAG2, Delta-like 1 (DLL1), DLL3, and DLL4. Leptin can induce the expression and activation of Notch in BC cells in vitro20. Moreover, leptin is also likely related to carcinogen-induced BC16.
We hypothesize that leptin could contribute to BC development through the activation of the Notch signaling pathway. Then, increased levels of leptin found in obesity may induce the expression of Notch and promote the growth of BC. To test this hypothesis, we investigated whether leptin induces Notch in E0771-BC cells and derived tumors hosted by lean and obese mice. The effects of abrogation of Notch signaling via inhibition of γ-secretase and forced expression of dominant negative RBP-Jk (CSL, an essential Notch transcription factor) on leptin-induced proliferation and migration of E0771 cells were determined. Additionally, we investigated the effects of leptin on Notch expression in ER+ breast cancer versus TNBC cell lines. To test whether increased levels of leptin found in obesity could be related to Notch expression and development of BC, lean and obese syngeneic C57BL/6J mice were implanted with E0771 cells and treated with a potent leptin antagonist (PEG-LPrA2)17. Present data strongly suggest that leptin-induced Notch signaling in BC is essential for proliferation, migration of BC cells and correlates to BC development in an obesity context.
Materials and Methods
Reagents and antibodies
Polyclonal Notch1 (sc-373891), Notch4 (sc-56594), Jagged1 (sc-8303), Ob-R-NH2 (sc-1834), leptin (sc-843) and RBP-Jk (Sc-8213) antibodies were obtained from Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA. Polyclonal Notch2 (ab-8926) and Notch3 (ab-23426) antibodies were from Abcam Incorporated, Cambridge, MA, USA. Polyclonal Survivin antibody (71G4B7) was from Cell Signaling, Danvers, MA, USA. Polyclonal anti-mouse and anti-rabbit antibodies horseradish peroxidase (HRP) conjugates were from Bio-Rad Laboratories, Hercules, CA, USA. Mouse leptin Quantikine ELISA Kit was purchased from R&D Systems Inc., Minneapolis, MN, USA. ECL-Western blot stripping buffer was from Thermo Scientific, Rockford, IL, USA. DMEM was obtained from Life Technologies, Grand Island, NY, USA. MTT assay kit was purchased from Promega Corporation, Fitchburg, WI, USA. Dual-luciferase assay system and pGL-3 plasmid were obtained from Promega (Madison, WI). RNeasy Mini kits, DNase kits and Superfect transfect reagents were obtained from Qiagen (Valencia, CA). Vectastin ABC-APK and Vectamount were obtained from Vector Laboratories, Burlingame, CA, USA. Hematoxilyn was purchased from Dako Corporation, Carpinteria, CA, USA. Monoclonal Notch1 (N6786) and β-actin (A5316) antibodies, protease inhibitor, phosphatase inhibitor cocktails 1 and 2, fetal bovine serum (FBS), DAPT [N-[N-(3,5-difluorophenacetyl)-L-alanyl]-S-phenylglycine t-butyl ester], DAPI (4',6-diamidino-2-phenylindole) and other chemicals were purchased from Sigma-Aldrich, St. Louis, MO, USA.
In vitro experiments
Cell cultures
Human MCF-7 (ER+) and MDA-MB231 (ER-) (ATCC, Manassas, VA) and mouse E0771 (ER+; provided by Dr. Mikhail Kolonin; Centre for Stem Cell Research, Institute of Molecular Medicine, University of Texas) breast cancer cell lines were used. The E0771 cell line was generated from an estrogen receptor positive (ER+) mammary adenocarcinoma isolated from a C57BL/6J mouse21. The cells (1.0×105cells/ml) were cultured on oncoated flat-bottomed plastic 24-well plates with DMEM supplemented with 10% FBS, containing 1% penicillin and streptomycin. Semi-confluent cells were cultured for 16-24h in basal medium (DMEM without FBS) and treated with different compounds. In all experiments triplicate wells, tubes and reactions were ran and repeated at least three times with different cell preparations.
Inhibition of leptin signaling
To inhibit leptin signaling in E0771 cells in vitro and in vivo, a potent inhibitor, leptin receptor peptide antagonist 2 (LPrA2) was used16-18, 22. LPrA2 shows high binding affinity for the leptin receptor (Ob-R; Ki ≈ 0.6×1010 M)23. To increase its solubility and half-life the peptide was coupled to polyethylene glycol (pegylated peptide: PEG-LPrA2; MW≈23000; half-life in mice via i.v.: unconjugated 1h versus pegylated 66h). In contrast to the unconjugated peptide, its derivative, PEG-LPrA, is water-soluble18. PEG-LPrA2 and a pegylated-scrambled peptide (PEG-Sc, for negative control) were synthesized and purified as previously described16.
Leptin dose-response effects on Ob-R and Notch expression
E0771 wild type cells were cultured in medium DMEM-FBS 10% until semi-confluent layers were achieved. Cells were starved for 24h in basal medium. Then, cells were cultured for 30 min in starvation medium containing 1.2 nM PEG-LPrA2 or PEG-Sc (pegylated peptide inactive control). Cells were cultured for additional 24h in basal medium containing leptin (0, 0.6, 1.2 and 6.2 nM, equivalent to 0, 10, 20, and 100 ng/ml). Conditioned media were harvested and cells were lysed as previously described to determine Notch and Ob-R via Western blot (WB) analysis20. Protein concentrations were determined using the Bio-Rad kit (Bio-Rad Lab.).
Inhibition of Notch signaling
To pharmacologically inhibit Notch signaling, a γ-secretase inhibitor, DAPT, was used. To specifically assess the role of RBP-Jk (CBS/CSL, an essential transcription factor for Notch signaling) a dominant negative construct pCMX-N/R218H (RIKEN, Tsukuba-city, Ibaraki, JAPAN) deposited by T. Honjo (University of Kyoto, Japan) was used23. R218H carries an R-to-H substitution at position 218, which is critical for the DNA binding activity of RBP-Jk. R218H was re-cloned into the pCMX vector and transfected into E0771 cells. To assess the inactivation of RBP-Jk gene expression by pCMX-N/R218H the cells were co-transfected with RBP-Jk-Luciferase reporter and Renilla control-plasmid (PGL3-CBF; Signosis, Inc.). Additionally, the levels of RBP-Jk protein in E0771-R218H cells were determined by WB after leptin challenge20.
Cell proliferation
Leptin dose-response effects on proliferation of E0771 wild type and E0771-R218H transfected cells were determined via MTT assay. E0771 cells were seeded 1×104 per well in a 96-well plate, starved for 24h and cultured for additional 24h in medium containing 0-1.2 nM leptin plus PEGLPrA2 (0 and 1.2nM). MTT was added (stock: 5 mg/ml, 20 μl/well) and cells were incubated for 4h at 37°C and lysed (200 μl/well DMSO). Absorbance was measured at 570 nm (Molecular Devices, CA)20.
Cell migration
E0771 wild type and E0771-R218H cells (5×104) were cultured in basal medium in the upper chamber of the Boyden chamber inserts (6.4-mm diameter, 8-μm pore size; BD Biosciences) as previously described20. To determine the effects of leptin and Notch signaling the cells were placed in a 24-well plate containing 0, 0.6 and 1.2 nM leptin, PEG-LPrA2 (0 and 1.2 nM) and DAPT (0 or 5 μM/0.1% DMSO). Migration assays were carried out for 24h. Cells that migrated to the lower side of the insert were fixed with 3.7% formaldehyde and stained with hematoxylin. Six randomly selected fields (×10 objective) were photographed, and the migrated and stained cells were counted20.
In vivo experiments
Animals and Experimental procedures
Fifty female C57BL/6J mice seven-week old (Jackson Laboratories, ME, USA) weighting approximately 18g were randomly allocated into two groups fed with either chow (lean mice; n=20) or high-fat diet (diet-induced-obesity mice, DIO; n=30), which were prepared by Harlan Laboratories Inc. (Indianapolis, IN, USA). Mice were housed five per cage in the animal facilities of Morehouse School of Medicine (MSM, Atlanta, GA, USA) in rooms maintained at 25°C with 10-15 air exchanges per hour. Artificial light was provided under a 12h/12h light/dark cycle. All housing materials, as well as food and water, were autoclaved prior to use. All experiments were performed according to the protocol approved by MSM-IACUC and NIH guide for the Care and Use of Laboratory Animals.
Composition of the experimental diets
The TD.06416 (chow diet: 10% Kcal from fat; 3.6 Kcal/g; Harlan Lab.) and TD.06414 (HFD; 60% Kcal from fat; 5.1 Kcal/g; Harlan Lab.) adjusted calories diets were used16.
Detection of obesity and breast tumor-take model
Obesity was identified at week 5 in mice showing body weights (BW) greater that 20% of BW of lean controls (DIO: BW> 120% BWL). Mice fed HFD that were not obese after week 5th were classified as obesity-resistant (BW< 120% BWL) and were withdrawn from the experiments. Lean and DIO-mice (obese) were inoculated into the left lower mammary pad (23-gauge needle) with E0771 wild type cells (1×105) suspended in phosphate saline (PBS)-0.3% matrigel solution (Sigma).
PEG-LPrA2 treatment
One week after E0771 cell inoculation, PEG-LPrA2 treatment was applied to mice (treated mice: lean; n=10 and DIO; n=10) via tail vein injections (50 μl/0.1 mM) once (‘I”) or two times (“II”) a week for 3 weeks. Control mice received no treatment or PEG-Sc (untreated mice: lean; n=10 and DIO; n=12). Lean and obese mice were randomly allocated into four subgroups each: lean and DIO receiving no treatment (L-Unt; n= 5 and DIO-Unt; n=7) or receiving PEG-Sc (L-Sc; n= 5 and DIO-Sc; n=5); and lean and DIO treated once (L-I; n=5 and DIO-I; n=5) or two times (L-II; n=5 and DIO-II; n=5) with PEG-LPrA2. Tumor development was detected by palpation of mammary glands. Food and caloric intake, BW, and general health status were recorded. After 3 weeks of treatment (week 9) the mice were sedated with halothane inhalation before the collection of blood samples via cardiac puncture. One ml blood samples were mixed with 200μl of EDTA (ethylenediaminetetraacetic acid; Sigma Aldrich St. Louis, MO). Plasma was collected by centrifugation and stored at −80°C. Final BW and carcass weight were determined. Mammary tumor tissues were dissected and stored −80°C.
RNA extraction and real-time PCR
RNA was extracted from mammary glands inoculated with E0771 cells and E0771-derived BC using RNeasy Mini Kit (Qiagen)20. Total RNA (0.7–1.0 μg) was used as template for cDNA synthesis. First-strand cDNA was synthesized (Applied Biosystems PCR system 2700 Thermal cycler; Life Technologies) from total RNA using SuperScript First-Strand Synthesis System with SuperScript II reverse transcriptase according to the manufacturer's protocols (Invitrogen, Carlsbad, CA). The cDNA was used as a template in real-time PCR reactions with QuantiTect SYBR-Green PCR mastermix (Bio-RAD) and was run on a Bio-RAD iCycler machine (Bio-RAD). Real-time quantitative PCR reactions consisted of 1× SybrGreen Supermix (Bio-Rad), 0.25mmol/L forward and reverse primers, and 10 ng cDNA. Specifics primers were used for Notch molecules as previously described20. Cycling conditions consisted of a three-step amplification and melt curve analysis using the iQ5 Real-time PCR Detection System (Bio-Rad). PCR conditions were: 1 cycle, 95°C for 3 min; 35 cycles, 95°C for 10 sec; 60°C for 30 sec and 72°C for 30 sec. Amplified cDNA from the reference sample detailed above was used in a 5-fold dilution series of 100 to 0.16 ng cDNA per reaction. Relative expression values (R) were calculated using the Ct adjusted method R = 2–(ΔΔCt). Values were normalized to the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression values20 and to the endogenous control values (untreated lean and DIO mice). Real-time PCR determinations were triplicated for each sample preparation.
Immunohistochemistry (IHC)
Unmasking of tissue antigens was performed in paraffin embedded sections from mammary tumors by heat treatment in sodium citrate buffer (pH 6, 10 mM) at 95°C for 15 min. Following primary antibodies diluted in PBS-0.1% BSA (bovine serum albumin): anti Notch1 (1:50); Notch4 (1:50); Jagged1 (JAG1; 1:50) and Survivin (1:400) were used. The tissues were incubated with a streptavidin-biotin peroxidase system (Vectastain, ABC-AP kit; Vector). Negative controls were also included in which the primary antibody was omitted or substituted with non-specific IgG. DAB substrate (Vector) was used and the sections were counterstained with hematoxylin. Positive cells were recorded and counted in randomly chosen fields.
Western blot (WB)
Total proteins from BC cells and E0771-derived BC were extracted using RIPA buffer containing an enzymatic inhibitor cocktail (Sigma). Thirty μg of protein lysates were used for WB analysis of Notch as previously described17. β–actin was used as the experimental loading control. For quantitative evaluation the NIH Image program18 was used. Protein concentrations of tissue lysates were determined using the Bradford method (Bio-Rad).
Leptin plasma levels
: Protein levels of leptin in plasma were determined using an ELISA kit according to the manufacturer's instructions (R&D Systems).
Data Analysis
Summary statistics were performed using Arithmetic means ± Standard deviation for normally distributed continuous data, and the Geometric mean with 95% Confidence Interval. Obesity and treatment status were defined as dichotomous variables with obese or DIO/lean and treated with PEG-LPrA/not treated with PEG-LPrA groups respectively. Differences between of final BW of lean and obese/DIO mice groups were tested using the student's t-test. Similarly, the student's t-test was used to test the difference in final BW between PEG-LPrA2 treated and non-PEG-LPrA2 treated mice, and antigen expression in cell cultures and tumor tissues. The likelihood ratio test was used to test the assumption that the differences in final BW between PEG-LPrA2 treated and non-PEG-LPrA2 treated differed by obesity status in a multiple regression model with an interaction term between obesity status and treatment status. Results of stratified analyses are presented when the test for interactions was statistically significant. All statistical tests were two-sided and p-values ≤ 0.05 were considered statistically significant. Chi-squared tests were used to test the association between tumor detection rates and obesity status. Similar analyses were used to test the association between tumor detection rates and treatment status. Fisher's exact test was utilized if expected values in any of the cells of the 2×2 tables were less than 5. The student's t-test was also used to test the differences in plasma leptin levels between mice with detectable tumors and mice without detectable tumors. Due to the skewed distribution of plasma leptin levels, the student's t-tests were performed on the natural logarithm values of plasma leptin. All statistical tests were performed using STATA SE version 11.
Results
Leptin-induces Notch in human MCF-7 and MDA-MB231 and, mouse E0771 breast cancer cells
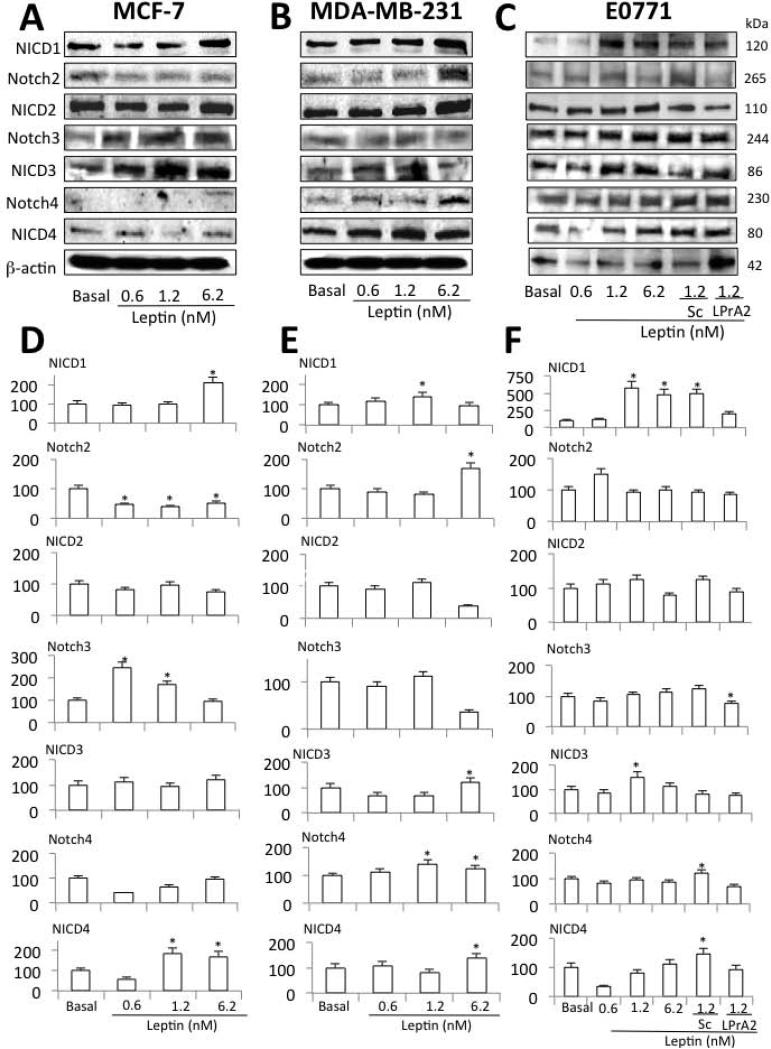
WB analysis showed that leptin induced the expression of several Notch receptors and activated molecules (NICD), in human and mouse breast cancer cells (Fig 1). NICD1 was increased by leptin in all cells. However, differential responsiveness for leptin-dose activation of Notch1 was as follows: E0771>MDA-MB231>MCF-7 cells. Notch3 expression was induced by leptin in MCF-7 cells (Fig 1A and D). A trend for leptin induction and activation of Notch3 and Notch4 was detected in E0771 cells (Fig 1C and F). Notch3 was significantly upregulated by leptin in MCF-7 cells (Fig 1A and D). In contrast, leptin significantly induced Notch4 expression in MDA-MB231 cells (Fig 1B and E). In addition, leptin activated Notch4 (NICD4) in MCF-7 and MDA-MB231 cells (Fig 1A and D and, B and E, respectively). Leptin did not affect Notch expression or activation (NICD2) in E0771 cells (Fig 1C and F). In contrast to MDA-MB231 cells (Fig 1B and E) leptin reduced Notch2 expression in MCF-7 cells (Fig 1A and D). Incubation of E0771 cells with PEG-LPrA2 and scrambled control (PEG-SC) demonstrated that the increased level of Notch expression was leptin-specific (Fig 1C and F). Furthermore, as it was previously found in MCF-7 and MDA-MB23118, E0771 cells expressed Ob-Rb (long isoform) and Ob-Ra (short isoform) (data not shown).
Fig 1. Leptin induces Notch in human and mouse breast cancer cells.
Representative Western blot (WB) results and quantitative analysis of leptin-induced effects on Notch 1, 2, 3 and 4 in human MCF-7 (A, D) and MDA-MB231 (B, E) and mouse E0771 (C, F) breast cancer cells. Cells were cultured for 24 h and leptin dose-induced (0, 0.6, 1.2 and 6.2 nM) effects were determined as described (see M &M). E0771 cells were also co-incubated with leptin and leptin inhibitor (leptin receptor antagonist 2, LPrA2) and inert control (scrambled peptide, Sc). The WB results were normalized to β-actin as loading control and densitometric analysis of bands for leptin-induced NICD1; Notch2; NICD2; Notch3; NICD3; Notch4 and NICD4 was carried-out with the image J software. (*) P<0.05 when comparing levels of protein to control (basal). Data (mean ± standard error) representative results derived from a minimum of 3 independent experiments.
Leptin induced cell proliferation and migration are Notch-dependent
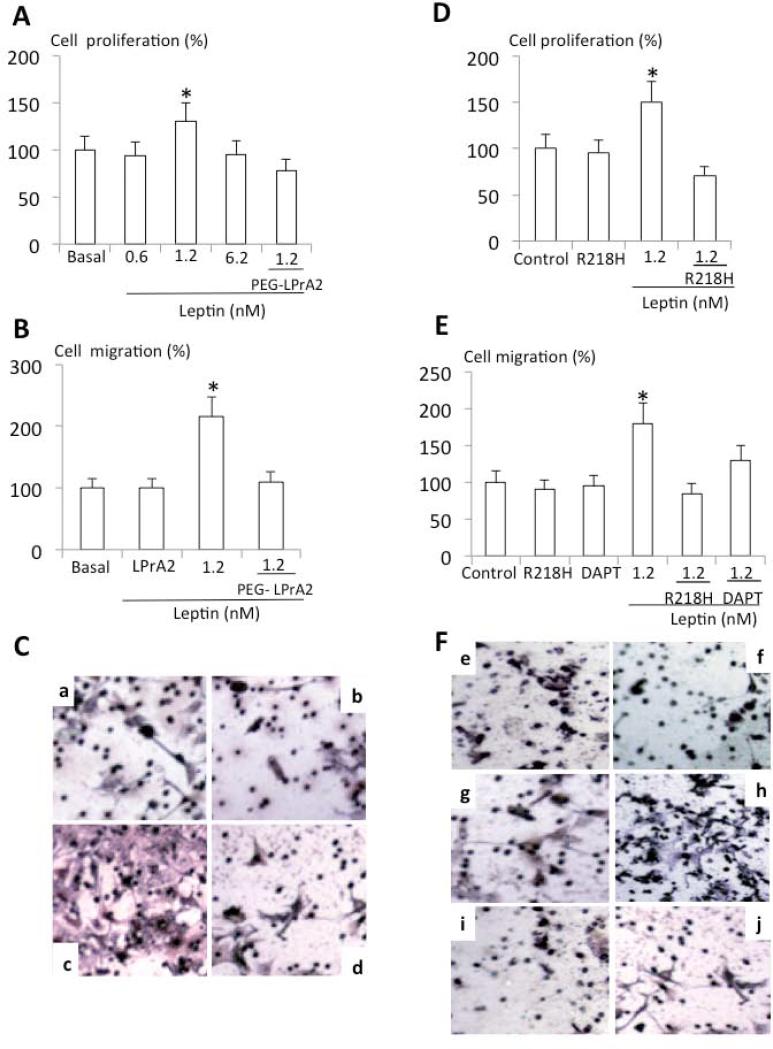
Leptin is a known proliferation factor for BC18, 20. Results from MTT assays showed that leptin significantly increased the proliferation of E0771 cells in vitro, which was completely abrogated by PEG-LPrA2 (Fig 2A). Furthermore, leptin also increased E0771 cell migration which was also inhibited by PEG-LPrA2 (Fig 2B and C). To investigate whether leptin-induced proliferation and migration of E0771 involves Notch signaling, the effects of leptin on proliferation of E0771 wild type and E0771-R218H mutant (expressing a non-functional and dominant negative RBP-Jk mutant) cells were compared. In sharp contrast to E0771 wild type cells (Fig 2A), leptin did not induce the proliferation of E0771-R218H cells (Fig 2D). Additionally, leptin-induced effects on migration of E0771 wild type cells (Fig 2B and C) were not found in E0771-R218H cells (Fig 2E and F). Also the addition of the γ-secretase inhibitor, DAPT, abrogated leptin-induced migration (Fig 2E and F). These data strongly suggest that leptin-induced proliferation and migration of E0771 cells requires a functional leptin-Notch signaling axis. These results led us to investigate whether obesity characterized by high levels of leptin can affect the development of E0771-derived BC hosted by obese mice through a similar mechanism.
Fig 2. Notch's loss-of–function completely inhibits leptin-induced proliferation and migration of E0771 cells.
Leptin-dose response effects on proliferation (A) and migration of E0771 wild type cells (B). Representative results of leptin and PEG-LPrA2 effects on migration of E0771 wild type cells (C). Detection of migration of E0771 wild type cells: basal (Ca); PEG-LPrA2 treated (Cb); leptin-treated (Cc) and leptin+PEG-LPrA2 treated (Cd). Leptin effects on proliferation (D) and migration (E) of E0771-R218H as compared to control E0771 wild type cells. Representative staining of migration of E0771 wild type and R218H expressing cells (F). Detection of migration: basal E0771 wild type cells (Fe); basal E0771-R218H cells (Ff); E0771 wild type cells treated with DAPT (Fg); E0771 wild type cells treated with leptin (Fh); E077-R218H cells treated with leptin+ DAPT (Fi) and E0771 wild type cells treated with leptin+ DAPT (Fj). Proliferation (MTT) and migration (Boyden chamber) assays were carried-out after 24h of incubation in different conditions and results were normalized to basal conditions (see Material and Methods). P<0.05 when comparing cell migration and proliferation to control (basal). Data (mean ± standard error) representative results derived from a minimum of 3 independent experiments. PEG-LPrA2: pegylated leptin receptor antagonist 2; R218H: dominant-negative RPB-Jk (CSL) plasmid; DAPT: [N-[N-(3,5-difluorophenacetyl)-L-alanyl]-S-phenylglycine t-butyl ester], a γ-secretase inhibitor.
Impact of diet and treatment on body weight, food and caloric intake
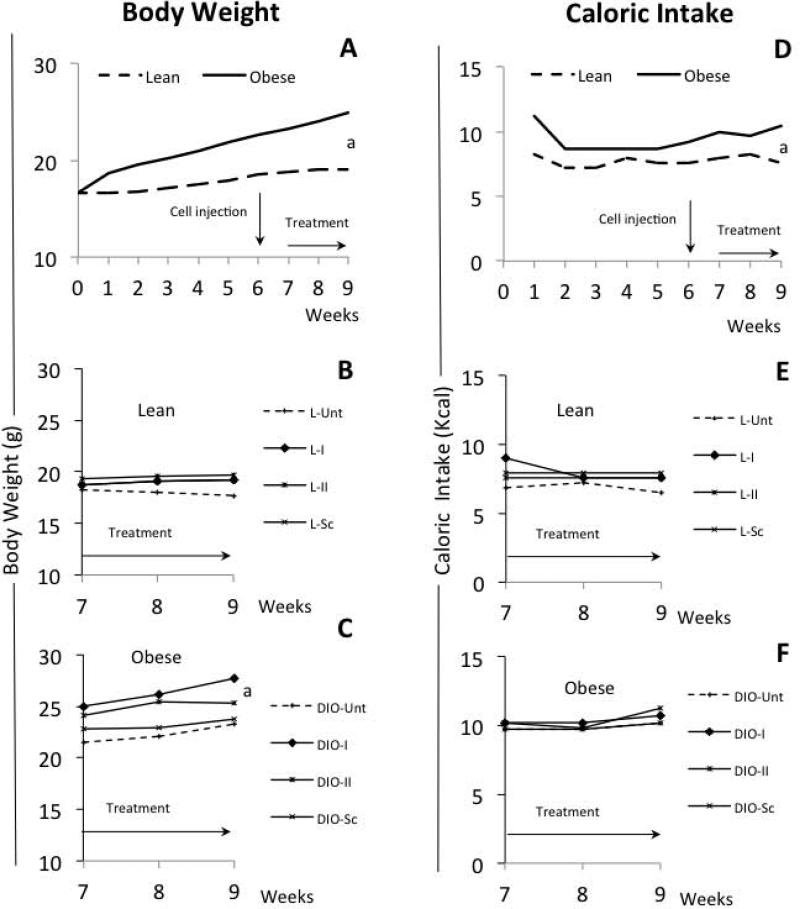
As expected, feeding a high-fat diet (HFD) significantly increased mouse BW over the first 5 weeks and continued until the end of the experimental period (DIO-mice: 22/30; 73 %) (Fig 3A and C) compared to lean mice fed a chow diet (Fig 3A and B). In contrast, food intake was stable and similar for both lean and DIO-mice (range: 1.7-2.4 g/day/mouse) during the experimental period (Fig 3D). However because the diets were not isocaloric, DIO-mice consumed more calories (Fig 3F) than the lean mice (Fig 3E). Statistical analysis showed a significant interaction between obesity status and treatment status, thus stratified analyses showed that DIO-mice (see Fig 3A and C) were significantly heavier than lean mice in both PEG-LPrA2 treated (p<0.0001) and non-PEG-LPrA treated mice (p=0.001) (see Fig 3A and B). However, DIO-mice treated with PEG-LPrA2 were significantly heavier than untreated DIO-mice (p=0.0013) at the end of the experimental period (see Fig 3C). Similarly, PEG-LPrA2 treatment increased carcass weight of both lean and DIO-mice (data not shown; p=0.008). PEG-LPrA2 effects on BW and carcass weight could be related to the improvement of the health of the mice due to the negative impact of PEG-LPrA2 on E0771-derived BC.
Fig 3. Effects of diets and PEG-LPrA2 treatment on body weight (BW) and caloric intake.
Weekly evaluation of BW of mice fed chow (lean; n=20 mice; normal diet 10% Kcal-fat) and high-fat diets (DIO, diet-induced-obesity mice; n=30 mice, 60% Kcal-fat) (A). Impact of PEG-LPrA2 treatment on BW of lean (B; n=20) and DIO-mice (C; n=22) mice after E0771 cell inoculation. Effects of consuming chow and high-fat diets on caloric intake of lean and DIO-mice (D). Caloric intake overtime of lean (E) and DIO-mice (F) after PEG-LPrA2 treatment. Obesity in DIO-mice (73%; 22/30) was evaluated at week 5 (see M&M). Mice were orthotopically inoculated with E0771 cells (1×105 cells). At week 6, lean and DIO-mice were allocated to 4 subgroups each. Mice were untreated (L-Unt; n=5 and DIO-Unt; n=7) or received inactive peptide (L-Sc; n=5 and DIO-Sc; n=5) or PEG-LPrA2 treatment [once (L-I; n=5 and DIO-I; n=5) or two times (L-II; n=5 and DIO-II; n=5) a week for 3 weeks]. (a) P<0.05 when comparing BW or caloric intake between lean and DIO mice. PEG-LPrA2: pegylated leptin receptor antagonist 2.
E0771-derived breast tumors and plasma levels of leptin
This tumor-take model showed that obesity tends to positively increase the detection rate of BC in DIO mice [DIO 17/22 (77%) vs Lean: 10/20 (50%); Pearson Chi2: p=006] (Table 1). Strikingly, mice receiving no treatment showed a significantly higher incidence of BC than those mice treated with PEG-LPrA2 [Lean no treatment: {(L-Unt +L-Sc): 7/10 (70%)} vs Lean treated:{L-I + L-II: 3/10 (30%)}, and obese no treatment: {DIO-Unt + DIO-Sc: 11/12 (92%)} vs obese treated: {DIO-I + DIO-II: 6/10 (60%)}; Pearson Chi2: p=0008] (see Table 1). Moreover, PEG-LPrA2 affected the development of BC in DIO-mice in a dose-response manner. The injection of two-doses per week of PEG-LPrA2 showed superior reduction of detectable BC in DIO-II (2/5; 40%) versus DIO-I mice (4/5; 80%). Present data confirm the notion that HFD intake and obesity are linked to the development of BC. Moreover, the results further suggest that leptin signaling is essential for the development of BC.
Table 1.
Impact of diet and treatment on breast cancer incidence
| Total | Treated | Untreated | ||||
|---|---|---|---|---|---|---|
| Groups | BC-free | BC | BC-free | BC | BC-free | BC |
| Lean (n=20) | 10 (50%) | 10 (50%)a | 7 (70%) | 3 (30%)a | 3 (30%) | 7 (70%) |
| DIO (n=22) | 5 (33%) | 17 (77%)a | 4 (40%) | 6 (60%)b | 1 (8%) | 11 (92%) |
Notes: Did: diet-induced-obesity; GC: breast cancer
p=0.006; Pearson Chi2 when compared DIO-BC to Lean-BC
p=0.0003; Pearson Chi2; when compared L-treated to untreated and, DIO-treated to untreated.
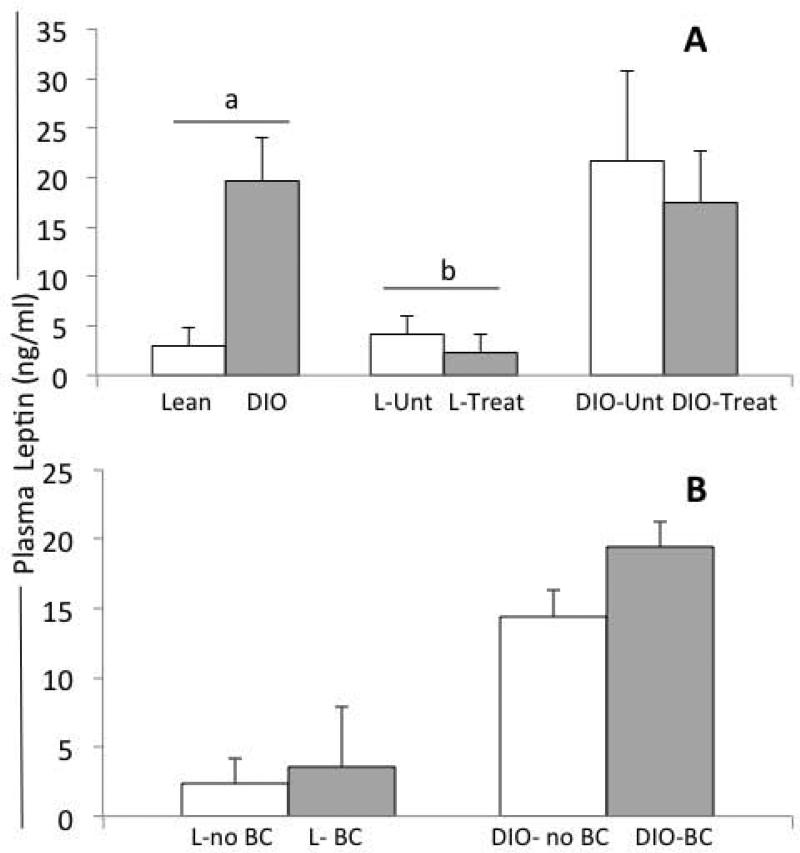
As predicted, DIO-mice showed higher plasma levels of leptin than lean mice (two-sample t test, p<0.00001; Fig 4A). Higher leptin levels in DIO and untreated lean mice correlated to BC detection (two-sample t test with equal variances; p=0.01). PEG-LPrA2 treatment significantly decreased the levels of leptin in lean mice compared to untreated lean mice (L-Unt +L-Sc vs L-I +L-II; p=0.02) (see Fig 4A). In contrast, no differences in plasma levels of leptin were found between treated and untreated DIO-mice (DIO-Unt +DIO-Sc vs DIO-I + DIO-II; p=0.72) (see Fig 4A). E0771-derived BCs were detected in both lean and DIO-mice. However, the presence of BC did not alter the levels of plasma leptin either in lean or DIO-mice (Fig 4B).
Fig 4. Effects of obesity, E0771-derived breast cancer and PEG-LPrA2 treatment on plasma levels of leptin in C57Bl/6J female mice.
Plasma levels of leptin at week 10 in lean (n=20; fed chow diet: 10% Kcal-fat) versus DIO-mice (diet-induced-obesity mice; n=22; fed high-fat diet, 60% Kcal-fat); lean-untreated (L-Unt; n=10) versus Lean-treated (Lean-Treat; n=10) mice and; DIO-untreated (DIO-Unt; n=12) versus DIO-treated (DIO-Treat; n=10) mice (A). Plasma levels of leptin in lean (L) and obese mice (DIO) with tumors (E0771-derived breast cancer; BC) or without breast cancer (no-BC) (B). Pearson Chi2: (a) P<0.00001; plasma levels of leptin in lean versus DIO mice; and (b) P=0.02, plasma levels of leptin in L-Unt versus L-Treat. Concentrations of plasma leptin (ng/ml; mean ± standard error) was determined by ELISA and; results are derived from 3 replicates. Mice were untreated (received no treatment or pegylated scrambled inactive control) or treated with pegylated leptin receptor antagonist 2 (PEG-LPrA2) for 3 weeks.
Notch expression in E0771-derived breast tumors
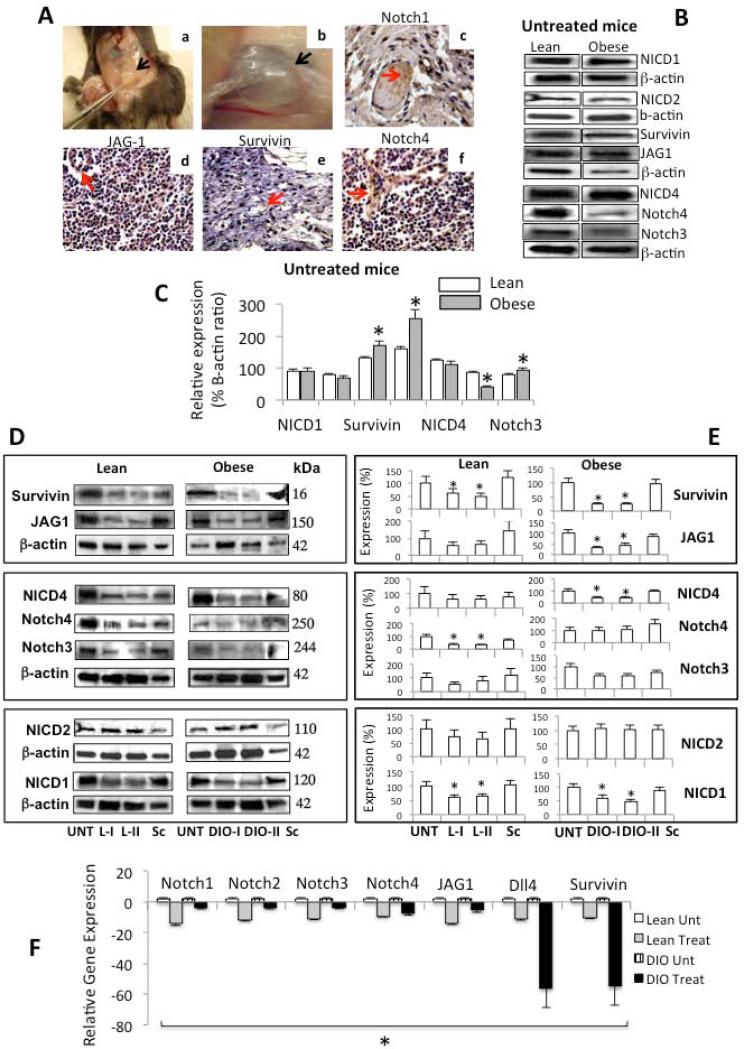
We sought to determine whether obesity and leptin signaling could also affect the expression of Notch in BC. IHC analysis detected Notch and target molecule, survivin, in paraffin sections. The use of IHC did not allow us to determine if these molecules were differentially expressed in lean or DIO-mice or whether treatment affected their levels of expression (Fig 5A). The use of a more sensitive method, WB, showed that BC hosted by DIO-untreated mice had higher levels of Notch3, JAG-1 and survivin (Fig 5B and C). However, Notch4 was overexpressed in BC hosted by lean mice. No effects of treatment or obesity on Notch2 expression and activation were found (Fig 5B and C). PEG-LPrA2 differentially affected the expression of Notch in BC from lean compared to DIO-mice (Fig 5D-F). PEG-LPrA2 reduced the levels of activated Notch1 (NICD1) and lowered the expression of Notch4 and survivin in lean mice (Fig 5D and E). Additionally, DIO-mice treated with PEG-LPrA2 showed reduced the activation of Notch1 (NICD1) and Notch 4 (NICD4) and decreased the levels of Notch3, JAG1 and survivin. Overall, it appears that PEGLPrA2 affected additional components of Notch system in DIO compared to lean mice (Fig 5D and E). In addition, obesity context and higher levels of leptin were related to increased Notch mRNA in E0771-derived BC. Real-Time RT-PCR demonstrated that PEG-LPrA2 treatment massively decreased the levels of mRNA for Notch receptors (Notch1-4), ligands (JAG1 and Dll4) and targeted molecule (survivin) (Fig 5F). These results suggest that obesity and leptin signaling were linked to the expression of Notch in E0771-derived BC.
Fig 5. Effects of obesity on Notch expression in E0771-derived breast cancer.
Representative pictures from the immunohistochemical determination of Notch in E0771-derived breast cancer (A). Dissection on an E0771-derived breast cancer; black arrow indicates the tumor burden (Aa). Enhanced view of an E0771-derived breast cancer; (Ab). Notch1 (Ac); JAG-1 (Ad); survivin (Ae) and Notch4 (Af). Representative results from Western blot (WB) analysis of NICD1, NICD2, survivin, JAG, Notch4, NICD4 and Notch3 in E0771-derived breast cancer hosted by untreated lean and obese mice (diet-induced-obesity, DIO) (B). Quantitative analysis of WB results expressed as percentage of levels of Notch in untreated mice (C). Representative results from WB analysis of Notch proteins in E0771-derived breast cancer hosted by lean (L) and obese (diet-induced-obesity, DIO) mice untreated and treated with PEGLPrA2 (D). Quantitative analysis of WB results expressed as percentage of levels of Notch in treated mice (E). Quantitative analysis of Notch mRNA expression (receptors Notch1-4; ligands JAG1 and Dll4 and; targeted gene survivin) as determined by real-time RT-PCR (F). The WB results were normalized to β-actin as loading control and densitometric analysis of bands was carried-out with the image J software. mRNA levels were normalized to GAPDH. At week 6, lean and DIO-mice were allocated to 4 subgroups each and were untreated (Unt), received inactive peptide (Sc) or received PEG-LPrA2 treatment [once (L-I and DIO-I) or two times (L-II and DIO-II) a week for 3 weeks]. (*) P<0.05 when comparing levels of Notch and survivin in breast cancer tissue from untreated lean and DIO-mice and when comparing levels of protein or mRNA in treated mice to control (Unt). Data (mean ± standard error) representative results derived from a minimum of 3 independent experiments. Black and red arrows show the tumor mass and positive staining, respectively. Magnification ×40. PEG-LPrA2: pegylated leptin receptor antagonist 2.
Discussion
According to the CDC (Center for Control Disease and Prevention, Atlanta, US), obesity is pandemic in US. In 2009-2010, it was reported that more than one-third of adults and nearly 17% of youth were obese, however no differences between genders were detected24. The World Health Organization (WHO) reported that approximately 700 million will be afflicted with this condition worldwide by 201525. Remarkably, BC risk is increased in obese women by a factor of 1.12 (1.08-1.16; 95% coefficient interval)3. Combined actions of inflammatory cues, insulin signaling, altered levels of lipids and changes in adipokine signaling have been suggested as potential factors promoting BC risk in obesity contexts3. Obese BC patients show higher mortality rates compared to non-obese patients. Obesity has been associated with bigger tumors, more advanced disease, poorer prognosis, and/or increased mortality in women with BC26. Indeed, women with increased adipose tissue had increased tumor growth rates27. These data may imply that tumors developed in obese individuals are more aggressive than those found in individuals with less adipose tissue28. Other studies have also shown that even post treatment of BC a decrease in BW may in fact lead to higher survival rates7. However, the molecular mechanisms involved in obesity-related cancer are not well-understood7,28.
Leptin, the most studied adipokine, shows increased levels in obese individuals. However, obesity is characterized by a leptin resistant status at the hypothalamic level29. High levels of circulating leptin can then impact normal and cancer tissues expressing Ob-R. Additionally, BC cells also secrete leptin and overexpress Ob-R. Abnormal leptin and Notch signaling are hallmarks of BC2,30. Notch signaling pathway is cell and context dependent and can influence cell fate by regulating programs leading to growth and differentiation. Furthermore, Notch receptors (Notch1-4) and ligands (JAG1/Dll-4) are not functionally redundant, as null mutations for each display unique phenotypes31. The crosstalk between oncogenic signaling pathways triggered by leptin, IL-1 and Notch (NILCO)20 found in BC could represent the integration of developmental, pro-inflammatory, and pro-angiogenic events critical for leptin-induced BC cell proliferation/migration and tumor angiogenesis2. Additionally, leptin-induced Notch seems to be related to DMBA (7,12-dimethylbenz[a]anthracene)-induced tumors in obese C57BL/6J mice16. Moreover, leptin induced angiogenic features and Notch signaling in endothelial cells32.
We hypothesize that the activation of a leptin-Notch signaling axis is instrumental and essential for the relationships between obesity and BC development. E0771 (ER+, PR+ and HER2-; data not shown) and MDA-MB231 (triple-negative) cells were more responsive to leptin-induced activation of Notch (NICD1; NICD3 and NICD4) than MCF-7 cells. MCF-7 cells downregulated Notch2, that could provide cell proliferation advantages33. This data corroborated previous reports showing higher Notch activity in triple-negative BC34. Inhibition of leptin or Notch signaling via pharmacologic inhibitors for leptin (PEG-LPrA2) and Notch signaling (DAPT for γ-secretase) abrogated E0771 cell proliferation and migration, as it was earlier shown in 4T1 cells20. However, DAPT has a broad range of inhibitory actions. Therefore, the inhibition of Notch signaling (RBP-Jk gene knockout) was also achieved by forcing the expression of dominant negative RBP-Jk gene (R218H mutant). R218H completely abrogated leptin-induced proliferation and migration of E0771 cells. These data strongly suggest that leptin-induced BC proliferation and migration requires a functional leptin-Notch signaling axis.
Data from present “tumor-take mouse model” corroborated that a HFD induces obesity and increases the incidence of BC in mice16,35,36. Importantly, PEG-LPrA2 treatment reduced the protein and mRNA levels of Notch components within BC tissues. Intriguingly, E0771-derived BC hosted by lean mice receiving no treatment showed higher levels of Notch4 that BC in untreated DIO-mice. However, PEG-LPrA2 reduced Notch4 levels within BC from lean and NICD4 from obese mice. Currently, we cannot provide a plausible explanation for these disparate results. Therefore, this should be further investigated. Overall, present results strongly suggest that the development of E0771-BC is linked to obesity and requires a functional leptin-Notch axis.
Notch signaling is considered as a major cause of BC development that increases BC stem cells37, relapse38,39 and drug resistance40. In addition to leptin, Notch signaling affected insulin sensitivity in insulin-resistant mice39 and enhanced the oncogenic potential of insulin growth factor-1 (IGF-1)42. However, insulin- or IGF-1 induced regulation of the Notch pathway has not been reported. Overall it is unclear whether these pathways are predominantly required for cancer in obese humans3.
Present findings further validate the idea that the leptin-Notch axis plays a role in BC development, which seems to be over activated in obese patients. The overall picture could be that obesity induces an increase in leptin levels, which can affect several processes linked to BC development, including accelerated angiogenesis, overexpression of VEGF/VEGFR-2, induction of oncogenic (Cyclin D1), and anti-apoptotic (Bcl-2) molecules2,17,37. Moreover, leptin is also able to transactivate VEGFR-2, even in absence of VEGF43. Potential involvement of leptin2, 44 and leptin-induced Notch in maintenance of BCSC and drug resistance37 may be involved in the poor prognosis of obese BC patients. However, we recognize the limitations of the data generated from the breast cancer mouse model used. Therefore, other factors may also contribute to obesity-related breast cancer.
Novelty & Impact Statement.
Pandemic obesity and breast cancer incidence strongly correlate, but the underlying mechanisms involved in these relationships are not well understood. Present data show an unveiled mechanism linking leptin and Notch signaling, which is mainly triggered in obesity contexts. Leptin-induced Notch signaling axis could contribute to the aggressiveness and poor prognosis of breast cancer. Present results could help to design novel strategies to prevent and treat breast cancer, which may be of utmost relevance for obese patients.
Acknowledgements
This work was partially funded by Grants from NIH/NCI 1SC1CA138658-04; and the Georgia Cancer Coalition Distinguished Cancer Scholar Award to R.R.G-P.; NIH/NCI 1R21CA153172-01A1 to MT-K, and facilities and support services at Morehouse School of Medicine (NIH RR03034 and 1C06 RR18386) and NIH/NCRR grant 1G12RR026250-03.
Contributor Information
Monica Battle, Department of Microbiology, Biochemistry & Immunology, Morehouse School of Medicine, Atlanta, GA 30310; mbattle88@gmail.com.
Corey Gillespie, Department ofMicrobiology, Biochemistry & Immunology, Morehouse School of Medicine, Atlanta, GA 30310; cgillespie@msm.edu.
Alexander Quarshie, Biomedical Informatics Program and Master of Science in Clinical Research Program, Clinical Research Center, Morehouse School of Medicine, Atlanta, GA 30310; aquarshie@msm.edu.
Viola Lanier, Department ofMicrobiology, Biochemistry & Immunology, Morehouse School of Medicine, Atlanta, GA 30310; vlanier@msm.edu.
Tia Harmon, Department ofMicrobiology, Biochemistry & Immunology, Morehouse School of Medicine, Atlanta, GA 30310; tharmon@msm.edu.
Kaamilah Wilson, Department of Microbiology, Biochemistry & Immunology, Morehouse School of Medicine, Atlanta, GA 30310; milahdances247@yahoo.com.
Marta Torroella-Kouri, Department of Microbiology and Immunology, University of Miami School of Medicine, Miami, FL 33101; MTorroella@med.miami.edu.
Ruben R. Gonzalez-Perez, Department of Microbiology, Biochemistry & Immunology, Morehouse School of Medicine, Atlanta, GA 30310.
References
- 1.2013 http://www.cancer.org/Cancer/BreastCancer/DetailedGuide/breast-cancer-key-statistics.
- 2.Guo S, Liu M, Wang G, Torroella-Kouri M, Gonzalez-Perez RR. Oncogenic role and therapeutic target of leptin signaling in breast cancer and cancer stem cells. Biochim Biophys Acta. 2012;1825:207–22. doi: 10.1016/j.bbcan.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khandekar MJ, Cohen P, Spiegelman BM. Molecular mechanisms of cancer development in obesity. Nat Rev Cancer. 2011;11:886–95. doi: 10.1038/nrc3174. [DOI] [PubMed] [Google Scholar]
- 4.Klein S, Wadden T, Sugerman HJ. AGA technical review on obesity. Gastroenterology. 2002;123:882–932. doi: 10.1053/gast.2002.35514. [DOI] [PubMed] [Google Scholar]
- 5.Rock CL Demark-Wahnefried W. Nutrition and survival after the diagnosis of breast cancer: a review of the evidence. J Clin Oncol. 2002;20:3302–16. doi: 10.1200/JCO.2002.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prieto-Hontoria PL, Perez-Matute P, Fernandez-Galilea M, Bustos M, Martinez JA, Moreno-Aliaga MJ. Role of obesity-associated dysfunctional adipose tissue in cancer: a molecular nutrition approach. Biochim Biophys Acta. 2011;1807:664–78. doi: 10.1016/j.bbabio.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 7.Nichols HB, Trentham-Dietz A, Egan KM, Titus-Ernstoff L, Holmes MD, Bersch AJ, Holick CN, Hampton JM, Stampfer MJ, Willett WC, Newcomb PA. Body Mass Index Before and After Breast Cancer Diagnosis: Associations with All-Cause, Breast Cancer, and Cardiovascular Disease Mortality. Cancer Epidemiology Biomarkers & Prevention. 2009;18:1403–9. doi: 10.1158/1055-9965.EPI-08-1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garofalo C, Surmacz E. Leptin and cancer. J.Cell.Physiol. 2006;207:12–22. doi: 10.1002/jcp.20472. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Y, Bellows CF, Kolonin MG. Adipose tissue-derived progenitor cells and cancer. World J Stem Cells. 2010;2:103–13. doi: 10.4252/wjsc.v2.i5.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Protani M, Coory M, Martin JH. Effect of obesity on survival of women with breast cancer: systematic review and meta-analysis. Breast Cancer Res Treat. 2010;123:627–35. doi: 10.1007/s10549-010-0990-0. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–32. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 12.Gonzalez RR, Simon C, Caballero-Campo P, Norman R, Chardonnens D, Devoto L, Bischof P. Leptin and reproduction. Hum Reprod Update. 2000;6:290–300. doi: 10.1093/humupd/6.3.290. [DOI] [PubMed] [Google Scholar]
- 13.Ando S, Catalano S. The multifactorial role of leptin in driving the breast cancer microenvironment. Nat Rev Endocrinol. 2011;8:263–75. doi: 10.1038/nrendo.2011.184. [DOI] [PubMed] [Google Scholar]
- 14.Cleary MP, Phillips FC, Getzin SC, Jacobson TL, Jacobson MK, Christensen TA, Juneja SC, Grande JP, Maihle NJ. Genetically obese MMTV-TGF-alpha/Lep(ob)Lep(ob) female mice do not develop mammary tumors. Breast Cancer Res Treat. 2003;77:205–15. doi: 10.1023/a:1021891825399. [DOI] [PubMed] [Google Scholar]
- 15.Cleary MP, Juneja SC, Phillips FC, Hu X, Grande JP, Maihle NJ. Leptin receptor-deficient MMTV-TGF-alpha/Lepr(db)Lepr(db) female mice do not develop oncogene-induced mammary tumors. Exp Biol Med (Maywood) 2004;229:182–93. doi: 10.1177/153537020422900207. [DOI] [PubMed] [Google Scholar]
- 16.Gillespie C, Quarshie A, Penichet M, Gonzalez-Perez RR. Potential Role of Leptin Signaling in DMBA-induced Mammary Tumors by Non-Responsive C57BL/6J Mice Fed a High-Fat Diet. J Carcinogene Mutagene. 2012;3:2. [Google Scholar]
- 17.Gonzalez RR, Cherfils S, Escobar M, Yoo JH, Carino C, Styer AK, Sullivan BT, Sakamoto H, Olawaiye A, Serikawa T, Lynch MP, Rueda BR. Leptin signaling promotes the growth of mammary tumors and increases the expression of vascular endothelial growth factor (VEGF) and its receptor type two (VEGF-R2). J Biol Chem. 2006;281:26320–8. doi: 10.1074/jbc.M601991200. [DOI] [PubMed] [Google Scholar]
- 18.Rene Gonzalez R, Watters A, Xu Y, Singh UP, Mann DR, Rueda BR, Penichet ML. Leptin-signaling inhibition results in efficient anti-tumor activity in estrogen receptor positive or negative breast cancer. Breast Cancer Res. 2009;11:R36. doi: 10.1186/bcr2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lorincz AM, Sukumar S. Molecular links between obesity and breast cancer. Endocr Relat Cancer. 2006;13:279–92. doi: 10.1677/erc.1.00729. [DOI] [PubMed] [Google Scholar]
- 20.Guo S, Gonzalez-Perez R. Notch, IL-1 and leptin crosstalk outcome (NILCO) is critical for leptin-induced proliferation, migration and VEGF/VEGFR-2 expression in breast cancer. PLoS One. 2011;6:e21467. doi: 10.1371/journal.pone.0021467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sugiura K, Stock CC. Studies in a tumor spectrum. I. Comparison of the action of methylbis(2-chloroethyl)amine and 3-bis(2-chloroethyl)aminomethyl-4-methoxymethyl-5-hydroxy-6-methylpyridine on the growth of a variety of mouse and rat tumors. Cancer. 1952;5:382–402. doi: 10.1002/1097-0142(195203)5:2<382::aid-cncr2820050229>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 22.Gonzalez RR, Leavis P. A peptide derived from the human leptin molecule is a potent inhibitor of the leptin receptor function in rabbit endometrial cells. Endocrine. 2003;21:185–95. doi: 10.1385/ENDO:21:2:185. [DOI] [PubMed] [Google Scholar]
- 23.Chung CN, Hamaguchi Y, Honjo T, Kawaichi M. Sitedirected mutagenesis study on DNA binding regions of the mouse homologue of Suppressor of Hairless, RBP-J kappa. Nucl Acids Res. 1994;22:2938–44. doi: 10.1093/nar/22.15.2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.2013 http://www.cdc.gov/obesity/data/adult.html.
- 25.WHO World Health Organization Fact Sheet for World Wide Prevalence of Obesity. 2006 http://www.who.int/mediacentre/factsheets/fs311/en/index.html.
- 26.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, Obesity, and Mortality from Cancer in a Prospectively Studied Cohort of U.S. Adults. N Engl J Med. 2003;348:1625–38. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 27.Daling JR, Malone KE, Doody DR, Johnson LG, Gralow JR, Porter PL. Relation of body mass index to tumor markers and survival among young women with invasive ductal breast carcinoma. Cancer. 2001;92:720–9. doi: 10.1002/1097-0142(20010815)92:4<720::aid-cncr1375>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 28.Whiteman MK, Hillis SD, Curtis KM, McDonald JA, Wingo PA, Marchbanks PA. Body Mass and Mortality After Breast Cancer Diagnosis. Cancer Epidemiology Biomarkers & Prevention. 2005;14:2009–14. doi: 10.1158/1055-9965.EPI-05-0106. [DOI] [PubMed] [Google Scholar]
- 29.Gautron L, Elmquist J. Sixteen years and counting: an update on leptin in energy balance. J Clin Invest. 2011;121:2087–93. doi: 10.1172/JCI45888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guo S, Liu M, Gonzalez-Perez RR. Role of Notch and its oncogenic signaling crosstalk in breast cancer. Biochim Biophys Acta. 2011;1815:197–213. doi: 10.1016/j.bbcan.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fei H, Okano HJ, Li C, Lee GH, Zhao C, Darnell R, Friedman JM. Anatomic localization of alternatively spliced leptin receptors (Ob-R) in mouse brain and other tissues. Proc Natl Acad Sci U S A. 1997;94:7001–5. doi: 10.1073/pnas.94.13.7001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lanier V, Gillespie C, McGlothen T, Dickson T, Guo S, Gonzalez-Perez RR. Leptin induces Notch in endothelial cells: role of VEGFR-2 transactivation. Cancer Research. 2012;72(Supplement 1) [Google Scholar]
- 33.Parr C, Watkins G, Jiang WG. The possible correlation of Notch-1 and Notch-2 with clinical outcome and tumour clinicopathological parameters in human breast cancer. Int J Mol Med. 2004;14:779–86. doi: 10.3892/ijmm.14.5.779. [DOI] [PubMed] [Google Scholar]
- 34.Reedijk M, Pinnaduwage D, Dickson BC, Mulligan AM, Zhang H, Bull SB, O'Malley FP, Egan SE, Andrulis IL. JAG1 expression is associated with a basal phenotype and recurrence in lymph node-negative breast cancer. Breast Cancer Res Treat. 2008;111:439–48. doi: 10.1007/s10549-007-9805-3. [DOI] [PubMed] [Google Scholar]
- 35.Wolff GL, Kodell RL, Cameron AM, Medina D. Accelerated appearance of chemically induced mammary carcinomas in obese yellow (Avy/A) (BALB/c X VY) F1 hybrid mice. J Toxicol Environ Health. 1982;10:131–42. doi: 10.1080/15287398209530237. [DOI] [PubMed] [Google Scholar]
- 36.Lane HW, Butel JS, Howard C, Shepherd F, Halligan R, et al. The role of high levels of dietary fat in 7,12-dimethylbenzanthracene-induced mouse mammary tumorigenesis: lack of an effect on lipid peroxidation. Carcinogenesis. 1985;6:403–7. doi: 10.1093/carcin/6.3.403. [DOI] [PubMed] [Google Scholar]
- 37.Korkaya H, Wicha MS. HER-2, notch, and breast cancer stem cells: targeting an axis of evil. Clin Cancer Res. 2009;15:1845–7. doi: 10.1158/1078-0432.CCR-08-3087. [DOI] [PubMed] [Google Scholar]
- 38.Clarke MF. Self-renewal and solid-tumor stem cells. Biol Blood Marrow Transplant. 2005;11:14–6. doi: 10.1016/j.bbmt.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 39.Clevers H. The cancer stem cell: premises, promises and challenges. Nat Med. 2011;17:313–9. doi: 10.1038/nm.2304. [DOI] [PubMed] [Google Scholar]
- 40.Wang Z, Li Y, Ahmad A, Azmi AS, Banerjee S, Kong D, FH S. Targeting Notch signaling pathway to overcome drug resistance for cancer therapy. Biochim Biophys Acta. 2010;1806:258–67. doi: 10.1016/j.bbcan.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pajvani UB, Shawber CJ, Samuel VT, Birkenfeld A L, Shulman GI, Kitajewski J, Accili D. Inhibition of Notch signaling ameliorates insulin resistance in a FoxO1-dependent manner. Nat Med. 2011;17:961–7. doi: 10.1038/nm.2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eliasz S, Liang S, Chen Y, De Marco MA, Machek O, Skucha S, Miele L, Bocchetta M. Notch-1 stimulates survival of lung adenocarcinoma cells during hypoxia by activating the IGF-1R pathway. Oncogene. 2010;29:2488–98. doi: 10.1038/onc.2010.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Garonna E, Botham KM, Birdsey GM, Randi AM, Gonzalez-Perez RR, Wheeler-Jones CP. Vascular endothelial growth factor receptor-2 couples cyclo-oxygenase-2 with pro-angiogenic actions of leptin on human endothelial cells. Plos one. 2011;6:e18823. doi: 10.1371/journal.pone.0018823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bartucci M, Svensson S, Ricci-Vitiani L, et al. Obesity hormone leptin induces growth and interferes with the cytotoxic effects of 5-fluorouracil in colorectal tumor stem cells. Endocr.Relat Cancer. 2010;17:823–33. doi: 10.1677/ERC-10-0083. [DOI] [PubMed] [Google Scholar]