Abstract
The death associated protein kinases (DAPK) are a phylogenetically widespread family of calcium-regulated serine/threonine kinases, initially identified from their roles in apoptosis. Subsequent studies, principally in vertebrate cells or models, have elucidated the functions of the DAPK family in autophagy and tumor suppression. Invertebrate genetic model organisms such as Drosophila and C. elegans have revealed additional functions for DAPK and related kinases. In the nematode C. elegans, the sole DAPK family member DAPK-1 positively regulates starvation-induced autophagy. Genetic analysis in C. elegans has revealed that DAPK-1 also acts as a negative regulator of epithelial innate immune responses in the epidermis. This negative regulatory role for DAPK in innate immunity may be analogous to the roles of mammalian DAPK in inflammatory responses.
Keywords: epidermis, epithelia, morphogenesis
Introduction
Death associated protein kinase (DAPK) was first discovered from a functional antisense RNA based screen in cell culture for suppressors of interferon (IFN)-induced cell death (1). DAPK is characterized by a conserved Ca2+/Calmodulin (CaM) regulatory domain, a serine/threonine kinase domain, and several other conserved noncatalytic domains (Figure 1). DAPK’s link to cell death was primarily deduced from in vitro experiments, in which over-expression of DAPK in cell culture resulted in cell membrane blebbing and other apoptotic features (2). DAPK is required for induction of cell death by various death signals (3). Further investigation showed that the mode of cell death was dependent on cell type. Over-expression of DAPK in certain cells, such as primary fibroblasts, induced caspase-dependent cell death, apoptosis-associated morphological changes (4), and DNA fragmentation (reviewed by (5)). In other cells, such as HeLa and MCF-7, overexpression resulted in the formation of autophagic vesicles and autolysosomes in the cytoplasm (2). Although autophagy can promote cell survival, it can also cause autophagic (type-II) programmed cell death. DAPK and related kinases are now recognized as playing a wide variety of roles in apoptotic and autophagic cell death and other processes in mammals. One challenge in defining the normal functions of DAPK has been the functional redundancy in the mammalian DAPK family. As C. elegans and Drosophila each encode only a single DAPK family member, genetic analyses in these genetic model organisms could provide insights into the functions of DAPK in normal development and physiology. Here we review findings on DAPK and related kinases from C. elegans and Drosophila and attempt to draw connections between these results and studies in mammalian models.
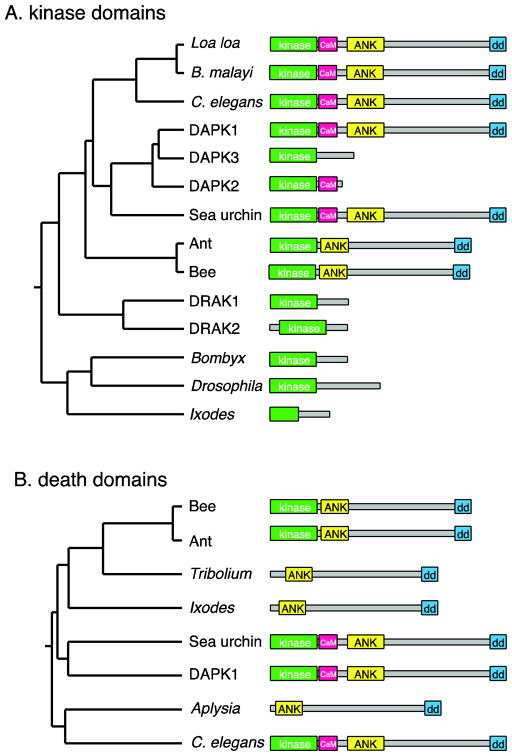
Figure 1. Phylogeny of the DAPK family.
A. Tree based on pairwise alignment (ClustalW) analysis of the amino acid sequences of the kinase domains of DAPK family members, along with cartoons of the protein domain architecture, approximately to scale. Human DAPK1 and DAPK family members are compared with related proteins predicted from genome sequences of various invertebrate species. B: Tree based on alignments of the death domains. To simplify the domain structures, the P-loops and ROCO domains are not shown. NCBI sequence accession numbers: C. elegans (O44997.2); Loa loa (EJD74144.1); Brugia malayi (XP_001896664.1), Human DAPK1 (AAI43760.1); Human DAPK3 (NP_001339.1); Human DAPK2 (Q9UIK4.1); sea urchin Strongylocentrotus purpuratus (XP_003723347.1); carpenter ant Camponotus floridanus (E1ZVV1); honey bee Apis florea (XP_003689717.1); Human DRAK1 (BAA34126.1), Human DRAK2 (BAA34127.1); silkworm Bombyx mori (E9JEH2); Drosophila Drak (E1JJH9); deer tick Ixodes scapularis (EEC12134.1). Kinase-less sequences: flour beetle Tribolium castaneum (XP_972597.2); I. scapularis (EEC05408.1); sea slug Aplysia californica (XP_005108326.1).
Phylogenetics of the DAPK family
DAPK-like proteins are found throughout the animal kingdom, but are by far best studied in mammals, i.e. mouse and human. Mammalian DAPK (now DAPK1) is a multidomain protein, consisting of a kinase domain, a calcium-calmodulin (Ca2+/CaM) regulatory domain, ankyrin repeats, P-loop motifs, a death domain, and additional conserved motifs (6). In addition to DAPK, mammals encode a family of ‘DAPK related’ kinases that share a closely related kinase domain, and in some cases, a Ca2+/CaM regulatory domain. The DAPK protein family consists of DAPK1 itself, DAPK2 (also known as DRP-1) and DAPK3 (previously known as Zipper interacting kinase, ZIPk). More divergent members of the DAPK family are the DAP kinase related apoptosis inducing protein kinases DRAK1 and 2. The DAPK family has been classified as part of the DMT subfamily of Ca2+/CaM-dependent kinases (7). All members of the DAPK group have been linked to cell death (8, 9). As the extracatalytic domains and biological function of these proteins differ drastically (3), suggesting that DAPK1 has unique roles compared to other DAPK-like proteins.
In general, invertebrates express fewer members of the DAPK family; ZIPK and DRP-1 are found only in mammals. Among invertebrates, nematodes, certain arthropods such as bees, ants, arachnids, and sea urchins have encode proteins similar to DAPK1 (that is, containing the noncatalytic domains such as the ankyrin repeats and a C-terminal death domain) (Figure 1A). C. elegans encodes a single family member, DAPK-1, overall 33% identical in sequence to human DAPK1 (10). Interestingly, Drosophila melanogaster lacks a canonical DAPK1 ortholog and instead encodes a single DRAK-like protein, Drak (11). The silkworm Bombyx also encodes a Drak-like protein. Other arthropod genomes, such as those of Tribolium (beetles) or arachnids, are predicted to encode proteins resembling truncated versions of DAPK1, lacking the kinase domain and Ca2+/CaM regulatory domains, but containing ankyrin repeats and a DAPK-like death domain (Figure 1B). Among the lophotrochozoa, Aplysia encodes a similar kinase-less protein. DAPK1-like proteins are evident in the genomes of echinoderms (sea urchin) and of various non-vertebrate chordates (not shown), but their functions remain unstudied.
C. elegans DAPK-1 regulates autophagy
The function of the C. elegans dapk-1 gene has been addressed by genetic mutations and RNA interference. Animals mutant for dapk-1 loss-of-function alleles are viable and fertile, with progressive defects in epidermal structure as described below. Developmentally programmed apoptotic cell deaths appear to occur in dapk-1 mutants, although a detailed analysis has not yet been reported. Thus, a subtle or redundant role for C. elegans DAPK-1 in apoptosis cannot be excluded. In contrast, DAPK-1 has been clearly linked to stress-induced autophagy. In C. elegans, starvation activates a MAPK pathway in the pharyngeal muscles via muscarinic acetylcholine receptor signaling, which acts to increase muscle activity (12). In mutants lacking the G protein β subunit GPB-2, starvation induces damage to the pharyngeal muscle via excessive autophagy, due to constitutive active muscarinic signaling (13). Autophagy of pharyngeal muscles renders the worms unable to feed, ultimately leading to lethality. Loss of function in the key autophagy factor beclin/bec-1 rescues the organismal death of gpb-2 mutants, confirming that elevated autophagy was the cause of death. By screening candidate cell death genes, Kang et al. showed that dapk-1 loss of function partly suppressed the starvation-induced autophagy and lethality of gpb-2 mutants (13). As loss of function in the key apoptotic caspase CED-3 did not rescue the starvation-induced death of gpb-2 mutants, DAPK-1’s effect is unlikely to be due to a role in apoptosis. Thus, it seems that dapk-1 acts downstream of or in parallel to muscarinic signaling to promote starvation-induced autophagy. Interestingly, DAPK(−/−) knockout mice are defective in ER stress-induced autophagic and apoptotic cell death (14). Thus, the DAPK family may play a conserved role in stress-induced cell death.
Roles in epidermal morphogenesis, epithelial integrity, and wound healing
C. elegans dapk-1 mutations were also independently identified in forward genetic screens for mutants displaying aberrant epidermal morphogenesis (10). dapk-1 loss of function mutants display progressive defects in the morphology of the epidermis and cuticle (Figure 2A). This aberrant morphology involves over-accumulation of collagens and other cuticle components, making the cuticle up to 5–10 times thicker than wild type. The cuticle over-secretion is first apparent in late larval stages and becomes progressively more severe during adult life; defects are also spatially confined to the head, tail, vulva, and interfacial regions of the epidermis. These morphological defects do not seem to result from mis-regulation of apoptosis or autophagy, as loss of function in core apoptosis or autophagy genes neither phenocopied nor suppressed the cuticle defects in dapk-1 mutants (10). The cuticle deposits of dapk-1 mutants contain autofluorescent aggregates similar to components of scars that form after skin wounding (15), suggesting dapk-1 might negatively regulate epidermal wound responses.
Figure 2. C. elegans DAPK-1 and epidermal development.
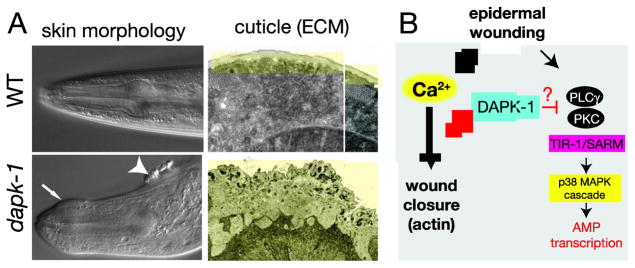
A. Images of the dapk-1 epidermal morphology phenotype, taken from Tong et al., 2009 (10). dapk-1(ju4) mutant adults display deformations of the epidermis (arrow and arrowhead, left panels) visible under DIC microscopy. Electron microscopy (right panels) reveals massive expansion of the cuticle (colored yellow) in the head region. B. Model for DAPK-1 function in wound responses as a negative regulator of the actin cytoskeleton and innate immunity (AMP transcription). See also (21).
DAPK-like kinases also play roles in epithelial morphogenesis or integrity in Drosophila. Mutants lacking the Drosophila Drak are viable and fertile and show normal levels of apoptosis. However drak null mutants display shortened and malformed appendages (wings and legs) reminiscent of rok mutants defective in Rho-associated protein kinase (11). drak displays dose-dependent genetic interactions with rok mutants, in that drak(−/−) rok(+/−) animals display defects in the tracheal and wing disk epithelia. These tissues are thinned and distorted, likely from cell loss due to high levels of apoptosis in the epithelia. Hence, the loss of epithelial integrity in drak mutants is mechanistically distinct from the apparently apoptosis-independent epidermal defects of C. elegans dapk-1 mutants. Furthermore, drak−/− rok−/− mutants displayed fully penetrant defects in head involution, a process in which dorsal and lateral epidermal cells spread to cover the anterior end of the embryo, suggesting Drak is important in epithelial morphogenesis at multiple stages of development. Drosophila Drak also functions redundantly with Rok to promote adherens junction remodeling during morphogenesis of ommatidia from the eye disk neuroepithelium (16). In summary, DAPK family members play either subtle or partly redundant roles in the morphology and integrity of epithelia of Drosophila and C. elegans.
DAPK-1 regulation of actin dynamics in epithelial development and wound healing
DAPK has long been known to associate with actin microfilaments (17). DAPK localizes to actin stress fibers in HeLa cells, and when over-expressed, induces extensive membrane protrusions and membrane blebbing (18). These cytoplasmic changes are a result of DAPK mediated phosphorylation of myosin-II regulatory light chain (RLC) and may contribute to DAPK’s pro-apoptotic activity. In vivo, DAPK family members play important roles in regulating actin dynamics. In Drosophila, Drak phosphorylates the fly myosin RLC (Sqh) to promote proper morphogenesis of epithelial tissues during development (11). Drak and Rok can each phosphorylate Sqh, explaining their synergistic lethality; expression of a constitutively activated Sqh can strongly rescue the elevated apoptosis and lethality of the drak rok double mutant.
The regulation of the actin cytoskeleton by DAPK is important in epidermal wound healing in C. elegans. The worm epidermis is a simple barrier epithelium that provides the first line of defense against pathogens and wounds (19, 20). As the nematode body is under hydrostatic pressure, it is imperative that damage to the epidermis be repaired rapidly for the worm to survive. Wounding causes massive calcium influx into the epidermal cytoplasm, triggering a Gq signaling cascade that results in actin-mediated wound closure (21). During wound closure, an actin ring forms around the wound site. In dapk-1 mutants, actin rings closed more rapidly around the wound site compared to wild type. Reduced Ca2+ signaling did not suppress dapk-1 fast wound closure, but loss of dapk-1 function does suppress the low post-wound survival of Gq mutants. These results support DAPK-1 acting as a negative regulator of wound closure dynamics in the epidermal epithelium, likely downstream of Ca2+ signals. Mammalian DAPK has also been studied in the context of wound repair, using the in vitro scratch assay. When NIH3T3 derivatives were assayed for wound-healing migration, over-expression of DAPK caused a significant delay in wound closure (22). Possibly, the impaired wound healing migration in DAPK over-expressing cells reflects misregulation of the actin cytoskeleton.
DAPK-1 is a negative regulator of epithelial innate immunity in C. elegans
In C. elegans as in other animals the skin is a barrier epithelium, forming the first line of defense against external pathogens and physical damage (20). C. elegans is now known to have a sophisticated and robust epidermal innate immune response that protects animals from infection via the skin. Infection by epidermis-penetrating fungi such as Drechmeria coniospora induces expression of numerous antimicrobial peptide (AMP) genes (23). Sterile skin wounding by needles or laser damage induces an overlapping set of responses (15). In dapk-1 mutants transcription of AMPs such as nlp-29 is constitutively up-regulated in the epidermis (10). This upregulation in dapk-1 mutants can be rescued by epidermal-driven DAPK-1 expression, suggesting dapk-1 acts cell autonomously to inhibit the innate immune response. Moreover, transient over-expression of DAPK-1 can suppress the up-regulation of nlp-29 in response to needle wounding, indicating that DAPK-1 can acutely block innate immune responses to damage.
Induction of the AMP nlp-29 by infection or wounding requires the SARM protein TIR-1 and a p38 MAPK cascade, acting in the epidermis (24) (Figure 2). TIR-1 and the p38 MAPK cascade are required for the constitutive induction of nlp-29 in dapk-1 mutants, indicating DAPK-1 acts upstream of the TIR-1/p38 signaling pathway. The TIR-1/p38MAPK pathway in epidermal AMP induction after infection is regulated by G-protein signaling and by TPA-1/Protein kinase C δ (25). Additional epistasis analysis is required to define where DAPK-1 intersects with the signaling cascade upstream of TIR-1. It will also be interesting to see what other pathways DAPK-1 interacts with to control the expression of the other AMPs that are upregulated after septic or sterile wounding.
Loss of function in the TIR-1/p38 MAPK cascade does not affect the cuticle hypertrophy phenotype of dapk-1 mutants. Conversely, suppression of dapk-1 cuticle hypertrophy by loss of function in the pre-mRNA 3′ end processing regulatory gene sydn-1 (26) did not affect the constitutive induction of epithelial innate immunity genes. Together these results suggest that DAPK-1 acts independently to regulate epidermal morphology and epithelial innate immunity.
DAPK and innate immunity in vertebrates
Several studies have pointed to roles for mammalian DAPK in regulating innate immune responses, specifically inflammatory responses. Initially, DAPK was found to act as a checkpoint in the macrophage inflammation program (27) and as a negative regulator of T-cell receptor-mediated activation of NFκB (28). Interestingly, the negative regulatory role of DAPK1 in NFκB activation may involve PKCθ, a homolog of TPA-1. In addition, DAPK knockout mice suffer from lung inflammation after lipopolysaccharide (LPS) challenge, due to the hypersecretion of cytokines such as IL-6 from macrophages and neutrophils (29). Recent work has elucidated a possible mechanism by which DAPK might regulate innate immunity (30). DAPK levels were elevated in ulcerative colitis-associated carcinoma (UCC), suggesting a protective role for DAPK in intestinal epithelial cells. DAPK binds and inhibits the transcription factor STAT3; conversely, STAT3 appears to repress DAPK transcription. This mutual negative regulation of STAT3 and DAPK may provide a finely balanced mechanism for regulating TNF-induced inflammation. Intriguingly, a C. elegans STAT-like protein STA-2 has been implicated in epidermal innate immunity. Loss of STA-2 activity by mutation or RNAi prevents up-regulation of nlp-29 after wounding (31). STA-2 acts downstream of the PMK-1 MAPK cascade, and may regulate transcription in the epidermis, but also localizes to endocytic vesicles in the cytoplasm. It will be interesting to learn if DAPK-1 and STA-2 interact in ways analogous to the DAPK/STAT3 circuit revealed in mammals. In summary, the role of mammalian DAPK as a negative regulator of inflammatory responses has intriguiing parallels to its function as a negative regulator of epithelial innate immune responses in C. elegans.
Conclusions and Questions
In contrast to the abundant evidence linking mammalian DAPK to cell death, DAPK family proteins in C. elegans and Drosophila appear largely dispensable for developmental apoptosis. C. elegans DAPK-1 promotes starvation-induced autophagy, but has not been linked more generally to autophagic cell death. It should be noted that mouse DAPK1 knockout mutants also display normal developmental apoptosis (14). It is possible that these different pictures of the role of the DAPK family in part reflect the differences between loss and gain of function studies.
Instead, C. elegans DAPK-1 functions in epithelial integrity, wound healing, and innate immunity; Drosophila DRAK also functions in epithelial development. C. elegans DAPK-1 acts as a coordinated negative regulator of epidermal innate immune defenses. It is not yet understood at a mechanistic level how C. elegans DAPK-1 regulates innate immunity, nor whether it is actively regulated during stresses such as epidermal infection or wounding. An important goal is to define where DAPK-1 intersects with the PKC/TIR-1/MAPK pathway in regulation of innate immunity.
Independently of its roles in innate immunity, DAPK-1 regulates the morphology of the epidermis. DAPK-1 is not required for embryonic epidermal morphogenesis, but appears to influence a post-embryonic epidermal maintenance process. It remains to be elucidated whether DAPK-1 primarily affects the epidermal cytoskeleton, polarity, secretion, or a combination of these. In wound repair DAPK-1 likely regulates the actin cytoskeleton via non-muscle myosin, reminiscent of the functions of Drosophila Drak and consistent with much evidence that DAPK1 regulates nonmuscle myosin. DAPK1 also interacts with the microtubule cytoskeleton; DAPK1 binds MAP1B (4), and has been shown to inhibit MT assembly via the MARK/PAR-1 kinase family (32). It will be interesting to determine whether the aberrant epithelial integrity of C. elegans dapk-1 mutants involves alterations in the microtubule cytoskeleton. Further studies of DAPK-1 in C. elegans may yield additional insights into the functions of this ancient family of kinases.
Acknowledgments
M.C. is supported by the UCSD Cellular and Molecular Genetics Training Grant (NIH T32 GM007240). Work in our laboratory on DAPK is supported by NIH R01 GM054657 to A.D.C.
References
- 1.Deiss LP, Feinstein E, Berissi H, Cohen O, Kimchi A. Identification of a novel serine/threonine kinase and a novel 15-kD protein as potential mediators of the gamma interferon-induced cell death. Genes & development. 1995;9:15–30. doi: 10.1101/gad.9.1.15. [DOI] [PubMed] [Google Scholar]
- 2.Inbal B, Bialik S, Sabanay I, Shani G, Kimchi A. DAP kinase and DRP-1 mediate membrane blebbing and the formation of autophagic vesicles during programmed cell death. The Journal of cell biology. 2002;157:455–468. doi: 10.1083/jcb.200109094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin Y, Hupp TR, Stevens C. Death-associated protein kinase (DAPK) and signal transduction: additional roles beyond cell death. Febs J. 2010;277:48–57. doi: 10.1111/j.1742-4658.2009.07411.x. [DOI] [PubMed] [Google Scholar]
- 4.Harrison B, Kraus M, Burch L, et al. DAPK-1 binding to a linear peptide motif in MAP1B stimulates autophagy and membrane blebbing. The Journal of biological chemistry. 2008;283:9999–10014. doi: 10.1074/jbc.M706040200. [DOI] [PubMed] [Google Scholar]
- 5.Gozuacik D, Kimchi A. DAPk protein family and cancer. Autophagy. 2006;2:74–79. doi: 10.4161/auto.2.2.2459. [DOI] [PubMed] [Google Scholar]
- 6.Bialik S, Kimchi A. Biochemical and functional characterization of the ROC domain of DAPK establishes a new paradigm of GTP regulation in ROCO proteins. Biochem Soc Trans. 2012;40:1052–1057. doi: 10.1042/BST20120155. [DOI] [PubMed] [Google Scholar]
- 7.Temmerman K, Simon B, Wilmanns M. Structural and functional diversity in the activity and regulation of DAPK-related protein kinases. FEBS J. 2013 doi: 10.1111/febs.12384. in press. [DOI] [PubMed] [Google Scholar]
- 8.Murata-Hori M, Fukuta Y, Ueda K, Iwasaki T, Hosoya H. HeLa ZIP kinase induces diphosphorylation of myosin II regulatory light chain and reorganization of actin filaments in nonmuscle cells. Oncogene. 2001;20:8175–8183. doi: 10.1038/sj.onc.1205055. [DOI] [PubMed] [Google Scholar]
- 9.Sanjo H, Kawai T, Akira S. DRAKs, novel serine/threonine kinases related to death-associated protein kinase that trigger apoptosis. The Journal of biological chemistry. 1998;273:29066–29071. doi: 10.1074/jbc.273.44.29066. [DOI] [PubMed] [Google Scholar]
- 10.Tong A, Lynn G, Ngo V, et al. Negative regulation of Caenorhabditis elegans epidermal damage responses by death-associated protein kinase. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:1457–1461. doi: 10.1073/pnas.0809339106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neubueser D, Hipfner DR. Overlapping roles of Drosophila Drak and Rok kinases in epithelial tissue morphogenesis. Molecular biology of the cell. 2010;21:2869–2879. doi: 10.1091/mbc.E10-04-0328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.You YJ, Kim J, Cobb M, Avery L. Starvation activates MAP kinase through the muscarinic acetylcholine pathway in Caenorhabditis elegans pharynx. Cell Metab. 2006;3:237–245. doi: 10.1016/j.cmet.2006.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kang C, You YJ, Avery L. Dual roles of autophagy in the survival of Caenorhabditis elegans during starvation. Genes & development. 2007;21:2161–2171. doi: 10.1101/gad.1573107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gozuacik D, Bialik S, Raveh T, et al. DAP-kinase is a mediator of endoplasmic reticulum stress-induced caspase activation and autophagic cell death. Cell Death Differ. 2008;15:1875–1886. doi: 10.1038/cdd.2008.121. [DOI] [PubMed] [Google Scholar]
- 15.Pujol N, Cypowyj S, Ziegler K, et al. Distinct Innate Immune Responses to Infection and Wounding in the C. elegans Epidermis. Curr Biol. 2008;18:481–489. doi: 10.1016/j.cub.2008.02.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robertson F, Pinal N, Fichelson P, Pichaud F. Atonal and EGFR signalling orchestrate rok- and Drak-dependent adherens junction remodelling during ommatidia morphogenesis. Development (Cambridge, England) 2012;139:3432–3441. doi: 10.1242/dev.080762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cohen O, Feinstein E, Kimchi A. DAP-kinase is a Ca2+/calmodulin-dependent, cytoskeletal-associated protein kinase, with cell death-inducing functions that depend on its catalytic activity. The EMBO journal. 1997;16:998–1008. doi: 10.1093/emboj/16.5.998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bialik S, Bresnick AR, Kimchi A. DAP-kinase-mediated morphological changes are localization dependent and involve myosin-II phosphorylation. Cell Death Differ. 2004;11:631–644. doi: 10.1038/sj.cdd.4401386. [DOI] [PubMed] [Google Scholar]
- 19.Ewbank JJ. Tackling both sides of the host-pathogen equation with Caenorhabditis elegans. Microbes Infect/Institut Pasteur. 2002;4:247–256. doi: 10.1016/s1286-4579(01)01531-3. [DOI] [PubMed] [Google Scholar]
- 20.Chisholm AD, Xu S. The epidermis as a model skin. II: differentiation and physiological roles. Wiley Interdiscip Rev Dev Biol. 2012;1:879–902. doi: 10.1002/wdev.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu S, Chisholm AD. A Galphaq-Ca(2)(+) signaling pathway promotes actin-mediated epidermal wound closure in C. elegans. Current biology : CB. 2011;21:1960–1967. doi: 10.1016/j.cub.2011.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuo JC, Wang WJ, Yao CC, Wu PR, Chen RH. The tumor suppressor DAPK inhibits cell motility by blocking the integrin-mediated polarity pathway. The Journal of cell biology. 2006;172:619–631. doi: 10.1083/jcb.200505138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Couillault C, Pujol N, Reboul J, et al. TLR-independent control of innate immunity in Caenorhabditis elegans by the TIR domain adaptor protein TIR-1, an ortholog of human SARM. Nature immunology. 2004;5:488–494. doi: 10.1038/ni1060. [DOI] [PubMed] [Google Scholar]
- 24.Pujol N, Zugasti O, Wong D, et al. Anti-fungal innate immunity in C. elegans is enhanced by evolutionary diversification of antimicrobial peptides. PLoS Pathogens. 2008;4:e1000105. doi: 10.1371/journal.ppat.1000105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ziegler K, Kurz CL, Cypowyj S, et al. Antifungal innate immunity in C. elegans: PKCdelta links G protein signaling and a conserved p38 MAPK cascade. Cell Host Microbe. 2009;5:341–352. doi: 10.1016/j.chom.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 26.Van Epps H, Dai Y, Qi Y, Goncharov A, Jin Y. Nuclear pre-mRNA 3′-end processing regulates synapse and axon development in C. elegans. Development (Cambridge, England) 2010;137:2237–2250. doi: 10.1242/dev.049692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mukhopadhyay R, Ray PS, Arif A, Brady AK, Kinter M, Fox PL. DAPK-ZIPK-L13a axis constitutes a negative-feedback module regulating inflammatory gene expression. Molecular cell. 2008;32:371–382. doi: 10.1016/j.molcel.2008.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chuang YT, Fang LW, Lin-Feng MH, Chen RH, Lai MZ. The tumor suppressor death-associated protein kinase targets to TCR-stimulated NF-kappa B activation. J Immunol. 2008;180:3238–3249. doi: 10.4049/jimmunol.180.5.3238. [DOI] [PubMed] [Google Scholar]
- 29.Nakav S, Cohen S, Feigelson SW, et al. Tumor suppressor death-associated protein kinase attenuates inflammatory responses in the lung. Am J Respir Cell Mol Biol. 2012;46:313–322. doi: 10.1165/rcmb.2011-0181OC. [DOI] [PubMed] [Google Scholar]
- 30.Chakilam S, Gandesiri M, Rau TT, et al. Death-associated protein kinase controls STAT3 activity in intestinal epithelial cells. Am J Pathol. 2013;182:1005–1020. doi: 10.1016/j.ajpath.2012.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 31.Dierking K, Polanowska J, Omi S, et al. Unusual regulation of a STAT protein by an SLC6 family transporter in C. elegans epidermal innate immunity. Cell Host Microbe. 2011;9:425–435. doi: 10.1016/j.chom.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 32.Wu PR, Tsai PI, Chen GC, et al. DAPK activates MARK1/2 to regulate microtubule assembly, neuronal differentiation, and tau toxicity. Cell Death Differ. 2011;18:1507–520. doi: 10.1038/cdd.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]