Abstract
Tumor Necrosis Factor Related Apoptosis Inducing Ligand (TRAIL) is a promising anti-cancer agent because it shows apoptosis-inducing activity in transformed, but not in normal cells. As with most anti-cancer agents, however, its clinical use is restricted by either inherent or acquired resistance by cancer cells. We demonstrate here that small-molecule SMAC mimetics that antagonize the Inhibitor of Apoptosis Proteins (IAPs) potently sensitize previously resistant human cancer cell lines, but not normal cells, to TRAIL-induced apoptosis, and that they do so in a caspase-8-dependent manner. We further show that the compounds have no cytotoxicity as single agents. Also, we demonstrate that several IAP family members likely participate in the modulation of cellular sensitivity to TRAIL. Finally, we note that the compounds that sensitize cancer cells to TRAIL are the most efficacious in binding to XIAP, and in inducing cIAP-1 and cIAP-2 degradation. Our studies thus describe valuable compounds that allow elucidation of the signaling events occurring in TRAIL resistance, and demonstrate that these agents act as potent TRAIL-sensitizing agents in a variety of cancer cell lines.
Keywords: TRAIL, XIAP, cIAP, caspase-8, apoptosis
INTRODUCTION
Members of the Tumor Necrosis Factor (TNF) superfamily are potent modulators of many cellular responses. Association of TNF, the prototypical family member, with its receptor TNFR1 results in receptor oligomerization and recruitment of adapter proteins, such as TNF Receptor Associated Death Domain protein (TRADD), to the receptor complex. Recruitment of Fas Associated Death Domain (FADD) in turn results in engagement of an apical caspase, such as caspase-8, leading to classical apoptosis induction (1, 2). Thus, the TNF-family receptor complex is capable of transducing either pro- or anti-apoptotic responses depending on the cellular context.
TNF Related Apoptosis Inducing Ligand (TRAIL, also known as Apo-2L or TNFSF10) is a promising potential anti-cancer agent due to its capability to induce apoptosis selectively in transformed cells, but not in normal cells (3). Accordingly, it is believed that TRAIL’s physiological role is in immune surveillance of cancerous cells in the body (4). This notion is supported by the observation that mice genetically deficient for TRAIL or its receptor are more susceptible to both induced and spontaneous tumor development (5, 6). Unlike other family members, TRAIL shows little or no toxicity when administered in vivo, further underscoring its potential utility as a novel anti-cancer therapy (7). As with many other anti-cancer agents, however, cancer cell resistance to TRAIL-induced apoptosis precludes its use in many cases (8, 9).
One mechanism by which cancer cells develop resistance to TRAIL-induced apoptosis is via upregulation of Inhibitor of Apoptosis Proteins (IAPs). Indeed, several members of the IAP family have been shown to be overexpressed in various cancers (10). IAP family proteins are characterized by the presence of an approximately 70 amino acid motif referred to as the Baculovirus IAP Repeat (BIR) domain (11, 12). The BIR domains mediate the IAPs’ direct binding to caspases, which are the proteases that are responsible for apoptosis, resulting in IAP-mediated inhibition of apoptosis (13). The most potent caspase inhibitor of the IAP family is X-linked IAP (XIAP), which directly binds to and inhibits caspases -3, -7 and -9 via its three BIR domains (14, 15, 16). Two other very similar IAP family members are the cellular-IAPs (cIAPs) -1 and -2. These proteins also possess three BIR domains, but are nevertheless weak direct binders and inhibitors of caspases.
Another level of signaling regulation is provided by the XIAP-binding protein SMAC (Second Mitochondrial Activator of Caspases, also known as DIABLO). SMAC competes directly with caspases for binding to XIAP BIR domains, and the release of SMAC from the mitochondria into the cytosol promotes apoptosis via release of caspases from XIAP and subsequent caspase activation (17). SMAC mediates association with XIAP via its N-terminal hydrophobic 4 amino acid sequence, AVPI. Synthetic compounds that mimic this SMAC tetrapeptide sequence have drawn much attention from the pharmaceutical industry due to their potential as inducers of apoptosis and as anti-cancer agents (e.g. 18, 19). Thus, SMAC mimetics sensitize a variety of human cancer cells to TNF- and TRAIL-induced apoptosis (20, 21). These mimetics are known to do so by binding to the BIR2 and BIR3 domains of XIAP to directly relieve their inhibition of caspases-3 and -7 or caspase-9, respectively (20, 22).
Importantly, SMAC mimetics also function as allosteric activators of the E3 ubiquitin ligase activity of cIAP-1 and cIAP-2 after binding to the BIR domains of these proteins, leading to their autodegradation (23, 24). While cIAP-1 and -2 are poor direct binders of caspases, they have been shown to associate with certain TNF family receptor complexes, including TRAIL, and ubiquitylate and thus target proteins in these complexes for proteasome-mediated degradation (25). One important c-IAP substrate in the complex is the NF-κB Inducing Kinase (NIK), which is involved in activation of the non-canonical NF-κB pathway downstream of the Death Receptors (e.g. 26). Furthermore, Smac mimetic-induced loss of cIAPs can lead to caspase-8 activation through the formation of the “riptosome” composed of RIPK1, FADD and caspase-8 in TNF-treated cells and in some other cellular conditions (27, 28, 29). Thus, at least in the case of TNF signaling pathways, SMAC mimetics are known to affect cellular signaling at multiple different levels.
We have previously described the design and synthesis of SMAC mimetics that are potent XIAP, ML-IAP, cIAP-1 and cIAP-2 binders and that modulate apoptosis (30, 31). Here, we demonstrate that these agents promote TRAIL-induced apoptosis in several cancer cell lines of varying TRAIL sensitivity, but are non-toxic as single agents. Importantly, normal cells are refractory to TRAIL even in the presence of these agents. Additionally, we show that administration of the compounds induces rapid cIAP-1 and -2 degradation, resulting in increased levels of NIK and subsequent non-canonical NF-κB2 pathway activation. Furthermore, we found that the compounds that sensitize cancer cells to TRAIL are the most efficacious in binding to XIAP, and in inducing cIAP-1 and cIAP-2 degradation. We have complemented these chemical genomics studies by the means of RNAi experiments, to further study the roles of XIAP, cIAP-1 and -2 in the modulation of TRAIL signaling.
MATERIALS AND METHODS
Reagents
Unless otherwise specified, all reagents were from Sigma-Aldrich (St. Louis, MO). Primocin and puromycin were obtained from InvivoGen (San Diego, CA), and TRAIL is from EMD/Calbiochem (La Jolla, CA). Small peptide caspase inhibitors are from BD Biosciences (La Jolla, CA) and 3-FC was from Santa Cruz Biotechnology (Santa Cruz, CA). Small molecule IAP antagonists MLS-0390969 (9h), MLS-0390982 (9f) and MLS-0391011 (9j) (30) and SB1-0636457 (10e) and SBI-0637142 (10f) (31) have been described previously. Nomenclature in parenthesis indicates terminology that was previously used (30, 31) to reference the compounds.
Cell culture
Caspase-8 deficient NB7 cells were a kind gift from Dr. Jill Lahti (St. Jude Children’s Research Hospital, Memphis, TN) and they and PC3M cells were maintained in RPMI 1640 supplemented with 10% (v/v) FBS, penicillin/streptomycin/L-Glutamine and Fungizone (Omega Scientific Inc, Tarzana, CA). MDA-MB-231, HeLa and normal human fibroblasts cells were maintained in DMEM with 10% (v/v) FBS, and penicillin/streptomycin/L-Glutamine and Fungizone. Patient derived breast cancer cells were obtained from SBMRI Tumor Analysis core facility with no identifying information provided. The cells were cultured in mammary epithelial basal medium (Lonza, Walkersville, MD), supplemented with penicillin, streptomycin, Fungizone, 4 μg/ml heparin (Sigma, St. Louis, MO), 20 ng/ml EGF (Sigma), 20 ng/ml bFGF (BD Bioscience, Bedford, MA) and B27 Supplement (Invitrogen-GIBCO, Grand Island, NY). MDA-MB-231 and HeLa cells are routinely sourced from ATCC and banked at early passage (P2). ATCC utilizes STR profiling at 17 loci plus Amelogenin with Promega PowerPlex® technology. Furthermore they, and any other cells we culture, are never cultured for more than 3 months or 12 further passages, whichever occurs sooner. NB7 cells were obtained directly from the Lahti/Kidd laboratory (St. Jude Children’s Research Hospital, Memphis, TN) and are maintained as per the ATCC lines described above. MDA-MB-231+Caspase-8 shRNA cells have been described previously (32). IAP shRNAmir DNAs were from OpenBiosystems (Lafayette, CO) and stable cell lines were generated by standard transfection with Fugene6 (Promega Corp., Madison, WI) followed by a two week selection with 1 μg/mL puromycin.
Cell survival and caspase activity assays
Cell viability was assessed using the CellTiter-Glo® Luminescent Cell Viability Assay (Promega Corp., Madison, WI). Briefly, cells are seeded at 5000 cells/well in 50 μL complete medium and allowed to attach overnight. 40 μL of fresh media containing the specified compound at the concentrations described is added before re-incubation of the cells at 37°C for 4 h. TRAIL is then added (as 10 μL) to the desired final concentration and the cells are again incubated at 37°C for 20 h. Plates are removed to room temperature for 30 min before addition of one half volume (50 μL) of freshly prepared CellTiterGlo reagent. The plates are gently shaken to ensure complete cellular lysis before luminescence is read on a Biotek Synergy 2 plate reader. All experiments were carried out in at least triplicate, at least three times.
Caspase activity was assessed utilizing CaspaseGlo® Assays (Promega Corp., Madison, WI). Cells are seeded as for CellTiterGlo (above) and treated as described. Caspase-8 activity is assessed as “LETD-ase” activity whilst caspase-3/7 activity is measured as “DEVD-ase” activity. The assays were carried out exactly as per manufacturer’s instructions before being read on a Biotek Synergy 2 plate reader utilizing Gen5 software.
Cell extracts and immunoblotting
Production of cellular protein extracts is essentially as described previously (33, 34). Primary antibodies used were: anti-pan-cIAP1/2 (Clone 315301, 1:1000, R&D Systems, Inc., Minneapolis, MN); anti-NF-κB2 (#4882, 1:2000), anti-NIK (#4994, 1:1000), anti-phospho-NF-κB2 (#4810, 1:1000), anti-XIAP (#2042, 1:2000), anti-Total Erk 1/2 (#9102, 1:5000) (all from Cell Signaling Technologies Inc. Beverly, MA); anti-β-Actin (A5441, 1:10000, Sigma-Aldrich, St. Louis, MO); or anti-Caspase-8 (C15, 1:500) (kind gift from Dr. Marcus Peter, Northwestern University, Chicago, IL). After incubation for 1 h with anti-rabbit IgG (111-035-003) or anti-mouse IgG (115-035-003) secondary antibodies conjugated to horseradish peroxidase (Jackson ImmunoResearch Laboratories Inc.), bands were detected using enhanced chemiluminescence (SuperSignal® West Pico Chemiluminescent substrate, #34080, Pierce, Rockford, IL). All analyses were performed at least three times.
RESULTS
Several cancer cell lines, but not normal cells, are TRAIL resistant but become TRAIL sensitive in the presence of the IAP inhibitors
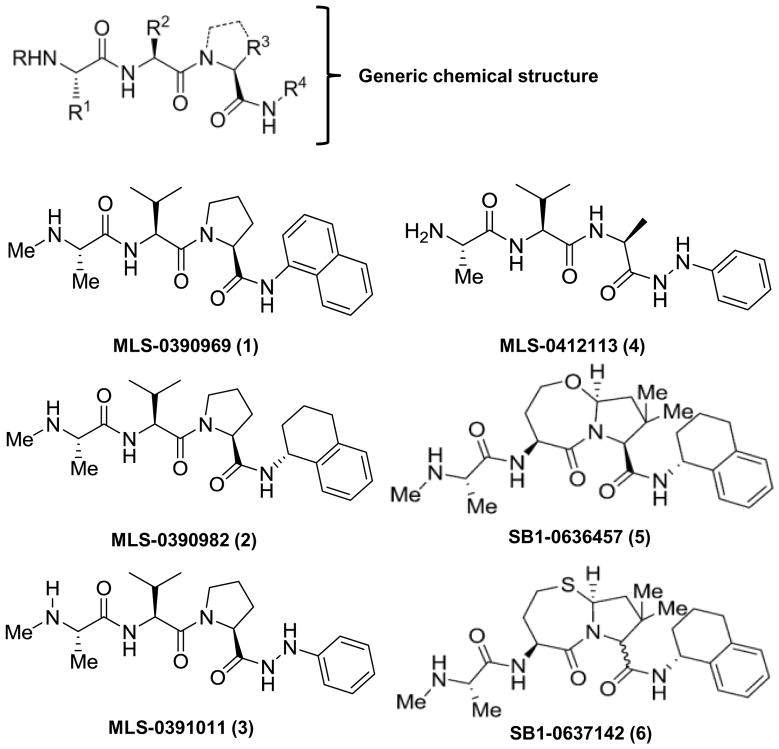
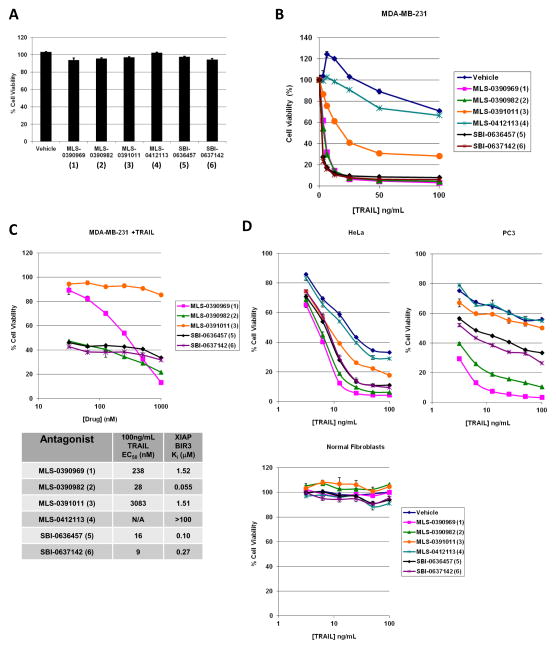
Fig. 1 shows the tripeptide pharmacophore of IAP inhibitors used in this study, in addition to the structure of the individual chemical agents. The synthesis of the compounds has been described in (30, 31). As shown in Fig. 2A, the IAP inhibitors are non-toxic in MDA-MB-231 breast adenocarcinoma cells as single agents. Indeed, the compounds demonstrate no cytotoxicity in BT474, BT549, MCF7 and MDA-MB-231 breast cancer cell lines up to a concentration of 20 μM (Supp. Fig. S1A). Administration of TRAIL alone to these cells similarly fails to induce appreciable cell death, up to a concentration of 100 ng/mL tested (Fig. 2B). Importantly, pre-treatment of the cells with 5 μM of several of the indicated IAP inhibitors for 4 h before addition of TRAIL sensitized them to TRAIL-mediated cell killing (Fig. 2B).
Figure 1. IAP antagonist structures.
Structure of the generic core and individual IAP inhibitors used in these studies. Numbering of the compounds from (1) to (6) is used in the subsequent figures as a quick reference.
Figure 2. Several cancer cell lines, but not normal cells, are TRAIL resistant but become TRAIL sensitive in the presence of the IAP inhibitors.
A) Cell viability assay on MDA-MB-231 cells treated with vehicle (0.1% DMSO) or 5 μM of each of the 6 IAP antagonists for 24 h. Data are averages +/− S.E.M. B) Concentration response curves to TRAIL-induced apoptosis (20 h) in the presence of vehicle or 5 μM of each of the 6 IAP antagonists. C) Upper panel, cell viability curves from MDA-MB-231 cells treated with varying concentrations of the IAP antagonists for 4 h before TRAIL-induced killing (100ng/mL) for 20 h. MLS-0412113 (4) was not tested as it showed no activity at 5μM. Lower panel, “EC50 values” of each compound required for 50% killing with 100 ng/mL TRAIL as compared to the binding affinities for the BIR3 domain of XIAP published before (30, 31). D) Cell viability assays of HeLa (upper left), PC3 (upper right) or normal human fibroblast cells (lower graph) pre-treated with vehicle or 5 μM of each of the 6 IAP antagonists for 4 h before treatment with TRAIL for a further 20 h. All concentration response curve studies in were carried out in at least triplicate at least three independent times and a representative graph is shown. Data values are averages +/− S.E.M. We note the S.E.M. values for some samples are extremely small and therefore may be difficult to see in some graphs.
In Fig. 2C, we performed a concentration-response analysis and “EC50” determination for the compounds by testing their sensitizing ability to a fixed TRAIL concentration (100 ng/mL) in MDA-MB-231 cells. One agent, MLS-0391011, showed less efficacy whilst another, MLS-0412113, devoid of a methyl group at the “R” position (Fig. 1), lacked TRAIL sensitizing ability altogether (Fig. 2B). Our previous studies have shown that the SMAC mimetic compounds bind, with varying affinities, to the BIR-domains of the IAP proteins (for details, see 30, 31). Therefore, the lower panel of Figure 2C shows the EC50 values for the compounds in a representative experiment with 100 ng/mL TRAIL (5.55 nM) as the killing concentration compared with the previous binding data for the BIR3 domain of XIAP (30, 31). A truncated concentration range is shown solely for clarity in the upper panel.
In order to confirm that the TRAIL-sensitizing abilities of small molecule IAP antagonists were not limited to breast cancer cell lines, we confirmed that these agents also demonstrate said activity in HeLa (cervical cancer) and PC3 (prostate cancer) cells (Fig. 2D, upper panels). HeLa cells were chosen to demonstrate a more sensitive cell line whilst PC-3 cells showed an intermediate phenotype relative to MDA-MB-231 cells. Further we show that primary cells derived from a breast cancer patient tumor sample are also sensitized to TRAIL by IAP antagonism (Supp. Fig. S1B).
The main draw of TRAIL as a potential anti-cancer therapy is its ability to induce apoptosis only in cancerous and not in non-transformed cells, and it was therefore of importance for us to test the TRAIL sensitizing ability of the IAP inhibitors in normal cells. Importantly, normal human fibroblasts were not sensitive to the combination of high concentrations of TRAIL and IAP antagonists (Fig. 2D, lower panel) that had resulted in profound killing of cancer cells (Fig. 2B and Fig. 2D, upper panels). Furthermore both normal mammary fibroblasts and normal mammary endothelial cells were refractory to TRAIL-induced apoptosis with or without the IAP antagonists, and no cytotoxicity was observed in these cells when the IAP inhibitors were applied as single agents (data not shown).
In sum, we show that the small-molecule IAP antagonists that we have previously described (30, 31) are non-toxic to cancer cells as single agents, but are efficacious as TRAIL-sensitizing agents in several previously TRAIL-resistant cancer cell lines. Importantly, the small molecule IAP antagonists exhibit no toxicity against normal cells, even in the presence of TRAIL, and thus demonstrate promise for their further development as TRAIL-sensitizing agents.
IAP inhibitor-mediated sensitization of cancer cell lines to TRAIL killing is caspase-8 dependent
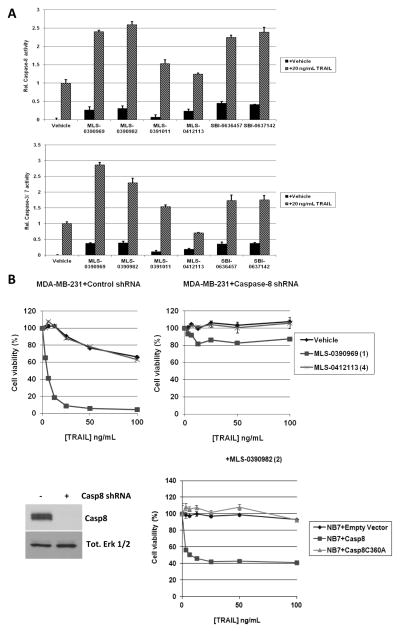
As shown in Fig. 3A, we observed that the IAP inhibitors are potent at promoting cellular activity of both caspase-3/7 (DEVDase) and caspase-8 (LETDase) in response to TRAIL in MDA-MB-231 cells. As noted in the Introduction, XIAP is a potent direct inhibitor of caspases-3/7, and thus activation of these caspases in response to the IAP antagonists in TRAIL-treated cells was expected. Consistent with this, the extent of caspase-3/7 activation (Fig. 3A) correlated with the potency of the compounds to bind to the BIR3 domain of XIAP, and with their capability to sensitize the cells to TRAIL-mediated killing (Fig. 2C).
Figure 3. IAP inhibitor-mediated sensitization of cancer cell lines to TRAIL killing is caspase-8 dependent.
A) Caspase activity assays in MDA-MB-231 cells pretreated with vehicle or 5 μM of each of the 6 IAP antagonists before treatment with 20 ng/mL TRAIL for 4 h. Activity is normalized to that of vehicle +TRAIL values. B) Cell viability assays of control shRNA-treated MDA-MB-231 cells (upper left panel), MDA-MB-231 cells with Caspase-8 shRNA (upper right panel), or NB7 cells expressing empty vector (NB7+Empty Vector), caspase-8 or inactive caspase-8 (C360A) (lower right panel) pretreated with vehicle or 5 μM of the indicated IAP antagonists for 4 h before treatment with TRAIL for a further 20 h. Lower left panel, immunoblot analysis of caspases-8 expression in MDA-MB-231 cells treated with control shRNA or caspase-8 shRNA. Total Erk1/2 immunoblot is used as a protein loading control.
The observed increase in cellular caspase-8 activity upon IAP inhibitor treatment in turn suggested that the cIAPs may also have some potential role in TRAIL resistance (see Introduction). In Fig. 3B, we studied MDA-MB-231 cells in which we had depleted caspase-8 by shRNAs (32) in TRAIL sensitization assays. While the control shRNA-treated cells were readily sensitized by a prototypical IAP antagonist, MLS-0390969, to TRAIL-mediated killing, the caspase-8-depleted MDA-MB-231 cells remained resistant (Fig. 3B, upper panels). Furthermore, caspase-8 null NB7 neuroblastoma cells (33) also displayed impaired TRAIL-induced apoptosis, and caspase-3/7 activity, in the presence of MLS-0390982, as compared to the same cells with caspase-8 re-constituted (Fig. 3B, lower panel). Importantly, caspase-8 null NB7 cells that had been reconstituted with an inactive caspase-8 protein (Casp8C360A) similarly failed to respond to TRAIL or activate effector caspases in the presence of the IAP inhibitor (Fig. 3B, lower panel and Supp. Fig. S1E). Taken together, our results suggest that caspase-8 activation is necessary for IAP inhibitor-mediated sensitization of cancer cell lines to TRAIL killing.
IAP antagonists result in rapid, concentration-dependent cIAP-1 and -2 degradation and NF-κB2 activation that is caspase-8 independent
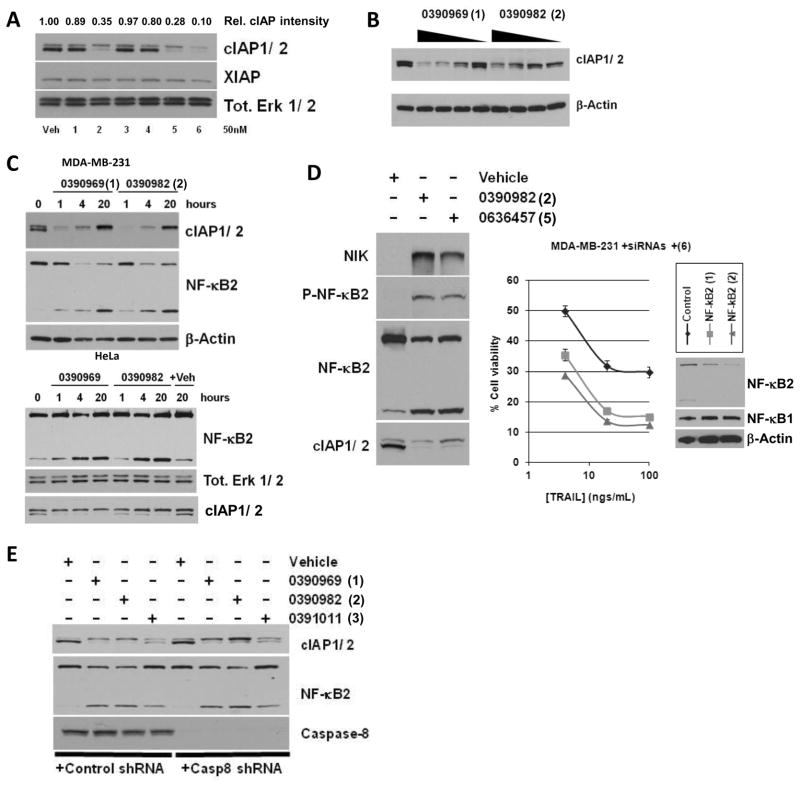
As noted in the Introduction, previous studies have demonstrated that SMAC mimetics are efficient in inducing cIAP autodegradation via a conformational change (23, 24). Consistent with this, several of the IAP antagonists utilized here were found to promote the degradation of cIAP-1 and -2 molecules in MDA-MB-231 cells at very low concentrations (50 nM), while no degradation of XIAP was observed (Fig. 4A). The observed cIAP-1 and -2 degradation at 50 nM appeared to correlate with the efficacy of the compounds to sensitize the cells to TRAIL-induced killing (Fig. 2C), and with the capability of the compounds to induce caspase-8 activation in TRAIL-stimulated cells (Fig. 3A). Our results suggest that IAP inhibitor-induced cIAP autodegradation may lead to the formation of a caspase-8-activating complex also in the context of TRAIL signaling, and that the subsequent caspase-8 activation is essential for IAP inhibitor-mediated TRAIL sensitization.
Figure 4. IAP antagonists induce a rapid, concentration-dependent cIAP-1 and -2 degradation and NF-κB2 activation that is caspase-8 independent.
A) Immunoblot analysis of cIAP-1, -2 (quantfied relative intensity of the cIAP protein levels is shown above the blot) and XIAP in MDA-MB-231 cells treated with vehicle or with low concentrations (50 nM) of each of the 6 IAP antagonists for 4 h. Erk 1/2 immunoblot is used as a loading control. B) Immunoblot analysis of cIAP-1/-2 in MDA-MB-231 cells treated with vehicle or with 10, 2 or 0.4 μM of MLS-0390969 or MLS-0390982 for 20 h. β-actin immunoblot is used as a loading control. C) Immunoblot analysis of cIAP-1/-2 and NF-κB2 in MDA-MB-231 (top panels) or HeLa (lower panels) cells untreated or treated with 5 μM MLS-0390969 or MLS-0390982 for 1, 4 or 20 h as indicated. β-actin or Erk 1/2 immunoblots are used as a loading control. D) Left panels, immunoblot analysis of NIK, phospho-NF-κB2, NF-κB2 and cIAP-1/-2 in MDA-MB-231 cells treated with vehicle or with 5 μM of the indicated IAP antagonists for 24 h. Erk 1/2 immunoblot is used as a loading control. Right panel, cell viability assays of MDA-MB-231 cells transfected with control or NF-κB2 siRNAs and pretreated with 5 μM IAP antagonist for 4 h before treatment with TRAIL for a further 20 h. Inset, immunoblot analysis of NF-κB2 “knockdown”. NF-κB1 and β-actin are shown as equal loading controls. E) Immunoblot analysis of cIAP-1/-2, NF-κB2 and caspase-8 in MDA-MB-231 cells harboring control shRNA (lanes 1–4) or Caspase-8 shRNA (lanes 5–8), and treated with vehicle or with 5 μM of the indicated IAP antagonists for 24 h. Erk 1/2 immunoblot is used as a loading control.
Previous studies by others have shown that cIAP autodegradation induced by IAP antagonists results in activation of the non-canonical NF-κB pathway. As shown in Fig. 4B, treatment of the MDA-MB-231 cells with the prototypic compounds MLS-0390969 and MLS-0390982 resulted in a concentration-dependent degradation of cIAPs in 4 h. A rapid, time-dependent, cIAP degradation was observed as early as after 1h of treatment of the cells with 5 μM of the IAP inhibitors (Fig. 4C). Significantly, this degradation was concomitant with the non-canonical NF-κB pathway activation, as judged by NF-κB2 processing in IAP inhibitor-treated MDA-MB-231 (breast) and HeLa (cervical) cancer cells (Fig. 4C). Interestingly whilst a rebound of cIAP-2 levels is observed at 20 h post IAP antagonism this is not sufficient to prevent TRAIL-induced apoptosis even at this 20 h time point (Supp. Fig. S1C).
Furthermore, by utilizing the compound MLS-0390982 and another potent prototypical compound SBI-0636457, we observed a profound induction of NIK levels, as well as enhanced phosphorylation and processing of NF-κB2 in 24 h in MDA-MB-231 cells, coinciding with cIAP degradation (Fig. 4D, left panels). In order to preliminarily examine the potential role of the NF-κB pathway activation in TRAIL signaling, we genetically “knocked down” NF-κB2 in MDA-MB-231 cells and pre-treated the cells with an IAP antagonist. As shown in Fig. 4D (right panel), NF-κB2 ablation also resulted in increased TRAIL-induced loss of cell viability. Also, a recently described chemical inhibitor of NF-κB signaling, 3-FC (35), results in sensitization to TRAIL and induces even greater sensitization with IAP inhibition (Supp. Fig. S1D). Thus, these preliminary results suggest that modulation of NF-κB pathway signaling may be another important intervention strategy in TRAIL-resistant cancers.
Whilst caspase-8 was found to be essential for TRAIL-induced apoptosis in the presence of the IAP inhibitors (Fig. 3), silencing the expression of this protease had no effect on the activation of the NF-κB pathway by the IAP antagonists. MDA-MB-231 cells treated with a control shRNA showed comparable NF-κB2 processing upon IAP antagonist treatment as the same cells with caspase-8 depleted by shRNA technology (Fig. 4E). Thus, we conclude that the non-canonical NF-κB pathway activation upon IAP inhibitor treatment is either independent of the caspase-8 status in the cells, or occurs upstream of caspase-8 activation in TRAIL signaling pathway.
Roles for XIAP, cIAP-1 and cIAP-2 in modulating TRAIL-induced apoptosis
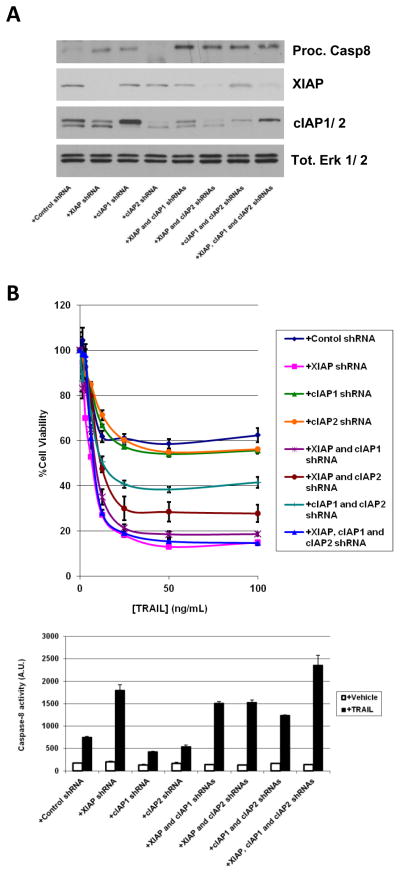
Whilst the majority of IAP antagonists in pharmaceutical development so far have targeted XIAP (e.g. 18, 36), our data above suggest that inhibition and subsequent degradation of cIAP-1 and -2 by IAP antagonists may also play a role in TRAIL sensitization. To complement our studies performed with the compounds, we studied the relative contribution of XIAP, cIAP-1 and cIAP-2 in TRAIL-induced apoptosis by genetic means. MDA-MB-231 cells were engineered to express shRNAs against each individual IAP and all combinations thereof and then treated with 100 ng/mL TRAIL for 4 h. Whilst the p18-processed subunit of capase-8 could only be detected with significant overexposure of an immunoblot, an intermediate processed form (indicating caspase-8 activity) could be faintly seen (Fig. 5A). This is consistent with caspase-8 activity assay results shown in Fig. 5B. Thus, caspase-8 is differentially induced in TRAIL-treated cells where either individual IAPs or combinations thereof had been genetically ablated. Again, the observed caspase-8 activity correlates with loss of cell viability (Fig. 5B). Although only tiny amounts of processed caspase-8 were detected (Fig. 5A), it is worth noting that caspase-8 is known to be active as an unprocessed dimer (37), and this point will be considered further in the Discussion.
Figure 5. Roles for XIAP, cIAP-1 and cIAP-2 in modulating TRAIL-induced apoptosis.
A) Immunoblot analysis of MDA-MB-231 cells stably transfected with control scrambled shRNA, with shRNAs for XIAP, cIAP-1 or cIAP-2 and all combinations thereof (as shown) and treated with 100 ng/mL TRAIL for 4 h. Erk 1/2; immunoblot is used as a loading control. B) Top panel, cell viability assays of MDA-MB-231 cells engineered to stably express the indicated shRNAs and treated with various concentrations of TRAIL as described for 20 h. Lower panel, caspase-8 activity assays in MDA-MB-231 cells with the indicated shRNAs (as above) treated with vehicle or 100 ng/mL TRAIL for 4 h. Activity is in arbitrary units.
Analysis of the relative contribution of each family member to TRAIL-induced apoptosis showed that reduced levels of XIAP most profoundly sensitized cells to TRAIL. The reduction in the levels of cIAP-1, and to an even lesser extent of cIAP-2, showed more moderate effects (Fig. 5B). Interestingly, whilst the effect of the combined knock-down of XIAP and either c-IAP was comparable to that of XIAP alone, the combined depletion of cIAP-1 and cIAP-2 showed a more profound sensitizing effect compared to depletion of either cIAP-1 or -2 alone (Fig. 5B). This is consistent with a significant induction of capase-8 activity in these cells in response to TRAIL (Fig. 5B, lower panel). Taken together, our studies are suggestive that all three IAP-proteins are likely involved in the regulation of the TRAIL pathway signaling (see Discussion).
DISCUSSION
We have previously described the design, synthesis and proof-of-concept testing of small molecule-based IAP antagonist compounds (30, 31). Here, we further extend our studies and report that these compounds effectively sensitize multiple previously TRAIL-resistant cancer cells, but not normal cells, to TRAIL-induced apoptosis.
We demonstrate here that our small molecule IAP antagonists are non-toxic as single agents against various cancer cells (as well as against normal cells). Previously, we have found these same compounds to demonstrate single agent toxicity in only one cancer cell line, the ovarian cancer cell line SKOV3 (31). Our results thus differ somewhat from those obtained with other IAP inhibitors, where single agent toxicity was observed in a subset of cancer cell lines (19, 38, 39). In these studies, IAP antagonists were found to induce autocrine TNF production in a restricted subset of cancer cells, followed by TNF-induced activation of the extrinsic apoptotic pathway and cell death (38). In our studies, we have failed to observe TNF production in all compound-treated cells, other than SKOV3 (31), which is consistent with the lack of single-agent toxicity by our compounds even at high concentrations in most cell lines we have studied. The reasons for these cell type-specific differences with respect to autocrine TNF production remain unclear and require further research.
Previous studies have demonstrated that the single agent toxicity and autocrine TNF production observed in certain cells results from IAP inhibitor-induced NF-κB activation (38). It was therefore of interest for us to assess NF-κB activation in our model systems. Notably, we failed to observe canonical NF-κB1 activation in our cell models upon IAP antagonist treatment. Instead, we found that non-canonical NF-κB2 processing takes place in response to our compounds. Thus, we postulate that differential NF-κB signaling in response to IAP antagonists, involving either the canonical or the non-canonical pathway activation, may explain the disparity with regard to autocrine TNF production and single-agent toxicity.
We observed that the non-canonical NF-κB2 processing occurs over a time course that is preceded by compound-induced cIAP degradation (Fig. 4C). Whilst a rebound of cIAP-2 levels is sometimes observed at 20 h post IAP antagonism (Fig. 3B) this is not sufficient to prevent TRAIL-induced apoptosis even at this 20 h time point (Supp. Fig. S1C). This is consistent as cIAP-2 alone has only a minor effect on TRAIL sensitization (Fig. 5). Indeed said rebound is probably due to loss of cIAP-2 degradation by cIAP-1. Furthermore, the non-canonical NF-κB2 pathway activation is concomitant with increased NIK levels and NF-κB2 phosphorylation (Figs. 4D and E). We next assessed what role, if any, the observed NF-κB2 pathway activation may have in our model systems. Consistent with the lack of canonical NF-κB1 activation, the use of the IKK inhibitor BAY 11-7082 failed to have any effect in our model systems (data not shown). Instead, siRNAs that target NF-κB2 further sensitized cancer cells to TRAIL-induced apoptosis when IAPs were antagonized (Fig. 4D). Taken together with findings by others that impairment of NF-κB signaling can sensitize cancer cells to TRAIL (40, 41), our studies suggest that concomitant development of NF-κB and IAP inhibitors may have therapeutic value.
We next interrogated the biological activity of our inhibitors to ascertain their mechanism-of-action as TRAIL sensitizers. Our studies with shRNAs targeting XIAP further underscored the notion that antagonism of XIAP represents a major mechanism by which these IAP inhibitors sensitize cancer cells to TRAIL (Fig. 5B). These findings are consistent with results obtained by others, noting the significant role of XIAP in regulating TRAIL-sensitivity (18, 36, 42).
Our studies are suggestive that IAP antagonist-induced cIAP degradation also plays a role in TRAIL sensitization. Thus, we observed a rapid and concentration-dependent cIAP degradation upon IAP inhibitor treatment (Fig. 4). A role for cIAPs is further supported by the notion that the combined genetic depletion of cIAP-1 and -2 also resulted in significant TRAIL sensitization and caspase-8 activation (Fig. 5B). The observed c-IAP degradation correlated with activation of caspase-8 in TRAIL-treated cancer cells, and we found that caspase-8 activation is absolutely essential for IAP inhibitor-mediated sensitization to TRAIL killing (Fig. 3). Interestingly, whilst cIAP-1 and -2 depletion resulted in a true sensitization, genetic depletion of all three IAPs was required to achieve the same level of TRAIL sensitivity as chemical inhibition (Fig. 5B). Thus, we postulate that inhibition of caspase-8 may take place in untreated cells as a result of a complex formation between the adapter proteins TRAF1 and/or TRAF2 and cIAPs (43). Compound-induced cIAP degradation, then, would result in a loss of caspase-8 inhibition at the receptor complex, thus rendering the cells susceptible to TRAIL-induced apoptosis. Whilst caspase-8 activity is consistent with loss of viability in response to TRAIL, we observed only very small amounts of processed caspase-8. We speculate that this apparent discrepancy could be reconciled by the fact that caspase-8 may be active as an unprocessed dimer (37); that simultaneous inhibition of multiple IAPs is required for robust caspase-8 activity (Fig. 5B); or that an undefined target for the small-molecule IAP antagonists is involved in caspase-8 activation and TRAIL sensitization.
In sum, we show that small-molecule IAP antagonists that are non-toxic alone can potently sensitize previously resistant cancer cell lines to the potentially important anti-cancer agent, TRAIL. Normal cells are refractory to this combination and caspase-8 is the essential apical protease involved in apoptosis induction. These probe compounds are expected to be useful in further elucidating TRAIL signaling pathways, and we have used them to demonstrate preliminarily that XIAP, cIAP-1 and cIAP-2 are involved in the modulation of TRAIL signaling and apoptosis. These agents are useful as lead compounds in a novel anti-cancer strategy in combination with TRAIL or derivatives thereof. Whereas both these IAP antagonists and TRAIL are expected to be non-toxic in vivo as single agents, our data suggest that they may be potentially powerful therapeutics in combination.
Supplementary Material
Acknowledgments
This work was supported by NIH grant MH095562 (to K. Vuori).
Footnotes
The authors declare that they have no conflict of interest.
References
- 1.Zheng L, Bidere N, Staudt D, Cubre A, Orenstein J, Chan FK, et al. Competitive control of independent programs of tumor necrosis factor receptor-induced cell death by TRADD and RIP1. Mol Cell Biol. 2006;26(9):3505–13. doi: 10.1128/MCB.26.9.3505-3513.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heyninck K, Beyaert R. Crosstalk between NF-kappaB-activating and apoptosis-inducing proteins of the TNF-receptor complex. Mol Cell Biol Res Commun. 2001;4(5):259–65. doi: 10.1006/mcbr.2001.0295. [DOI] [PubMed] [Google Scholar]
- 3.Walczak H, Miller RE, Ariail K, Gliniak B, Griffith TS, et al. Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo. Nat Med. 1999;5:157–163. doi: 10.1038/5517. [DOI] [PubMed] [Google Scholar]
- 4.Takeda K, Smyth MJ, Cretney E, Hayakawa Y, Kayagaki N, Yagita H, et al. Critical role for tumor necrosis factor-related apoptosis-inducing ligand in immune surveillance against tumor development. J Exp Med. 2002;195(2):161–9. doi: 10.1084/jem.20011171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finnberg N, Klein-Szanto AJ, El-Deiry WS. TRAIL-R deficiency in mice promotes susceptibility to chronic inflammation and tumorigenesis. J Clin Invest. 2008;118(1):111–23. doi: 10.1172/JCI29900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cretney E, Takeda K, Yagita H, Glaccum M, Peschon JJ, Smyth MJ. Increased susceptibility to tumor initiation and metastasis in TNF-related apoptosis-inducing ligand-deficient mice. J Immunol. 2002;168(3):1356–61. doi: 10.4049/jimmunol.168.3.1356. [DOI] [PubMed] [Google Scholar]
- 7.Ashkenazi A, Pai RC, Fong S, Leung S, Lawrence DA, et al. Safety and antitumor activity of recombinant soluble Apo2 ligand. J Clin Invest. 104:155–162. doi: 10.1172/JCI6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mahalingam D, Oldenhuis CN, Szegezdi E, Giles FJ, de Vries EG, de Jong S, et al. Targeting trail towards the clinic. Curr Drug Targets. 2011;12(14):2079–90. doi: 10.2174/138945011798829357. [DOI] [PubMed] [Google Scholar]
- 9.Thorburn A, Behbakht K, Ford H. TRAIL receptor-targeted therapeutics: resistance mechanisms and strategies to avoid them. Drug Resist Updat. 2008;11(1–2):17–24. doi: 10.1016/j.drup.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dean EJ, Ranson M, Blackhall F, Dive C. X-linked inhibitor of apoptosis protein as a therapeutic target. Expert Opin Ther Targets. 2007;11(11):1459–71. doi: 10.1517/14728222.11.11.1459. [DOI] [PubMed] [Google Scholar]
- 11.Deveraux QL, Reed JC. IAP family proteins-suppressors of apoptosis. Genes Dev. 1999;13(3):239–52. doi: 10.1101/gad.13.3.239. [DOI] [PubMed] [Google Scholar]
- 12.LaCasse EC, Mahoney DJ, Cheung HH, Plenchette S, Baird S, Korneluk RG. IAP-targeted therapies for cancer. Oncogene. 2008;27(48):6252–75. doi: 10.1038/onc.2008.302. [DOI] [PubMed] [Google Scholar]
- 13.Pop C, Salvesen GS. Human caspases: activation, specificity, and regulation. J Biol Chem. 2009;284(33):21777–81. doi: 10.1074/jbc.R800084200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Riedl SJ, Renatus M, Schwarzenbacher R, Zhou Q, Sun C, Fesik SW. Structural basis for the inhibition of caspase-3 by XIAP. Cell. 2001;104(5):791–800. doi: 10.1016/s0092-8674(01)00274-4. [DOI] [PubMed] [Google Scholar]
- 15.Chai J, Shiozaki E, Srinivasula SM, Wu Q, Datta P, Alnemri ES, et al. Structural basis of caspase-7 inhibition by XIAP. Cell. 2001;104(5):769–80. doi: 10.1016/s0092-8674(01)00272-0. [DOI] [PubMed] [Google Scholar]
- 16.Srinivasula SM, Hegde R, Saleh A, Datta P, Shiozaki E, Chai J. A conserved XIAP-interaction motif in caspase-9 and Smac/DIABLO regulates caspase activity and apoptosis. Nature. 200;410(6824):112–6. doi: 10.1038/35065125. [DOI] [PubMed] [Google Scholar]
- 17.Shiozaki EN, Shi Y. Caspases, IAPs and Smac/DIABLO: mechanisms from structural biology. Trends Biochem Sci. 2004;29(9):486–94. doi: 10.1016/j.tibs.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 18.Oost TK, Sun C, Armstrong RC, Al-Assaad AS, Betz SF, Deckwerth TL, et al. Discovery of potent antagonists of the antiapoptotic protein XIAP for the treatment of cancer. J Med Chem. 2004;47(18):4417–26. doi: 10.1021/jm040037k. [DOI] [PubMed] [Google Scholar]
- 19.Zobel K, Wang L, Varfolomeev E, Franklin MC, Elliott LO, Wallweber HJ, et al. Design, synthesis, and biological activity of a potent Smac mimetic that sensitizes cancer cells to apoptosis by antagonizing IAPs. ACS Chem Biol. 2006;1(8):525–33. doi: 10.1021/cb600276q. [DOI] [PubMed] [Google Scholar]
- 20.Li L, Thomas RM, Suzuki H, De Brabander JK, Wang X, Harran PG. A small molecule Smac mimic potentiates TRAIL- and TNFalpha-mediated cell death. Science. 2004;305(5689):1471–4. doi: 10.1126/science.1098231. [DOI] [PubMed] [Google Scholar]
- 21.Petersen SL, Wang L, Yalcin-Chin A, Li L, Peyton M, Minna J, et al. Autocrine TNFalpha signaling renders human cancer cells susceptible to Smac-mimetic-induced apoptosis. Cancer Cell. 2007;12(5):445–56. doi: 10.1016/j.ccr.2007.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chai J, Du C, Wu JW, Kyin S, Wang X, Shi Y. Structural and biochemical basis of apoptotic activation by Smac/DIABLO. Nature. 2000;406(6798):855–62. doi: 10.1038/35022514. [DOI] [PubMed] [Google Scholar]
- 23.Dueber EC, Schoeffler AJ, Lingel A, Elliott JM, Fedorova AV, Giannetti AM, et al. Antagonists induce a conformational change in cIAP1 that promotes autoubiquitination. Science. 2011;334(6054):376–80. doi: 10.1126/science.1207862. [DOI] [PubMed] [Google Scholar]
- 24.Feltham R, Bettjeman B, Budhidarmo R, Mace PD, Shirley S, Condon SM, et al. Smac mimetics activate the E3 ligase activity of cIAP1 protein by promoting RING domain dimerization. J Biol Chem. 2011;286(19):17015–28. doi: 10.1074/jbc.M111.222919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun M, Song L, Li Y, Zhou T, Jope RS. Identification of an antiapoptotic protein complex at death receptors. Cell Death Differ. 2008;15(12):1887–900. doi: 10.1038/cdd.2008.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun SC. Non-canonical NF-κB signaling pathway. Cell Res. 2011;21(1):71–85. doi: 10.1038/cr.2010.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang L, Du F, Wang X. TNF-alpha induces two distinct caspase-8 activation pathways. Cell. 2008;133(4):693–703. doi: 10.1016/j.cell.2008.03.036. [DOI] [PubMed] [Google Scholar]
- 28.Feoktistova M, Geserick P, Kellert B, Dimitrova DP, Langlais C, Hupe M, et al. cIAPs block Ripoptosome formation, a RIP1/caspase-8 containing intracellular cell death complex differentially regulated by cFLIP isoforms. Mol Cell. 2011;43(3):449–63. doi: 10.1016/j.molcel.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tenev T, Bianchi K, Darding M, Broemer M, Langlais C, Wallberg F, et al. The Ripoptosome, a signaling platform that assembles in response to genotoxic stress and loss of IAPs. Mol Cell. 2011;43(3):432–48. doi: 10.1016/j.molcel.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 30.González-López M, Welsh K, Finlay D, Ardecky RJ, Ganji SR, Su Y, et al. Design, synthesis and evaluation of monovalent Smac mimetics that bind to the BIR2 domain of the anti-apoptotic protein XIAP. Bioorg Med Chem Lett. 2011;21(14):4332–6. doi: 10.1016/j.bmcl.2011.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vamos M, Welsh K, Finlay D, Lee PS, Mace PD, Snipas SJ, et al. Expedient Synthesis of Highly Potent Antagonists of Inhibitor of Apoptosis Proteins (IAPs) with Unique Selectivity for ML-IAP. ACS Chem Biol. 2013;8(4):725–732. doi: 10.1021/cb3005512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Finlay D, Richardson RD, Landberg LK, Howes AL, Vuori K. Novel HTS strategy identifies TRAIL-sensitizing compounds acting specifically through the caspase-8 apoptotic axis. PLoS One. 2010;5(10):e13375. doi: 10.1371/journal.pone.0013375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Finlay D, Vuori K. Novel Non-catalytic Role for Caspase-8 in Promoting Src Mediated- Adhesion and Erk Signaling in Neuroblastoma Cells. Cancer Res. 2007;67:11704–11711. doi: 10.1158/0008-5472.CAN-07-1906. [DOI] [PubMed] [Google Scholar]
- 34.Finlay D, Howes A, Vuori K. Critical Role for Caspase-8 in EGF signaling. Cancer Res. 2009;69:5023–5029. doi: 10.1158/0008-5472.CAN-08-3731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yadav VR, Prasad S, Gupta SC, Sung B, Phatak SS, Zhang S, et al. 3-Formylchromone interacts with cysteine 38 in p65 protein and with cysteine 179 in IκBα kinase, leading to down-regulation of nuclear factor-κB (NF-κB)-regulated gene products and sensitization of tumor cells. J Biol Chem. 2012;287(1):245–56. doi: 10.1074/jbc.M111.274613. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 36.Park CM, Sun C, Olejniczak ET, Wilson AE, Meadows RP, Betz SF, et al. Non-peptidic small molecule inhibitors of XIAP. Bioorg Med Chem Lett. 2005;15(3):771–5. doi: 10.1016/j.bmcl.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 37.Boatright KM, Renatus M, Scott FL, Sperandio S, Shin H, Pedersen IM, et al. A unified model for apical caspase activation. Mol Cell. 2003;11(2):529–41. doi: 10.1016/s1097-2765(03)00051-0. [DOI] [PubMed] [Google Scholar]
- 38.Varfolomeev E, Blankenship JW, Wayson SM, Fedorova AV, Kayagaki N, Garg P, et al. IAP antagonists induce autoubiquitination of c-IAPs, NF-kappaB activation, and TNFalpha-dependent apoptosis. Cell. 2007;131(4):669–81. doi: 10.1016/j.cell.2007.10.030. [DOI] [PubMed] [Google Scholar]
- 39.Sun H, Liu L, Lu J, Qiu S, Yang CY, Yi H, et al. Cyclopeptide Smac mimetics as antagonists of IAP proteins. Bioorg Med Chem Lett. 2010;20(10):3043–6. doi: 10.1016/j.bmcl.2010.03.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huerta-Yepez S, Vega M, Jazirehi A, Garban H, Hongo F, Cheng G, et al. Nitric oxide sensitizes prostate carcinoma cell lines to TRAIL-mediated apoptosis via inactivation of NF-kappa B and inhibition of Bcl-xl expression. Oncogene. 2004;23(29):4993–5003. doi: 10.1038/sj.onc.1207655. [DOI] [PubMed] [Google Scholar]
- 41.Ammann JU, Haag C, Kasperczyk H, Debatin KM, Fulda S. Sensitization of neuroblastoma cells for TRAIL-induced apoptosis by NF-kappaB inhibition. Int J Cancer. 2009;124(6):1301–11. doi: 10.1002/ijc.24068. [DOI] [PubMed] [Google Scholar]
- 42.Allensworth JL, Aird KM, Aldrich AJ, Batinic-Haberle I, Devi GR. XIAP Inhibition and Generation of Reactive Oxygen Species Enhances TRAIL Sensitivity in Inflammatory Breast Cancer Cells. Mol Cancer Ther. 2012;11(7):1518–27. doi: 10.1158/1535-7163.MCT-11-0787. [DOI] [PubMed] [Google Scholar]
- 43.Wang CY, Mayo MW, Korneluk RG, Goeddel DV, Baldwin AS., Jr NF-kB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science. 1998;281:1680–1683. doi: 10.1126/science.281.5383.1680. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.