Abstract
Rationale
There is a substantial body of literature documenting the deleterious effects of both alcohol consumption and age on driving performance. There is, however, limited work examining the interaction of age and acute alcohol consumption.
Objectives
The current study was conducted to determine if moderate alcohol doses differentially affect the driving performance of older and younger adults.
Methods
Healthy older (55 – 70) and younger (25 – 35) adults were tested during a baseline session and again following consumption of one of three beverages (0.0% (placebo), 0.04% or 0.065% target breath alcohol concentration). Measures of driving precision and average speed were recorded.
Results
Older adults performed more poorly on precision driving measures and drove more slowly than younger adults at baseline. After controlling for baseline performance, interactions between alcohol and age were observed following beverage consumption on two measures of driving precision with older adults exhibiting greater impairment as a result of alcohol consumption.
Conclusions
These data provide evidence that older adults may be more susceptible to the effects of alcohol on certain measures of driving performance. An investigation of mechanisms accounting for alcohol’s effects on driving in older and younger adults is required. Further evaluation using more complex driving environments is needed to assess the real-world implication of this interaction.
Keywords: Moderate alcohol consumption, aging, simulated driving
Introduction
Driving while under the influence of alcohol accounts for a number of negative consequences for both individuals and society at large. It is estimated that 37% of all fatal motor vehicle crashes in the US are the result of intoxicated driving, accounting for over 12,000 deaths annually (NHTSA, 2012). In 2009, Zaloshnja & Miller determined that the total cost of all alcohol related motor vehicle crashes in the US amounted to over $66 billion.
Previous case-control studies have concluded that the risk of causing a traffic collision increases exponentially beginning at the .08% blood alcohol concentration (BAC) level (Blomberg et al., 2005; Borkenstein et al., 1974). However, a closer look at these data reveals that even BACs below .08% are associated with significant risks to drivers. For example, 15% of all alcohol related fatal motor vehicle crashes are caused by drivers with BACs below the .08% level (NHTSA, 2012) and BACs as low as .04% result in significant increases in the risk of causing a crash (Blomberg et al., 2005; Borkenstein et al., 1974). In addition to crash risk, recent evidence suggests that the severity (e.g. fatal injury vs. incapacitating injury) of motor vehicle crashes is significantly increased in individuals with BACs below the .08% legal limit compared to sober drivers (Phillips & Brewer, 2011).
In laboratory settings, driving simulators are a safe and useful tool for characterizing the complex set of cognitive and behavioral deficits contributing to the dangers of drinking and driving. Although gross measures such as crash events can be used for such investigations, refined measures of the precision of driving maneuvers can detect the more subtle effects of experimental manipulations. Importantly, these measures of driving precision have been shown to predict real-world driving behaviors (Lew et al., 2005; Shechtman et al., 2009). Within the alcohol literature, doses producing BACs above .08% on precision driving measures have been shown to be modulated by environmental factors such as level of distraction (Allen et al., 2009) and response conflict (Fillmore et al., 2008), and by driver specific factors such as impulsivity (Weafer et al., 2008). Research evaluating the effects of low to moderate doses of alcohol on driving performance is less common. Previous studies have documented reduced vehicle control at BACs of .05% using similar precision driving measures to those evaluated in the higher dose studies (Mets et al., 2011). Impairments in more complex driving scenarios such as increased response times to hazards have also been observed at this same BAC level (West et al., 1993).
In addition to alcohol consumption, the effects of aging on driving performance have been studied using simulators. Drivers over 65 years of age exhibited deficits in precision driving (Bunce et al., 2012; Reimer et al., 2009;) and specific visual-spatial attention (Lavalliere et al., 2011). In a previous study by Harrison & Fillmore (2005), poorer baseline driving performance was found to predict an increase in the susceptibility to the impairing effects of alcohol. Therefore, the presence of these age-related driving impairments may put older adults at an increased risk for alcohol related deficits compared to younger adults. In addition, differential age effects of moderate alcohol doses have been observed on more traditional neurocognitive assessments of psychomotor (Gilbertson et al., 2009;Tupler et al., 1995) and visual-spatial (Sklar et al., 2012) abilities. Because the mean age of the older subjects in these studies was between 55 and 60, these studies also suggest that alcohol might exacerbate subtle age-related changes in adults traditionally considered to be too young to be included in studies of cognitive aging.
To date, the majority of studies exploring potential age differences in sensitivity to alcohol’s effects on driving performance have focused on risk taking behaviors in young drivers (Leung & Starmer, 2005; Peck et al., 2008). These studies typically compare groups of young drivers at or below the legal drinking age (i.e. 21) to adults only modestly older (e.g. 25 – 35) and therefore provide little insight into the effects of alcohol across the adult lifespan. To our knowledge, Quillian et al. (1999) have conducted the only study exploring the effects of alcohol consumption on driving performance employing a broad age sample (i.e. 30 – 77 years old). A significant interaction between age group (middle aged (30 – 50) vs. older (60 – 77)) and alcohol was observed for one of the thirteen dependent driving measures (i.e. inappropriate braking). However, a relatively small sample size (N=28) and the administration of a common alcohol dose across all subjects regardless of factors known to alter pharmacokinetics (e.g. age and sex) may have resulted in significant variability within groups and reduced the opportunity to observe other significant interactions.
The present study employed a driving simulator to compare the performance of younger (25 – 35) and older (55 – 70) adults on four basic components of driving ability before and after the consumption of moderate doses of alcohol. We expected to observe baseline impairments in older relative to younger adults on measures of precision driving as well as alcohol induced deficits based on previous experiments using simulated driving tasks. Of greater interest, however, was the potential interaction between the effects of age and alcohol consumption on driving performance. We expected the differential age effect of alcohol observed in previous behavioral studies to extend to simulated driving performance. In addition, we wanted to explore the presence of a dose threshold on these effects by administering low (target peak BrAC of .04% (i.e. 40 mg/dl)), moderate (target peak BrAC of .065% (i.e. 65 mg/dl)), and placebo doses.
Methods
Participants
Sixty-seven younger and thirty-six older community dwelling social drinkers completed the study. From this larger group of younger adults, thirty-six were randomly selected within each dose assignment (12 placebo, 13 low, and 11 moderate dose subjects). This resulted in an equal numbers of older and younger adults included in our analysis (N=72; 36 younger and 36 older).
All participants completed two separate screening sessions. During the first, they provided demographic information, alcohol use histories (Quantity Frequency Index (QFI: average daily absolute ethanol consumption over the past six months in ounces) [Cahalan et al., 1969]; Max-QFI (maximum QFI for a single day in the past six months); maximum length of sobriety in past 6 months), a brief physical health history and a list of current medications via self-report questionnaires. Participants also completed questionnaires to assess driving experience (lifetime and current), levels of state anxiety (Spielberger State Anxiety Inventory (STAI) [Spielberger, 1983]), as well as age-appropriate measures of depressive symptomatology (Beck Depression Inventory-II (BDI-II) [Beck et al., 1996]; Geriatric Depression Scale [Yesavage et al., 1982]). During the second screening session, a computerized clinical research interview based on DSM-IV criteria (computerized Diagnostic Interview Schedule (cDIS); [Robins et al., 1995]) was administered to provide probabilistic Axis I psychiatric diagnoses. Although a formal clinical evaluation was not performed, the participants’ mental status was examined using the Mini-Mental State Exam (Folstein et al., 1975) and Hopkins Verbal Learning Test (Benedict et al., 1998) to confirm the absence of dementia (MMSE≥26 and HVLT total immediate recall >15).
Participants were excluded if they 1) were not moderate drinkers (USDA/USDHHS, 2010), 2) met criteria for a current Axis I disorder, 3) suffered from a medical condition or were taking a medication which contraindicated the use of alcohol, 4) were smokers, 5) had a positive drug (tetrahydrocannabinol, cocaine, benzodiazepines, morphine, and methamphetamine) or pregnancy urine screen on the morning of testing, or 6) did not have an active driver’s license. Smokers were not included in the study to eliminate the effects of nicotine withdrawal as a potential confound and in response to a growing literature on the effects of chronic smoking on neurocognitive function (Durazzo et al., 2007). The University of Florida Medical Institutional Review Board approved all procedures used. Participants provided consent prior to both screening and lab testing sessions and were compensated for their time at a rate of $15/hour.
Simulated Driving Task
The driving task was conducted in a sound attenuated booth using STISIM Drive simulator technology (Systems Technology Inc., Hawthorne, CA). The apparatus consists of three elevated monitors intended to provide the participant with frontal and peripheral perspectives. A speedometer as well as rear and side-view mirrors were displayed on the monitors. Participants controlled the virtual vehicle using a steering wheel console and accelerator and brake pedals. Vehicle sounds (e.g. engine noises) were presented over a speaker system.
Driving data were collected using a 16,000 ft (~3 miles) long stretch of road in a rural setting intended to produce stable measures of driving precision. Oncoming traffic was presented periodically throughout the drive on the two lane road (each 12 ft wide), but participants did not encounter other vehicles in their own lane. The driver did not encounter any intersections or other events that would demand an alteration in driving behavior. Participants were instructed to stay in the center of their lane, maintain their speed at the posted speed limit (55 mph), and follow the normal rules for safe driving (e.g. keeping both hands on the steering wheel). The scenario took approximately 3 min to complete, depending on the speed of the driver.
For the purposes of this investigation, we considered four measures commonly used in similar investigations (e.g., Fillmore et al., 2008; Howard et al., 2007; Mets et al., 2011). These measures were derived by the driving simulator software in an automated fashion. The first, steering rate (SR) depicts how rapidly the driver attempts to adjust their lane position. It is measured in the radians of change in the steering wheel position per second. Alcohol has been shown to be associated with quicker, more abrupt corrections resulting in higher steering rates (Fillmore et al., 2008). Deviation in lane position (LPSD), is the second indicator of driving precision. It provides an indication of a driver’s ability to maintain a consistent position within their lane and is sensitive to both acute alcohol (Mets et al., 2011) and aging (Bunce et al., 2012) effects. The LPSD is calculated as the standard deviation in a driver’s average within-lane position throughout the drive. The third measure of driving precision was the standard deviation in average speed (SD-Speed). Alcohol consumption has previously been shown to impair the ability to maintain a constant speed in simulated driving tasks resulting in increased SD-Speed (Howard et al., 2007). In addition to these measures of precision driving, the average speed of the drivers over the course of the drive was recorded.
Trail Making Test (Parts A and B)
The Trail Making Test (TMT) Form A and B are subsets of the Halstead Reitan Neuropsychological Test Battery (Reitan & Wolfson, 1986; Strauss et al., 2006). TMT Part A is a psychomotor task requiring participants to draw a line connecting 25 numbers arranged on a page in ascending order. In addition to psychomotor performance, TMT Part B assesses set-shifting abilities by requiring participants to draw a line connecting 25 numbers and letters in an alternating fashion (e.g. 1-A-2-B-3-C). The dependent variable of interest for both TMT Part A and B is time to completion. Errors were committed too infrequently in the current sample to allow for a proper analysis. The primary purpose for the inclusion of the TMT in the present study was to explore possible relationships between psychomotor performance and our precision driving measures. This comparison was of interest for two primary reasons. First, our previous work in an independent sample of older and younger adults showed a differential age effect of alcohol on the TMT (i.e. better performance among younger adults and worse performance among older adults) (Gilbertson et al., 2009). Second, poorer performance on the TMT has previously been associated with driving impairment in older adults (Shanmugaratnam et al., 2010).
Alcohol Administration
Participants within each age group were randomly assigned to one of three dose conditions: placebo, low, and moderate. Participants received an alcohol dose intended to produce a peak breath alcohol concentration (BrAC) of either .04% or .065% for the low and moderate groups respectively. This dose was calculated for each participant individually using a modified version of the Widmark Equation (Watson et al., 1980). Alcohol doses were diluted with 355 ml of diet, sugar-free, non-caffeinated lemon-lime soda. Placebo beverages consisted of the same volume of soda misted with a negligible amount of alcohol to control for alcohol expectancy effects. BrACs of 0.00% were recorded for each participant in the placebo group throughout the testing session. Participants had 5 min to consume both beverages.
A booster beverage was also administered 25 min after the original beverage. For participants in the placebo group, this drink consisted of half of the volume of their original beverage. For participants in the active dose conditions, the booster depended on their BrAC at the 25 min time-point. If BrACs were below 50% of target (i.e. .02% or .0325% for .04% and .065% respectively), booster beverages contained half of the original alcohol dose. A placebo booster was served if BrACs were above 50% of target. This approach resulted in the administration of 5 total active dose boosters (2 younger adult in the low dose group, 2 older adults in the low dose group, and 1 older adult in the moderate dose group). Active booster dose administration produced a shift in the BrAC curves of these individuals leading to task performance during the ascending rather than descending limb. Participants receiving active boosters did not differ significantly from their age and dose-matched non-booster counterparts on any of the driving measures of interest (p’s>.05). This analysis, however, should not be considered evidence for a lack of BrAC limb effect on driving as it is not adequately powered for this purpose.
Procedure
Participants performed a simulator practice scenario followed by a baseline driving session at the end of the second screening session. The practice scenario consisted of a brief (6000 ft) drive with intersections requiring right and left turns intended to familiarize the participants with the controls of the simulator. After demonstrating proficiency on the practice scenario (e.g. appropriate stops, no crashes, etc.), participants performed the driving task described above. Participants unable to complete the baseline driving task due to symptoms of simulator sickness were discontinued from the study. Simulator sickness resulted in the exclusion of 22 older and 3 younger adults. Our final sample (36 younger and 36 older adults) did not include data from any participant who could not complete the driving task due to simulator sickness.
On a separate day, participants returned to the laboratory for a testing session that began at 9:30 AM. They were instructed to fast for a minimum of 4hrs prior to the session and were given a small snack (~220kcal) after providing consent for the laboratory procedures. Research assistants performed a urine drug screen for all participants and pregnancy test for female participants of child-bearing age prior to beverage administration. Participants performed the simulated driving task 60 min post-consumption. This time-point was chosen in order to reflect a real world episode of moderate drinking in which the operation of a motor vehicle would occur during the descending limb of the alcohol curve. Breath samples were taken 10, 25, 60, 75, and 85min. post-consumption (Intoxilyzer, Model 400; CMI, Inc., Owensboro, KY). Participants were asked to rate their level of intoxication using a 10-point Likert scale prior to the driving task and to indicate whether or not they believed they received alcohol following the completion of the task to assess placebo effectiveness.
Data Analysis Strategy
Main effects of age, dose, and their interaction were tested using a general linear model (GLM) approach for all demographic and alcohol use variables of interest. Data collected for each dependent measure during the baseline driving session were analyzed using GLMs which included age group, dose condition and an interaction term as independent factors. These analyses allowed us to detect main effects of age on driving performance prior to alcohol consumption as well as any pre-test differences among the randomly assigned dose groups. Effects of dose and interactions between dose condition and age group were tested using the data collected during the testing session (i.e. following beverage consumption) with separate GLMs which included baseline driving performance as covariates. P-values ≤ 0.05 were considered significant for all effects of age, dose, and their interaction. As justified by our a priori hypothesis of a differential age effect of alcohol, planned comparisons between dose conditions within the age groups (e.g., older adults receiving placebo vs. the moderate dose) and between age groups within each dose condition (e.g., younger vs. older adults in the moderate dose condition) were conducted when a significant interaction was observed. Individual group differences and their effect sizes (Cohen’s d) are reported for all simple main effects. Pearson correlations were also conducted across age group and dose assignment to determine associations between scores on psychomotor tasks and driving performance during the testing session. SAS Version 9.3 (SAS Institute, Inc., Cary, NC) was used for all statistical operations.
Results
Demographics
In addition to age (expected difference), age-corrected state anxiety (STAI) scores (t70=3.76, p<.001; 41.9±6.2 vs. 37.3±4.0), body-mass index (BMI) (t70=1.99, p=.051; 27.1±5.1 vs. 24.8±4.7), and average weekly driving distance (t70=2.29, p=.03; 21.2±19.5 vs. 12.3±11.4) were significantly higher among older adults (Table 1). Older adults also reported fewer years of education (t70=2.38, p=.02; 16.0±1.7 vs. 16.9±1.1) and lower Max-QFIs (t70=5.71, p<.001; 1.9±1.4 vs. 3.8±1.4) than younger adults. None of the demographic, alcohol use, or driving history variables correlated with any driving measures within the two age groups (p’s>.05). These differences were therefore not subjected to additional analyses.
Table 1.
Demographic and baseline driving data (Mean (SD)) by age and dose condition
| Younger | Older | |||||
|---|---|---|---|---|---|---|
| Placebo (n=12; 4♀) |
.04% (n=13; 5♀) |
.065% (n=11; 5♀) |
Placebo (n=12; 5♀) |
.04% (n=13; 5♀) |
.065% (n=11; 7♀) |
|
| Age* | 27.75 (2.1) | 28.69 (3.3) | 27.18 (2.0) | 62.25 (4.5) | 58.54 (2.8) | 60.55 (4.1) |
| Education* | 17.00 (0.7) | 17.00 (1.4) | 16.63 (1.3) | 16.33 (1.4) | 15.62 (2.0) | 16.18 (1.8) |
| GDS/BDI-IIa,b | 00.64 (1.8) | 02.00 (3.2) | 00.82 (1.5) | 01.67 (1.8) | 01.46 (1.8) | 02.55 (2.3) |
| STAIc* | 37.25 (5.4) | 36.92 (2.6) | 37.72 (4.0) | 41.33 (4.6) | 40.15 (5.9) | 44.36 (7.8) |
| QFId | 00.34 (0.2) | 00.44 (0.3) | 00.35 (0.2) | 00.44 (0.4) | 00.26 (0.2) | 00.21 (0.2) |
| Max-QFIe* | 03.75 (1.7) | 03.99 (1.4) | 03.55 (1.1) | 01.77 (1.0) | 02.55 (1.8) | 01.43 (0.8) |
| Soberf | 25.92 (24) | 19.23 (14) | 22.55 (10) | 11.17 (8.5) | 22.23 (16) | 21.82 (19) |
| BMIg* | 26.49 (3.9) | 25.39 (5.6) | 22.47 (3.6) | 26.54 (5.0) | 26.86 (4.7) | 27.84 (6.1) |
| Driving Distanceh* | 09.73 (8.3) | 12.18 (10) | 14.92 (16) | 23.42 (24) | 22.90 (19) | 17.25 (13) |
| SR (radians/sec)i* | 01.79 (1.1) | 01.99 (0.8) | 01.72 (0.4) | 02.72 (1.1) | 02.80 (1.0) | 02.39 (1.2) |
| LPSD (ft)j* | 00.90 (0.2) | 00.84 (0.1) | 00.87 (0.2) | 00.99 (0.4) | 01.07 (0.2) | 01.11 (0.3) |
| SD-Speed (MPH)k* | 09.00 (1.0) | 09.28 (1.2) | 09.09 (1.0) | 09.53 (1.1) | 09.60 (1.8) | 10.21 (1.1) |
| Avg Speed (MPH)l* | 51.76 (1.8) | 52.64 (3.9) | 51.75 (2.7) | 49.79 (3.2) | 49.76 (5.7) | 50.52 (3.5) |
Significant differences between older and younger participants (p<.05)
Geriatric Depression Scale (Yesavage et al, 1982);
Beck Depression Inventory-II (Beck et al, 1996);
Spielberger State Anxiety Inventory (Spielberger, 1983);
Quantity-frequency Index (average daily consumption of ethanol in ounces; 1 drink=~.6) (Cahalan et al, 1969);
Maximum QFI recorded during a single day in the past 6 months;
Number of the most consecutive non-drinking days in the past 6 months;
Body-Mass Index;
Average distance driven per week;
Steering rate (radians/sec);
Lane position standard deviation (ft.);
Standard deviation of speed (MPH);
Average speed (MPH)
No differences were observed among the three dose conditions on any of the demographic, alcohol use, or driving history variables. All participants maintained an active driver’s license for at least three years prior to participation.
Breath Alcohol Concentration
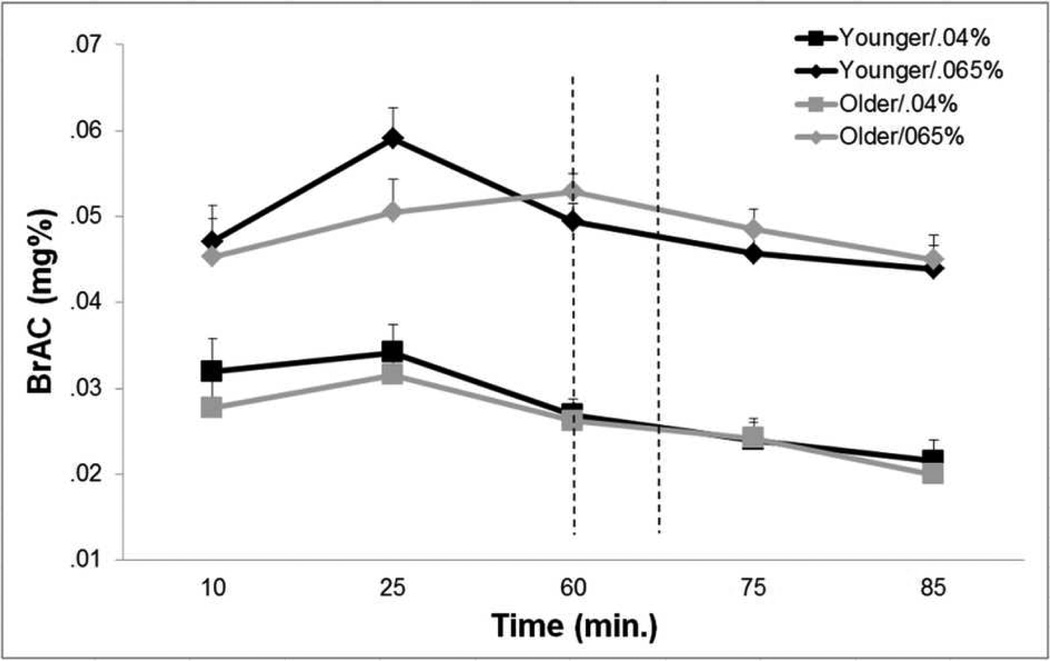
As expected, there was a significant main effect of dose on BrACs over the course of the testing session (F1,41=197, p<.001) (Figure 1). There was no main effect of age (F1,41=0.6, p=.45) or interaction between age and dose group (F1,41=0.1, p=.71) on the BrAC curves.
Fig 1.
Breath alcohol concentration (BrAC) curves for older and younger adults in both alcohol dose conditions. Dashed lines represent the start and approximate end of the driving task. No effect of age group or interaction between age and dose condition was observed. Error bars represent SEM.
Placebo Effect and Subjective Intoxication
Although the placebo was effective in a greater portion of older adults (58%) compared to younger adults (33%), this difference did not reach statistical significance when subjected to a Chi-Square test (χ2=1.51, p=.22).
As expected, a significant main effect of dose group was observed on ratings of intoxication prior to driving (F2,62=45.3, p<.0001) with individuals in the low (t23=3.03, p=.004) and moderate (t21=4.74, p<.0001) dose groups indicating higher levels of intoxication than those receiving a placebo beverage. No main effect of age (F1,62=6.2, p=.21) or interaction between age group and dose (F2,62=0.16, p=.85) was observed.
Steering Rate (SR)
Baseline Session
There was a significant main effect of age on SR (F1,66=11.9, p=.001) during the baseline driving session (Table 1). Older adults (2.6±0.8 radians/sec) adjusted their lane position using more rapid displacements in steering wheel position (i.e. turning the wheel faster) than younger adults (1.8±1.1 radians/sec), resulting in higher steering rates (t70=3.45, p=.001, d=0.82). Confirming a lack of pre-consumption dose group differences, no main effect of dose (F2,66=0.72, p=.5) or interaction between age group and dose condition (F2,66=0.10, p=.9) was observed.
Testing Session
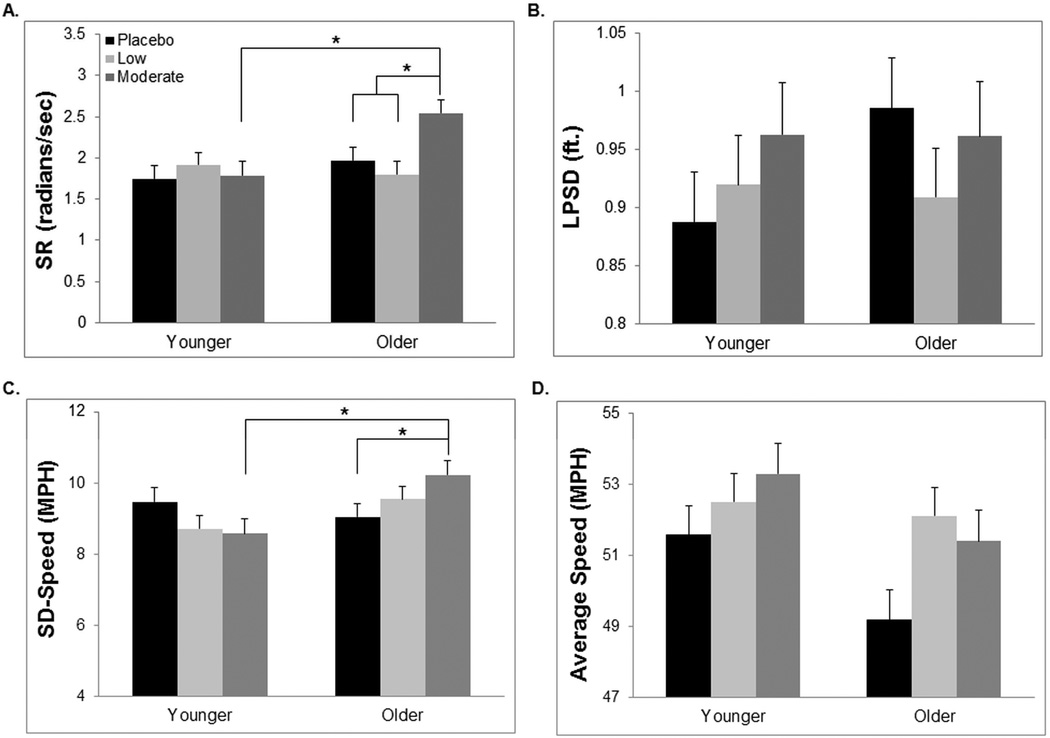
An interaction between dose condition and age group (F3,65=3.80, p=.01) was observed post-consumption after controlling for baseline performance. Older adults exhibited greater impairment as a result of alcohol consumption with those in the moderate dose condition performing significantly worse than older adults in the placebo (t21=2.50, p=.01, d= 1.09) and low dose groups (t22=3.31, p=.002, d=1.41). Older adults receiving the moderate dose also recorded higher SRs than younger adults in the moderate dose group (t20=3.20, p=.002, d=1.43). On the contrary, no differences were observed between the three dose conditions among younger adults (Figure 2). A main effect of dose was not observed during the testing session (F2,65=2.37, p=.1).
Fig 2.
Driving performance following beverage consumption. A: Older adults in the moderate dose condition recorded significantly higher steering rates than those in the placebo and low dose conditions, as well as younger adults in the moderate dose condition. B: A significant interaction between age group and dose on lane position standard deviation was not observed. C: Older adults receiving the moderate alcohol dose exhibited more variation in speed than those receiving a placebo beverage or younger adults in the moderate dose group. D: A significant interaction between age group and dose on average speed was not observed. *p<.05. Error bars represent SEM.
Lane Position Standard Deviation (LPSD)
Baseline Session
Our analysis of LPSD revealed a significant main effect of age (F1,66=15.4, p<.001) during baseline (Table 1). Older adults (1.1±0.2 ft) as a group exhibited a significantly higher LPSD than younger adults (0.9±0.2 ft) (t70=3.92, p<.001, d=0.94). The absence of pre-consumption differences in dose groups was confirmed by the lack of a significant dose effect (F2,66=0.43, p=.7) or interaction between age group and dose condition (F2,66=1.17, p=.3) was observed.
Testing Session
Controlling for baseline performance, there was no interaction between dose condition and age (F3,65=0.89, p>.4) or main effect of dose (F2,65=0.61, p>.5) on LPSD during the test session (Figure 2).
Standard Deviation of Speed (SD-Speed)
Baseline Session
A significant main effect of age group was observed on participants’ SD-Speed over the course of the baseline drive (F1,66=4.87, p=.03) (Table 1) with older adults (9.8±1.4 MPH) exhibiting more deviation than younger adults (9.1±1.1 MPH) (t70=2.21, p=.03, d=0.53). Confirming a lack of pre-consumption dose group differences, no main effect of dose (F2,66=0.54, p=.6) or interaction between dose and age group (F2,66=0.64, p=.5) was observed at baseline.
Testing Session
There was a significant interaction between age group and dose condition during the testing session after controlling for baseline performance (F3,65=3.65, p=.02). Older adults receiving the moderate alcohol dose exhibited more deviation in speed compared to those receiving the placebo beverage (t21=2.09, p=.04, d=0.91) as well as the group of younger adults in the moderate dose condition (t20=2.81, p=.007, d=1.26). In contrast, no differences were observed between the three dose conditions among younger adults (Figure 2). No significant main effect of dose condition was observed (F2,65=0.78, p=.78).
Average Speed
Baseline Session
There was a main effect of age on the speed of drivers at baseline (F1,66=5.27, p=.02) (Table 1). Older adults (50.0±4.3 MPH) drove more slowly than younger adults (52.1±2.9 MPH) (t70=2.30, p=.02, d=0.55). There were no significant effects of dose condition (F2,66=0.09, p=.9) or interaction between dose and age (F2,66=0.29, p=.7) during the baseline session, ensuring the absence of any pre-consumption dose group differences.
Testing Session
A significant interaction between dose group and age group was not observed (F3,65=2.16, p=.1) during the testing session after controlling for baseline performance (Figure 2). There was, however, a significant main effect of dose (F2,65=3.72, p=.03). Individuals in the low (t23=2.39, p=.02, d=1.00) and moderate (t21=2.33, p=.02, d=1.02) dose groups drove significantly faster compared to the placebo group.
Psychomotor Performance (TMT Parts A & B)
A significant main effect of age group on both Part A (F1,66=11.9, p=.001) and B (F1,66=17.9, p<.001) was observed. Older adults took longer to complete Part A (33.06±10.2 sec) and Part B (79.85±32.2 sec) than younger adults (TMT A: 24.55±11 sec; TMT B: 52.55±19.9 sec). However, neither a main effect of dose (A: F2,66=1.85, p=.17; B: F2,66=0.48, p=.62) nor an interaction between age group and dose condition (A: F2,66=1.41, p=.25; B: F2,66=0.17, p=.84) were observed on either TMT Parts A or B for this sample. Significant, positive correlations were observed during the testing session between TMT Part A and SD-Speed (r70=.23, p=.02) as well as between TMT Part B and LPSD (r70=.25, p=.01) and SR (r70=.20, p=.04) (i.e. worse performance on psychomotor tasks associated with impairment on driving measures).
Discussion
The purpose of the present study was to examine the impact of age, moderate alcohol consumption, and their interaction on driving performance. Older (55 – 70) and younger (25 – 35) social drinkers completed a simulated driving task prior to consuming alcohol as well as the same driving task and a psychomotor task following beverage administration. In general, older adults performed more poorly on measures of driving precision and drove more slowly than younger adults prior to the consumption of alcohol. Older adults also exhibited greater impairment as a result of alcohol consumption on two precision driving measures.
Significant interactions between age and dose were observed for both SR and SD-Speed after adjusting for baseline performance. The moderate dose altered the performance of older adults resulting in increased SR and SD-Speed compared to the placebo condition. In contrast, the effects of alcohol on younger adults were negligible. In addition to dose differences within age group, the interactions between age and alcohol were also driven by the significant impairments among older compared to younger adults in the moderate dose condition for both measures. This difference in performance was observed despite a lack of differences between the two age groups in the placebo and low dose conditions.
These interactions suggest a greater sensitivity to alcohol among older drinkers, a finding unlikely to be the result of differences in the subjective effects of the beverages (i.e. no age effect or interaction between age and dose groups on perceived level of intoxication at time of driving task; see Results). Similar interactions between alcohol dose and age have previously been reported using neuropsychological tests of psychomotor function (Gilbertson et al., 2009; Tupler et al., 1995). As expected, performance on similar psychomotor tasks administered in the present study (TMT Part A and B) correlated with our precision driving measures which rely heavily on motor stability and perceptual-motor integration. These associations raise the possibility that alcohol’s differential age effects on SR and SD-Speed might be partially accounted for by its effects on psychomotor function.
Impairments in divided attention represent another possible explanation for the differential effects of alcohol on the SD-Speed measure. The ability to maintain a constant speed requires a driver to periodically check their current speed by diverting their attention from the road to their dashboard speedometer. Divided attention tasks have been shown to impair driving performance in both older adults (Chaparro et al., 2004) and intoxicated individuals (Harrison & Fillmore, 2011) despite there being no effect of divided attention on driving in control groups. Unfortunately, divided attention was not explicitly tested in the present study. A systematic investigation of neurocognitive functions and driving performance is needed to provide for a more complete understanding of the mechanisms underlying these interactions.
Main effects of dose were also observed during the testing session for average speed after controlling for baseline performance. Overall, alcohol consumption resulted in higher average speeds for both active dose conditions compared to placebo. The effect of alcohol on drivers’ speed might be related to its tendency to increase risk-taking behaviors previously observed during simulated driving tasks, even at low to moderate dose levels (Burian et al., 2002). However, this interpretation is weakened by the fact that the average speed for all groups in the present study was below the posted speed limit (55 mph) during both sessions.
The absence of an alcohol main effect on our precision driving measures was likely due to the simplicity of the task which placed few demands on the driver, the relatively low dose levels used (BrACs=.026% and .05% for low and moderate groups respectively at the start of the driving task), and the examination of driving performance during the descending limb of the BrAC curve (with the exception of the five subjects who received a booster). A variety of measures examining motor control (Beirness & Vogel-Sprott, 1984), cognitive performance (Schweizer et al., 2005), and subjective intoxication (Schweizer et al., 2004) have been shown to exhibit acute tolerance (i.e. a greater sensitivity to alcohol on the ascending as opposed to the descending limb). However, a recent report which found measures of driving performance to be less susceptible to acute tolerance (Weafer & Fillmore, 2012) reduces the likelihood of this explanation. In the case of SR and SD-Speed, the presence of an interaction between age group and dose also makes the absence of alcohol main effects more difficult to interpret.
Limitations
Despite targeted recruitment efforts, older adults comprised only about one-third of our total sample. The lack of older adults can partially be attributed to higher attrition rates resulting from an increased susceptibility to simulator sickness in this group. To correct for our unbalanced sample, we randomly selected a group of younger adults within each dose condition to produce the current sample of 72 moderate drinkers. As a result of our reduced sample, we were unable to perform meaningful analyses of sex on driving performance, a particularly relevant issue in light of a recent finding which observed a greater susceptibility to age related driving impairments among women (Yan et al., 2007).
The notable variability in the effects of acute alcohol on performance and subjective intoxication among the general population (Fillmore & Vogel-Sprott, 1998; Tolentino et al., 2011) represents a potential limitation. However, we believe our strict selection criteria and control of potentially confounding pharmacokinetic variables mitigate this concern by reducing potential sources of variability within groups. In addition, our measure of quantity and frequency of alcohol consumption (i.e. QFI) and subjective ratings of intoxication prior to task performance did not differ between the three dose conditions or two age groups.
Finally, it is important to acknowledge the limitations of driving simulators in predicting real-world driving performance and crash risk. Due to a lack of non-visual sensory feedback and altered control sensitivity (e.g. steering wheel and brake), the simulated experience is quite different from actual driving. In addition, our analysis focused on behavioral measures associated with real-world safe driving (Lew et al., 2005; Shechtman et al., 2009) rather than actual simulator crash events as the brevity and simplicity of our driving scenario did not allow for this outcome. As a result, despite evidence supporting the external validity of certain simulated driving measures, caution should be applied when interpreting our driving simulator data and its ability to predict actual traffic events.
Conclusion
In addition to confirming previously observed age related driving impairments, these data suggest that certain driving behaviors of older adults may be more susceptible to the effects of moderate alcohol doses than those of younger adults. While many of the interactions reported are subtle, they are consistent with a growing body of literature suggesting a differential age effect of alcohol consumption (Gilbertson et al., 2009; Lewis et al., 2013; Sklar et al., 2012). Furthermore, the fact that these effects were observed despite the relative simplicity and brevity of the driving task speaks to the stability of this interaction. By highlighting potential risks associated with individual episodes of moderate consumption, these findings add an important perspective to the literature documenting the health benefits in older adults associated with a moderate drinking lifestyle (Balsa et al., 2008; Djousse et al., 2009). Unfortunately, the subtle change in the effects of alcohol on performance over time may lead older adults to ignore these risks as they are unlikely to have experienced significant consequences or impairment resulting from this level of consumption at earlier points in their lives.
Due to the understudied nature of this question and its potential impact on a growing percentage of the population, further investigation is needed. For example, because the complexity of the driving scenario differentially impacts the performance of older and younger adults (Bunce et al., 2012), increasing the cognitive demands of the driving task is likely to affect our observed relationship between age and alcohol variables. Sex differences in the effects of alcohol consumption and aging also merit investigation, particularly within the driving literature where they have received little attention to date.
Acknowledgments
Support for this project was provided by R01AA019802 (S.J. Nixon, PI); F30AA021315 (A.L. Sklar, PI; S.J. Nixon, Sponsor); F31AA0919862 (J. Boissoneault, PI; S.J. Nixon, Sponsor).
Footnotes
None of the authors maintain any financial agreements that would constitute a conflict of interest.
References
- Allen AJ, Meda SA, Skudlarski P, Calhoun VD, Astur R, Ruopp KC, Pearlson GD. Effects of alcohol on performance on a distraction task during simulated driving. Alcohol Clin Exp Res. 2009;33(4):617–625. doi: 10.1111/j.1530-0277.2008.00876.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsa AI, Homer JF, Fleming MF, French MT. Alcohol consumption and health among elders. Gerontologist. 2008;48(5):622–636. doi: 10.1093/geront/48.5.622. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Beck Depression Inventory. Second Edition. San Antonio: The Psychological Corporation; 1996. [Google Scholar]
- Benedict RHB, Schretlen D, Groninger L, Brandt J. Hopkins verbal learning test-revised: Normative data and analysis of inter-form and test-retest reliability. Clinical Neuropsychologist. 1998;12(1):43–55. [Google Scholar]
- Beirness D, Vogel-Sprott M. The development of alcohol tolerance: acute recovery as a predictor. Psychopharmacology. 1984;84:398–401. doi: 10.1007/BF00555220. [DOI] [PubMed] [Google Scholar]
- Blomberg RD, Peck RC, Moskowitz H, Burns M, Fiorentino D. Crash Risk of Alcohol Involved Driving: A Case-Control Study. Stamford CT: Dunlap and Associates Inc.; 2005. [Google Scholar]
- Borkenstein RF, Crowther RF, Shumate RP, Zylman R. The role of the drinking driver in traffic accidents (The Grand Rapids Study) Blutalcohol. 1974;11:s7–s13. [Google Scholar]
- Bunce D, Young MS, Blane A, Khugputh P. Age and inconsistency in driving performance. Accid Anal Prev. 2012;49:293–299. doi: 10.1016/j.aap.2012.01.001. [DOI] [PubMed] [Google Scholar]
- Burian SE, Liguori A, Robinson JH. Effects of alcohol on risk-taking during simulated driving. Hum Psychopharmacol. 2002;17(3):141–150. doi: 10.1002/hup.384. [DOI] [PubMed] [Google Scholar]
- Cahalan D, Cissin L, Crossley H. American Drinking Practices: A National Study of Drinking Behaviors and Attitudes (Monograph No. 6) New Brunswick, NJ: Rutgers Center of Alcohol Studies; 1969. [Google Scholar]
- Chaparro A, Wood JM, Carberry T. Effects of age and auditory and visual dual tasks on closed-road driving performance. Optometry Vision Sci. 2005;82(8):747–754. doi: 10.1097/01.opx.0000174724.74957.45. [DOI] [PubMed] [Google Scholar]
- Djousse L, Lee IM, Buring JE, Gaziano JM. Alcohol consumption and risk of cardiovasular disease and death in women: potential mediated mechanisms. Circulation. 2009;120:237–244. doi: 10.1161/CIRCULATIONAHA.108.832360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Rothlind JC, Gazdzinski S, Banys P, Meyerhoff DJ. Chronic smoking is associated with differential neurocognitive recovery in abstinent alcoholic patients: a preliminary investigation. Alcohol Clin Exp Res. 2007;31(7):1114–1127. doi: 10.1111/j.1530-0277.2007.00398.x. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Blackburn JS, Harrison EL. Acute disinhibiting effects of alcohol as a factor in risky driving behavior. Drug Alcohol Depend. 2008;95(1–2):97–106. doi: 10.1016/j.drugalcdep.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillmore MT, Vogel-Sprott M. Behavioral impairmen under alcohol: cognitive and pharmacokinetic factors. Alcohol Clin Exp Res. 1998;22(7):1476–1482. [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-Mental State: A practical method for grading the cognitive state of patients for the clinician. J Psychiat Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gilbertson R, Ceballos NA, Prather R, Nixon SJ. Effects of acute alcohol consumption in older and younger adults: perceived impairment versus psychomotor performance. J Stud Alcohol Drugs. 2009;70(2):242–252. doi: 10.15288/jsad.2009.70.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison EL, Fillmore MT. Are bad drivers more impaired by alcohol? Sober driving precision predicts impairment from alcohol in a simulated driving task. Accid Anal Prev. 2005;37(5):882–889. doi: 10.1016/j.aap.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Harrison EL, Fillmore MT. Alcohol and distraction interact to impair driving performance. Drug Alcohol Depend. 2011;117(1):31–37. doi: 10.1016/j.drugalcdep.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard ME, Jackson ML, Kennedy GA, Swann P, Barnes M, Pierce RJ. The interactive effects of extended wakefulness and low-dose alcohol on simulated diving and vigilance. Sleep. 2007;30(10):1334–1340. doi: 10.1093/sleep/30.10.1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavalliere M, Laurendeau D, Simoneau M, Teasdale N. Changing lanes in a simulator: effects of aging on the control of the vehicle and visual inspection of mirrors and blind spot. Traffic Inj Prev. 2011;12(2):191–200. doi: 10.1080/15389588.2010.548426. [DOI] [PubMed] [Google Scholar]
- Leung S, Starmer G. Gap acceptance and risk-taking by young and mature drivers, both sober and alcohol-intoxicated, in a simulated driving task. Accid Anal Prev. 2005;37(6):1056–1065. doi: 10.1016/j.aap.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Lew HL, Poole JH, Lee EH, Jaffe DL, Huang HC, Brodd E. Predictive validity of driving-simulator assessments following traumatic brain injury: a preliminary study. Brain Injury. 2005;19(3):177–188. doi: 10.1080/02699050400017171. [DOI] [PubMed] [Google Scholar]
- Lewis B, Boissoneault J, Gilbertson R, Prather R, Nixon SJ. Neurophysiological correlates of moderate alcohol consumption in older and younger social drinkers. Alc Cin Exp Res. doi: 10.1111/acer.12055. (ePub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mets MA, Kuipers E, Senerpont Domis LM, Leenders M, Olivier B, Verster JC. Effects of alcohol on highway driving in the STISIM driving simulator. Hum Psychopharmacol Clin Exp. 2011;26:434–439. doi: 10.1002/hup.1226. [DOI] [PubMed] [Google Scholar]
- National Highway Traffic Safety Administration (NHTSA) Washington, DC: U.S. Department of Transportation, National Highway Traffic Safety Administration; 2012. Traffic Safety Facts 2010; Alcohol, DOT HS 811 606. [Google Scholar]
- Peck RC, Gebers MA, Voas RB, Romano E. The relationship between blood alcohol concentration (BAC), age, crash risk. J Safety Res. 2008;39(3):311–319. doi: 10.1016/j.jsr.2008.02.030. [DOI] [PubMed] [Google Scholar]
- Phillips DP, Brewer KM. The relationship between serious injury and blood alcohol concentration (BAC) in fatal motor vehicle accidents: BAC=0.01% is associated with significantly more dangerous accidnets than BAC=0.00% Addiction. 2011;106:1614–1622. doi: 10.1111/j.1360-0443.2011.03472.x. [DOI] [PubMed] [Google Scholar]
- Quillian WC, Cox DJ, Kovatchev BP, Phillips C. The effects of age and alcohol intoxication on simulated driving performance, awareness and self-restraint. Age Ageing. 1999;28(1):59–66. doi: 10.1093/ageing/28.1.59. [DOI] [PubMed] [Google Scholar]
- Reimer B, Mehler B, Son J, Pohlmeyer E, Orszulak J, Long J, Coughlin J. Proc. 5th Int. Symp. Human Factors in Driver Assessment, Training and Vehicle Design. Big Sky, MT: 2009. A cross-cultural comparison of younger and older adults’ simulated highway driving performance under single and dual task conditions; pp. 206–213. [Google Scholar]
- Reitan RM, Wolfson D. The Halstead-Reitan Neuropsychological Test Battery. In: Wedding D, Horton AM, editors. The neuropsychology handbook: behavioral and clinical perspectives. Vol. 1986. New Yourk: Springer Publishing Co.; 1986. pp. 134–160. [Google Scholar]
- Robins LN, Cottler L, Bucholz KK, Compton W. The Diagnostic Interview Schedule, Version IV. St. Louis: Washington University; 1995. [Google Scholar]
- Schweizer TA, Vogel-Sprott M, Danckert J, Roy EA, Skakum A, Broderick CE. Neuropsychological profile of acute alcohol intoxication during ascending and descending blood alcohol concentrations. Neuropsychopharmacol. 2005;31:1301–1309. doi: 10.1038/sj.npp.1300941. [DOI] [PubMed] [Google Scholar]
- Schweizer TA, Jolicoeur P, Vogel-Sprott M, Dixon MJ. Fast, but error-prone, responses during acute alcohol intoxication: effects of stimulus-response mapping complexity. Alcohol Clin Exp Res. 2004;28(4):643–649. doi: 10.1097/01.alc.0000121652.84754.30. [DOI] [PubMed] [Google Scholar]
- Shanmugaratnam S, Kass SJ, Arruda JE. Age differences in cognitive and psychomotor abilities and simulated driving. Accid Anal Prev. 2010;42:802–808. doi: 10.1016/j.aap.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Shechtman O, Classen S, Awadzi K, Mann W. Comparison of driving errors between on-the-road and simulated driving assessment: A validation study. Traffic Inj Prev. 2009;10:379–385. doi: 10.1080/15389580902894989. [DOI] [PubMed] [Google Scholar]
- Sklar AL, Gilbertson R, Boissoneault J, Prather R, Nixon SJ. Differential Effects of Moderate Alcohol Consumption on Performance Among Older and Younger Adults [Epub ahead of print] Alcohol Clin Exp Res. 2012 doi: 10.1111/j.1530-0277.2012.01833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD. Manual for State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- Strauss E, Sherman EMS, Spreen O. A Compendium of Neuropsychological Tests: Administration, Norms and Commentary. 3 ed. New York, NY: Oxford University Press; 2006. [Google Scholar]
- Tolentino NJ, Wierenga CE, Hall S, Tapert SF, Paulus MP, Liu TT, Smith TL, Schuckit MA. Alcohol effects on cerebral blood flow in subjects with low and high responses to alcohol. Alcohol Clin Exp Res. 2011;35(6):1034–1040. doi: 10.1111/j.1530-0277.2011.01435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tupler LA, Hege S, Ellinwood EH., Jr Alcohol pharmacodynamics in young-elderly adults contrasted with young and middle-aged subjects. Psychopharmacology. 1995;118(4):460–470. doi: 10.1007/BF02245947. [DOI] [PubMed] [Google Scholar]
- United States Department of Agriculture, & United States Department of Health and Human Services. Dietary guidelines for Americans, 2010. 7th Ed. Washington, D.C.: U.S. Government Printing Office; 2010. Dec, [Google Scholar]
- Watson PE, Watson ID, Batt RD. Prediction of blood alcohol concentrations in human subjects. Updating the Widmark Equation. J Stud Alcohol. 1981;42(7):547–556. doi: 10.15288/jsa.1981.42.547. [DOI] [PubMed] [Google Scholar]
- Weafer J, Camarillo D, Fillmore MT, Milich R, Marczinski CA. Simulated driving performance of adults with ADHD: comparisons with alcohol intoxication. Exp Clin Psychopharmacol. 2008;16(3):251–263. doi: 10.1037/1064-1297.16.3.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weafer J, Fillmore MT. Acute tolerance to alcohol impairment of behavioral and cognitive mechanisms related to driving: drinking and driving on the descending limb. Psychopharmacology. 2012;220(4):697–706. doi: 10.1007/s00213-011-2519-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West R, Wilding J, French D, Kemp R, Irving A. Effects of low and moderate doses of alcohol on driving hazard perception latency and driving speed. Addiction. 1993;88:527–532. doi: 10.1111/j.1360-0443.1993.tb02059.x. [DOI] [PubMed] [Google Scholar]
- Yan X, Radwan E, Guo D. Effects of major-road vehicle speed and driver age and gender on left-turn gap acceptance. Accid Anal Prev. 2007;39:843–852. doi: 10.1016/j.aap.2006.12.006. [DOI] [PubMed] [Google Scholar]
- Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, Leirer VO. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17(1):37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- Zaloshnja E, Miller TR. Cost of crashes related to road conditions, United States, 2006. Ann Adv Automot Med. 2009;53:141–153. [PMC free article] [PubMed] [Google Scholar]