Summary
Endothelial function typically precedes clinical manifestations of cardiovascular disease and provides a potential mechanism for the associations observed between cardiovascular disease and sleep quality. This study examined how subjective and objective indicators of sleep quality relate to endothelial function, as measured by brachial artery flow-mediated dilation (FMD). In a clinical research center, 100 non-shift working adults (mean age: 36 years) completed FMD testing and the Pittsburgh Sleep Quality Index, along with a polysomnography assessment to obtain the following measures: slow wave sleep, percentage rapid eye movement (REM) sleep, REM sleep latency, total arousal index, total sleep time, wake after sleep onset, sleep efficiency, and apnea hypopnea index. Bivariate correlations and followup multiple regressions examined how FMD related to subjective (i.e., Pittsburgh Sleep Quality Index scores) and objective (i.e., polysomnography-derived) indicators of sleep quality. After FMD showed bivariate correlations with Pittsburgh Sleep Quality Index scores, percentage REM sleep, and REM latency, further examination with separate regression models indicated that these associations remained significant after adjustments for sex, age, race, hypertension, body mass index, apnea hypopnea index, smoking, and income (p's<0.05). Specifically, as FMD decreased, scores on the Pittsburgh Sleep Quality Index increased (indicating decreased subjective sleep quality) and percentage REM sleep decreased, while REM sleep latency increased (p's<0.05). Poorer subjective sleep quality and adverse changes in REM sleep were associated with diminished vasodilation, which could link sleep disturbances to cardiovascular disease.
Keywords: endothelial function, sleep, vasodilation, subjective sleep quality, polysomnography
Introduction
Research has linked poor sleep quality (e.g., insufficient sleep duration, self-reported low sleep quality) to the incidence of cardiovascular disease (CVD), primarily systemic hypertension, and possibly myocardial infarction, congestive heart failure, and stroke(Hoevenaar-Blom et al.,2011;Wolk et al.,2005). While the physiological underpinnings of the sleep and CVD relationship are not yet well defined, one plausible mechanism may be endothelial dysfunction, which is found in the early stages of atherosclerosis(Wolk et al.,2005). Circulating markers of endothelial cell activation and damage (e.g. von Willebrand factor, endothelin-1) are increased among patients with obstructive sleep apnea(OSA) compared to healthy controls(Phillips et al.,1999;Zamarrón-Sanz et al.,2006;El Solh et al.,2008) and among relatively healthy adults with increased sleep disturbance according to both self-reports (von Känel et al.,2010) and polysomnography-derived indices of sleep (e.g., latency in rapid eye movement or REM sleep)(Mills et al.,2007;von Känel et al.,2007;von Känel et al.,2010).
Although circulating biomarkers are useful indicators of endothelial conditions, flow-mediated dilation(FMD) offers a more direct way to assess endothelial dysfunction (Moens et al.,2005). FMD refers to endothelium-dependent vasodilation following a short period of increased shear stress generated by reactive hyperemia (Corretti et al.,2002). In endothelial dysfunction, the endothelium responds to reactive hyperemia with decreased FMD, mainly suggesting diminished availability of endothelium-derived nitric oxide(Corretti et al.,2002; Celermajer et al.,1992). FMD is predictive of adverse cardiovascular outcomes and is inversely related to cardiovascular risk (Yeboah et al.,2007).
Studies suggest that certain populations known to have disrupted sleep, such as patients with diagnosed OSA and workers with night shift jobs, not only have higher risk for CVD (Wolk et al.,2005;Wang et al.,2011), but also have reduced endothelial function (Amir et al.,2004; Patt et al.,2010). However, little is known about how endothelial function, as measured by FMD, relates to sleep quality among the general population of adults. Self-reported poor sleep quality and polysomnographic-assessed sleep disturbances (e.g., OSA) have been linked with cardiovascular outcomes (Chien et al.,2010;Hoevenaar-Blom et al.,2011;Wolk et al.,2005;Young et al.,2008), but it is unclear whether such subjective and objective measures of sleep quality also exhibit links to endothelial function in adults sampled from the general population.
The aim of this study was to assess how subjective and objective indices of sleep quality relate to endothelial function (as measured by FMD) among relatively healthy, employed (non-shift work) adults. We hypothesized that lower FMD (i.e., poorer endothelial function) would be associated with lower self-reported sleep quality and greater objectively assessed sleep disturbance as measured by polysomnography.
Methods
Participants
The sample included 100 employed (30+ hrs/wk; non-shift work) adults of upper, middle, and lower socioeconomic status, who were recruited via community advertisements or referrals between 2005 and 2010 to participate in a larger cardiovascular investigation. Excluded were participants with secondary hypertension, diabetes (or a fasting glucose >120mg/dL), known sleep disorders, current drug or alcohol abuse, ongoing psychiatric treatment, creatinine levels >1.4 mg/dl, proteinuria or hematuria by dipstick analysis, or renal bruit upon examination. For women, additional exclusions were current pregnancy, postmenopausal status, oral contraceptive use, and premenopausal syndrome. Volunteers were eligible if the assessment (i.e., physical exam and medical history) by the study's physician indicated that they were ostensibly healthy with no prescription medication use and no major medical/conditions. The sample included twelve non-medicated individuals with hypertension, who either had not been taking antihypertensive medications (n=10) or had undergone a 3-week closely monitored drug-tapering program during which their non-medicated blood pressure had remained <180/110 mmHg to qualify for participation in the study (n=2). In addition, the sample included eight light smokers (smoked less than one pack per day). Written informed consent was obtained from all participants in accordance with the Institutional Review Board of the University of California San Diego.
Procedures
The study procedures for the larger study have been previously reported (von Känel et al.,2007). To summarize aspects of the protocol relevant to the current analyses, demographic and psychosocial data were collected, along with measures of resting blood pressure (BP) and body mass index (BMI) during the initial visit. Later, participants underwent a 5:00 pm admission to the University of California San Diego's General Clinical Research Center for a 2-night stay at the Gillin Laboratory of Sleep and Chronobiology. Set-up for sleep monitoring with standard polysomnography (von Känel et al.,2007) took place between 8:00 pm and 9:00 pm, then lights out at 10:00 pm for an adaptation sleep study during the first night that ended with awakening at 6:00 am and completion of psychosocial questionnaires. Upon the participants' return to the unit that evening at 5:00 pm, overnight sleep monitoring with polysomnography was repeated from 10:00 pm to 6:00 am for data collection. After a light (low fat, no caffeine) standard breakfast, the subjects underwent FMD testing at 9:00 am. Prior to the FMD testing session, participants had been on the unit for over 12 hours and had refrained from smoking, exercise, and consumption of caffeine, alcohol, and foods containing high fat or nitrates.
Measures
Endothelial Function
FMD
A single examiner blinded to the hypotheses conducted noninvasive assessments of endothelial functioning using the guidelines for FMD testing as described by Celermajer and colleagues (Celermajer et al.,1992). The study's examiner (co-author M.S.M.) has extensive experience in brachial artery imaging and providing reliable measurements (e.g., intra-observer reliability based on two blinded evaluations of 20 randomly selected recordings: intraclass correlation 0.96;95% confidence interval: 0.89–0.98;coefficient of baseline values was 16%). Prior to testing, participants relaxed quietly in the supine position for 30 minutes in a dimmed, temperature-controlled room (22-25°C) to limit the potential influence of mental or environmental stress on vasodilator responses. The right brachial artery was visualized in longitudinal section 2 to 8 cm proximal to the antecubital fossa with a high-resolution 5-12 MHz broadband linear array transducer (ATL HDI5000 SonoCT System, Philips). Arterial diameter was measured from the anterior to the posterior intima line at end diastole, synchronous with ECG R wave. Baseline diameter was determined by averaging three measurements taken immediately before inflation of cuff on upper arm. Then, the cuff was inflated to pressure 50 mmHg above the systolic BP. After 5 minutes, the cuff was deflated and ultrasound scans were acquired for post-occlusion diameter measurements at 15-second intervals for 1 minute and then at 30-second intervals for an additional 9 minutes. FMD was calculated using following formula FMD=[(D(max)/D(baseline)–1)*100] where FMD is maximum percent change in diameter, D(max) is maximum postoclussion diameter in centimeters and D(baseline) is average preoclussion diameter in centimeters.
Sleep Quality: Subjective Measure
Pittsburgh Sleep Quality Index (PSQI)
Subjective sleep quality was measured with the PSQI (Buysse et al.,1989). The PSQI includes 19 items (e.g.,“wake up in the middle of the night or early morning”) that assess perceived sleep disturbance over the preceding four weeks. Seven component scores (i.e., sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleep medication, daytime dysfunction) are summed to derive a PSQI global score of subjective sleep quality (range 0–21 points). Higher scores on the PSQI indicate poorer sleep quality.
Sleep Quality: Objective Measures
Polysomnography
Sleep was recorded using the Grass Heritage (model PSG36-2;West Warwick, RI) sleep recording system, which recorded central and occipital electroencephalogram, bilateral electro-oculogram, submental and tibialis anterior electromyogram, electrocardiogram, nasal airflow (nasal cannula and pressure transducer), oral airflow (thermistor), respiratory effort (chest and abdominal piezoelectric belts), and oxyhemoglobin saturation. Sleep staging was scored according to the criteria of Rechtshaffen and Kales (Rechtshaffen & Kales,1968) by technicians with interrater reliabilities above 90%. Apneas were defined as decrements in airflow ≥90% from baseline for a period ≥10 seconds. Hypopneas were defined as decrements in airflow ≥50% but <90% from baseline for a period ≥10 seconds. Airflow was measured using a pressure transducer and thermistor simultaneously. The pressure transducer was used as the primary channel to score apneas and hypopneas. The thermistor was used primarily to detect oral breathing, and served as a confirmatory adjunct to the pressure transducer measurement.
Based on prior studies (Mills et al.,2007;von Känel et al.,2007;von Känel et al.,2010), we selected a panel of eight sleep parameters that reflect the different domains of sleep that might reasonably be expected to show associations with endothelial function. They were slow wave sleep (SWS)(i.e., stage 3 plus stage 4 sleep), percentage rapid eye movement (%REM) sleep, REM sleep latency, total arousal index (TAI), total sleep time (TST), Wake After Sleep Onset (WASO), sleep efficiency (TST/time in bed × 100), and apnea hypopnea index (AHI). [Given the potential confounding of undiagnosed sleep apnea, AHI also was included as a covariate in the analyses of FMD and other sleep quality measures.]
Covariate Measures
The relationship of endothelial function to subjective and objective sleep quality has a variety of potential confounders, including socioeconomic status (Mezick et al.,2008). Accordingly, in addition to adjusting the analyses for age, sex, race, BMI, hypertension status, AHI, and smoking (self-reported current smoker: yes/no), we also accounted for socioeconomic status (i.e., self-reported annual income). Exploratory analyses will additionally consider perceived stress as a potential confounder.
Hypertension Status
Hypertensive participants (systolic BP>140 mmHg and/or a diastolic BP>90 mmHg) were identified based on the average of three BP measures [Dinamap 1846× monitor (Critikon;Tampa, FL)] obtained after 5 minutes of seated rest.
Income
Participants answered the following question in reference to their employment: “What is your total annual wage or salary from this job?”
Stress [Exploratory Analyses]
Analyses explored the impact of adjusting for perceived stress, because of the literature suggesting that stress may influence both sleep and endothelial function (Åkerstedt,2006; Toda & Nakanishi-Toda 2011). Self-reported stress levels in the past 30 days were measured with the Perceived Stress Scale(PSS) (Cohen et al.,1983). The PSS asks respondents to indicate on a 5-point Likert scale [0(never) to 4(very often)] how often they have experienced each of the 10 items (eg, “How often have you felt difficulties were piling up so high that you could not overcome them?) during the past month. Total scores may range from 0 to 40, with higher scores indicating higher levels of perceived stress.
Data Analysis
The relationships of FMD to subjective sleep quality (PSQI-Global) and to objective sleep measures (i.e., SWS, %REM sleep, REM sleep latency, TAI, TST, WASO, sleep efficiency, AHI) were analyzed with correlational and multiple regression analyses using PASW/SPSS 18.0 software. After examining FMD for bivariate correlations (p≤.05) with subjective and objective sleep measures, these noted correlations were further examined with separate multiple regressions that tested the significance of the respective association after adjustment for a standard set of covariates: sex, age, race, BMI, hypertension, AHI, smoking, and income. Covariates were selected based on prior literature (Mezick et al.,2008; Moens et al.,2005), and/or simple correlations with the variables of interest.
In the adjusted regression models, covariates were entered on the first step, followed by the sleep measure of interest entered on the last step. Exploratory regression analyses were conducted to assess whether the previously described adjusted regression models remained significant after further adjusting for the potential psychosocial confounder of perceived stress, as measured by the PSS.
The scores for PSQI Global, REM latency, FMD, and income were log10 transformed to reduce skew. Continuous scores were used to represent all variables, with the exception of categorical variables for sex, race, smoking, and hypertension. One extreme outlier (standardized residual >-4.5; Cook's distance over 8 times the 4/n threshold) was excluded from the analyses, resulting in a sample size of 100. However, three missing PSQI-Global scores reduced the sample size to 97 for analyses of subjective sleep quality and FMD.
Results
Sample Characteristics
As shown in Table 1, the sample included more men than women (57% vs. 43%) and a greater proportion of Caucasians than African Americans (63% vs. 37%). Participants were predominantly normotensive and non-smoking. On average, the sample exhibited a 14% change in brachial artery diameter. The mean PSQI score was 4.9, falling slightly below the cutoff of 5, which is used to screen for sleep disturbance (approximately 36% of participants had PSQI scores of ≥6). Even though this was an ostensibly healthy sample from the general population, we found the mean AHI was somewhat high (mean AHI=8.1).
Table 1. Sample Characteristics (n=100).
| Variable | MEAN (SD) or % | Range |
|---|---|---|
| Flow Mediated Dilation % | 13.50% (5.89%) | 2.70 – 28.6 |
| Sex (% male:% female) | 57%: 43% | |
| Age (yrs) | 35.66 (9.54) | 19 – 53 |
| Race (% Caucasian:% African American) | 63%: 37% | |
| BMI (kg/m2) | 26.24 (4.11) | 18.92–42.44 |
| [Obesity: BMI≥30 kg/m2] | [19%] | |
| Hypertension (% normotensive:% hypertensive) | 88%: 12% | |
| [Systolic Blood Pressure (mmHg)] | [120.75 (14.09)] | [91.33-153.67] |
| [Diastolic Blood Pressure (mmHg)] | [71.70 (9.94)] | [53.67-102.67] |
| Smoking (% nonsmokers:% smokers) | 92%:8% | |
| Annual Income | $31,519 ($19,067) | $300–$100,000 |
| Perceived Stress Scale | 13.42 (6.11) | 0-28 |
| Pittsburgh Sleep Quality Inventory Global Score | 4.88 (2.89) | 0-16 |
| Total Sleep Time (minutes) | 395.77 (49.46) | 241.3 - 557.4 |
| Wake After Sleep Onset (minutes) | 23.31 (20.87) | 2.5 – 117.0 |
| REM Sleep % | 22.73 (5.79) | 8.58–39.19 |
| REM Sleep Latency from Sleep Onset (minutes) | 87.50 (48.50) | 3.5–271.0 |
| Slow Wave Sleep (minutes) | 80.27 (39.91) | 0 –182.0 |
| Sleep Efficiency % | 91.89 (6.20) | 70.04–99.12 |
| Total Arousal Index (events/hour) | 8.09 (5.43) | 0 –33.70 |
| Apnea-hypopnea Index (events/hour) | 8.09 (8.35) | 0.17 – 38.64 |
| [No OSA: AHA 0-4.9] | [48%] | |
| [Undiagnosed Mild OSA: AHI 5.0-14.9] | [36%] | |
| [Undiagnosed Moderate OSA: AHI 15.0-29.9] | [12%] | |
| [Undiagnosed Moderate OSA: AHI 15.0-29.9] | [4%] |
Bivariate Correlations
As indicated by the simple correlations provided in Table 2, decreasing FMD was associated with decreasing %REM sleep (p<0.01) and with increasing REM latency (p<0.05). FMD did not show bivariate associations with any other objective sleep measure derived from polysomnography. However, decreasing endothelial function also showed a correlation with poorer subjective sleep quality, such that FMD was inversely associated with PSQI-Global scores (p=0.05). Higher PSQI-Global scores (indicating poorer sleep quality) were correlated with lower SWS(p=0.05), higher perceived stress(p<0.01), hypertension (p<0.01), and African American race (p<0.05). Lower SWS and lower sleep efficiency were both correlated with African American race(p's<0.01). Other simple correlations revealed that increased %REM sleep was associated with increasing income and age (p's<0.05) in this young to middle-aged sample.
Table 2. Simple Correlations (r) among Variables.
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. FMD | - | .36** | .20* | −.17 | .04 | .09 | .02 | .04 | −.09 | −.20† | .03 | .02 | .27** | −.21* | −.18 | −.05 | −.02 | −.01 |
| 2. Sex | -- | −.04 | .04 | .03 | .05 | −.11 | −.07 | −06 | −.08 | .03 | −.15 | .06 | .05 | .21* | .13 | −.14 | −.08 | |
| 3. Age | -- | −.28** | .40** | .24* | .09 | .16 | .02 | .16 | −.15 | .34** | .43** | −.10 | .52** | −.36** | .32** | .24* | ||
| 4. Race | -- | −.38** | −.23* | −.16 | −.03 | −.14 | −.21* | .09 | −.14 | −.14 | −.05 | .42** | .28** | −.02 | −.13 | |||
| 5. BMI | -- | .49** | −.10 | .17 | .14 | .13 | .01 | .25* | .18 | −.05 | -.30** | −.19† | .10 | .21* | ||||
| 6. Hypertension | -- | .01 | .01 | .02 | .29** | −.04 | .11 | −.04 | .08 | .24* | −.18 | .03 | .15 | |||||
| 7. Smoking | -- | −.03 | .17 | .13 | −.17 | .12 | .13 | .02 | −.26** | −.12 | .14 | −.03 | ||||||
| 8. Income | -- | −.02 | .02 | .12 | −.06 | .22* | −.18 | −.10 | .07 | .10 | .22* | |||||||
| 9. Perceived Stress | -- | .40** | −.07 | .11 | .03 | .08 | −.17 | −.15 | −.01 | −.08 | ||||||||
| 10.PSQI-Global | -- | −.12 | .17 | −.10 | −.02 | −.20† | −.13 | .07 | .11 | |||||||||
| 11. TST | -- | −.35** | .08 | −.08 | .17 | .55** | −.15 | .18 | ||||||||||
| 12. WASO | -- | .09 | −.02 | −.27** | −.83** | .26* | .08 | |||||||||||
| 13. %REM Sleep | -- | −.39** | −.45** | −.15 | .28** | .38** | ||||||||||||
| 14. REM Latency | -- | .14 | −.11 | −.26** | −.16 | |||||||||||||
| 15. SWS | -- | .34** | −.28** | −.16 | ||||||||||||||
| 16. Sleep Efficiency | -- | −.12 | −.07 | |||||||||||||||
| 17. TAI | -- | .33** | ||||||||||||||||
| 18. AHI | -- |
FMD = Flow mediated dilation; BMI=body mass index; PSQI-Global=Pittsburgh Sleep Quality Inventory Global Score; TST =total sleep time; WASO = Wake After Sleep Onset; REM =rapid eye movement; SWS=slow wave sleep; TAI= total arousal index; AHI= apnea hypopnea index
p<.01;
p<.05;
p=.05 (2-tailed).
Regression Analyses: Subjective Sleep Quality and FMD
The simple correlation found between poorer subjective sleep quality (i.e., increasing PSQI-Global scores) and poorer endothelial function (i.e., decreasing FMD) was further examined with a regression of PSQI-Global on FMD(see Table 3). After adjustment for sex, age, race, BMI, hypertension, AHI, smoking, and income, the inverse association between PSQI-Global scores and FMD remained significant(p<0.05). PSQI-Global scores accounted for 4% of the variance in FMD explained by the final adjusted model[F(9,87)=4.05;p<.001, R2 =.30].
Table 3. Regression (final step) of the Pittsburgh Sleep Quality Index–Global on flow-mediated dilation (n=97): poorer subjective sleep quality correlated with poorer vasodilation.
| Predictors | ΔR2 | ΔF | ΔF p value | B (SE) | Standard Beta | Beta p value |
|---|---|---|---|---|---|---|
| Step 1: Covariates | .25 | 3.72 | .001 | |||
| a) Sex | .160(.04) | 0.39 | .000 | |||
| b) Age | .004(.00) | 0.21 | .047 | |||
| c) Race | -.056(.02) | −0.26 | .012 | |||
| d) Hypertension | .107(.07) | 0.17 | .115 | |||
| e) Body Mass Index | -.010(.01) | −0.20 | .096 | |||
| f) Apnea-hypopnea Index | -.002(.00) | −0.06 | .507 | |||
| g) Income | .043(.05) | 0.08 | .405 | |||
| h) Current Smoker | −.010(.07) | −0.01 | .883 | |||
| Step 2: Pittsburgh Sleep Quality Index–Global | .04 | 5.26 | .024* | −.164(.07) | −0.22 | 024* |
To better understand these findings, the association between PSQI-Global and FMD was deconstructed by assessing the bivariate correlations of FMD with the PSQI's seven components/subscales (data not shown). Only the PSQI's daytime dysfunction component/subscale (e.g., “During the past month, how often have you had trouble staying awake while driving, eating meals, or engaging in social activity”) was correlated with FMD(r= −.26, p<0.05), which suggests that subjective daytime dysfunction was driving the relationship found between PSQI-Global and FMD.
Regression Analyses: Objective Sleep Quality and FMD
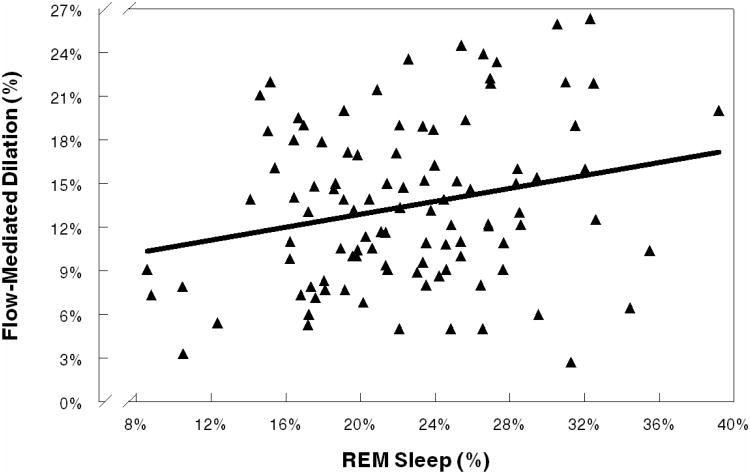
Regression analyses were conducted to further examine the observed simple correlations of FMD with the objective sleep measures of %REM sleep and REM latency. In a multiple regression adjusted for sex, age, race, BMI, hypertension, AHI, smoking, and income (Table 4), a significant association was observed between decreased FMD and decreased %REM sleep(p<0.05). Approximately 4% of the variance in FMD was explained by %REM sleep in the final adjusted model [F(9,90)=4.22;p<0.001,R2 =.30]. The relationship between FMD and %REM sleep is illustrated in Figure 1.
Table 4. Regression (final step) of REM Sleep % on FMD (n=100): lower %REM correlated with lower endothelial funtion.
| Predictors | ΔR2 | ΔF | ΔF p value | B (SE) | Standard Beta | Beta p value |
|---|---|---|---|---|---|---|
| Step 1: Covariates | .25 | 3.88 | .001 | |||
| a) Sex | .153(.04) | 0.37 | .000 | |||
| b) Age | .002(.00) | 0.10 | .368 | |||
| c) Race | −.051(.02) | −0.24 | .016 | |||
| d) Hypertension | .100(.07) | 0.16 | .132 | |||
| e) Body Mass Index | −.010(.01) | −0.21 | .078 | |||
| f) Apnea-Hypopnea Index | −.004(.00) | −0.15 | .143 | |||
| g) Income | .031(.05) | 0.06 | .541 | |||
| h) Current Smoker | .053(.07) | −0.07 | .454 | |||
| Step 2: %REM Sleep | .04 | 5.42 | .022* | .009(.00) | 0.25 | .022* |
Figure 1.
Flow-Mediated Dilation and REM Sleep Percentage: As endothelial functioning increased, percentages of REM sleep increased.
The results of multiple regression analyses indicated that decreases in FMD were accompanied by significant increases in REM latency(p<0.05), after adjustment for sex, age, race, BMI, hypertension, AHI, smoking, and income(Table 5). REM latency explained 4% of the variance in FMD accounted for by the full model [F(9,90)= 4.09;p<0.001, R2=0.29].
Table 5. Regression (final step) of REM Latency on FMD (n=100): greater REM latency correlated with lower endothelial function.
| Predictors | ΔR2 | ΔF | ΔF p value | B (SE) | Standard Beta | Beta p value |
|---|---|---|---|---|---|---|
| Step 1: Covariates | .25 | 3.88 | .001 | |||
| a) Sex | .168(.04) | 0.41 | .000 | |||
| b) Age | .004(.00) | 0.17 | .095 | |||
| c) Race | −.055(.02) | −0.26 | .010 | |||
| d) Hypertension | .084(.07) | 0.13 | .202 | |||
| e) Body Mass Index | −.010(.01) | −0.20 | .084 | |||
| f) Apnea-Hypopnea Index | −.002(.00) | −0.10 | .312 | |||
| g) Income | .031(.05) | 0.06 | .553 | |||
| h) Current Smoker | −.029(.07) | −0.39 | .674 | |||
| Step 2: REM Sleep Latency | .04 | 4.53 | .036* | −.115(.05) | −0.20 | .036* |
Exploratory Regression Analyses
A series of exploratory regressions were conducted to test whether adjustments for Perceived Stress Scale scores, obesity (i.e., BMI<30 kg/m2 vs. BMI≥30 kg/m2), levels of undiagnosed OSA severity, or oxygen saturation measures would attenuate the observed associations of FMD with PSQI global scores, %REM sleep, and REM sleep latency. Levels of undiagnosed OSA severity were assessed by an AHI variable categorizing OSA as: none (0-4.9); mild (5.0-14.9); moderate (15.0-29.9); and severe (≥30.0). Measures of oxygen saturation included mean, lowest, and percentage of time in bed with oxyhemoglobin saturation (Spo2)<90%. None of these exploratory covariates were significant in the models and none of them altered the significant associations found for FMD with PSQI, %REM sleep or REM sleep latency.
Discussion
Data showed that as FMD decreased, self-reported sleep quality (PSQI-Global) worsened, %REM sleep decreased, and REM latency increased, after adjusting for sex, age, race, BMI, hypertension, AHI, smoking, and income. Thus, diminished endothelial function was associated with both subjective and objective indicators of poor sleep quality after adjustment for confounders.
No other studies were found in the literature that investigated how FMD relates to either subjective sleep quality or to the objective sleep measures examined in the current study. Thus, it is challenging to integrate our findings with the existing literature. Several studies suggest that FMD may be reduced among groups with disrupted sleep, such as adults doing night shift work and patients with confirmed OSA and (Amir et al.,2004; Patt et al.,2010), with other work reporting that patients with OSA who were treated with continuous positive airway pressure (CPAP) showed increased FMD (Panoutsopoulos et al.,2012). The current study extends those findings by finding associations between sleep quality and FMD among non-shift working adults sampled from the general population, who did not have a previously diagnosed sleep disorder. To our knowledge, this is the first study that has linked reduced FMD to poorer subjective sleep quality and to objective indicators of poorer REM sleep quality. Diminished endothelial function could be a mechanism underlying the observed associations between poor sleep quality and adverse cardiovascular health (Chien et al.,2010;Wolk et al.,2005;Young et al.,2008).
While the literature has no comparable studies of subjective sleep quality and endothelial function, as measured by FMD, a previous study by our group (von Känel et al.,2010) found that poorer subjective sleep quality was associated with endothelial dysfunction, as measured by von Willebrand factor, in a sample of chronically stressed older adults and controls. The earlier study(von Känel et al.,2010) noted that poor subjective sleep quality could be a surrogate marker for stress, but similar to the current study, it found that adjustment for psychosocial stress did not attenuate the relationship observed between poorer perceived sleep quality and poorer endothelial function. Although mental stress is related to both perceived sleep quality and endothelial function (Toda & Nakanishi-Toda,2011), these results suggest that perceived sleep quality is associated with endothelial function independent of psychosocial stress. This association also seems to be independent of AHI, which showed no bivariate correlation with PSQI and did not attenuate the relationship between PSQI-Global scores on FMD in the regression analyses in the present study. Poorer subjective sleep quality showed a significant bivariate correlation with higher diastolic BP in the current sample (data not shown). Autonomic dysfunction may be a mechanism linking subjective sleep disturbances to poor cardiovascular health (von Kanel et al.,2010;Wolk et al.,2005). Given that autonomic changes also are associated with REM sleep (Dang-Vu et al.,2010;Wolk et al.,2005), further research is needed to better understand how autonomic functioning may relate to subjective sleep quality, particularly daytime dysfunction, as well as REM sleep.
It is intriguing that not only was decreased FMD associated with poorer subjective sleep quality, particularly subjective daytime dysfunction, but that it also was associated with decreased %REM sleep and with increased latency of REM sleep. Although no direct investigations of its potential associations with cardiovascular risk have been reported, REM sleep has been shown to be important in optimal learning and memory, as well as daytime functioning(Dang-Vu et al.,2010). While nitric oxide in the brain facilitates REM sleep(Cespuglio et al.,2012), FMD is a functional measure of nitric oxide-mediated vasodilation (Corretti et al.,2002). In our previous study (Mills et al.,2007), increased REM latency among healthy adults was associated with increased endothelin-1, which is another marker of endothelial dysfunction and nitric oxide dysregulation. Research has found that patients with OSA exhibit diminished nitric oxide and FMD (Wolk et al.,2005). However, given that the current analyses were adjusted for AHI, the findings for endothelial function and REM sleep measures are likely independent of undiagnosed OSA. It is unclear why FMD showed associations with the REM indicators, but not with any of the other objective sleep measures in the current study. However, FMD did have a marginal bivariate association with SWS, which may have become significant if more statistical power was available. Interestingly, SWS and REM are both partially facilitated by nitric oxide (Cespuglio et al.,2012). Additional research is needed to elucidate the links between endothelial function, REM sleep, SWS, and nitric oxide-mediated pathways.
Systemic low-grade inflammation that is predictive of CVD might be another important mechanism linking REM sleep to endothelial dysfunction. Inflammatory processes contribute to and also are affected by endothelial dysfunction (Trepels et al.,2006). Greater REM latency has been associated with elevated plasma levels of the proinflammatory cytokine interleukin(IL)-6 (Mills et al.,2007). Subcutaneous administration of low-dose recombinant IL-6 was found to decrease REM sleep (Späth-Schwalbe et al.,1998). FMD and IL-6 have shown an inverse correlation (Esteve et al.,2007). IL-6 might induce endothelial dysfunction through impairment of the vasodilating effects of insulin mediated by the endothelial nitric oxide synthase (eNOS) pathway and by directly inhibiting eNOS expression (Andersen & Petersen,2008).
One of the strengths of this study is that it assessed both subjective and objective measures of sleep to give a broader picture of relations between FMD and sleep quality. Moreover, it appears that this is the first investigation of these relations in a relatively healthy sample from the general population. Another strength is the study's limiting of the potential effect of confounders on the results through relatively strict exclusion criteria, physician confirmation of health status, and testing in a controlled inpatient setting. Other strengths include adjustments for varying levels of several traditional and psychosocial risk factors for CVD.
Still, our results should be interpreted within the limitations of the study design and sample. Our findings should be replicated with larger samples. The current study examined a sample from the general population that was ostensibly healthy. However, the findings may be limited by the sample's relatively high mean level of AHI. Although roughly 4% of this sample had an AHI ≥30 (suggesting severe sleep disordered breathing), this prevalence is similar to samples of other population-based studies (Young et al.,2008). We did not exclude participants with high AHI levels due to sample size considerations. The significant findings reported were based on primary analyses adjusted for AHI (continuous scores) and exploratory analyses adjusted for AHI categorized according to AHI levels of OSA severity. Further, AHI did not reach significance as a covariate in any of the regression models for PSQI, %REM Sleep, or REM Latency on FMD. However, it is possible that undiagnosed OSA is influencing our findings and that the high levels of AHI in the current sample will limit the generalizability of our findings to non-OSA samples.
Similarly, we did not exclude hypertensive participants, but they all were unmedicated and we adjusted for hypertension status in the regression analysis. The sample also included several smokers, but this was addressed with an adjustment in the analyses and participant restriction from smoking in the hours before FMD testing. Approximately 19% of the sample had BMI≥30 kg/m2. However, our analyses were adjusted for BMI and exploratory analyses that included obesity as a alterative covariate did not alter the results. Like other samples (Moens et al.,2005;Yeboah et al.,2007), FMD in the current sample was greater among women than men. Although the analyses included an adjustment for sex, the findings could be limited by our inability to account for possible variations in endothelial function or sleep that might have been related to hormonal changes (e.g., estradiol) associated with the menstrual cycle in premenopausal women. While the inclusion/exclusion criteria likely reduced variance in this otherwise healthy, unmedicated, young-to-middle aged sample, these findings might have limited generalizability to other age groups and to populations in which comorbidity/medication use is prevalent. Our polysomnography data were obtained during a single night (after an adaptation night), as is common in the literature, but an assessment involving several nights of recordings might have produced different results. In addition, as indicated in Figure 1, participants' %REM sleep values varied notably, ranging from relatively low values (8%–11%) to a relatively high value (39%). Given that none of these values were statistical outliers and that their omission from the sample in exploratory regressions (data not shown) did not substantially change the results, we retained these participants in the sample to maintain statistical power. Finally, no causal or temporal determinations can be made from this cross-sectional, correlational study.
In summary, reduced endothelial function was related to poor subjective sleep quality and to potentially adverse decreases in %REM sleep and increases in REM latency. These findings suggest that endothelial dysfunction could be a pathway linking subjective and objective measures of poor sleep quality to CVD.
Acknowledgments
National Institutes of Health Grants HL36005, RR00827, AG08415, and P60 MD00220 supported this research.
Abbreviations
- AHI
apnea hypopnea index
- BMI
body mass index
- BP
blood pressure
- CVD
cardiovascular disease
- FMD
flow-mediated dilation
- OSA
obstructive sleep apnea
- PSQI
Pittsburgh Sleep Quality Index
- REM
rapid eye movement
- SWS
slow wave sleep
- TAI
total arousal index
- TST
total sleep time
- WASO
wake after sleep onset
Footnotes
Conflicts of Interest: Authors D.C.C, M.G.Z, M.S.M, S.A-I, P.J.M, J.S.L, R.vK, J.E.D.: None
Publisher's Disclaimer: Disclaimer: The views expressed in this article are solely those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs, the University of California San Diego, or the University of Bern.
References
- Åkerstedt T. Psychosocial stress and impaired sleep. Scand J Work Environ Health. 2006;32:493–501. [PubMed] [Google Scholar]
- Andersen K, Pedersen BK. The role of inflammation in vascular insulin resistance with focus on IL-6. Horm Metab Res. 2008;40:635–639. doi: 10.1055/s-0028-1083810. [DOI] [PubMed] [Google Scholar]
- Amir O, Alroy S, Schliamser JE, et al. Brachial artery endothelial function in residents and fellows working night shifts. Am J Cardiol. 2004;93:947–949. doi: 10.1016/j.amjcard.2003.12.032. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Celermajer DS, Sorensen KE, Gooch VM. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992;340:1111–1115. doi: 10.1016/0140-6736(92)93147-f. [DOI] [PubMed] [Google Scholar]
- Cespuglio R, Amrouni D, Meiller A, Buguet A, Gautier-Sauvigné S. Nitric oxide in the regulation of the sleep-wake states. Sleep Med Rev. 2012;16:265–279. doi: 10.1016/j.smrv.2012.01.006. [DOI] [PubMed] [Google Scholar]
- Chien KL, Chen PC, Hsu HC, et al. Habitual sleep duration and insomnia and the risk of cardiovascular events and all-cause death: report from a community-based cohort. Sleep. 2010;33:177–184. doi: 10.1093/sleep/33.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- Corretti MC, Anderson TJ, Benjamin EJ, et al. International Brachial Artery Reactivity Task Force. International Brachial Artery Reactivity Task Force: Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39:257–265. doi: 10.1016/s0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- Dang-Vu TT, Schabus M, Desseilles M. Functional neuroimaging insights into the physiology of human sleep. Sleep. 2010;33:1589–1603. doi: 10.1093/sleep/33.12.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Solh AA, Akinnusi ME, Berim IG, Peter AM, Paasch LL, Szarpa KR. Hemostatic implications of endothelial cell apoptosis in obstructive sleep apnea. Sleep Breath. 2008;12:331–337. doi: 10.1007/s11325-008-0182-x. [DOI] [PubMed] [Google Scholar]
- Esteve E, Castro A, Lopez-Bermejo A, Vendrell J, Ricart W, Fernandez-Real JM. Serum interleukin-6 correlates with endothelial dysfunction in healthy men independently of insulin sensitivity. Diabetes Care. 2007;30:939–945. doi: 10.2337/dc06-1793. [DOI] [PubMed] [Google Scholar]
- Hoevenaar-Blom MP, Spijkerman AM, Kromhout D, van den Berg JF, Verschuren WM. Sleep duration and sleep quality in relation to 12-year cardiovascular disease incidence: the MORGEN study. Sleep. 2011;34:1487–1492. doi: 10.5665/sleep.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezick EJ, Matthews KA, Hall M, et al. Influence of race and socioeconomic status on sleep: Pittsburgh SleepSCORE project. Psychosom Med. 2008;70:410–416. doi: 10.1097/PSY.0b013e31816fdf21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills PJ, von Känel R, Norman D, Natarajan L, Ziegler MG, Dimsdale JE. Inflammation and sleep in healthy individuals. Sleep. 2007;30:729–735. doi: 10.1093/sleep/30.6.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moens AL, Goovaerts I, Claeys MJ, Vrints CJ. Flow-mediated vasodilation: a diagnostic instrument, or an experimental tool? Chest. 2005;127:2254–2263. doi: 10.1378/chest.127.6.2254. [DOI] [PubMed] [Google Scholar]
- Panoutsopoulos A, Kallianos A, Kostopoulos K, et al. Effect of CPAP treatment on endothelial function and plasma CRP levels in patients with sleep apnea. Med Sci Monit. 2012;18:CR747–751. doi: 10.12659/MSM.883603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patt BT, Jarjoura D, Haddad DN, et al. Endothelial dysfunction in the microcirculation of patients with obstructive sleep apnea. Am J Respir Crit Care Med. 2010;182:1540–1545. doi: 10.1164/rccm.201002-0162OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips BG, Narkiewicz K, Pesek CA, Haynes WG, Dyken ME, Somers VK. Effects of obstructive sleep apnea on endothelin-1 and blood pressure. J Hypertens. 1999;17:61–6. doi: 10.1097/00004872-199917010-00010. [DOI] [PubMed] [Google Scholar]
- Rechtshaffen A, Kales A. National Institutes of Health. 1968. A manual of standardized terminology, techniques, and scoring system for sleep stages of human subjects. Publication No. 204. [Google Scholar]
- Spath-Schwalbe E, Hansen K, Schmidt F, et al. Acute effects of recombinanthuman interleukin-6 on endocrine and central nervous sleep functions in healthy men. J Clin Endocrinol Metab. 1998;83:1573–1579. doi: 10.1210/jcem.83.5.4795. [DOI] [PubMed] [Google Scholar]
- Toda N, Nakanishi-Toda M. How mental stress affects endothelial function. Pflugers Arch. 2011;462:779–794. doi: 10.1007/s00424-011-1022-6. [DOI] [PubMed] [Google Scholar]
- Trepels T, Zeiher AM, Fichtlscherer S. The endothelium and inflammation. Endothelium. 2006;13:423–429. doi: 10.1080/10623320601061862. [DOI] [PubMed] [Google Scholar]
- von Känel R, Ancoli-Israel S, Dimsdale JE, et al. Sleep and biomarkers of atherosclerosis in elderly Alzheimer caregivers and controls. Gerontology. 2010;56:41–50. doi: 10.1159/000264654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Känel R, Loredo JS, Ancoli-Israel S, Mills PJ, Natarajan L, Dimsdale JE. Association between polysomnographic measures of disrupted sleep and prothrombotic factors. Chest. 2007;131:733–739. doi: 10.1378/chest.06-2006. [DOI] [PubMed] [Google Scholar]
- Wang XS, Armstrong ME, Cairns BJ, Key TJ, Travis RC. Shift work and chronic disease: the epidemiological evidence. Occup Med (Lond) 2011;61:78–89. doi: 10.1093/occmed/kqr001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolk R, Gami AS, Garcia-Touchard A, Somers VK. Sleep and cardiovascular disease. Curr Probl Cardiol. 2005;30:625–662. doi: 10.1016/j.cpcardiol.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Yeboah J, Crouse JR, Hsu FC, Burke GL, Herrington DM. Brachial flow-mediated dilation predicts incident cardiovascular events in older adults: the Cardiovascular Health Study. Circulation. 2007;115:2390–2397. doi: 10.1161/CIRCULATIONAHA.106.678276. [DOI] [PubMed] [Google Scholar]
- Young T, Finn L, Peppard PE, et al. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep. 2008;31:1071–1078. [PMC free article] [PubMed] [Google Scholar]
- Zamarrón-Sanz C, Ricoy-Galbaldon J, Gude-Sampedro F, Riveiro-Riveiro A. Plasma levels of vascular endothelial markers in obstructive sleep apnea. Arch Med Res. 2006;37:552–555. doi: 10.1016/j.arcmed.2005.10.011. [DOI] [PubMed] [Google Scholar]