Abstract
The wall of the ventral third ventricle is composed of two distinct cell populations: tanycytes and ependymal cells. Tanycytes regulate many aspects of hypothalamic physiology, but little is known about the transcriptional network that regulates their development and function. We observed that the retina and anterior neural fold homeobox transcription factor (Rax) is selectively expressed in hypothalamic tanycytes, and showed a complementary pattern of expression to markers of hypothalamic ependymal cells, such as Rarres2 (retinoic acid receptor responder). To determine whether Rax controls tanycyte differentiation and function, we generated Rax haploinsufficient mice and examined their cellular and molecular phenotype in adulthood. These mice appeared grossly normal, but careful examination revealed a thinning of the third ventricular wall and reduction of both tanycyte and ependymal markers. These experiments show that Rax is required for hypothalamic tanycyte and ependymal cell differentiation. Rax haploinsufficiency also resulted in the ectopic presence of ependymal cells in the α2 tanycytic zone, where few ependymal cells are normally found, suggesting that Rax is selectively required for α2 tanycyte differentiation. These changes in the ventricular wall were associated with reduced diffusion of Evans Blue tracer from the ventricle to the hypothalamic parenchyma, with no apparent repercussion on the gross anatomical or behavioral phenotype of these mice. In conclusion, we have provided evidence that Rax is required for the normal differentiation and patterning of hypothalamic tanycytes and ependymal cells, as well as for maintenance of the cerebrospinal fluid-hypothalamus barrier.
INTRODUCTION
The hypothalamus regulates many physiological processes essential for life, including feeding, reproduction, sleep, circadian rhythms, stress response, blood pressure and core body temperature (Simerly, 1994; Sternson, 2013). In addition to the functions performed by diverse and distinct hypothalamic neuronal subtypes, a still poorly understood glial cell population called tanycytes also regulates hypothalamic function. Hypothalamic tanycytes show elongated radial glial-like morphology, with cell bodies localized in the ventricular layer of the third ventricle and in direct contact with cerebrospinal fluid (CSF). A single long basal process emerges from the tanycyte cell body. The basal processes of more dorsally located α1 and α2 tanycytes extend into the hypothalamic parenchyma, where they make contact with endothelial cells, glia and neurons. In contrast, the basal processes of more ventrally located β1 and β2 tanycytes extend to the external layer of the median eminence or all the way to the pial surface (Millhouse, 1971; Bleier, 1971; Rodriguez et al., 1979). In addition to their morphological differences, these tanycyte subtypes also exhibit distinct gene expression profiles (Rodriguez et al., 2005).
Another cell population present in the wall of the third ventricle is ependymal cells. These cells do not have a long process and are characterized by the presence of multiple cilia projecting to the ventricular lumen where they promote CSF flow and neuroblast migration during development (Doetsch et al., 1997; Sawamoto et al., 2006). Ependymal cells are commingled with tanycytes at the hypothalamus-ventricle interface, and the relative abundance of these two cell types divides the third ventricular wall in the tuberal hypothalamus into three dorsoventral zones: the most ventral tanycytic zone, composed almost exclusively of β1, β2 and α2 tanycytes; the transition zone, which contains α1 tanycytes intercalated with ependymal cells; and the most dorsal ependymal zone, which is composed only of ependymal cells (Mathew, 2008).
Tanycytes have been implicated in regulating multiple processes within the adult hypothalamus, including active transport of growth factors between CSF and hypothalamic parenchyma (Fernandez-Galaz et al., 1996; Duenas et al., 1994); acting as both a barrier to diffusion and facilitating the diffusion of small molecules between blood, CSF and hypothalamic parenchyma (Mullier et al., 2010); sensing blood glucose levels (Frayling et al., 2011; Orellana et al., 2012); regulating neuropeptide release (Prevot et al., 1999; Sanchez et al., 2009); and acting as neural progenitor cells (Xu et al., 2005; Li et al., 2012; Lee et al., 2012; Lee and Blackshaw, 2012; Li et al., 2012; Haan et al., 2013). Tanycytes are able to regulate the activity of hypothalamic neurons, leading to changes in hypothalamic functions such as reproduction (Prevot et al., 1999; Nakao et al., 2008) and energy balance (Coppola et al., 2007; Marsili et al., 2011; Bolborea and Dale, 2013).
In contrast to our rapidly increasing knowledge of tanycyte function, tanycyte development remains poorly understood. Studies in the rat and mouse have shown that tanycytes and ependymal cells of the third ventricle begin to be generated at the end of hypothalamus development apparently from local radial glia (Altman and Bayer, 1978; Rutzel and Schiebler, 1980; Hajos and Basco, 1984). In the rat, ependymogenesis occurs between embryonic day (E)16 and E19 from the caudal to rostral direction along the neural tube, with maturation continuing until the end of the first postnatal week (Altman and Bayer, 1978). Hypothalamic tanycytes start forming at the very end of the rat embryonic development (E19), but some continue to be generated during the first and even the second postnatal week (Altman and Bayer, 1978). Based on cytological, histochemical and ultrastructural criteria, tanycytes reach their complete morphological and functional maturity during the first month of life (Monroe and Paull, 1974; Altman and Bayer, 1978; Walsh et al., 1978; Rutzel and Schiebler, 1980; Seress, 1980). The stage at which terminal differentiation of tanycytes occurs seems to vary among different subtypes. It has been shown that ventral tanycytes (α2 tanycytes) acquire adult morphology as early as the first postnatal week, and the most dorsal tanycytes (α1 tanycytes) take longer to mature (Walsh et al., 1978).
Genetic fate mapping of Shh-expressing progenitors in the mouse hypothalamus indicates that a subset of tanycytes in the median eminence arise from Shh expressing progenitors as early as E8.5 (Alvarez-Bolado et al., 2012). The discrepancy between early birth dating studies and this recent repot might arise from the different species and techniques used. Virtually nothing is known about the molecular mechanisms that control tanycyte specification and differentiation, however.
We and others have previously reported that Rax mRNA is prominently and selectively expressed in hypothalamic progenitor cells (Mathers et al. 1997; Shimogori, et al. 2010). By E11.5, Rax is expressed along the ventral lateral walls of the hypothalamic primordium and is co-expressed with Shh in a localized ventral region of the midline. From E11.5 to E16.5, Rax is broadly expressed in ventral hypothalamic progenitors (Shimogori et al., 2010; Lu et al., 2013). By E16.5, Rax mRNA is expressed along the entire wall of the hypothalamic third ventricle, and absent from all other hypothalamic regions (Shimogori et al., 2010). Rax-deficient mice have lethal abnormalities of the anterior neural tube, including complete absence of hypothalamus, craniofacial defects, and anophthalmia (Voronina et al., 2005). In addition, reduced Rax expression has being associated with SCN abnormalities in the eyeless inbred mouse strain ZRDCT (Tucker et al., 2001). A recent mouse study, in which hypothalamic expression of a conditional Rax allele had been disrupted using Shh:Cre, observed that Rax is required for both proliferation of mediobasal hypothalamic progenitors and differentiation of neurons in the ventromedial hypothalamic nucleus (VMH) and the arcuate nucleus (ArcN) (Lu, et al. 2013). However, though this study also reported Rax mRNA expression along the ventricular wall of the hypothalamic primordium, the effects of loss of function of Rax on tanycyte differentiation were not characterized.
Based on the prominent expression of Rax mRNA in late-stage hypothalamic progenitor cells and its known role in regulating hypothalamic cell fate specification, we hypothesized that Rax might also control the development of hypothalamic tanycytes, and addressed this question by characterizing the cellular and molecular phenotype of adult mice heterozygous for Rax.
MATERIALS AND METHODS
Animals
All mice used in these studies were maintained and euthanized according to protocols approved by the Institutional Animal Care and Use Committee at the Johns Hopkins School of Medicine. Mice were kept in a 12:12 day cycle in a temperature-controlled room 23°C. Water and food were available ad libitum.
Six week old C56BL/6 female and male wild type mice were purchased from Charles Rivers, USA and used for the characterization of wild type Rax, Rarres2 and Gpr50 chromogenic in situ or fluorescent in situ hybridization (fISH) and vimentin immunohistochemistry (IHC) expression.
Raxf/f mice were a generous donation from Dr. Peter Mathers and had a mixed background (C56BL/6, Sv129, CD1). These mice were bred to the germline Cre mouse line Ella-Cre (C56BL/6 background), donated by Dr. Jeremy Nathans, to generate Rax+/− animals. Rax+/− animals were mated to generate Rax+/− and Rax +/+ littermates, which were then used for all the experiments described in the study.
Male P45 Rax+/+ mice and Rax+/− male were used for extraction and quantification of RNA from hypothalamus. Female P45 Rax+/+ and Rax+/− mice were used for intracerebroventricular injections (i.c.v) of Evans Blue (EB).
Rax and Rarres2 fISH for quantification were performed in male and female P45 Rax+/+ and Rax+/− mice.
Tissue preparation
Brain tissue collection for fISH and IHC was performed as follows. Mice were anesthetized with 100μl of Nembutal and subjected to cardiac perfusion with 4% paraformaldehyde (4% PFA) in 1X sodium phosphate buffer pH 7.5 (PBS 1X). The pH of the PFA 4%-PBS solution was adjusted to a pH 7 with sodium hydroxide or hydrochloric acid. Brains were removed from the skull and immediately post-fixed in 4% PFA with rotation for 18–20 hours at 4°C. Brains were then were washed in PBS 1X, cryoprotected in 30% sucrose for 48h, embedded in Tissue-Tek® O.C.T. Compound, (Sakura® Finetek) and stored at −80 °C. Coronal free-floating sections were collected by sectioning in a cryostat at 40 μm, starting from the middle of the anterior hypothalamus to the end of the posterior hypothalamus. Sections were floated in RNase free PB 0.5X serially distributed in individual wells of an RNase free 48 well plate (Falcon). After cutting, sections where examined under the light microscope to select sections containing only the tuberal hypothalamus and the anterior region of the posterior hypothalamus for a total of ten to twelve sections per brain. Sections where mounted in four Superfrost Plus slides (Fisher) per brain organized as follow; alternate sections were mounted in the first group of two slides and the remaining alternate sections were mounted in the second group of two slides. Each group of two slides was used for a different in situ hybridization and/or IHC staining experiment. Mounted sections were dried overnight at room temperature. Next day slides where stored at −80°C until they were used for the experiments.
Brain tissue collection for EB diffusion analysis was performed as follows. Mice were decapitated with scissors when they were still under anesthesia and 1 minute after the EB infusion was finished. Brains were removed from the skull, embedded in O.C.T. compound and stored at −80°C. Fresh frozen coronal hypothalamic sections from these brains were collected by sectioning in a cryostat at 40μm starting from the middle of the anterior hypothalamus to the end of the posterior hypothalamus. Sections from each brain were immediately mounted in Superfrost Plus slides (Fisher) and allowed to dry at room temperature in the dark for around 20 minutes. Slides were photographed and stored immediately at −80°C.
Hypothalamus dissections for RNA extraction were performed as follows. Mice were sacrificed by CO2 asphyxia, and their brains were immediately dissected. Brains were placed ventral side up, immobilize with needles and submerged in PBS 1X during dissection. An initial incision was made in the midline, followed by two more lateral cuts made ~2mm away from the midline originating two rectangular pieces of tissue. Each tissue piece was placed in its side to performed two cuts, a rostral cut made just anterior to the occulomotor nerve (nervus occulomotorius) and a caudal cut made posterior to the red nucleus. Hypothalami were placed in Eppendorf tubes containing RNAlater (Life technologies, Invitrogen) and stored at −80°C for RNA extraction.
Antibodies
Primary antibodies used in this study are described in Table 1. Detyrosinated alpha tubulin was used as a cilia marker and has been previously characterized (Gundersen et al., 1984). It has shown to stain primary cilia in vitro (Gundersen and Bulinski, 1986), apical cilia of ependymal cells in vivo (Paturle-Lafanechere et al., 1994; Mullier et al., 2010). The vimentin antibody was used as a cell marker and stained hypothalamic tanycytes and ependymal cells as previously reported by others (Mullier et al., 2010; Sanchez et al., 2009). Primary antibodies used for detecting RNA probes labeled with DIG were conjugated to either alkaline phosphatase (AP) for chromogenic in situ hybridization or horseradish peroxidase (POD) for fISH. RNA probes labeled with fluorescein were detected with antibodies conjugated with POD. In all cases, RNA probes used for fISH were detected with the Cy3 or Fluorescein Tyramide signal amplification (TSA) kits (Roche NEL744001 and NEL741001 respectively). To test the specificity of RNA hybridization, we performed control experiments in which no labeled probe was added. No signal was detected in the absence of the labeled probe (data not shown).
Table 1.
Primary antibodies used in this study
| Antibody | Immunogen | Catalog No./Distributor | Host Species and clonality | Dilution |
|---|---|---|---|---|
| Anti-Vimentin | Recombinant Syrian gold hamster vimentin | AB5733/Millipore | Chicken IgY polyclonal | 1:1,500 |
| Anti-Tubulin, detyrosinated | N-GEEEGEE-C synthetic peptide corresponding to the seven C-terminal aminoacids | AB3201/Millipore | Rabbit IgG polyclonal | 1:500 |
| Anti-DIG- POD, Fab fragment | Digoxigenin | 11207733910/Roche | Sheep IgG polyclonal | 1:10,000 |
| Anti-FL- POD, Fab fragments | Fluorescein | 11426338910/Roche | Sheep IgG polyclonal | 1:5,000 |
| Anti-DIG- AP, Fab fragments | Digoxigenin | 11093274910/Roche | Sheep IgP polyclonal | 1:2,500 |
AP: Alcaline phosphatase, POD: Horseradish peroxidase, DIG: Digoxigenin, FL: Fluorescein
Secondary antibodies used were AlexaFluor 633 goat anti-chicken IgG 1:500 dilution (Invitrogen, A21103) and AlexaFluor 488 donkey anti-rabbit 1:500 dilution (Invitrogen, A21206). See Table 1 for a full list of antibodies used in this study.
Genotyping
DNA for mice genotyping was obtained from tail tips collected carefully with new razor blades to avoid cross contamination. Tails were incubated at 55°C overnight in lysis solution containing 0.1μg/μl of Proteinase K (Roche). Rax flox and null alleles were genotyped as previously described (Voronine et al. 2005) The Rax null allele was genotyped using the sense primer 5′-AGGAGCTCCAGGAGCTCGAAAGAGC-3′, and the antisense primer 5′-CGAGTATCCCTACTGCCTGGAAATC-3′ and the wildtype and floxed alleles were genotyped using the sense primer AGGAGCTCCAGGAGCTCGAAAGAGC-3′ and the antisense primer 5′-GGACGTGCTTCTCCTTGCTCCTTGG-3′. Rax PCR genotyping protocol was: 94°C for 5 min, 94°C for 30 sec, 55°C for 30 sec, 72°C for 1.3 min, steps 2 to 4 were repeated 30 times, 72°C for 5 min, 4°C hold.
Probe labeling for in situ hybridization
Clones carrying cDNA for Rax BE951347, Gpr50 BI289437 and Rarres2 AW048638 were amplified with T3/T7 primers using a Taq PCR Master Mix (Sigma). The amplified PCR product was checked for size and quality using agarose electrophoresis. PCR products that yielded a single band of the correct size were then used for generating a labeled RNA probe. Probe labeling with DIG or fluorescein was conducted under RNAse-free conditions and using an RNA DIG and fluorescein labeling kits (Roche) as recommended by the manufacturer. After RNA amplification the labeling reaction was precipitated by overnight incubation with 4M lithium chloride (LiCl) and 100% ethanol. Pellets were washed with 70% ethanol and resuspended in 100 μl of 10mM EDTA, pH 8.0 (Sigma). RNA probe size was then checked using agarose electrophoresis. Labeled probes were stored at −80°C.
Chromogenic in situ hybridization
In situ hybridizations were performed under RNase free conditions, using diethyl pyrocarbonate (DEPC) (Sigma) treated water (DEPC water) for all washes and solutions used prior to probe hybridization. Brain sections were allowed to defrost at room temperature and fixed in 4% PFA prepared in 1X PBS pH 7.5 for 10 minutes. Then, they were permeabilized in PBT (1X PBS and 0.1% Tween-20) twice for 5 minutes followed by treatment with 1 μg/ml of Proteinase K solution (PK and 1X PBS) at 37°C. Slides were washed again in PBT twice for five minutes and post-fixed in 4% PFA for 5 minutes. Background reduction was performed by submerging the slides in acetylation solution (0.1M triethanolamine, 24.5% acetic anhydride and DEPC water) for 10 minutes at room temperature (RT). RNA probes were added to the hybridization solution (10mM Tris pH7.5, 600mM NaCl, 1mM EDTA, 0.25% SDS, 10% Dextran Sulfate (American Bioanalytical 50% solution), 1X Denhardt’s, 200mg/ml yeast tRNA (Gibco), 50% formamide) and incubated overnight at 68°C in a hybridization chamber humidified with 50% formamide/5X saline-sodium citrate (SSC). After hybridization, slides were submerged in TNE (Tris pH 7.5 10mM, NaCl 500mM, EDTA 1mM) for 10 minutes at 37°C, followed by treatment with RNAse solution (0.02μ/ml of RNase A prepared in TNE) for 25 minutes at 37°C. After the RNase treatment, slides were washed again with TNE for 10 minutes at RT followed by stringency washes in SCC at 65°C as follows; 2X SSC once for 20 minutes, 0.2X SSC twice for 20 minutes each. Then, slides were washed in B1T twice (2M Tris pH7.5, 3M NaCl and 0.1% Tween-20) at RT for 5 minutes and blocked with 20% blocking solution (20% Heat Inactivated Sheep Serum, HISS diluted in B1T) for 1 hour and incubated overnight with an antibody solution (Anti-DIG AP 1:2,500 diluted in B1T) at 4°C. Next day, slides were washed in B1T three times for 5 minutes and NTMT for 10 minutes (100mM Tris HCl, pH = 9.5; 100mM NaCl, 50mM MgCl2, 1% Tween 20). The riboprobe/mRNA duplexes were detected with a solution containing 0,033 μg/μl of NBT (4-Nitro blue tetrazolium chloride) (Roche) and 50mg/ml of BCIP (5-Bromo-4-chloro-3-indolyl-3-phosphate) (Sigma) prepared in NTMT. After 1 to 4 hours of incubation, slides were rinsed with 1XPBS1, stained with DAPI (4′,6-diamidino-2-phenylindole) (Roche) solution 1:5,000 prepared in PBS 1X, post-fixed in 4%PFA and coverslipped with Gelvatol.
Single-color fluorescence in situ hybridization
We combined the chromogenic in situ hybridization protocol with the Tyramide Signal Amplification (TSA) (Perkin Elmer) reaction to perform fluorescent in situ hybridization (fISH). Pre-hybridization, hybridization and post-hybridization steps were performed as the chromogenic in situ hybridization described above. However, after the post hybridization washes with SSC, we performed quenching of endogenous peroxidase activity incubating the slides in 3% hydrogen peroxide (H2O2) in PBS 1X for 30 min at RT and blocking in TNB (0.1 M Tris HCl pH 7.5, 0.15 M NaCl and 0.5% w/v Blocking buffer from Perkin Elmer) 1 hour at RT. Slides were incubated overnight with Anti DIG-POD diluted in TNB 1:10,000 at 4°C in a humidified chamber. Fluorescence visualization of riboprobe-mRNA duplexes was done using Cy3 diluted 1:50 in the amplification buffer provided in the TSA kit (Perkin Elmer). The optimal time for the tyramide amplification reaction was determine experimentally; Rax 16–18 hours and Rarres2 one hour. The reaction was stopped by washing the slides with B1T three times 5 minutes at RT. One color fISH was followed by immunohistochemistry.
Two-color fluorescence in situ hybridization
Two color fluorescence in situ hybridization was performed as the single color except that the overnight hybridization was done using two riboprobes added simultaneously, one riboprobe was DIG-labeled (Rarres2 or Gpr50) and the other riboprobe was fluorescein labeled (Rax). The DIG labeled riboprobe was developed first (Rarres2, 1 hour or Gpr50, 1 hour) using Cy3 diluted 1:50 in the amplification buffer provided in the TSA kit. The reaction was stopped with three washes of B1T for 5 minutes at RT and it was followed by quenching the peroxidase activity in 1% H2O2 for 15 min. Then, the fluorescein labeled riboprobe was developed (Rax, 16 hours) using fluorescein diluted 1:50 in the amplification buffer provided in the TSA kit. The second color reaction was stopped with B1T. The two colors in situ hybridization was followed by immunohistochemistry.
Immunohistochemistry
Permeabilization/blocking was performed using three washes with PBS plus solution for 5 minutes at RT (0.3% Triton 100X and 5% normal horse serum, 0.1M 1X PBS) and followed by blocking in Superblock (ScyTek) for 5 minutes. When the anti-tubulin detyrosinated antibody was used, the blocking was preceded by antigen retrieval with L.A.B. Solution (Liberate Antibody Binding Solution) (Polyscience) during 20 minutes at RT. After blocking, slides were incubated overnight at 4°C with primary antibodies prepared in PBS plus. Next day, slides were incubated with Alexa secondary antibodies diluted in PBS plus (1:500) for 2 hours at RT. DAPI staining was performed by submerging the slides in a DAPI solution 1:5,000 prepared in 1X PBS. When the staining was complete, 100μl of Vectashield hard-set mounting medium with DAPI (Vector laboratories) was applied to the slides and covered with a cover slip. Vectashield was allowed to dry in the dark for 1 hour and stored at 4°C until the image acquisition.
Cannulation and intracerebroventricular injections of Evans Blue dye
Evans Blue powder dye content 75% (Sigma) was dissolved in sterile 0.9% saline solution to a 1% final concentration. The EB solution was prepared fresh and kept in the darkness at RT.
P45 female Rax+/+ and Rax+/− mice were anesthetized with an imp injection of a Dormitory (medetomidine, selective α-2-receptor adrenergic agonist) (1mg/kg) and Ketamine (NMDA antagonist) (80 mg/kg) cocktail in a 5:2 ratio (Dormitor:Ketamine). The scalp incision area of the anesthetized mice was prepared for surgery by shaving off their hair, followed by swabbing their skin with a Povidone-Iodine swab stick, an antiseptic germicide, and 70% ethanol. The mice were then placed in a stereotaxic instrument (Kopf) by adjusting the incisor and ear bars an elevating the mice to a skull level position. An incision was made into the skin along the midline of the scalp starting at a point slightly posterior from between the eyes to a point approximately 1 mm caudal to lambda reference point. Then, a hole was drilled into the skull 0.6 mm caudal to Bregma and 1.2 mm lateral to midline. In order to allow for the cannula to adhere into place, the surface of the skull was etched with a sterile scalpel, coated with super-glue and etched again with a scalpel when the glue was dry. An extra-thin walled 24-gauge stainless steel cannula was inserted 2.2 mm below the skull surface, and secured in place using dental cement. A 30-guage obturator was inserted into the cannula to maintain patency. The wound around the base of the dental cement was closed using Sofsilk sutures. To prevent hypothermia and death, the mice were kept on a heating pad set at 40°C. Finally, mice received an i.c.v injection of 1μl of 1% EB into the lateral ventricle using a disinfected stainless steel injector Hamilton Syringe connected by polyethylene tubing. Administration of EB lasted around 3–4 seconds and the injector was kept inside the cannula for 1 minute after the EB was infused. The injector was then removed and the mice were decapitated with scissors. The brains were collected and immediately embedded and frozen in O.C.T and stored at −80°C.
RNA extraction and qRT-PCR
mRNA was extracted from dissected hypothalami using RNeasy Mini kits (Qiagen). RNA was quantified using NanoDrop 1000 spectrophotometer (Thermo Scientific), and Superscript II reverse transcriptase (Invitrogen) was used to create cDNA from 1000 ng of RNA. Levels of mRNA for specific genes were quantified with reverse transcription polymerase chain reaction (qRT-PCR) using Power SYBR green PCR master mix (Applied Biosystems). The qRT-PCR output generated a Ct value which was transformed to ΔCt by normalizing each sample to 18S gene (sense primer 5′-GCAATTATTCCCCATGAACG-3′ and antisense primer 5′-GGCCTCACTAAACCATCCAA-3′. The ΔΔCt value was then calculated relative to the average control ΔCt value. The ΔΔCt values were analyzed for statistical significance using unpaired t-test considering p<0.05 as significant.
Primers were obtained from the validated pools of primers in the PrimerBank website http://pga.mgh.harvard.edu/primerbank/ (Spandidos et al., 2008; Spandidos et al., 2010) except for Hes5 primers which were donated by Dr. Nicholas Gaiano. Primer sequences used for qRT-PCR are described in Table 2.
Table 2.
RT-PCR primers used in this study
| Gene | Forward primer | Reverse primer |
|---|---|---|
| Rax | 5′-TGGGCTTTACCAAGGAAGACG-3′ | 5′-GGTAGCAGGGCCTAGTAGCTT-3′ |
| Gpr50 | 5′-AGAGCAACATGGGACCTACAA-3′ | 5′ GCCAGAATTTCGGAGCTTCTTG-3′ |
| Hes1 | 5′-CCAGCCAGTGTCAACACGA-3′ | 5′-AATGCCGGGAGCTATCTTTCT-3′ |
| Hes5 | 5′-AGAAAAACCGACTGCGGAAGCC-3′ | 5′-CGCGGCGAAGGCTTTGCT-3′ |
| Foxj1 | 5′-GCCTCCCTACTCGTATGCCA-3′ | 5′-GCCGACAGGGTGATCTTGG-3′, |
| Rarres2 | 5′-GCTGATCTCCCTAGCCCTATG-3′ | 5′-CCAATCACACCACTAACCACTTC-3′ |
| Vimentin | 5′-CGTCCACACGCACCTACAG-3′ | 5′-GGGGGATGAGGAATAGAGGCT-3′ |
| GFAP | 5′-CCCTGGCTCGTGTGGATTT-3′ | 5′-GACCGATACCACTCCTCTGTC-3′ |
Image acquisition
Low-resolution images of chromogenic in situ hybridization and fISH were obtained in an Axioskop-2 microscope (Carl Zeiss) equipped with an Axiovision software and using a 10X objective.
Z-stack images were taken in a Zeiss LSM510 Meta confocal microscope using a 63X or 100X objective and digital zoom 0.7 or 1 and equipped with Zen 2009 software. To ensure that the z-stack images to be used for quantification in the two genotype groups (Rax+/+ and Rax +/−) were taken in identical conditions, we collected them together during the same confocal session, using the same zoom, pinhole, gain, contrast and optical slice interval.
The landmarks used for the identification of the different tanycytes along the ventro-dorsal axis of the medial hypothalamus were the following: Medial zone of the ME (β2 tanycytes), lateral evagination of the infundibular recess (β1 tanycytes), immediately ventral the point where the infundibular recess starts opening (α2 tanycytes). We termed this point the deflection point, located immediately dorsal to the deflection point (α1 tanycytes) and roof of the third ventricle (ependymal cells).
Identification of Bregma points along the anterior-posterior axis was done comparing our images with the Allen Mouse Brain Atlas (Sunkin et al., 2013) and using the shape of the ME, the lateral region of pars tuberalis and the shape of the different hypothalamic nuclei stained with DAPI as landmarks.
Low-resolution images of brain sections containing EB were obtained with an Axioskop-2 mot (Carl Zeiss) equipped with Axiovision software and using a 5X and 10X objective. Two sets of pictures were taken in these sections, the first set was taken right after the slides were dried and used to examine the general distribution of EB in the third ventricle. The second set was taken 4 months after the initial set of pictures and it was used to obtain images for pixel quantification. Slides were frozen at −80°C during the period of time between the two set of pictures and defrosted at room temperature in the dark for 15 minutes prior to image acquisition.
Image analysis and statistics
Quantification of z stack images was done by one blind evaluator and using the Imaris software version 7.1.1. Rax and Rarres2 fISH signal was quantified using the automatic three-dimensional quantification of spots followed by editing for excess or lack of signal detection. Spot quantifications were performed in three mice per genotype group and we used a two-tailed unpaired t-test to analyze statistical significance considering p<0.05 as significant.
Quantification of DAPI volume was done semi-automatically using the cell tool of the Imaris software and manually adjusting the threshold to correspond to the area of interest. Visualization of volume reconstruction was done with the surface tool of Imaris and visualization of the two-dimensional images of the z stacks were obtained by grouping the z stack in the ImageJ software. Volume quantifications were performed in three mice per genotype group and we used a two-tailed unpaired t-test to analyze statistical significance considering p<0.05 as significant.
Cilia quantification was performed in brain sections from Rax+/+ and Rax+/− stained with G-TUB, which labels cilia, and Vim, which labels ependymal cells and tanycytes. Confocal z-stack images of the α2 tanycytic zone were analyzed for the presence of multiple cilia as follows: each digital slide of the confocal z-stack was visualized in the slice gridded viewer of the Imaris software. One blind observer manually counted the number of cilia longer than 2μm present in each square of the grid for each digital slide. Cilia shorter than 2μm were excluded because it most likely corresponded to primary cilia. When a cilium was clearly part of a cilia cluster it was counted only once. Cilia quantifications were performed in three Rax+/+ mice and four Rax+/− mice and we used a two-tailed unpaired t-test to analyze statistical significance considering p<0.05 as significant.
Quantification of EB signal was performed as follows. All 10X images from the entire medial hypothalamus of each mouse were used for pixel quantification using the ImageJ software. In imageJ, all images had the same background subtraction and brightness adjustment before converting them in binary images (0 or 255 pixels). The threshold used for all image was the same and was set automatically after choosing the maxentropy thresholding method. Then, two different regions of interest (ROI) were drawn in each image for quantification: ROI(b) was a fixed rectangular box that contained all pixels in the third ventricle and the adjacent hypothalamic parenchyma where EB diffused. ROI(a) was an irregular shaped ROI different for each image and contained only the pixels in the parenchyma. When there were no pixels in the parenchyma we assigned a default number of pixels that was the lowest number of pixels in the entire sample (359) to be able to calculate a ratio. The total amount of pixels for each ROI was the “area measurement” obtained from the analyze measure tool of the software. Finally, the distal diffusion of EB was calculated as a ratio R of the EB in the parenchyma (ROIa) divided by the total amount of EB in the medial hypothalamus (ROIb), where R=(ROIa/ROIb). The R value for each image obtained from the Rax+/+ mice (n=3, total images=44) was compared with the ratio for each image obtained from Rax+/− mice (n=3, total images=41) and analyzed for statistical significant difference using an unpaired non parametric two tail Mann-Whitney test using p< 0.05 as significant.
All z-stacks images were stored in the Zen 2009 software as lsm images. To generate figures for publication, z-stack images were processes with Image J using the following tools: autocontrast, split color and group z project which generated flatten images of individual channels. Then, the merge channel tool was used to generate images of the merged channels. Images of individual channels and merged channels were converted to tiff and processed in Photoshop version 10.0.1 to adjust image size. These images were then moved to Illustrator version 13.0.2 to build the figures. When necessary, the figure was converted from illustrator file to a tiff file and processed in photoshop to adjust the input level of each channel in all panels of the figure equally and simultaneously. Sometimes, shadows, highlight and contrast were also modified in order to improve the quality of the figure.
EB images were stored in the Axionvision software and converted to tiff images to prepared figures for publication. These images were processed in Photoshop version 10.0.1 to adjust size and to pseudocolor them in black and white. Figures were built in Illustrator and exported to tiff again to be able to modified input levels in the entire figure equally and simultaneously using Photoshop.
RESULTS
Rax mRNA is expressed in terminally differentiated hypothalamic tanycytes
Using in situ hybridization, we characterized Rax expression in mature hypothalamus and found that in postnatal day (P) 40 mice, Rax mRNA expression was restricted to cells lining the ventral hypothalamic third ventricle, closely matching the distribution of hypothalamic tanycytes (Figure 1). In anterior hypothalamus, Rax was expressed in a small region of the ventral midline (Figure 1). In the medial hypothalamus, the zone of Rax expression expanded dorsally, reaching the walls of the third ventricle adjacent to the VMH and DMH. In the posterior hypothalamus, Rax was strongly expressed in the ventral and lateral walls of the third ventricle. Rax expression was absent from the dorsal, purely ependymal portion of the third ventricular wall (Figure 1). Rax expression appears to be specific to tanycytes of the mediobasal hypothalamus, as expression was not detected in any other circumventricular organ, where cells with tanycyte-like morphology have been detected (PMID:23649873).
Figure 1. Rax mRNA expression in adult hypothalamus is restricted to the wall of the third ventricle.
Chromogenic Rax in situ hybridization in brain sections from adult C57BL/6 wild type mice (P40). (A) At low resolution, Rax expression (black) is restricted to the wall of the third ventricle (black arrow head). (B–D) At higher resolution, Rax mRNA expression is observed only in the ventral portion of the wall of the third ventricle of the anterior (B), medial (C) and posterior hypothalamus (D). Rax is also present in the median eminence (bracket in C).. Rax signal is not observed in the dorsal portion of the third ventricle (black arrows). Scale -bar 200μm. 3V: third ventricle, ME: median eminence.
To confirm that Rax mRNA was selectively expressed in hypothalamic tanycytes, we performed fluorescent in situ hybridization (fISH) for Rax followed by immunohistochemistry (IHC) for the intermediate filament protein Vimentin (Vim). Vimentin is enriched in tanycyte cell bodies and basal processes, as well as ependymal cell bodies (Rodriguez et al., 2005). We observed that Rax was co-expressed with Vimentin in cell bodies lining the wall of the hypothalamic third ventricle. Rax mRNA was selectively expressed in tanycytes, which were readily identifiable by the presence of both a Vim+ cell body and a vimentin-positive basal process that extends into the hypothalamic parenchyma. In contrast, Rax was completely absent from the ependymal cells, identified by a Vim+ cell body lacking a basal process (Figure 2).
Figure 2. Rax is co-expressed with vimentin in the ventral portion of the third ventricle wall.
Confocal z-stack reconstruction of Rax fluorescent in situ hybridization (fISH) (magenta) and vimentin (Vim) immunohistochemistry (green) counterstained with DAPI in brain sections of adult C57BL/6 wild type mice (P45). (A–T) Rax is expressed along the anteroposterior axis of the ventral hypothalamus and co-localizes with vimentin in the ventral portion of the wall of the third ventricle (white arrow heads). Numbers in left bottom corner correspond to different Bregma points. Bregma −1.555mm (A–D) represents the anterior hypothalamus, Bregmas −1.655mm to −1.955 mm (E–P) represent the medial hypothalamus and Bregma −2.055mm (Q–T) represents the posterior hypothalamus. Scale bar: 100μm. 3V: third ventricle.
As an additional test for Rax mRNA enrichment in tanycytes, we performed double-fluorescence in situ hybridization for Rax and the G protein-coupled receptor 50 (Gpr50), a known tanycyte marker (Batailler et al. 2012) in combination with immunohistochemistry for Vimentin. We found that Rax mRNA expression co-localized with Gpr50 mRNA in hypothalamic ventricular cells (Figure 3). We also observed that Gpr50 mRNA was prominently expressed in neurons of the dorsomedial hypothalamic nucleus (DMH), as previously reported (Batailler et al., 2012), but that Rax mRNA was not detectable in these cells (Figure 1).
Figure 3. Rax and Gpr50 are expressed in tanycytes.
Confocal z-stack reconstruction of double fISH for Rax (green), Gpr50 (magenta) mRNA and vimentin (Vim) immunohistochemistry (white) counterstained with DAPI (blue) in brain sections of adult C57BL/6 wild type mice (P45). Rax and Gpr50 are expressed by α1 (F–J), α2 (K–O), β1(P–T) and β2 (U–Y) tanycytes (Vim+ cells with process, white arrow heads) and they are absent from ependymal cells (A–E) (Vim+ cells without process, yellow arrow heads). Rax and Gpr50 are also expressed in the median eminence (U–W). 3V: third ventricle. Median eminence: ME. Scale bar: 20μm.
In addition to ventricular tanycytes, we detected Rax expression in the median eminence of the tuberal hypothalamus (Figures 1B, 3, 4). Using confocal imaging, we observed that Rax mRNA was not only localized in cell bodies of β2 tanycytes in the ependymal layer of median eminence, but was also expressed in cells whose nuclei were localized in deeper layers of ME (Figure 4). In addition, some Rax mRNA was not associated with any nuclei, but was associated with tanycyte processes (Figure 4). Interestingly, Gpr50 showed a similar pattern of expression in ME, suggesting that Rax+, Gpr50+ cells may correspond to astrocytic tanycytes previously identified using Golgi staining, which lack identified molecular markers (Millhouse, 1971).
Figure 4. Rax and Gpr50 are expressed within the median eminence.
Orthogonal view of Rax (green) and Gpr50 (magenta) double fISH combined with vimentin (Vim) immunohistochemistry (white) counterstained with DAPI (blue) in the median eminence of adult C57BL/6 wild type mice (P45) (A) Rax and Gpr50 mRNAs are not only expressed in β tanycytes located in the ependymal layer (EL) of the ME (white arrow heads), but also in other layers of the ME in close association with tanycyte processes (yellow arrows). This localization suggests that Rax and Gpr50 are translated in tanycyte processes. (B–K) Orthogonal view of the dashed square area showing a Rax+, Gpr50+ nucleus in close association with Vim staining. These Rax+, Gpr50+ cells might correspond to “astrocytic tanycytes” EL, Ependymal layer, SE, subependymal layer, FL, fiber layer, RL, reticular layer, PL, palisade layer. 3V: third ventricle. Scale bar: 20 μm.
Rarres2 mRNA is selectively expressed in ependymal cells
In order to differentiate tanycytes from ependymal cells, we used the Allen Brain Atlas (Sunkin et al., 2013) to identify genes expressed exclusively in ependymal cells. Using this approach, we identified Rarres2 as a candidate ependymal marker. Rarres2 encodes chemerin, a secreted protein which functions as an adipokine and is involved in immune response and inflammation (Ernst and Sinal, 2010). Using in situ hybridization, we found that Rarres2 mRNA expression in the hypothalamus was restricted to the dorsal ventricular wall of the anterior, medial and posterior hypothalamus (Figure 5A). Rarres2 expression was absent from the ventral portion of the hypothalamic ventricular zone, where tanycytes are located (Figure 5B). Since ependymal cells, unlike tanycytes, have multiple apical cilia, we used a cilia marker, detyrosinated alpha tubulin (G-TUB) to label ependymal cells (Mullier et al., 2010). To confirm that Rarres2 was expressed in ependymal cells, we used, and examined the presence of Rarres2 in ciliated cells. We found that Rarres2 expression matched G-TUB expression in the anterior, medial and posterior hypothalamus (Figure 6A). Moreover, Rarres2 mRNA was located in cell nuclei right beneath the multiple cilia of ependymal cells of the dorsal third ventricle (Figure 6B).
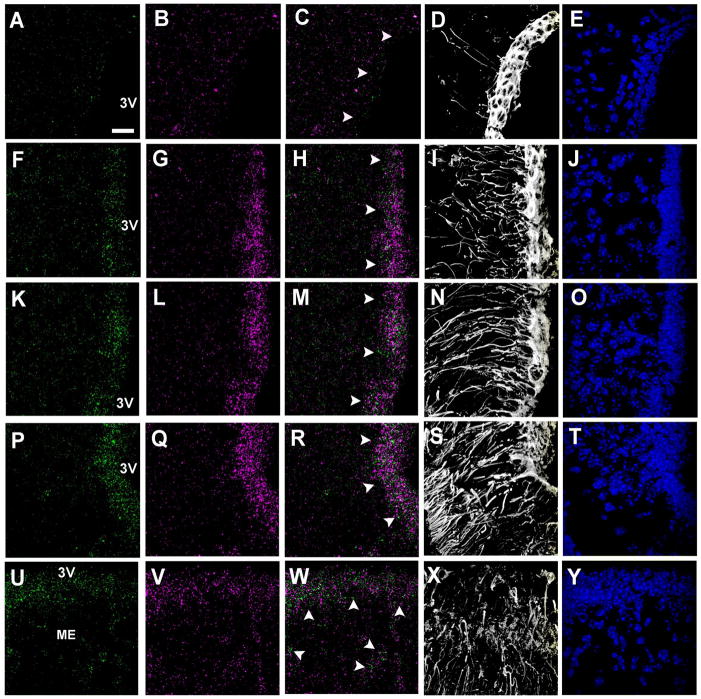
Figure 5. Rax and Rarres2 show complementary expression patterns in hypothalamic ependymal cells.
mRNA expression of Rax and Rarres2 along the hypothalamic anteroposterior axis (A–F) and ventrodorsal axis (G–Z). Top panels show Rarres2 (A–C) and Rax (D–F) chromogenic in situ hybridization in the anterior (A, D), medial (B, E) and posterior (C, F) hypothalamus of adult C57BL/6 wild type mice (P45). Rarres2 and Rax mRNAs are not expressed in the same zones of the third ventricular wall along the anteroposterior axis of the hypothalamus. Rarres2 mRNA is absent at the ventral portion of the wall of the third ventricle where tanycytes are located (white arrow heads). Rax mRNA expression is absent at the dorsal portion of the wall of the third ventricle were ependymal cells are located (black arrow heads). Scale bar: 200μm. (G–Z) Bottom panels show confocal z-stack reconstruction of double fISH for Rarres2 (magenta) and Rax (green) mRNA and vimentin (Vim) immunohistochemistry (white). Panels correspond to ependymal zone (G–J), transition zone (K–N), dorsal α2 tanycytic zone (O–R), β1 tanycytes zone (S–V) and β2 tanycyte zone (W–Z). Rarres2 and Rax mRNAs are not expressed in the same zones along the ventrodorsal axis of the wall of the third ventricle. Rarres2 mRNA is predominantly expressed in the most dorsal region, where ependymal cells are the exclusive cell population and in the transition zone where tanycytes are intermingled with ependymal cells (G–N) (yellow arrow heads). Rarres2 mRNA expression decreases ventrally where tanycytes are the predominant cell population (O–Z) (white arrow heads). There is some expression of Rarres2 in the dorsal α2 tanycytic zone close to the transition zone were some ependymal cells intercalate with tanycytes (O–R). Rax mRNA is predominantly expressed in the ventral region of the wall of the third ventricle where tanycytes are abundant (S–Z) and it progressively decreases dorsally where ependymal cells become the predominant cell population (G–N). 3V: third ventricle. Scale bar: 20μm.
Figure 6. Rarres2 is expressed in hypothalamic ependymal cells.
Confocal z-stack reconstruction of Rarres2 fISH (magenta) and immunohistochemistry for detyrosinated α tubulin (G-TUB) (green) counter stained with DAPI (blue) in the anterior (A), medial (B) and posterior (C) hypothalamus of adult C57BL/6 wild type mice (P45). (A–C) Top panels show that G-TUB is expressed in the dorsal region of the wall of the third ventricle along the anteroposterior axis similar to Rarres2 expression (white arrow heads). Scale bar: 100μm. Bottom panels (D–K) show an orthogonal view of the box in (A) where G-TUB (D, E) and Rarres2 (F, G) signal is observed in ependymal cells identified by the expression of G-TUB in their multiple cilia projecting to the third ventricle (green). Rarres2 signal is underneath G-TUB+ multiple cilia (J–L). G-TUB signal in hypothalamic parenchyma corresponds to primary cilia. 3V: third ventricle. Scale bar: 20 μm.
Reduced expression of both tanycytic and ependymal markers is seen in Rax+/− mice
Having shown that Rax is selectively expressed in tanycytes and Rarres2 is selectively expressed in ependymal cells, we next investigated whether loss of function of Rax disrupted the development of cells of the hypothalamic ventricular zone. To avoid early lethality seen in Rax null animals (Mathers, et al. 1997), we examined Rax heterozygous mice (Rax+/−). As expected, adult Rax+/− mice showed an approximately two-fold reduction in Rax mRNA expression compared to wild-type controls, as assessed by both quantification of Rax fISH signal in α2 tanycytes (Figure 7) and qRT-PCR analysis of hypothalamic mRNA (Figure 8). In addition, mRNA levels of other genes expressed in developing and terminally differentiated tanycytes such as Hes1, Hes5, and Gpr50 (Lee, et al. 2012) were significantly reduced in Rax+/− hypothalamus as determined by qRT-PCR (Figure 8). Interestingly, mRNA levels of genes specific to ependymal cells such as the forkhead transcription factor J1 (Foxj1) (Yu et al., 2008) were also reduced in Rax+/− hypothalamus. In contrast, levels of Vim and Gfap mRNA did not show any significant difference in expression between Rax+/+ and Rax+/− mice (Figure 8).
Figure 7. Rax mRNA is reduced in Rax heterozygote mice.
Confocal z-stack reconstruction of Rax fISH (magenta) and vimentin (Vim) immunohistochemistry (green) counterstained with DAPI (blue) in the α2 tanycytic zone of the medial hypothalamus (Bregma −1.655mm). Rax mRNA signal is reduced in Rax heterozygotes mice (Rax+/−) (E) compared to Rax wild type mice (Rax+/+) (A). (I) Digital quantification of Rax fISH signal in the α2 tanycytic zone using a three dimensional reconstruction of the signal with the spot tool of the Imaris software Unpaired t-test (n=3), p=0.01. 3V: third ventricle. Scale bar 20μm.
Figure 8. Rax heterozygotes show a two-fold reduction in tanycyte and ependymal markers.
qRT-PCR quantification of relative mRNA obtained from hypothalami dissected from adult mice (P45). Two fold reduction of relative Rax mRNA in heterozygous mice (Rax+/−) p=0.05. Relative mRNA of other genes expressed by terminally differentiated tanycytes is also reduced (Gpr50 p= 0.01, Hes1 p=0.01, Hes5 p=0.04.). Ependymal genes Foxj1 (p=0.03) and Rarres2 (p=0.03) are significantly reduced in Rax+/−. There is no significant reduction of Gfap and vimentin relative mRNA. Unpaired t-test Rax+/+ (n=5), Rax+/− (n=6).
Rax+/− mice show reduced cell volume of the third ventricular wall
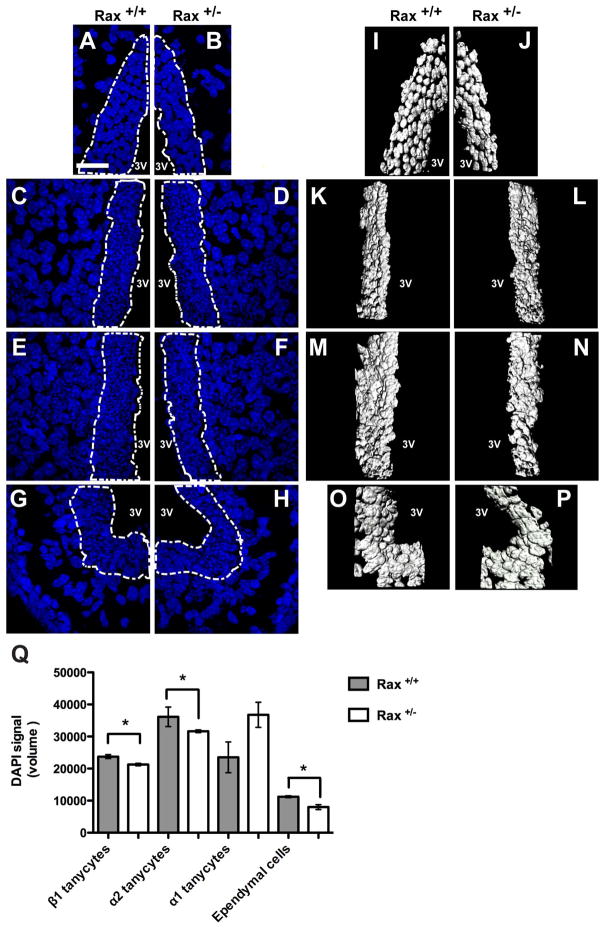
Reduced mRNA levels for multiple tanycyte and ependymal markers in Rax+/− hypothalamus suggested that these mice might have fewer cells in the ventricular zone of the third ventricle. Quantification of total cell number in the walls of the third ventricle using confocal images was not possible due to the tight packing of tanycytes and ependymal cells. However, the cell nuclei of the ventricular layer, which are comprised exclusively of tanycytes and/or ependymal cells, can be easily separated from the subventricular zone and hypothalamic parenchyma, and its volume can be measured using digital analysis. To do this, we measured the volume of the wall of the third ventricle using digital quantification of confocal z-stack reconstruction images of DAPI staining in the ventricular layer. We measured the volume of the cell nuclei present in the ventricular wall of the tuberal hypothalamus located at the lateral evaginations of the infundibular recess containing (LEIR) β1 tanycytes, the region ventral to the deflection point where the infundibular recess starts opening (α2 tanycytes), the region dorsal to the deflection point (α1 tanycytes), and the ependymal zone. We found that Rax heterozygous mice showed a significant reduction in third ventricle wall volume in the LEIR, the α2 tanycytic zone and the ependymal zone. Interestingly, the transition zone where α1 tanycytes are located did not show any significant difference in volume (Figure 9). β2 tanycytes were not examined, since most sections lost the ME during sectioning or staining.
Figure 9. Rax heterozygotes show a reduction in volume in the third ventricle wall.
(A–H) Confocal z-stack reconstruction of DAPI staining along the third ventricular wall of the medial hypothalamus (dotted area) in adult mice (P45). (I–P) Digital three-dimensional reconstruction of the dotted areas in (A). (Q) Digital volume quantification of the wall of the third ventricle using the cell tool of the Imaris software. Rax heterozygotes have reduced volume of the ventricular wall at the ependymal zone (B, J, Q) as well as at the α2 (F, N, Q) and β1 (H, P, Q) tanycytic zones. Unpaired t-test (n=3), β1 tanycytes p=0.01, α2 tanycytes p=0.03, α1 tanycytes p=0.08, ependymal cells p=0.02. 3V: third ventricle. Scale bar: 50μm.
Rax+/− mice show a ventralization of Rarres2 expression and ectopic ependymal cells in the α2 tanycytic zone
After quantifying Rarres2 mRNA in fISH confocal images, we found that Rax+/− mice selectively showed significantly higher levels of Rarres2 mRNA in the ventricular wall of the α2 tanycytic zone at Bregma −1.655 (Figure 10). This difference was not observed individually in the α2 tanycytic zone at other anterioposterior points examined inside the tuberal hypothalamus (Bregma −1.755 and −1.855), although a significant trend towards increased Rarres2 expression in overall Rax+/− α2 tanycytic zone was detected when data from all Bregma points was combined (Figure 10).
Figure 10. Rax heterozygotes show a ventralization of Rarres2 expression.
(A–H) Confocal z-stack reconstruction of Rarres2 fISH (magenta) and vimentin (Vim) immunohistochemistry (green) counterstained with DAPI (blue) in adult mice (P45). Rarres2 mRNA is more abundant in the α2 ventral tanycytic zone (immediately ventral to the deflection point) of Rax+/− mice (E) compared to Rax+/+ controls (A). (I) Digital quantification of Rarres2 fISH signal in the ventral α2 tanycytic zone at three different Bregma points using a three-dimensional reconstruction of Rarres2 fISH signal with the spot tool of the Imaris software. Cumulative indicates the sum of Rarres2 signal along the three different Bregma points (from 1.655 mm to −1.855 mm). Unpaired t-test (n=3), Bregma −1.655 mm p=0.05, Cumulative p=0.04. 3V: third ventricle. Scale bar: 20μm.
Having previously found that Rarres2 is expressed in ependymal cells of the third ventricle, we used immunohistochemical analysis to visualize the presence of multiple cilia in the α2 tanycytic zone in order to confirm the presence of ependymal cells (Mullier et al., 2010). Confocal z-stack visualization of G-TUB in the α2 tanycytic zone showed an increased in cilia projecting to the ventricular lumen (Figure 11). However, we noticed that G-TUB was present not only in the motile cilia of the ependymal cells (multiple cilia), but also in the non-motile cilia (primary cilia) which are detectable in some α2 tanycytes, but more abundant in β1 and β2 tanycytes (data not shown).
Figure 11. Rax heterozygotes show increased detyrosinated α tubulin-positive cilia in the α2 tanycytic zone.
(A, B) Confocal optical slides of immunohistochemistry for vimentin (Vim) immunohistochemistry (magenta) and detyrosinated α tubulin (G-TUB) (green). G-TUB+ Primary cilia are more abundant in the α2 tanycytic zone of Rax+/− mice (white arrow heads in B), compared to Rax+/+ mice (A). (C) Quantification of multiple cilia clusters in each optical slice of z-stack confocal pictures at three different Bregma points. The cumulative total indicates the sum of multiple cilia along the three different Bregma points (from −1.555 mm to −1.755 mm). Unpaired t-test Rax+/+ (n=4), Rax+/− (n=3). Bregma −1.655 mm p= 0.001, Bregma −1.755 mm p=0.02, cumulative p=0.04. Scale bar: 20 μm.
Primary cilia in tanycytes and motile cilia of the ependymal cells can be differentiated by their length and arrangement. Primary cilia are shorter than motile cilia, <1 μm for primary cilia and >2–8 μm for motile cilia (O’Callaghan et al., 1999) and, more importantly for discrimination between the two, motile cilia are found in clusters, whereas primary cilia are found as solitary cilia arising from the cell body (Satir and Christensen, 2007). Taking these differences in account, we quantified the clusters of cilia longer than 2 μm in the tanycytic zone of three Bregma points in each slide of our confocal z-stacks. We found that there was a significant increase in the number of multiple cilia clusters in the tanycytic zone of Rax+/− mice at Bregma −1.655, where we found the ventralization of Rarres2, as well as at Bregma −1.755mm (Figure 11).
Rax+/− mice display reduced distal diffusion of Evans Blue
Since tanycyte and ependymal cells form the CSF-brain barrier in the hypothalamus, and since we observed both fewer tanycytes and ependymal cells in the third ventricle and ectopic ependymal cells in the α2 tanycytic zone, we hypothesized that Rax+/− mice would exhibit altered tracer diffusion from CSF to the hypothalamic parenchyma. To test this hypothesis, we injected Evans Blue (EB) dye in the lateral ventricle of Rax+/+ and Rax+/− mice. Since EB is a low-molecular-mass tracer with high albumin affinity and that readily diffuses across paracellular space (Fry et al., 1977), we quantified EB signal in the hypothalamic parenchyma 1 minute after intracerebroventricular injection (Figure 12).
Figure 12. Rax heterozygotes show reduced distal diffusion of Evans Blue.
(A) Schema of Evans Blue i.c.v injection protocol. EB was injected in the lateral ventricle of adult mice (P45) that were sacrificed 1 min after the injection. (B–G). EB distribution in the brain. There was more distal diffusion in the anterior (B, E), medial (C, F) and posterior hypothalamus (D, G) (white arrow heads) than in other brain regions. Some distal diffusion is present in the lateral ventricles (yellow arrow head in B) and the dorsal third ventricle (yellow arrow head in C). (H–M). Closer view of. EB distribution of in the hypothalamus. The EB diffusion in the anterior hypothalamus was only proximal (H, K). Distal diffusion was observed in the medial (I, L) and posterior hypothalamus (J, M). In the medial hypothalamus distal diffusion is present in the ArcN, VMH and DMH (I, L). In the posterior hypothalamus distal diffusion is present in the ArcN (J, M). (N) Quantification of distal diffusion of the EB in the medial hypothalamus. The distal diffusion was calculated as a ratio between the amount of EB, measured as pixels, in the parenchyma of each brain section (ROIa) divided by the total amount of EB (parenchyma and ventricle) in the same brain section (ROIb). In the medial hypothalamus of Rax+/− mice the distal diffusion was significantly lower than the diffusion in Rax+/+ mice. Unpaired t-test of distal EB diffusion in the medial hypothalamus Rax +/+ (n= 3, 44 sections) vs. Rax +/− n=3, 41 sections) p (ROIa/ROIb)=0.001. Unpaired t-test Rax +/+ (n= 3, 44 sections) vs. Rax +/− n=3, 41 sections). Scale bars: 500μm (B–G) and 100μm (H–M) ArcN: Arcuate nucleus, VMH: ventromedial nucleus, DMH: Dorsomedial nucleus, i.c.v: intracerebroventricular.
In Rax+/+ mice, we found that EB distal diffusion was higher in the hypothalamus compared to other brain regions adjacent to ventricular compartments (Figure 12B). Also, within the medial hypothalamus, EB diffusion varied along the anteroposterior axis in both genotypes, with no distal diffusion in the anterior hypothalamus, and more distal diffusion in the medial and posterior hypothalamus (Figure 12B and 12C). When we compared the EB signal in Rax+/− and Rax+/+ animals by simple visualization, we found a consistent difference only in the medial hypothalamus. Based on this result, and also owing to desire to compare our results with the previous report of EB diffusion in wild type mice (Mullier et al. 2010), we quantified the total amount of EB in all brain sections of the medial hypothalamus, as well as EB in the parenchyma, using the pixel quantification ImageJ tool. Distal diffusion was calculated as a ratio between the levels of EB in the parenchyma of each brain section divided by the total amount of EB in the same brain section. In Rax+/− mice, distal diffusion into medial hypothalamic parenchyma was significantly lower than in Rax+/+ mice (Figure 12D). Most diffusion in both genotypes was located ventrally near the ArcN and dorsally near the DMH, where ependymal cells are abundant.
DISCUSSION
Previous studies have shown that Rax mRNA is expressed in progenitors located along the ventricular wall of the third ventricle. Our data showed that Rax expression persisted through adulthood, when its expression became restricted to hypothalamic tanycytes and the median eminence (Figures 1 to 4). Characterization of Rax+/− mice showed that Rax haploinsufficiency during development leads to a reduction in both tanycyte and ependymal cell numbers in the hypothalamic third ventricle in adult animals, as demonstrated by reduced marker expression for both cell types and reduced volume of the ventricular wall (Figures 8 and 9). Along with the overall reduction of ependymal cells in Rax+/− mice, we observed that some ependymal cells were ectopically located in the α2 tanycytic zone, where few of these cells are usually found (Figures 10 and 11). Furthermore, we found that Rax+/− mice displayed changes in the hypothalamic CSF-brain barrier that resulted in reduced distal diffusion of EB from the CSF to the parenchyma of the medial hypothalamus (Figure 12). Our main findings are summarized in Figure 13.
Figure 13. Schematic representation of tanycyte phenotype seen in Rax heterozygote animals.

(A) Reduction of Rax gene dose during the development of Rax+/− mice leads to a thinner ventricular wall and ectopic presence of ependymal cells in the α2 tanycytic zone suggesting that Rax probably participates in tanycyte and ependymal cell progenitor proliferation and differentiation. Hypocellularity of the ventricular wall leads to a reduced distal diffusion of the Evans blue tracer. Ep: Ependymal cells, Tan: Tanycytes. EB: Evans Blue.
Rax haploinsufficiency leads to reduced volume of the third ventricular wall
Rax+/− mice showed reduced ventricular wall volume due to a reduction in both tanycyte and ependymal cell numbers (Figure 8 and 9). This likely arises at least in part from Rax haploinsufficiency defects in late-stage hypothalamic progenitor cells, which give rise to both cell types (Mathers et al., 1997). The mechanism by which Rax controls hypothalamic progenitor proliferation is unknown, although recent studies of Xrx1, the Xenopus ortholog of Rax, results in smaller eye and brain size due to reduced proliferation (Andreazzoli et al., 2003; Casarosa et al., 2003). Terada and Furukawa have shown that Xrx1 binds to the chromatin modulator Xhmgb3 (Xenopus high mobility group 3) and to the Six family transcription factor XOptx2 (Six6) to promote cell proliferation in the eye and brain. Xhmgb3 in turn binds to the SUMO E2 ligase UBC9 and inactivates transcription of the kinase inhibitor p27Xic1, leading to increased proliferation of retinal progenitor cells (Terada and Furukawa, 2010). Both Six6 and Hmgb2, a close homologue of Hmgb3, are prominently and selectively expressed in hypothalamic progenitor cells (Shimogori et al., 2010), suggesting that Rax might be regulating the proliferation of hypothalamic ventricular radial glia through a similar mechanism, with reduction of Rax expression leading to increased p27 levels and suppression of tanycyte progenitor proliferation.
Rax haploinsufficiency is associated with an increased fraction of cells with multiple cilia in the tanycytic zone
Under normal conditions, there are few ependymal cells in the ventral third ventricle zone where α2 tanycytes are located. However, we found that in Rax+/− mice there was both a substantial increase in the number of cells with multiple cilia, characteristic of ependymal cells, and a ventral extension of ependymal cell marker Rarres2 expression in this region (Figure 10). Persistent and selective expression of Rax mRNA in mature hypothalamic tanycytes but not ependymal cells suggests that, in addition to regulating late-stage hypothalamic progenitor proliferation, Rax may actively promote tanycyte differentiation at the expense of ependymal identity.
Alternatively, it has been shown that motile cilia can develop from single ciliated cells during development of airway epithelial cells (Jain et al., 2010). This opens the possibility that cells in the ventricular zone with single apical cilia, such as tanycytes, could develop motile cilia in response to cell-autonomous and non-cell-autonomous cues similar to what happens in the airway. Two key cell-autonomous regulators of multiciliogenesis, multicilin and miR449, promote cilia formation in skin, kidney, and airway by blocking Notch signaling (Marcet et al., 2011; Stubbs et al., 2012). Interestingly, Notch signaling components are expressed in developing and mature tanycytes, but not ependymal cells (Shimogori et al, 2010), and show reduced expression in Rax+/− mice, in line with previous reports that Rax stimulates Notch signaling in retinal progenitor cells (Furukawa et al., 2000). These data suggest that Rax haploinsufficiency may trigger the formation of multiple cilia through a reduction of Notch signaling.
The multiciliated cells observed in the tanycytic zone might correspond to a population of hybrid tanycytes with both ependymal cell and tanycyte characteristics, similar to hybrid cells previously described after conditional loss of function of the transcription factor Six3 during ependymal cell maturation (Lavado and Oliver, 2011). Based on their location, morphology and immunoreactivity, the postnatal radial glia described by the authors of this study correspond to tanycytes. When Six3 is absent, the transition from “radial glia” to ependymal cells was defective.
Using Vimentin staining alone, we are unable to discriminate between a hybrid, multiciliated tanycyte and an ependymal cell located directly on top of a tanycyte cell body, giving the appearance of a multiciliated tanycyte. Analysis of individual tanycyte morphology using mice expressing fluorescent reporter genes, in combination with co-labeling with cilia markers, will ultimately allow us to determine whether such hybrid tanycyte-ependymal cells exist in Rax+/− mice.
The cellular composition of the ventricular wall influences the distal diffusion of CSF-derived EB in Rax+/− mice
We investigated permeability of the ventricular wall in Rax+/− mice, which have a reduced overall number of ependymal cells and tanycytes along the hypothalamic third ventricular wall and ectopic ependymal cells in the α2 tanycytic zone. We found that in Rax+/− mice there was reduced distal diffusion of EB from CSF to the medial hypothalamic parenchyma (Figure 12). We observed proximal EB diffusion all along the anterior to posterior hypothalamic axis, but distal EB diffusion was only present in the medial and posterior hypothalamus of Rax+/+ mice. This might be related to the variable composition of the hypothalamic ventricular wall along this axis. The ventricular zone of the ventral anterior hypothalamus is composed exclusively of ependymal cells, while tanycytes are only present in a small area at the floor of the third ventricle. In contrast, the medial and posterior hypothalamus features more tanycytes than ependymal cells (Rodriguez et al., 2005).
Our observation suggests that distal diffusion of EB is higher in hypothalamic areas enriched with tanycytes. Both tanycytes and ependymal cells can form gap junctions that permit proximal diffusion of a tracer, but only tanycytes have long processes that project distally from the ventricle. Tanycytes may facilitate distal diffusion of EB by endocytosis of the EB followed by transcellular transport. We also observed that in Rax+/+ mice, EB distribution was similar to that seen in previous studies that reported lateral diffusion of ventricular EB in the ArcN (α2 tanycytic zone) and in the ependymal zone of the medial hypothalamus (Mullier et al., 2010). However, we also observed lateral EB diffusion in Rax+/+ mice in the transition zone at the level of VMH and DMH, where both α1 tanycytes and ependymal cells are present, which was not previously reported. In the referenced study, EB diffusion in ArcN was reported at only a single Bregma point (Mullier et al., 2010). Since we observed that EB diffusion varies along the anterioposterior axis, it is possible that this study missed spatially restricted domains of lateral EB diffusion in the α1 tanycytic zone.
Finally, we found reduced distal diffusion of EB in the medial hypothalamus of Rax+/− mice. If distal EB diffusion were facilitated by the presence of tanycytes, the reduction of tanycytes in Rax+/− mice would explain a reduction in distal EB diffusion. However, reduced EB diffusion was not only observed ventrally in the tanycytic zone but also dorsally in the ependymal region of the hypothalamus. This suggests that there might be other factors that affect hypothalamic ventricular wall permeability beyond simply the number of tanycytes, such as altered expression of tight and gap junction proteins. There are abundant tigth and gap junctions between ependymal cells and tanycytes of the hypothalamic third ventricular wall (Peruzzo et al., 2000; Rodriguez et al., 2005; Mullier et al., 2010). Since paracellular permeability depends on the type of intercellular junctions, with gap junctions facilitating diffusion while tight junctions restrict diffusion, reduced distal diffusion of EB in Rax+/− mice suggests that a possible compensatory increase of tight junctions resulting from a reduced number of cells along the third ventricular wall may have occurred. However, we were unable to successfully perform staining with antibodies against tight or gap junction proteins in conjunction with EB visualization, owing to the instability and highly hydrophilic nature of EB.
Disrupted EB diffusion between CSF and hypothalamic parenchyma seen in Rax+/− mice may have physiological consequences. Protein-mediated signaling between the CSF and adjacent neurons is important for normal brain development and function (Dziegielewska et al., 2000; Sharma and Johanson, 2007). Signaling may be receptor-mediated or may occur by paracellular transport (Agnati et al., 1995). It has been suggested that based on the expression of cell-cell junctions and the pattern of EB diffusion in the ArcN, there is an open communication between the CSF and this nucleus (Mullier et al., 2010). Molecules such as T3 and leptin have been proposed to diffuse through the CSF to regulate the activity of neurons of the ArcN (Rodriguez et al., 2010). As a result, it would be expected that changes in protein permeability might affect hypothalamic function. Although the Rax+/− mice used in this study did not display any gross anatomical or behavioral abnormalities, this does not exclude the presence of more subtle changes in hypothalamic function, such as altered neuropeptide release, or the effects of compensatory changes that minimize the effects of Rax haploinsufficiency on tanycyte and hypothalamus function.
Acknowledgments
We thank Peter Mathers for donating the Raxf/f mice, Dr. Jeremy Nathans for donating the Ella-Cre mice; David Ginty, Nicholas Gaiano and Adam Kaplin for advice on experimental design; Joseph Bedont and Daniel Lee for comments on the manuscript; Jimmy de Melo for technical advice and comments on the manuscript; and Scot C. Kuo, Barbara Smith, Michael Delannoy and Michele Pucak for guidance on image acquisition and analysis. This work was supported by NIH R21NS067393, and grants from National Association for Research in Schizophrenia and Depression (NARSAD) and the Klingenstein Fund. S.B. was a W.M. Keck Distinguished Young Scholar in Medical Research.
Footnotes
Conflict of interest
None
Role of Authors:
Study concept and design: ALMA, SB. Acquisition of data: ALMA, SB, MB, JM, HW. Analysis and interpretation of data: ALMA, SB, MB. Drafting of the manuscript: ALMA, SB. Critical revision of the manuscript for important intellectual content: ALMA, SB. Statistical analysis: ALMA, SB, MB. Obtained funding: SB. Administrative, technical and material support: ALMA, SB, MB, JM, HW. Study supervision: SB
LITERATURE CITED
- Agnati LF, Zoli M, Stromberg I, Fuxe K. Intercellular communication in the brain: wiring versus volume transmission. Neuroscience. 1995;69:711–726. doi: 10.1016/0306-4522(95)00308-6. [DOI] [PubMed] [Google Scholar]
- Altman J, Bayer SA. Development of the diencephalon in the rat. III. Ontogeny of the specialized ventricular linings of the hypothalamic third ventricle. J Comp Neurol. 1978;182:995–1015. doi: 10.1002/cne.901820513. [DOI] [PubMed] [Google Scholar]
- Alvarez-Bolado G, Paul FA, Blaess S. Sonic hedgehog lineage in the mouse hypothalamus: from progenitor domains to hypothalamic regions. Neural Dev. 2012;7:4–7. doi: 10.1186/1749-8104-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreazzoli M, Gestri G, Cremisi F, Casarosa S, Dawid IB, Barsacchi G. Xrx1 controls proliferation and neurogenesis in Xenopus anterior neural plate. Development. 2003;130:5143–5154. doi: 10.1242/dev.00665. [DOI] [PubMed] [Google Scholar]
- Batailler M, Mullier A, Sidibe A, Delagrange P, Prevot V, Jockers R, Migaud M. Neuroanatomical distribution of the orphan GPR50 receptor in adult sheep and rodent brains. J Neuroendocrinol. 2012;24:798–808. doi: 10.1111/j.1365-2826.2012.02274.x. [DOI] [PubMed] [Google Scholar]
- Bleier R. The relations of ependyma to neurons and capillaries in the hypothalamus: a Golgi-Cox study. J Comp Neurol. 1971;142:439–463. doi: 10.1002/cne.901420404. [DOI] [PubMed] [Google Scholar]
- Bolborea M, Dale N. Hypothalamic tanycytes: potential roles in the control of feeding and energy balance. Trends Neurosci. 2013;36:91–100. doi: 10.1016/j.tins.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casarosa S, Amato MA, Andreazzoli M, Gestri G, Barsacchi G, Cremisi F. Xrx1 controls proliferation and multipotency of retinal progenitors. Mol Cell Neurosci. 2003;22:25–36. doi: 10.1016/s1044-7431(02)00025-8. [DOI] [PubMed] [Google Scholar]
- Coppola A, Liu ZW, Andrews ZB, Paradis E, Roy MC, Friedman JM, Ricquier D, Richard D, Horvath TL, Gao XB, Diano S. A central thermogenic-like mechanism in feeding regulation: an interplay between arcuate nucleus T3 and UCP2. Cell Metab. 2007;5:21–33. doi: 10.1016/j.cmet.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J Neurosci. 1997;17:5046–5061. doi: 10.1523/JNEUROSCI.17-13-05046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duenas M, Luquin S, Chowen JA, Torres-Aleman I, Naftolin F, Garcia-Segura LM. Gonadal hormone regulation of insulin-like growth factor-I-like immunoreactivity in hypothalamic astroglia of developing and adult rats. Neuroendocrinology. 1994;59:528–538. doi: 10.1159/000126702. [DOI] [PubMed] [Google Scholar]
- Dziegielewska KM, Knott GW, Saunders NR. The nature and composition of the internal environment of the developing brain. Cell Mol Neurobiol. 2000;20:41–56. doi: 10.1023/a:1006943926765. [DOI] [PubMed] [Google Scholar]
- Ernst MC, Sinal CJ. Chemerin: at the crossroads of inflammation and obesity. Trends Endocrinol Metab. 2010;21:660–667. doi: 10.1016/j.tem.2010.08.001. [DOI] [PubMed] [Google Scholar]
- Fernandez-Galaz MC, Torres-Aleman I, Garcia-Segura LM. Endocrine-dependent accumulation of IGF-I by hypothalamic glia. Neuroreport. 1996;8:373–377. doi: 10.1097/00001756-199612200-00073. [DOI] [PubMed] [Google Scholar]
- Frayling C, Britton R, Dale N. ATP-mediated glucosensing by hypothalamic tanycytes. J Physiol. 2011;589:2275–2286. doi: 10.1113/jphysiol.2010.202051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry DL, Mahley RW, Weisgraber KH, Oh SY. Simultaneous accumulation of Evans blue dye and albumin in the canine aortic wall. Am J Physiol. 1977;233:H66–H79. doi: 10.1152/ajpheart.1977.233.1.H66. [DOI] [PubMed] [Google Scholar]
- Furukawa T, Mukherjee S, Bao ZZ, Morrow EM, Cepko CL. rax, Hes1, and notch1 promote the formation of Muller glia by postnatal retinal progenitor cells. Neuron. 2000;26:383–394. doi: 10.1016/s0896-6273(00)81171-x. [DOI] [PubMed] [Google Scholar]
- Gundersen GG, Bulinski JC. Distribution of tyrosinated and nontyrosinated alpha-tubulin during mitosis. J Cell Biol. 1986;102:1118–1126. doi: 10.1083/jcb.102.3.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen GG, Kalnoski MH, Bulinski JC. Distinct populations of microtubules: tyrosinated and nontyrosinated alpha tubulin are distributed differently in vivo. Cell. 1984;38:779–789. doi: 10.1016/0092-8674(84)90273-3. [DOI] [PubMed] [Google Scholar]
- Haan N, Goodman T, Najdi-Samiei A, Stratford CM, Rice R, El AE, Bellusci S, Hajihosseini MK. Fgf10-expressing tanycytes add new neurons to the appetite/energy-balance regulating centers of the postnatal and adult hypothalamus. J Neurosci. 2013;33:6170–6180. doi: 10.1523/JNEUROSCI.2437-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajos F, Basco E. The surface-contact glia. Adv Anat Embryol Cell Biol. 1984;84:1–79. doi: 10.1007/978-3-642-69623-7. [DOI] [PubMed] [Google Scholar]
- Jain R, Pan J, Driscoll JA, Wisner JW, Huang T, Gunsten SP, You Y, Brody SL. Temporal relationship between primary ad motile ciliogenesis in airway epithelial cells. Am J Respir Cell Mol Biol. 2010;43:731–739. doi: 10.1165/rcmb.2009-0328OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavado A, Oliver G. Six3 is required for ependymal cell maturation. Development. 2011;138:5291–5300. doi: 10.1242/dev.067470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DA, Bedont JL, Pak T, Wang H, Song J, Miranda-Angulo A, Takiar V, Charubhumi V, Balordi F, Takebayashi H, Aja S, Ford E, Fishell G, Blackshaw S. Tanycytes of the hypothalamic median eminence form a diet-responsive neurogenic niche. Nat Neurosci. 2012;15:700–702. doi: 10.1038/nn.3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DA, Blackshaw S. Functional implications of hypothalamic neurogenesis in the adult mammalian brain. Int J Dev Neurosci. 2012;30:615–621. doi: 10.1016/j.ijdevneu.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Tang Y, Cai D. IKKbeta/NF-kappaB disrupts adult hypothalamic neural stem cells to mediate a neurodegenerative mechanism of dietary obesity and pre-diabetes. Nat Cell Biol. 2012;14:999–1012. doi: 10.1038/ncb2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu F, Kar D, Gruenig N, Zhang ZW, Cousins N, Rodgers HM, Swindell EC, Jamrich M, Schuurmans C, Mathers PH, Kurrasch DM. Rax is a selector gene for mediobasal hypothalamic cell types. J Neurosci. 2013;33:259–272. doi: 10.1523/JNEUROSCI.0913-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcet B, Chevalier B, Luxardi G, Coraux C, Zaragosi LE, Cibois M, Robbe-Sermesant K, Jolly T, Cardinaud B, Moreilhon C, Giovannini-Chami L, Nawrocki-Raby B, Birembaut P, Waldmann R, Kodjabachian L, Barbry P. Control of vertebrate multiciliogenesis by miR-449 through direct repression of the Delta/Notch pathway. Nat Cell Biol. 2011;13:693–699. doi: 10.1038/ncb2241. [DOI] [PubMed] [Google Scholar]
- Marsili A, Sanchez E, Singru P, Harney JW, Zavacki AM, Lechan RM, Larsen PR. Thyroxine-induced expression of pyroglutamyl peptidase II and inhibition of TSH release precedes suppression of TRH mRNA and requires type 2 deiodinase. J Endocrinol. 2011;211:73–78. doi: 10.1530/JOE-11-0248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathers PH, Grinberg A, Mahon KA, Jamrich M. The Rx homeobox gene is essential for vertebrate eye development. Nature. 1997;387:603–607. doi: 10.1038/42475. [DOI] [PubMed] [Google Scholar]
- Mathew TC. Regional analysis of the ependyma of the third ventricle of rat by light and electron microscopy. Anat Histol Embryol. 2008;37:9–18. doi: 10.1111/j.1439-0264.2007.00786.x. [DOI] [PubMed] [Google Scholar]
- Millhouse OE. A Golgi study of third ventricle tanycytes in the adult rodent brain. Z Zellforsch Mikrosk Anat. 1971;121:1–13. doi: 10.1007/BF00330913. [DOI] [PubMed] [Google Scholar]
- Monroe BG, Paull WK. Ultrastructural changes in the hypothalamus during development and hypothalamic activity: the median eminence. Prog Brain Res. 1974;41:185–208. doi: 10.1016/S0079-6123(08)61907-X. [DOI] [PubMed] [Google Scholar]
- Mullier A, Bouret SG, Prevot V, Dehouck B. Differential distribution of tight junction proteins suggests a role for tanycytes in blood-hypothalamus barrier regulation in the adult mouse brain. J Comp Neurol. 2010;518:943–962. doi: 10.1002/cne.22273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao N, et al. Thyrotrophin in the pars tuberalis triggers photoperiodic response. Nature. 2008;452:317–322. doi: 10.1038/nature06738. [DOI] [PubMed] [Google Scholar]
- O’Callaghan C, Sikand K, Rutman A. Respiratory and brain ependymal ciliary function. Pediatr Res. 1999;46:704–707. doi: 10.1203/00006450-199912000-00005. [DOI] [PubMed] [Google Scholar]
- Orellana JA, Saez PJ, Cortes-Campos C, Elizondo RJ, Shoji KF, Contreras-Duarte S, Figueroa V, Velarde V, Jiang JX, Nualart F, Saez JC, Garcia MA. Glucose increases intracellular free Ca(2+) in tanycytes via ATP released through connexin 43 hemichannels. Glia. 2012;60:53–68. doi: 10.1002/glia.21246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paturle-Lafanechere L, Manier M, Trigault N, Pirollet F, Mazarguil H, Job D. Accumulation of delta 2-tubulin, a major tubulin variant that cannot be tyrosinated, in neuronal tissues and in stable microtubule assemblies. J Cell Sci. 1994;107 (Pt 6):1529–1543. doi: 10.1242/jcs.107.6.1529. [DOI] [PubMed] [Google Scholar]
- Peruzzo B, Pastor FE, Blazquez JL, Schobitz K, Pelaez B, Amat P, Rodriguez EM. A second look at the barriers of the medial basal hypothalamus. Exp Brain Res. 2000;132:10–26. doi: 10.1007/s002219900289. [DOI] [PubMed] [Google Scholar]
- Prevot V, Croix D, Bouret S, Dutoit S, Tramu G, Stefano GB, Beauvillain JC. Definitive evidence for the existence of morphological plasticity in the external zone of the median eminence during the rat estrous cycle: implication of neuro-glio-endothelial interactions in gonadotropin-releasing hormone release. Neuroscience. 1999;94:809–819. doi: 10.1016/s0306-4522(99)00383-8. [DOI] [PubMed] [Google Scholar]
- Rodriguez EM, Blazquez JL, Guerra M. The design of barriers in the hypothalamus allows the median eminence and the arcuate nucleus to enjoy private milieus: the former opens to the portal blood and the latter to the cerebrospinal fluid. Peptides. 2010;31:757–776. doi: 10.1016/j.peptides.2010.01.003. [DOI] [PubMed] [Google Scholar]
- Rodriguez EM, Blazquez JL, Pastor FE, Pelaez B, Pena P, Peruzzo B, Amat P. Hypothalamic tanycytes: a key component of brain-endocrine interaction. Int Rev Cytol. 2005;247:89–164. doi: 10.1016/S0074-7696(05)47003-5. [DOI] [PubMed] [Google Scholar]
- Rodriguez EM, Gonzalez CB, Delannoy L. Cellular organization of the lateral and postinfundibular regions of the median eminence in the rat. Cell Tissue Res. 1979;201:377–408. doi: 10.1007/BF00236998. [DOI] [PubMed] [Google Scholar]
- Rutzel H, Schiebler TH. Prenatal and early postnatal development of the glial cells in the median eminence of the rat. Cell Tissue Res. 1980;211:117–137. doi: 10.1007/BF00233728. [DOI] [PubMed] [Google Scholar]
- Sanchez E, Vargas MA, Singru PS, Pascual I, Romero F, Fekete C, Charli JL, Lechan RM. Tanycyte pyroglutamyl peptidase II contributes to regulation of the hypothalamic-pituitary-thyroid axis through glial-axonal associations in the median eminence. Endocrinology. 2009;150:2283–2291. doi: 10.1210/en.2008-1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satir P, Christensen ST. Overview of structure and function of mammalian cilia. Annu Rev Physiol. 2007;69:377–400. doi: 10.1146/annurev.physiol.69.040705.141236. [DOI] [PubMed] [Google Scholar]
- Sawamoto K, Wichterle H, Gonzalez-Perez O, Cholfin JA, Yamada M, Spassky N, Murcia NS, Garcia-Verdugo JM, Marin O, Rubenstein JL, Tessier-Lavigne M, Okano H, Alvarez-Buylla A. New neurons follow the flow of cerebrospinal fluid in the adult brain. Science. 2006;311:629–632. doi: 10.1126/science.1119133. [DOI] [PubMed] [Google Scholar]
- Seress L. Development and structure of the radial glia in the postnatal rat brain. Anat Embryol (Berl) 1980;160:213–226. doi: 10.1007/BF00301862. [DOI] [PubMed] [Google Scholar]
- Sharma HS, Johanson CE. Blood-cerebrospinal fluid barrier in hyperthermia. Prog Brain Res. 2007;162:459–478. doi: 10.1016/S0079-6123(06)62023-2. [DOI] [PubMed] [Google Scholar]
- Shimogori T, Lee DA, Miranda-Angulo A, Yang Y, Wang H, Jiang L, Yoshida AC, Kataoka A, Mashiko H, Avetisyan M, Qi L, Qian J, Blackshaw S. A genomic atlas of mouse hypothalamic development. Nat Neurosci. 2010;13:767–775. doi: 10.1038/nn.2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simerly R. The rat nervous system. San Diego: Academic Press; 1994. [Google Scholar]
- Spandidos A, Wang X, Wang H, Dragnev S, Thurber T, Seed B. A comprehensive collection of experimentally validated primers for Polymerase Chain Reaction quantitation of murine transcript abundance. BMC Genomics. 2008;9:633. doi: 10.1186/1471-2164-9-633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spandidos A, Wang X, Wang H, Seed B. PrimerBank: a resource of human and mouse PCR primer pairs for gene expression detection and quantification. Nucleic Acids Res. 2010;38:D792–D799. doi: 10.1093/nar/gkp1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternson SM. Hypothalamic survival circuits: blueprints for purposive behaviors. Neuron. 2013;77:810–824. doi: 10.1016/j.neuron.2013.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubbs JL, Vladar EK, Axelrod JD, Kintner C. Multicilin promotes centriole assembly and ciliogenesis during multiciliate cell differentiation. Nat Cell Biol. 2012;14:140–147. doi: 10.1038/ncb2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunkin SM, Ng L, Lau C, Dolbeare T, Gilbert TL, Thompson CL, Hawrylycz M, Dang C. Allen Brain Atlas: an integrated spatio-temporal portal for exploring the central nervous system. Nucleic Acids Res. 2013;41:D996–D1008. doi: 10.1093/nar/gks1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terada K, Furukawa T. Sumoylation controls retinal progenitor proliferation by repressing cell cycle exit in Xenopus laevis. Dev Biol. 2010;347:180–194. doi: 10.1016/j.ydbio.2010.08.023. [DOI] [PubMed] [Google Scholar]
- Tucker P, Laemle L, Munson A, Kanekar S, Oliver ER, Brown N, Schlecht H, Vetter M, Glaser T. The eyeless mouse mutation (ey1) removes an alternative start codon from the Rx/rax homeobox gene. Genesis. 2001;31:43–53. doi: 10.1002/gene.10003. [DOI] [PubMed] [Google Scholar]
- Voronina VA, Kozlov S, Mathers PH, Lewandoski M. Conditional alleles for activation and inactivation of the mouse Rx homeobox gene. Genesis. 2005;41:160–164. doi: 10.1002/gene.20109. [DOI] [PubMed] [Google Scholar]
- Walsh RJ, Brawer JR, Lin PL. Early postnatal development of ependyma in the third ventricle of male and female rats. Am J Anat. 1978;151:377–407. doi: 10.1002/aja.1001510305. [DOI] [PubMed] [Google Scholar]
- Xu Y, Tamamaki N, Noda T, Kimura K, Itokazu Y, Matsumoto N, Dezawa M, Ide C. Neurogenesis in the ependymal layer of the adult rat 3rd ventricle. Exp Neurol. 2005;192:251–264. doi: 10.1016/j.expneurol.2004.12.021. [DOI] [PubMed] [Google Scholar]
- Yu X, Ng CP, Habacher H, Roy S. Foxj1 transcription factors are master regulators of the motile ciliogenic program. Nat Genet. 2008;40:1445–1453. doi: 10.1038/ng.263. [DOI] [PubMed] [Google Scholar]