Abstract
Background
Colorectal cancer screening (CRCS) reduces morbidity and mortality; however, the positive benefits might be partially offset by long-term distress following positive screening results. We examined relationships among CRC-specific worry and situational anxiety after positive fecal occult blood tests [FOBT (+)] compared to receipt of negative results.
Methods
2,260 eligible members of Group Health, an integrated healthcare delivery system, completed baseline surveys and received FOBT screening kits, with 1,467 members returning the kits. We matched FOBT (+) patients (n = 55) 2:1 on age and sex with FOBT (−) respondents (n = 110). Both groups completed follow-up surveys at 7–14 days and 4-months post screening. We assessed situational anxiety (State-Trait Anxiety Inventory, STAI), CRC worry frequency, and mood disturbance.
Results
Mean age was 59 years, majority were women (62%) and white (89%). After adjusting for age, sex and baseline worry, at 7–14 days post screening, FOBT (+) group was 3.82 (95% CI: 1.09, 13.43) times more likely to report CRC-related mood disturbances and significantly higher mean STAI scores than FOBT (−) group, (mean =38.8 versus 30.9, p=0.007). At 4 months post-test, mood disturbances and situational anxiety appeared to drop to baseline levels for FOBT (+). No colon cancer worry frequency was observed.
Conclusions
FOBT (+) results are associated with short-term situational anxiety and CRC-specific mood disturbances.
Impact
Distress from FOBT (+) result declined to near baseline levels by 4 months. Additional studies are needed to clarify the relationship between long-term distress and follow-up colonoscopy.
Introduction
Colorectal cancer screening (CRCS) reduces morbidity and mortality, however little is known about the psychological consequences of receiving a positive fecal occult blood test (FOBT) screening result. Distress after a positive screen is evident for cervical, prostate and breast cancer, with anxiety lasting for short time periods including 2 weeks post-test (1–5) and longer periods including several months (2,6). For breast cancer, the deleterious effects include heightened anxiety and worry (6).
The relationship between receipt of a positive FOBT screen result and associated negative psychological consequences is less well-studied; the few available studies have reported heightened distress immediately following a positive outcome with no evidence of sustained distress beyond 4 months post-test (7–9). Only one study (8) assessed psychological status both prior to and after receiving screening, and none assessed the relationship between CRCS result and colon cancer-specific worry (7, 8). It is important to understand the implications of participating in a colon cancer screening program and the experience of adverse psychological events. The objective of the current study is to evaluate the relationship between receiving a positive FOBT screen result and patients’ experience of short and longer-term cancer-specific worry and situational anxiety.
Materials and Methods
Participants of this longitudinal study were members of Group Health, an integrated health insurance and care delivery system in Washington State. The Group Health Institutional Review Board approved all study documents and procedures.
Recruitment occurred between January and February 2011. Members were 50–74 years of age, continuously enrolled in Group Health for at least 2 years, received care at one of 21 Group Health Medical Centers in Western Washington, and due for CRC screening based on administrative and clinical data. Eligibility criteria based on CRCS were as follows: no evidence of a FOBT in the last 8 months, no flexible sigmoidoscopy in the last 4 years, and no colonoscopy in the last 9 years. We also used clinical data to exclude prospective participants based on the following factors: 1) history of CRC, inflammatory bowel disease, or total colectomy; 2) a myocardial infarction in the previous 12 months; 3) end-stage or organ failure diseases; and, 4) active treatment for cancer with chemotherapy.
We mailed an invitation letter, baseline surveys and a $2 incentive to 9,922 Group Health patients. Participants who returned baseline questionnaires and provided informed consent were further checked for eligibility based on their response to the survey. Patients who reported prior CRC and a first degree relative diagnosed with CRC at age 60 or younger were excluded, as were those who self-reported having a colonoscopy in the last 9 years. Prospective respondents who were eligible after the baseline surveys were randomly assigned to receive one of three types of mailed FOBT kits (1-sample OC-Auto® fecal immunochemical test (FIT); 2-sample InSure® FIT; 3-sample guaiac Hemoccult SENSA®) in addition to instructions and postage paid return envelope.
Participants returning the FOBT kits and with FOBT positive results were matched by age and sex to two FOBT negative participants and mailed a second wave of questionnaires within two business days of the FOBT screen result (7–14 days post-test), (Figure 1). Non-responders received telephone reminders to complete questionnaires and we excluded individuals who completed questionnaires more than 21 days post FOBT screen result because of our interest in measuring short-term distress related to screening. GHC provides patients with FOBT positive outcome, a copy of their lab results with a mailed standard letter that explains the result. Patients are also asked to contact their physician’s office by phone if they have not already been contacted.
Figure 1.
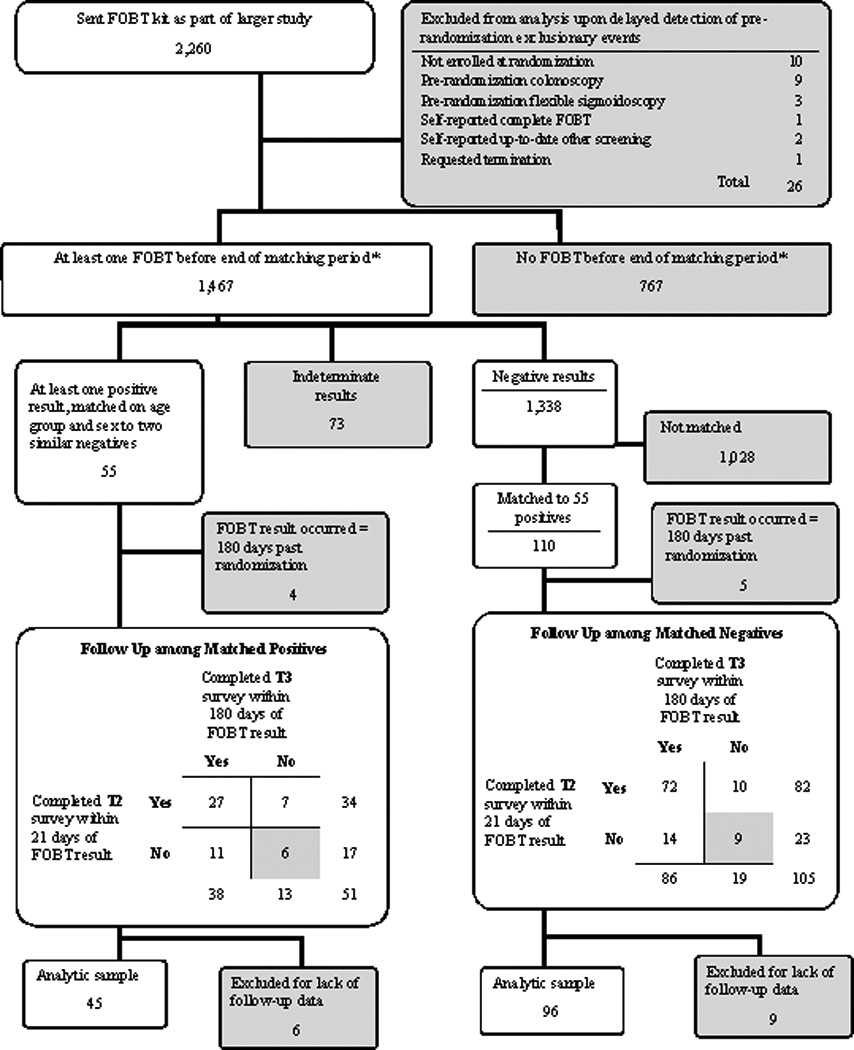
Recruitment of study participants for psychological distress and FOBT study
We mailed out the third wave of questionnaires (T3) to the FOBT positive patients and their matched controls at 4 months after receipt of the screening test result. All participants received the T3 follow-up surveys even if they did not return T2 follow-up surveys. Similar to T2, we prompted T3 survey non-responders by providing telephone reminders and allowed two months for returning the surveys before excluding the participant. Participants received $20.00 after completion of the T2 and T3 surveys.
Participants who were excluded due to length of time past randomization (N=9) or lack of completion of the follow-up surveys (N=15) did not differ significantly on any baseline characteristics compared to those who remained in the analyses
Measures
Demographic characteristics and relevant background information (from baseline surveys) included: health history and previous CRC diagnosis; CRC screening behaviors and future intention to be screened; CRC knowledge, confidence about screening and perceived barriers to screening; and CRC worry frequency and situational anxiety.
Colon cancer-specific worry – we assessed CRC-specific worry at baseline (T1), 7–14 days post FOBT screen result (T2), and 4 months post FOBT screen result (T3) by asking two questions. The first question queried about worry frequency – “how often do you worry about getting colon cancer?” and the item was rated on a 5-point Likert scale ranging from “never”= 1 to “all the time” = 5. We designated respondents as “no worry” by combining the following results (never = 1 and rarely = 2) and “yes, at least some worry” by combining the following results (sometimes = 3, most of the time = 4, and all of the time = 5). Similar studies have used this single-item scale to assess worry frequency related to a cancer diagnosis (14).
The second question assessed the effect of recent CRC worry on mood – “during the past two weeks, how often have thoughts about getting colorectal cancer affected your mood?” The question was rated on a 5-point Likert scale from “never” = 1, to “all the time” = 5. We designated respondents as “no impact on mood” by combining the following results (not at all = 1 and rarely = 2) and “yes, at least some impact on mood” by combining the following results (sometimes = 3, most of the time = 4, and all of the time = 5).
Situational anxiety – we assessed situational anxiety at all three time points using the 40-item State component of the State-Trait Anxiety Inventory (STAI). This measure captures symptoms of anxiety that the respondent experienced at a single moment in time, and is rated on a 4-point scale with score range of 20–80, with higher scores indicating more anxiety (15).
Statistical Analyses
We calculated frequency distributions for categorical variables (frequency of cancer worry and effect of recent cancer worry on mood) for which binary categories of “yes” and “no” were created, and then calculated means and standard deviations for continuous variables (State component of STAI). Chi-squared analyses were employed to assess differences among categorical variables and t-tests implemented to compare age in years, allowing unequal variances.
We analyzed binary outcomes at baseline with log-linear regression models that accommodated clustering within matched positives and negatives. Continuous variables at baseline were modeled with linear regression models which similarly accommodated clustering within matched sets. Though FOBT positive and negative subjects were matched on age and sex, we found that drop-out during follow-up was not uniform across the matched sets, rendering adjustment only through matching inadequate to control for potential confounding. Therefore, all regression models were adjusted for age in years and sex. To evaluate differences at T2 and T3, we fit longitudinal mixed effects regression models that controlled for the baseline value of the outcome and modeled random intercepts for each subject to accommodate correlations among survey responses by the individual.
Results
Recruitment
Figure 1 shows the study recruitment process. We distributed FOBT kits and background questionnaires to 2,260 eligible respondents and 1,467 FOBT kits were returned – a 64.9% participation rate. We matched 55 FOBT (+) respondents with 110 FOBT (−) respondents. We excluded 4 FOBT (+) and 5 FOBT (−) individuals because their screen test results occurred later than 180 days past randomization. We further excluded 6 FOBT (+) and 9 FOBT (−) subjects who did not complete at least one of the two follow up surveys. Our final sample consisted of 45 FOBT (+) and 96 FOBT (−) respondents.
Study Sample Characteristics
Table 1 presents the demographic and background characteristics of the study population. The mean age of study participants was 59 years. The majority were women (62%), white (89%), and married or living with a partner (69%). FOBT (−) patients were more likely to be white (92% vs. 84%), slightly more likely than FOBT (+) to have a bachelor’s degree or higher (47% vs. 36%) and to be employed full or part time (69% vs. 56%), but none of these differences were significant.
Table 1.
Demographic and background characteristics of study sample at Baseline
| Demographic and background characteristics |
FOBT(+) N = 45 % |
FOBT(−) N=96 % |
P-value |
|---|---|---|---|
| Age | |||
| 50–64 | 87 | 81 | |
| 65+ | 13 | 19 | 0.43 |
| Mean age | 59.8 | 58.6 | |
| Gender | |||
| Female | 67 | 60 | |
| Male | 33 | 40 | 0.48 |
| Race | |||
| White | 84 | 92 | |
| African-American/Black | 7 | 0 | |
| Asian Pacific Islander | 2 | 6 | |
| American Indian/Native American | 0 | 1 | |
| Multiple races endorsed | 5 | 0 | |
| Other | 2 | 1 | 0.04 |
| Marital Status | |||
| Married or living with partner | 67 | 69 | |
| Not married or living with a partner | 33 | 31 | 0.83 |
| Education | |||
| High school grad/GED | 18 | 16 | |
| Some college, no degree | 47 | 37 | |
| Bachelor’s degree or higher | 36 | 47 | 0.44 |
| Employment Status | |||
| Full or part-time | 56 | 69 | |
| Retired | 33 | 22 | |
| Other | 11 | 9 | 0.29 |
| Family history of CRC | |||
| Yes | 9 | 2 | |
| No | 78 | 95 | |
| Don’t know | 13 | 3 | 0.13 |
P-values are from chi-square tests of homogeneity for categorical variables, and from t-test for age in years, accommodating unequal variances.
Note: Participants who did not respond are as follows:
Race: 2 FOBT(+)and 8 FOBT(−) participants
Marital Status: 2 FOBT(+) and 8 FOBT (−) participants
Education: 2 FOBT(−) participants
Employment Status: 2 FOBT (−) participants
Family History: 1 FOBT (−) group participant.
Psychological Characteristics for FOBT Screening Test
Table 2 presents results for colon cancer worry, mood disturbance and situational anxiety for FOBT (+) and FOBT (−) group at baseline, 7–14 days and 4 months post FOBT screen result.
Table 2.
Results for psychological outcomes for FOBT (+) and FOBT (−) groups over time
| Baseline | 7 – 14 days post FOBT | 4 Months post FOBT | ||||||
|---|---|---|---|---|---|---|---|---|
| Binary Outcomes | FOBT(+) N= 45 % |
FOBT(−) N= 96 % |
FOBT(+) N= 34 % |
FOBT(−) N= 82 % |
Adjusted RR (95% CI) |
FOBT(+) N= 38 % |
FOBT(−) N= 86 % |
Adjusted RR (95% CI) |
| Colon Cancer Worry Frequency | 17.8 | 14.6 | 35.3 | 15.9 | 2.03 (0.92, 4.50) | 29.0 | 18.6 | 1.76 (0.81, 3.83) |
| Colon Cancer Mood disturbance | 4.4 | 4.2 | 20.6 | 4.9 | 3.82 (1.09, 13.43)* | 5.7 | 2.4 | 5.07 (0.96, 26.75) |
| Continuous outcomes | FOBT(+) Mean | FOBT(−) Mean | FOBT(+) Mean | FOBT(−) Mean | Adjusted change difference since baselinea(95% CI) | FOBT(+) Mean | FOBT(−) Mean | Adjusted change difference since baselinea(95% CI) |
| Situational Anxiety (STAI) | 35.6 | 32.5 | 38.8 | 30.9 | 4.59 (1.23, 7.95)* | 34.6 | 30.6 | 1.71 (−1.56, 4.98) |
To evaluate differences at T2 and T3, longitudinal mixed effects regression models were used that controlled for baseline outcome values. All regression models were adjusted for age in years and gender
For continuous outcomes, differences shown are age and gender adjusted estimates of the change in FOBT(+) group minus the change in the FOBT(−) group
Indicates significant differences.
RR = Relative Risk
Colon Cancer-Specific Worry: Worry Frequency (1-Item Worry Frequency Measure)
After adjusting for baseline status, age, and sex, the FOBT (+) group was 2.03 (95% CI: 0.92, 4.50) times more likely than FOBT (−) group to report frequent colon cancer worry at 7–14 days post FOBT result and 1.76 (95% CI: 0.81, 3.83) times more likely than FOBT (−) to report frequent colon cancer worry at 4 months; both results were not significant.
Colon Cancer-Specific Worry: Mood Disturbance (1-Item Recent CRC Worry Measure)
The FOBT (+) group was 3.82 (95% CI: 1.09, 13.43) times more likely than the FOBT (−) group to report mood disturbance associated with recent CRC worry at 7–14 days post-test. For the FOBT (+) group, mood disturbance increased almost five-fold from baseline to 7–14 days post (4.4% to 20.6%) then decreased to near baseline levels by 4 months post. The disparity in mood disturbance between FOBT (+) and FOBT (−) was greater at 4 months post than at baseline (baseline =4.4 versus 4.2, and 4 months post = 5.7 versus 2.4 respectively); the result due to lowered mood disturbance among FOBT (−) at 4 months post compared to baseline.
Situational Anxiety (STAI)
After adjusting for baseline status, age and sex, the FOBT (+) group reported significantly higher situational anxiety compared to FOBT (−) group at T2 (mean STAI score = 38.8 vs. 30.9 respectively), but not at 4 months. Although FOBT (+) persons reported higher situational anxiety, the mean level of experienced anxiety was not clinically meaningful (STAI >= 54 for clinical status) at any of the three time points.
Follow-up Colonoscopy
A total of 41 of the 45 (91.1%) FOBT (+) patients received follow-up colonoscopy within 12 months of the receipt of their positive result. Of these, 34 returned T3 surveys. Among the 34 who returned T3 surveys, 30 patients received a follow-up colonoscopy before returning T3 surveys, and 4 received a follow-up colonoscopy after returning T3 surveys. The mean STAI score for patients with a follow-up colonoscopy before completing T3 surveys was 32.93 (sd = 11.16) and mean STAI score for patients with a follow-up colonoscopy after completing T3 surveys was 41.75 (sd = 24.66). Two (6.6%) of the 30 FOBT (+) patients who received colonoscopy before returning T3 surveys reported recent cancer worry and 1 (25%) of the 4 FOBT (+) patients who received colonoscopy after returning T3 surveys reported recent cancer worry. All 4 patients who did not complete follow-up colonoscopies returned T3 surveys. The data suggest elevated colon cancer-specific worry and situational anxiety among the FOBT (+) patients who did not have a colonoscopy before completing T3 surveys versus FOBT (+) patients who already had the colonoscopy at the time of T3 surveys. However, due to insufficient sample size, we did not conduct formal statistical testing of the differences observed.
Discussion
In this study, patients receiving a positive fecal occult blood test result [FOBT (+)] experienced significantly elevated short-term mood disturbances and situational anxiety compared to patients who received a negative screen result. By four months post-test, situational anxiety decreased towards baseline levels. Although cancer-specific mood disturbance also subsided to baseline levels among FOBT (+) patients, the differences appeared larger between FOBT (+) and FOBT (−) at 4 months, and this is attributed to a lowering of mood disturbance among FOBT (−) respondents.
The findings demonstrating a short-term negative psychological impact of receiving a positive FOBT result are consistent with several other studies showing short-term psychological distress associated with positive screen test outcome (7, 8). In comparison to these analyses, we included measures of colon cancer-specific distress thus providing a targeted assessment. The targeted approach may have enabled us to detect the substantial short-term impact of a positive screen test outcome, as evidenced by FOBT (+) patients reporting more elevated colon cancer-related mood disturbance relative to situational anxiety at 7–14 days post screen result.
Although short-term mood disturbances and situational anxiety were pronounced at 7 days post-test, the association disappeared as emotional responses declined towards baseline values by four months post-test. Although the numbers were too small to infer statistical significance, it is potentially noteworthy that 30 out of 34 FOBT (+) patients who had a follow-up colonoscopy before completing T3 on average reported lower colon cancer worry and situational anxiety compared to respondents completing the colonoscopy after the survey. Further study of the psychological consequences of receiving a positive FOBT screen result without follow-up assessment is warranted.
Factors shown to reduce the experience of psychological distress in stressful situations (e.g., receipt of a positive screen result) are patients’ perception of risk and subsequent appraisal of the potentially stressful event. Risk appraisal research has shown that an event that one perceives as personally impactful (stressful) will lead to elevated situational anxiety (16, 17); however, perceived control over the stressful event contributes to the development and implementation of favorable coping strategies that might minimize the distress (18, 19, 20, 21, 22). The above might be relevant to the subset of FOBT (+) patients.
Study Limitations
The limitations of the study include the small numbers of positive FOBT results, and missing psychological status data for 7 patients out of the 41 who completed follow-up colonoscopies. In spite of these limitations we compared longitudinally the psychological status of FOBT (+) and FOBT (−) respondents, and obtained important preliminary data of the psychological status of patients pre and post follow-up colonoscopy.
Summary
Our study is possibly the first in the US to assess colon cancer-specific psychological harms associated with receiving a positive FOBT screening result. The targeted assessment and repeated measures enabled the detection of a robust short-term negative psychological impact.
The observed colon cancer-specific mood disturbance and situational anxiety appeared to decline by 4 months post screen for FOBT (+) patients, however we cannot rule out persistent psychological distress among those who do not receive follow up colonoscopy. Additional studies with larger sample sizes might provide clarification on the long-term implications of receiving a positive fecal occult blood test result.
Acknowledgments
Funding disclosure:
This research study was supported by the National Cancer Institute, National Institutes of Health (an American Recovery and Reinvestment Act supplemental award to the Systems of Support to Increase Colorectal Cancer Screening and Follow-up Trial – R01CA121125-03S1) grant award. The content is the sole responsibility of the authors and does not represent the official views of the NIH.
Footnotes
Conflict of Interest
The authors have no relationships/conditions/circumstances that present potential conflict of interest.
References
- 1.Hellsten C, Sjostrom K, Lindqvist P. A 2-year follow-up study of anxiety and depression in women referred for colposcopy after an abnormal cervical smear. BJOG. 2008;115:212–218. doi: 10.1111/j.1471-0528.2007.01609.x. [DOI] [PubMed] [Google Scholar]
- 2.Katz DA, Jarrard DF, McHorney C, Hillis SL, Wiebe DA, Fryback DG. Health perceptions in patients who undergo screening and workup for prostate cancer. Urology. 2007;69(2):215–220. doi: 10.1016/j.urology.2006.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gray NM, Sharp L, Cotton SC, Masson LF, Little J, Walker LG, et al. Psychological effects of a low-grade abnormal cervical smear test result: anxiety and associated factors. Br J Cancer. 2006;94(9):1253–1262. doi: 10.1038/sj.bjc.6603086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McNaughton-Collins M, Fowler FJ, Jr, Caubet JF, Bates DW, Lee JM, Hauser A, et al. Psychological effects of a suspicious prostate cancer screening test followed by a benign biopsy result. Am J Med. 2004;117:719–725. doi: 10.1016/j.amjmed.2004.06.036. [DOI] [PubMed] [Google Scholar]
- 5.Lampic C, Thurfjell E, Bergh J, Sjödén PO. Short-and long-term anxiety and depression in women recalled after breast cancer screening. Eur J Cancer. 2001;37(4):463–469. doi: 10.1016/s0959-8049(00)00426-3. [DOI] [PubMed] [Google Scholar]
- 6.Brewer NT, Salz T, Lillie SE. Systematic review: The long-term effects of false positive mammograms. Ann Intern Med. 2007;146(7):502–510. doi: 10.7326/0003-4819-146-7-200704030-00006. [DOI] [PubMed] [Google Scholar]
- 7.Brasso K, Ladelund S, Frederiksen BL, Jørgensen T. Psychological distress following fecal occult blood test in colorectal cancer screening – a population-based study. Scand J Gastroenterol. 2010;45(10):1211–1216. doi: 10.3109/00365521.2010.485355. [DOI] [PubMed] [Google Scholar]
- 8.Parker MA, Robinson MH, Scholefield JH, Hardcastle JD. Psychiatric morbidity and screening for colorectal cancer. J Med Screen. 2002;9(1):7–10. doi: 10.1136/jms.9.1.7. [DOI] [PubMed] [Google Scholar]
- 9.Mant D, Fitzpatrick R, Hogg A, Fuller A, Farmer A, Verne J, et al. Experiences of patients with false positive results from colorectal cancer screening. Br J Gen Pract. 1990;40(339):423–425. [PMC free article] [PubMed] [Google Scholar]
- 10.Aro AR, Pilvikki Absetz S, van Elderen TM, van der Ploeg E, van der Kamp LJ. False-positive findings in mammography screening induces short-term distress – breast cancer-specific concern prevails longer. Eur J Cancer. 2000;36(9):1089–1097. doi: 10.1016/s0959-8049(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 11.Lipkus IM, Klein WM, Rimer BK. Communicating breast cancer risks to women using different formats. Cancer Epidemiol Biomarkers Prev. 2001;10:895–898. [PubMed] [Google Scholar]
- 12.Zheng YF, Saito T, Takahashi M, Ishibashi T, Kai I. Factors associated with intentions to adhere to colorectal cancer screening follow-up exams. BMC Public Health. 2006;6:272. doi: 10.1186/1471-2458-6-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O'Donnell S, Goldstein B, Dimatteo MR, Fox SA, John CR, Obrzut JE. Adherence to mammography and colorectal cancer screening in women 50–80 years of age: The role of psychological distress. Womens Health Issues. 2010;20(5):343–349. doi: 10.1016/j.whi.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 14.Trask PC, Paterson AG, Wang C, Hayasaka S, Milliron KJ, Blumberg LR, et al. Cancer-specific worry interference in women attending a breast and ovarian cancer risk evaluation program: impact on emotional distress and health functioning. Psychooncology. 2001;10(5):349–360. doi: 10.1002/pon.510. [DOI] [PubMed] [Google Scholar]
- 15.Spielberger CD, Gorsuch RL, Lushene RE. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1970. [Google Scholar]
- 16.Lazarus RS, Folkman S. Stress, Appraisal and Coping. New York: Springer and Publishing Company; 1984. [Google Scholar]
- 17.Lynch BM, Steginga SK, Hawkes AL, Pakenham KI, Dunn J. Describing and predicting psychological distress after colorectal cancer. Cancer. 2008;112(6):1363–1370. doi: 10.1002/cncr.23300. [DOI] [PubMed] [Google Scholar]
- 18.Taylor SE, Kemeny ME, Aspinwall LG, Schneider SG, Rodriguez R, Herbert M. Optimism, coping, psychological distress, and high-risk sexual behavior among men at risk for acquired immunodeficiency syndrome – AIDS. J Pers Soc Psychol. 1992;63:460–473. doi: 10.1037//0022-3514.63.3.460. [DOI] [PubMed] [Google Scholar]
- 19.Suls J, Fletcher B. The relative efficacy of avoidant and nonavoidance coping strategies: meta-analysis. Health Psychology. 1985;4:249–288. doi: 10.1037//0278-6133.4.3.249. [DOI] [PubMed] [Google Scholar]
- 20.Carver CS, Pozo C, Harris SD, Noriega V, Scheier MF, Robinson DS, et al. How coping mediates the effect of optimism on distress: a study of women with early stage breast cancer. J Pers Soc Psychol. 1993;65(2):375–390. doi: 10.1037//0022-3514.65.2.375. [DOI] [PubMed] [Google Scholar]
- 21.Dracup K, Westlake C, Erickson VS, Moser DK, Caldwell ML, Hamilton MA. Perceived control reduces emotional stress in patients with heart failure. J Heart Lung Transplant. 2003;22(1):90–93. doi: 10.1016/s1053-2498(02)00454-0. [DOI] [PubMed] [Google Scholar]
- 22.Cohen M. First-degree relatives of breast-cancer patients: cognitive perceptions, coping, and adherence to breast self-examination. Behav Med. 2002;28(1):15–22. doi: 10.1080/08964280209596394. [DOI] [PubMed] [Google Scholar]


