Abstract
Critics have suggested that neoadjuvant chemotherapy (NACT) followed by interval debulking may select for resistant clones or cancer stem cells when compared to primary cytoreduction. β-tubulins are chemotherapeutic targets of taxanes and epothilones. Class III β-tubulin overexpression has been linked to chemoresistance and hypoxia. Herein, we describe changes in class III β-tubulin in patients with advanced ovarian carcinoma in response to NACT, in relationship to clinical outcome, and between patients who underwent NACT versus primary debulking; we characterize in vitro chemosensitivity to paclitaxel/patupilone of cell lines established from this patient population, and class III β-tubulin expression following repeated exposure to paclitaxel. Using immunohistochemistry, we observed among 22 paired specimens obtained before/after NACT decreased expression of class III β-tubulin following therapy within stroma (p=0.07), but not tumor (p=0.63). Poor median overall survival was predicted by high levels of class III β-tubulin in both tumor (HR 3.66 [1.11,12.05], p=0.03) and stroma (HR 4.53 [1.28,16.1], p=0.02). Class III β-tubulin expression by quantitative-real-time-polymerase-chain-reaction was higher among patients who received NACT (n=12) compared to primary cytoreduction (n=14) (mean±SD fold-change: 491.2±115.9 vs 224.1±55.66, p=0.037). In vitro subculture with paclitaxel resulted in class III β-tubulin upregulation, however, cell lines that overexpressed class III β-tubulin remained sensitive to patupilone. Overexpression of class III β-tubulin in patients dispositioned to NACT may thus identify an intrinsically aggressive phenotype, and predict poor overall survival and paclitaxel resistance. Decreases in stromal expression may represent normalization of the tumor microenvironment following therapy. Epothilones warrant study for patients who have received neoadjuvant carboplatin and paclitaxel.
Keywords: neoadjuvant chemotherapy, ovarian cancer, epothilone, tubulin, paclitaxel resistance
Introduction
In patients with stage IIIC/IV ovarian carcinoma, neoadjuvant chemotherapy (NACT) followed by interval debulking results in equivalent hazard ratios [1] for death and progressive disease, often with less surgical morbidity and greater likelihood of optimal cytoreduction [2][3][4] compared to primary debulking followed by chemotherapy. Nevertheless, critics have expressed concern that NACT followed by interval debulking may select for resistant clones or cancer stem cells when compared to cytoreduction followed by chemotherapy [5][6]. Choice of subsequent adjuvant regimen may become problematic in instances of poor response to the standard regimen of neoadjuvant carboplatin and paclitaxel, even if optimal debulking is achieved. There are currently few biomarkers identified to guide additional therapy.
Class III β-tubulin overexpression has been linked to hypoxia [7] and chemoresistance through reduced binding of paclitaxel [8] and modulation of prosurvival pathways [9]. Class III β-tubulin is one of 9 β-isoforms [10] capable of heterodimerizing with α subunits to form microtubules critical to cell division. Paclitaxel binds preferentially to class I β-tubulin isoforms [8], which differ from class III β-tublin at paclitaxel binding site positions 275 (Ser → Ala) and 364–365 (Ala-Val → Ser-Ser) [10]. High class III β-tubulin expression correlates with poorer overall survival in non-small cell lung [11], breast [12], colon [13] and unknown primary cancers [14]. Epothilones (EPOxide THIazoLe ketONEs; e.g., ixabepilone, patupilone, sagopilone) are microtubule-stabilizing macrolides isolated from Sorangium cellulosum [15] with activity in paclitaxel-resistant malignancies putatively due to their unique ability to bind class III and I isoforms with at least equal affinity [8].
In this study, we
describe changes of class III β-tubulin expression in response to NACT, in relationship to oncologic outcome, and comparatively between patients who underwent NACT with interval debulking versus primary debulking followed by chemotherapy
examine in vitro chemoresponsiveness to paclitaxel and patupilone of ovarian serous carcinoma cell lines in relationship to class III β-tubulin expression
describe changes in class III β-tubulin expression in serous cell lines after prolonged in vitro exposure to paclitaxel.
Materials and methods
Tissue procurement, establishment of cell lines, and characterization of cellular growth rate
As approved by the institutional review board at Yale University, paraffin-embedded tissues and patient characteristics were obtained at time of biopsy and debulking. For immunohistochemistry analyses, all cases representing stage IIIC/IV disease with sufficient matched solid tissue as identified from an institutional cancer registry from 2003–2012 were included. Patients with non-solid tissue specimens at time of initial diagnosis (i.e., ascites or pleural fluid) were excluded. Disposition to NACT was at the surgeon’s discretion given imaging and/or physical exam findings suggestive of low likelihood of optimal debulking and/or desire to avoid significant surgical morbidity required to achieve optimal cytoreduction given disease burden, as opposed to medical comorbidity rendering the patient a non-surgical candidate. Tumors were staged according to criteria established by the American Joint Committee on Cancer 7th edition and the most recent FIGO (Fédération Internationale de Gynécologie et d’Obstétrique) staging schema [16]. Fresh-frozen samples included in the PCR analyses were chosen at random from a laboratory database. Carcinoma and normal human ovarian surface epithelial cell lines were established from tissue biopsies and maintained as described previously [17][18]. Cellular growth rate was determined per established protocol [19]. Survival data were obtained through review of electronic medical and/or public records.
RNA extraction, purification, and reverse-transcription
Total RNA extraction from cell culture lysates and macrodissected fresh-frozen tissues was performed using AllPrep DNA/RNA/Protein Minikit (Qiagen, Germantown, MD). Only samples containing at least 70% tumor epithelial cells as assessed by a staff pathologist were used for total RNA extraction. Quantitative real time polymerase chain reaction (qRT-PCR) was performed with a 7500 RealTime PCR System per the manufacturer’s protocol (Applied Biosystems, Foster City, CA). All RNA samples were treated with Turbo DNase enzyme (Applied Biosystems). Total RNA (5μg) was reverse-transcribed using Superscript III (Invitrogen, Carlsbad, CA). Five microliters of reverse-transcribed RNA was amplified using Taqman PCR Master Mix (Applied Biosystems). Class III β-tubulin (Hs00964962_g1) specific primers were obtained (Applied Biosystems), with GAPDH (Hs99999905_m1) as an internal control. Gene expression was analyzed using the comparative threshold method and normalized to levels observed in a lymphoid cell line with minimal expression of class III β-tubulin (ΔCT = 14.8) obtained from a healthy donor. Use of this particular calibrator allowed simultaneous relative characterization of class III β-tubulin among carcinomas and normal tissues.
Immunohistochemistry
All slides were reviewed by an unblinded gynecologic pathologist (NB). Representative formalin-fixed sections were cut at 4 m for each patient. Slides were stained with anti-class III β-tubulin monoclonal antibody (TUJ1; Covance, Berkeley, CA) at 1:500 dilution with the appropriate positive and negative controls. This commercially available antibody has also been validated for immunohistochemistry by other researchers [20], and against PCR by our group [21,22]. Staining of tumor and stroma were assessed using the following scoring system: 0 (<5%), 1+ (5–30%), 2+ (31–70%), 3+ (>70%) [23]. Sections were chosen so that consistent and sufficient proportions of tumor and stroma were examined in pre- and post-NACT comparisons.
Chemosensitivity assays and paclitaxel subcultures
Patupilone (Novartis Pharma, Basel, Switzerland) and paclitaxel (T7402: Sigma-Aldrich, St. Louis, MO) were dissolved in dimethyl sulfoxide (Sigma-Aldrich, St. Louis, MO) as stock solutions protected from light exposure and stored at −20°C. Drugs were serially diluted to achieve concentrations of 1 nm to 1 μM in culture medium before exposure to cells. Cells at log phase of growth were seeded in 6-well microtiter plates at optimum density and exposed to drug at 24 hours. After 48–72 hours of additional incubation, well contents were harvested in entirety, centrifuged then stained with propidium iodide (2 μL of a 500 μg/mL stock solution in PBS with 0.1% sodium azide and 2% fetal bovine serum) for flow cytometric counts. To study the effects of repeated exposure to paclitaxel on class III β-tubulin expression, established (i.e., > 30 passages at time of initial drug exposure) ovarian (OSPC) and uterine (USPC) serous carcinoma cell lines containing >99% epithelial cells by microscopic inspection were exposed to increasing concentrations of paclitaxel at time of each passage. Surviving clones were subcultured in RPMI and allowed to grow to 80–90% confluence prior to next passage and drug exposure. At time of each passage, approximately 5–10% of the total detached cell volume was re-plated.
Statistical analyses
Dose response curves were analyzed using GraphPad Prism version 5.0 (GraphPad Software, Inc., LaJolla, CA). IC50 was defined as the absolute inhibitory concentration of drug required to result in reduction of cell viability to 50% of untreated control. IC50 was determined through interpolation of sigmoidal curves fit with a standard Hill slope of negative 1.0. Kaplan-Meier curves were generated and analyzed with the log-rank Mantel-Cox test with a consistent hazard ratio. Overall survival was calculated from the interval between date of diagnosis to date of last follow-up or death. All student’s t-tests and Pearson correlations employed 2-tails and assumed equal variance. Non-parametric Wilcoxon matched pairs analyses were applied to examine the distributions of immunohistochemistry scores in which ties were eliminated from the analysis. For all analyses, p values < 0.05 were considered statistically significant.
Results
Neoadjuvant chemotherapy administration is associated with a trend towards decreased stromal expression of class III β-tubulin
A total of 22 patients who had received neoadjuvant chemotherapy for clinical stage IIIC/IV ovarian carcinoma from 2003–2012 were identified retrospectively. Diagnosis was made through core biopsy in all patients except four who instead had undergone exploratory laparotomy with aborted debulking. Patient and treatment characteristics are provided in Table 1. None of the patients received bevacizumab as part of their adjuvant consolidation regimen; of patients who did not receive adjuvant consolidation with carboplatin/paclitaxel (i.e., ‘other’), regimens consisted of either pegylated liposomal doxorubicin or docetaxel in combination with carboplatin. Strength of immunohistochemistry staining for class III β-tubulin within tumor and the surrounding stroma was quantified among matched specimens representing biopsies obtained prior to NACT administration and those obtained following NACT at the time of interval debulking. Class III β-tubulin decreased within stroma with a trend towards statistical significance (p=0.07), but was unchanged in tumor (p=0.63) following treatment (Figure 1). The Spearman correlation for this set was 0.68 (p = 0.002), indicative of effective pairing. Normal ovary did not overexpress class III β-tubulin. Representative immunohistochemistry specimens are shown in Figure 2. Staining was homogeneous and cytoplasmic. We have previously shown good correlation between strength of immunohistochemistry staining and PCR [21–22].
Table 1.
Demographic and treatment characteristics for patients included in survival analyses
| MEAN ± SD | % | n | |
|---|---|---|---|
| AGE, years | 65.0 ± 12.6 | ||
| NACT CYCLES OF CARBOPLATIN/PACLITAXEL | 5.6 ± 0.9 | ||
| DEBULKING STATUS | |||
| no residual disease | 68.2 | 15 | |
| ≤ 5 mm residual or miliary disease | 22.7 | 5 | |
| 6–10 mm residual disease | 4.5 | 1 | |
| ≥ 2 cm residual disease | 4.5 | 1 | |
| HISTOLOGY | |||
| serous, high-grade | 72.7 | 16 | |
| clear cell | 0 | 0 | |
| endometrioid | 4.5 | 1 | |
| mixed | 4.5 | 1 | |
| undifferentiated | 18.2 | 4 | |
| ADJUVANT CONSOLIDATION REGIMEN | |||
| carboplatin/paclitaxel | 63.6 | 14 | |
| other | 22.7 | 5 | |
| treatment at outside facility/unknown | 13.6 | 3 | |
Fig 1.

Following treatment with NACT, a trend towards decreased strength of stromal (p = 0.07) but not tumoral staining (p = 0.76) was observed. Horizontal bars represent mean values
Fig 2.

Following NACT, class III β-tubulin staining by immunohistochemistry was unchanged in tumor, but decreased in surrounding stroma. Representative slides are shown at original magnification of 100–200x and illustrate 3+ stromal staining before NACT (left) and 1+ stromal staining after NACT (middle); corresponding scores for tumor staining in this specimen are 3+ and 2+, respectively. Normal ovary does not stain for class III β-tubulin (right)
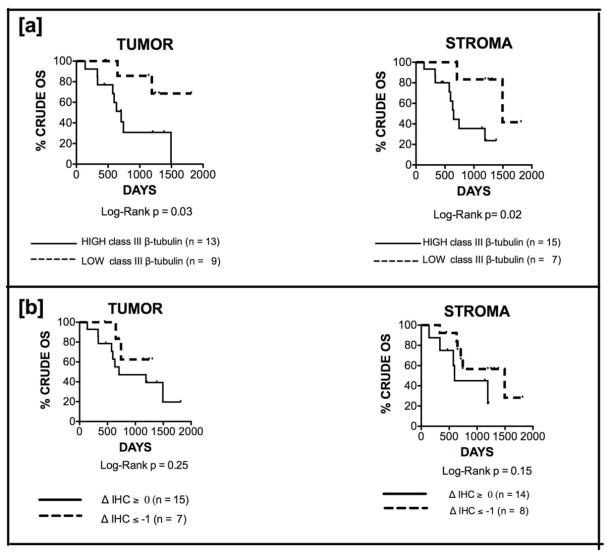
Class III β-tubulin expression at time of interval debulking predicts crude overall survival
We next examined the prognostic significance of class III β-tubulin score at time of interval debulking. We have previously shown in uterine and ovarian serous carcinomas that class III β-tubulin prior to chemotherapy predicts overall survival [21–22]. In this manuscript, reduced median crude overall survival (OS) was predicted by increased class III β-tubulin staining by both tumor (high [2+,3+] vs low [0,1+]: 707 days vs not reached, HR 3.66 [1.11,12.1], p=0.03) (Figure 3a-left) and stroma (high [1+,2+,3+] vs low [0]: 649 vs 1494 days, HR 4.53 [1.28,16.1], p=0.02) (Figure 3a-right) at time of interval debulking. The ‘high’ and ‘low’ expression cutoff were defined arbitrarily. Interestingly, a decrease in class III β-tubulin expression following NACT in both tumor (ΔIHC score ≤ −1 vs ≥ 0: not reached vs 707 days, HR 2.116 [0.58, 7.69], p = 0.25) (Figure 3b-left) and stroma (ΔIHC score ≤ −1 vs ≥ 0: 1494 vs 596 days, HR 2.73 [0.693, 10.72], p= 0.15) (Figure 3b-right) appeared to confer some survival benefit, though this failed to reach statistical significance. Cytoreduction to no residual disease was achieved in 85% versus 50% of patients with a decrease in stromal staining compared to those with no change or an increase in class III β-tubulin (p = 0.09), respectively. Median follow-up interval within the cohort was 649 days (range: 57-1668). There were a total of 11 deaths during this time.
Fig 3.
a Class III β-tubulin staining by both tumor (left) and stroma (right) predicts crude overall survival (OS). Median OS among patients with high [2+,3+] versus low [0,1+] expression by tumor was 707 days versus not reached (HR 3.66 [1.11,12.1], p=0.03). Similarly, median OS among patients with high [1+,2+,3+] versus low [0] expression by stroma was 649 versus 1494 days (HR 4.53 [1.28,16.1], p=0.02). b Decreased expression of class III β-tubulin expression following NACT in both tumor (ΔIHC score ≤ 1 vs ≥ 0: not reached vs 707 days, HR 2.116 [0.58, 7.69], p = 0.25) (left) and stroma (ΔIHC score ≤ −1 vs ≥ 0: 1494 vs 596 days, HR 2.73 [0.693, 10.72], p= 0.15) (right) appeared to confer survival benefit, though statistical significance was not reached
Patients who are dispositioned to neoadjuvant chemotherapy exhibit higher levels of class III β-tubulin reflective of an inherently aggressive tumor phenotype
Using a separate cohort of patients identified retrospectively and restricted to a laboratory database of those with stage IIIC/IV high-grade serous ovarian carcinoma for whom fresh-frozen tissue was available from time of debulking, we then compared class III β-tubulin expression using qRT-PCR among those who received carboplatin and paclitaxel in neoadjuvant fashion against those who underwent primary debulking followed by chemotherapy. Sample size was limited by the number of patients who received NACT; there were otherwise no pre-determined selection criteria and the comparison group consisting of patients who underwent primary debulking were essentially chosen at random. These groups did not differ by mean age (primary debulking versus NACT: 58.1 ± 12.5 versus 61.3 ± 11.8, respectively; p = 0.5); all patients exhibited high-grade serous histology, and all except one were Caucasian. No information regarding BRCA mutation status was known. We have shown previously that class III β-tubulin staining by IHC reflects expression by qRT-PCR among patients who underwent primary debulking for whom formalin-fixed paraffin-embedded and fresh-frozen samples were obtained simultaneously [21, 22], an important validation given the potential discordance between protein expression and mRNA transcripts in mammalian cells due to post-transcriptional modifications [24]. We have additionally shown in these prior studies that qRT-PCR expression levels stratify overall survival in patients with serous carcinoma of the uterus [21] and clear cell carcinoma of the ovary [22]. In the present work, class III β-tubulin was higher at time of debulking among patients who received NACT followed by interval debulking (n=12) compared to those who underwent primary cytoreduction followed by chemotherapy (n=14) (mean ± SD fold-change: 491.2 ± 115.9 vs 224.1 ± 55.66, p=0.037) (Figure 4). These values greatly exceed levels expressed by normal ovary, endometrium, and myometrium (mean ± SEM: 32.63 ± 6.51, n=3; data not shown). In this cohort of patients, a statistical difference between overall survival of patients who underwent primary debulking versus NACT (p = 0.54) did not exist. When stratified by qRT-PCR expression values, however, overall survival was improved among patients with low (< 150; n = 8) compared to high (>150; n= 9) class III β-tubulin (2877 vs 1077 days, HR 1.06 [0.15–7.7], p = 0.003) based on cutoffs that allowed for equal numbers of patients in each group. Of note, sufficient follow-up was available for <70% of patients, therefore these results should be interpreted with caution.
Fig 4.

Patients dispositioned to NACT exhibit higher class III β-tubulin levels compared to those who undergo primary debulking. At time of cytoreduction, among 12 patients treated with NACT and 14 who underwent primary debulking class III β-tubulin expression values were 491.0 ± 115.7 and 224.1 ± 55.66, respectively
Paclitaxel subculture results in upregulation of class III β-tubulin
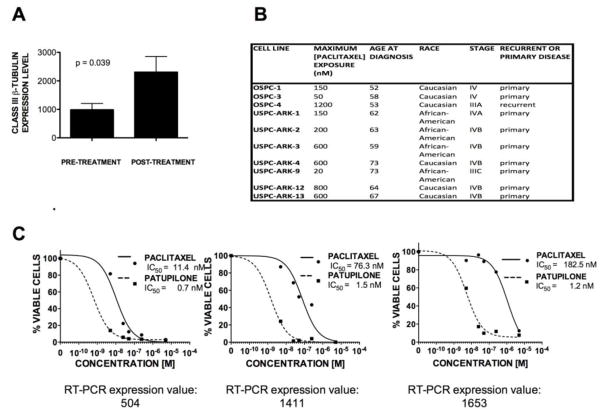
We then mimicked neoadjuvant chemotherapy administration by exposing serous carcinoma cell lines to successively higher concentrations of paclitaxel over the course of 18 weeks (i.e., equivalent to approximately 6 cycles of carboplatin/paclitaxel). A total of 10 serous carcinoma cell lines (3 ovarian, 7 uterine) were serially subcultured using a mean ± SD maximum paclitaxel dose of 430 ± 385 nM across a range of 9–25 passages. Mean ± SD for class III β-tubulin expression values increased from baseline at 985.8 ± 219 to 2306 ± 550 (p = 0.039) (Figure 5a). Cell line characteristics are provided in Figure 5b.
Fig 5.
a Paclitaxel subculture of 10 cell lines results in upregulation of class III β-tubulin (from 985.8 ± 219 before treatment to 2306 ± 550 after treatment; p = 0.039). b Characteristics of cell lines exposed to paclitaxel subculture. c Ovarian serous cell lines that overexpress class III β-tubulin are exquisitely sensitive to patupilone; representative chemoresponsiveness to paclitaxel and patupilone among three different chemotherapy-naïve cell lines is shown in relationship to baseline class III β-tubulin expression. Molar concentration (x-axis) is shown against percentage viable cells (y-axis).
Cell lines that overexpress class III β-tubulin remain exquisitely sensitive to patupilone
We subsequently examined chemosensitivity of ovarian serous carcinoma cell lines to various microtubule-stabilizing agents. Cell lines previously unexposed to chemotherapy in vitro with high class III β-tubulin expression showed exquisite sensitivity to patupilone relative to paclitaxel. As illustrated in Figure 5c, cell lines with class III β-tubulin expression values ranging from 504-1653 were 16–>100 times more sensitive to patupilone compared to paclitaxel. Doubling times for the representative cell lines shown ranged from 22 to 37 hours.
Discussion
Neoadjuvant chemotherapy with carboplatin and paclitaxel for ovarian carcinoma is an acceptable alternative to primary surgical cytoreduction in instances of advanced disease otherwise felt to be non-debulkable or medical comorbidity preclusive of immediate surgical intervention [1–4]. Though maximum cytoreduction remains the single most important independent prognostic factor for outcome [25–30], choice of adjuvant regimen may be problematic in cases where response to NACT is poor, even if optimal debulking is achieved.
Kavallaris and colleagues first noted upregulation of class III β-tubulin in paclitaxel-resistant ovarian cancer cell lines as early as 1997 [31]. It has since been shown to be a marker of poor outcome in a variety of non-gynecologic [11][12][14] and gynecologic cancers [23, 32]. This group has shown previously that uterine carcinosarcomas which overexpress class III β-tubulin may be significantly more sensitive to patupilone than paclitaxel as well as more sensitive to patupilone than ovarian carcinosarcomas which express lower levels of class III β-tubulin [19]. Similarly, we have also previously implicated class III β-tubulin overexpression in the identification of a subset of chemo-naïve patients with uterine serous [21] and ovarian clear cell [22] carcinoma at risk of poor outcome secondary to paclitaxel resistance, with retention of responsiveness to epothilones. We now propose that class III β-tubulin overexpression at time of interval debulking following NACT may be used in the prediction of poor clinical course and to guide choice of additional chemotherapeutic agents. We have shown the prognostic relevance of overexpression by both tumor (p=0.03) and stroma (p=0.04) on overall survival and have found that NACT may lead to decreased stromal expression (p=0.07), presumably coincident with restoration of normal tumor microenvironment or decreased tumor bulk as a result of therapy due to either the taxane or platinum component. This may support the emerging role for class III β-tubulin beyond conferring drug resistance through altered microtubule function, but as a mediator of prosurvival pathways and constitutive expression in hypoxic conditions of the tumor microenvironment via a HIF-1α mechanism among others [33][7]. The trend towards statistical significance observed for decreased stromal staining following NACT would likely be improved with a larger sample size, which would also allow performance of multi-variate analyses. Change in immunohistochemistry score of tumor and stroma following neoadjuvant treatment failed to predict crude overall survival and was not found to be a sensitive marker of outcome, but is limited by small numbers. Additionally, it is impossible to eliminate inherent variations in stromal characteristics between pre-NACT specimens (often consisting of metastatic disease to the omentum) and post-NACT specimens (often representing primary tumor), which may have skewed this effect. Stronger β-tubulin staining in metastatic lymph nodes relative to primary tumor has been shown in some studies [34], but not others [35]. As with other reports employing a semi-quantitative scoring system, our immunohistochemistry analysis may be limited by rater subjectivity [36].
Overall, our observations are consistent with a previous publication by Ferrandina and colleagues (2006) [37], who also described a decrease in β-tubulin positivity of tissues following NACT, however, we report for the first time separately tumoral and stromal staining. The current study differs from the investigation by Mozzetti and colleagues (2005) [20] as the baseline specimen in the majority of our patients was obtained by image-guided biopsy as opposed to surgery; this is important difference as some evidence suggests the inflammatory milieu brought upon by surgery may play an important role in stimulating tumor renewal through progenitor cells [38]. In accordance with existing reports, we have shown upregulation of class III β-tubulin overexpression in serous cell lines through application of in vitro selection pressure [31, 39–40]. Of note, we included both uterine and ovarian serous carcinoma cell lines for our experiments with repeated exposure to paclitaxel as we have previously shown that class III β-tubulin overexpression similarly serves as a poor prognostic factor in both uterine and ovarian serous carcinomas [21–22]. Furthermore, despite molecular differences, the standard of care for treatment of both advanced ovarian and uterine serous carcinomas includes platinum/taxane combinations [41] and in both instances, neoadjuvant therapy has been used successfully [42–44]. Nevertheless, these results must be interpreted with caution.
The mechanisms by which class III β-tubulin overexpression leads to paclitaxel resistance remain incompletely elucidated. We have previously reported enhanced sensitivity of ovarian/uterine serous carcinoma cell lines [21, 22] to paclitaxel following siRNA-mediated knockdown of class III but not class II β-tubulin. Data in non-small cell lung carcinoma suggest reductions in class III β-tubulin may also improve sensitivity to other chemotherapeutic agents including epothilones by predisposing cells to apoptosis through mechanisms other than reduction in mitotic block induced by chemotherapeutic agent [45–46]. Additional studies are required to fully understand these processes, as well as the contribution of platinum resistance in patients receiving neoadjuvant carboplatin/paclitaxel. To better understand the potential efficacy of carboplatin/epothilone doublets in patients who have received neoadjuvant carboplatin/paclitaxel, future inquiries are also required to explore sensitivity to epothilones in cell lines after repeated in vitro exposure to carboplatin. Mechanisms leading to carboplatin resistance, including decreased uptake and increased efflux of drug, activation of alternative pro-survival pathways, and enhanced efficiency of DNA repair are not currently thought to specifically overlap with epothilones but must be explored.
Importantly, using qRT-PCR we have shown that patients dispositioned to NACT exhibit higher levels of class III β-tubulin compared to those who undergo primary cytoreduction, possibly reflective of intrinsic biologic aggressiveness. Due to small sample size and retrospective nature of the design, we cannot exclude systematic bias as a source of these differences. Furthermore, despite their paclitaxel resistance, cell lines that overexpressed class III β-tubulin remained exquisitely sensitive to patupilone. In light of previous work suggesting that class III β-tubulin may represent a marker for responsiveness to epothilones [19, 21, 22, 47], examination for overexpression at the time of interval debulking may identify patients who are candidates for these novel microtubule-stabilizing agents. This suggests a potential role for epothilones for use in treatment of patients who have previously received NACT with carboplatin/paclitaxel, possibly even in the immediate adjuvant setting following interval debulking. Epothilones are currently under evaluation for treatment of gynecologic malignancies, but have not yet been studied explicitly in the setting of NACT. In GOG-126M, an overall response rate of 14.3% and disease stabilization rate of 40.8% was achieved in 49 patients with platinum/taxane-resistant recurrent ovarian cancer using 20 mg/m2 on days 1, 8 and 15 of a 28 day cycle [48]. GOG-129P evaluated 50 patients with recurrent or persistent endometrial cancer who received one prior line of taxane-based chemotherapy; an overall response rate of 12% was achieved using 40 mg/m2 every 21 days; disease stabilization for at least 8 weeks occurred in 60%. Median progression-free and overall survival was 2.9 months and 8.7 months, respectively [49]. Ixabepilone is currently under evaluation investigation as first-line therapy with carboplatin and bevacizumab in stage III/IV primary or recurrent endometrial cancers (GOG–86P) [50].
CONCLUSIONS
Overexpression of class III β-tubulin in patients dispositioned to NACT compared to primary cytoreduction likely reflects intrinsic tumor phenotype, though repeated exposure to paclitaxel in vitro–and conceivably through NACT administration–may also cause upregulation of class III β-tubulin. Importantly, cells that overexpress class III β-tubulin remain sensitive to patupilone despite paclitaxel resistance. Decreases in stromal expression that occur following NACT administration may represent normalization of the tumor microenvironment as a result of therapy. Class III β-tubulin expression by either tumor and stroma at time of interval debulking predicts OS following NACT, and may be used to guide choice of adjuvant therapy. Patients who exhibit high expression of class III β-tubulin in both tumor and stroma may be candidates for treatment using an epothilone with or without carboplatin. Single-agent epothilones and carboplatin-epothilone doublets have been examined at the phase II level in platinum-resistant [51] and -sensitive settings [52–53] in non-small cell lung and ovarian cancers. Along these lines, we believe epothilones warrant study in subsequent treatment of ovarian cancer patients who have received NACT with carboplatin/paclitaxel and overexpress class III β-tubulin.
ABBREVIATIONS
- NACT
neoadjuvant chemotherapy
- FIGO
Fédération Internationale de Gynécologie et d’Obstétrique
- qRT-PCR
quantitative real time polymerase chain reaction
- OSPC
ovarian serous papillary carcinoma
- USPC
uterine serous papillary carcinoma
- OS
overall survival
- ΔIHC
change in immunohistochemistry score
Footnotes
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest to declare.
SOURCES
- 1.Vergote I, Tropé CG, Amant F, Kristensen GB, Ehlen T, Johnson N, Verheijen RHM, van der Burg MEL, Lacave AJ, Benedetti-Panici P, Kenter GG, Casado A, Mendiola C, Coens C, Verleye L, Stuart GC, Pecorelli S, Reed NS. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. New Engl J Med. 2010;363:943–953. doi: 10.1056/NEJMoa0908806. [DOI] [PubMed] [Google Scholar]
- 2.Kang S, Nam BH. Does neoadjuvant chemotherapy increase optimal cytoreduction rate in advanced ovarian cancer? Meta-analysis of 21 studies. Ann Surg Oncol. 2009;16:2315–2320. doi: 10.1245/s10434-009-0558-6. [DOI] [PubMed] [Google Scholar]
- 3.Crawford SC, Vasey PA, Paul J, Hay A, Kaye SB. Does aggressive surgery only benefit patients with less advanced cancer? Results from an international comparison within the SCOTROC-1 Trial. J Clin Oncol. 2005;23:8802–8811. doi: 10.1200/JCO.2005.02.1287. [DOI] [PubMed] [Google Scholar]
- 4.Glasgow MA, Yu H, Rutherford TJ, Azodi M, Silasi DA, Santin AD, Schwartz PE. Neoadjuvant chemotherapy (NACT) is an effective way of managing elderly women with advanced stage ovarian cancer (FIGO Stage IIIC and IV) J Surg Oncol. 2013;107(2):195–200. doi: 10.1002/jso.23171. [DOI] [PubMed] [Google Scholar]
- 5.Menczer J, Usviatzov I, Ben-Shem E, Golan A, Levy T. Neoadjuvant chemotherapy in ovarian, primary peritoneal and tubal carcinoma: can imaging results prior to interval debulking predict survival? J Gynecol Oncol. 2011;22(3):183–187. doi: 10.3802/jgo.2011.22.3.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bristow RE, Chi DS. Platinum-based neoadjuvant chemotherapy and interval surgical cytoreduction for advanced ovarian cancer: A meta-analysis. Gynecol Oncol. 2006;103:1070–1076. doi: 10.1016/j.ygyno.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 7.Raspaglio G, Fillipetti F, Prislei F, Penci R, De Maria I, Cicchillitti K, Mozzetti S, Scambia G, Ferlini C. Hypoxia induces class III beta-tubulin gene expression by HIF-1 alpha binding to its 3′ flanking region. Gene. 2008;409(1–2):100–108. doi: 10.1016/j.gene.2007.11.015. [DOI] [PubMed] [Google Scholar]
- 8.Magnani M, Ortuso F, Soro S, Alcaro S, Tramontano A, Botta M. The β-I/β-III- tubulin isoforms and their complexes with antimitotic agent: docking and molecular dynamics studies. FEBS J. 2006;273:3301–3310. doi: 10.1111/j.1742-4658.2006.05340.x. [DOI] [PubMed] [Google Scholar]
- 9.De Donato M, Mariani M, Petrella L, Martinelli E, Zannoni GF, Vellone V, Ferrandina G, Shahabi S, Scambia G, Ferlini C. Class III β-tubulin and the cytoskeletal gateway for drug resistance in ovarian cancer. J Cell Physiol. 2012;227:1034–1041. doi: 10.1002/jcp.22813. [DOI] [PubMed] [Google Scholar]
- 10.Ferlini C, Raspaglio G, Cicchillitti L, Mozzetti S, Prislei S, Bartollino S, Scambia G. Looking at drug resistance mechanisms for microtubule interacting drugs: does TUBB3 work? Curr Cancer Drug Targets. 2007;7(8):704–712. doi: 10.2174/156800907783220453. [DOI] [PubMed] [Google Scholar]
- 11.Sève P, Dumontet C, Reiman T. Role of tubulin-β-III in predicting chemoresistance in non-small cell lung cancer. Lung Cancer. 2010;67(2):136–143. doi: 10.1016/j.lungcan.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 12.Tommasi S, Mangia A, Lacalamita R, Bellizzi A, Fedele V, Chiriatti A, Thomssen C, Kendzierski N, Latorre A, Lorusso V, Schittulli F, Zito F, Kavallaris M, Paradiso A. Cytoskeleton and paclitaxel sensitivity in breast cancer: the role of β-tubulins. Int J Cancer. 2007;120:2078–2085. doi: 10.1002/ijc.22557. [DOI] [PubMed] [Google Scholar]
- 13.Mariani M, Zannoni G, Sioletic S, Sieber S, Martino C, Martinelli E, Coco C, Scambia G, Shahabi S, Ferlini C. Gender influences the Class III and V beta-tubulin ability to predict poor outcome in colorectal cancer. Clin Cancer Res. 2012;18(10):2964–2975. doi: 10.1158/1078-0432.CCR-11-2318. [DOI] [PubMed] [Google Scholar]
- 14.Sève P, Reiman T, Lai R, Hanson J, Santos C, Johnson L, Dabbagh L, Sawyer M, Dumontet C, Mackey JR. Class III β-tubulin is a marker of paclitaxel resistance in carcinomas of unknown primary site. Cancer Chemother Pharmacol. 2007;60:27–34. doi: 10.1007/s00280-006-0343-1. [DOI] [PubMed] [Google Scholar]
- 15.Bollag DM, McQueney PA, Zhu J, Hensens O, Koupal L, Liesch J, Goetz M, Lazarides E, Woods CM. Epothilones, a new class of microtubule-stabilizing agents with a taxol-like mechanism of action. Cancer Res. 1995;55:2325–2333. [PubMed] [Google Scholar]
- 16.Tavassoli FA, Devilee P. World Health Organization: Tumours of the Breast and Female Genital Organs. IARC Press; Lyon: 2003. [Google Scholar]
- 17.El-Sahwi K, Bellone S, Cocco E, Cargnelutti M, Casagrande F, Bellone M, Abu-Khalaf M, Buza N, Tavassoli FA, Hui P, Silasi DA, Azodi M, Schwartz PE, Rutherford TJ, Pecorelli S, Santin AD. In vitro activity of pertuzumab in combination with trastuzumab in uterine serous papillary adenocarcinoma. Br J Cancer. 2010;102(1):134–143. doi: 10.1038/sj.bjc.6605448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tassi RA, Bignotti E, Rossi E. Overexpression of mammaglobin B in epithelial ovarian carcinomas. Gynecol Oncol. 2007;105:578–585. doi: 10.1016/j.ygyno.2007.01.043. [DOI] [PubMed] [Google Scholar]
- 19.Carrara L, Guzzo F, Roque DM, Bellone S, Cocco E, Sartori E, Pecorelli S, Schwartz PE, Rutherford TJ, Santin AD. Differential in vitro sensitivity to patupilone versus paclitaxel in uterine and ovarian carcinosarcoma cell lines is linked to tubulin-beta-III expression. Gynecol Oncol. 2012;125(1):231–236. doi: 10.1016/j.ygyno.2011.12.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mozzetti S, Ferlini C, Concolino P, Filippetti F, Raspaglio G, Prislei S, Gallo D, Martinelli E, Ranelletti F, Ferrandina G, Scambia G. Class III β-tubulin overexpression is a prominent mechanism of paclitaxel resistance in ovarian cancer patients. Clin Cancer Res. 2005;11:298–305. [PubMed] [Google Scholar]
- 21.Roque DM, Bellone S, English DP, Buza N, Cocco E, Gasparrini S, Bortolomai I, Ratner E, Silasi DA, Azodi M, Rutherford TJ, Schwartz PE, Santin AD. Tubulin-β-III overexpression by uterine serous carcinomas is a marker for poor overall survival after platinum/taxane chemotherapy and sensitivity to epothilones. Cancer. 2013 doi: 10.1002/cncr.28017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roque DM, Bellone S, Buza N, Romani C, Cocco E, Bignotti E, Ravaggi A, Rutherford TJ, Schwartz PE, Pecorelli S, Santin AD. Class III β-tubulin overexpression in ovarian clear cell and serous carcinoma as a marker for poor overall survival after platinum/taxane chemotherapy and sensitivity to patupilone. Am J Obstet Gynecol. 2013 doi: 10.1016/ajog.2013.04.017. [DOI] [PubMed] [Google Scholar]
- 23.Umezu T, Shibata K, Kajiyama H, Terauchi M, Ino K, Nawa A, Kikkawa F. Taxol resistance among the different histological subtypes of ovarian cancer may be associated with the expression of class III beta-tubulin. Int J Gynecol Pathol. 2008;27(2):207–212. doi: 10.1097/PGP.0b013e318156c838. [DOI] [PubMed] [Google Scholar]
- 24.Tian Q, Stepaniants SB, Mao M, Weng L, Feetham MC, Doyle MJ, Yi EC, Dai H, Thorsson V, Eng J, Goodlett D, Berger JP, Gunter B, Linseley PS, Stoughton RB, Aebersold R, Collins SJ, Hanlon WA, Hood LE. Integrated genomic and proteomic analyses of gene expression in mammalian cells. Mol Cell Proteomics. 2004;3:960–969. doi: 10.1074/mcp.M400055-MCP200. [DOI] [PubMed] [Google Scholar]
- 25.Vergote I, De Wever I, Tjalma W, Van Gramberen M, Decloedt J, van Dam P. Neoadjuvant chemotherapy or primary debulking surgery in advanced ovarian carcinoma: a retrospective analysis of 285 patients. Gynecol Oncol. 1998;71:431–436. doi: 10.1006/gyno.1998.5213. [DOI] [PubMed] [Google Scholar]
- 26.Zivanovic O, Eisenhauer EL, Zhou Q, Iasonos A, Sabbatini P, Sonoda Y, Abu-Rustum NR, Barakat RR, Chi DS. The impact of bulky upper abdominal disease cephalad to the greater omentum on surgical outcome for stage IIIC epithelial ovarian, fallopian tube, and primary peritoneal cancer. Gynecol Oncol. 2008;108:287–292. doi: 10.1016/j.ygyno.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 27.Aletti GD, Dowdy SC, Podratz KC, Cliby WA. Surgical treatment of diaphragm disease correlates with improved survival in optimally debulked advanced stage ovarian cancer. Gynecol Oncol. 2006;100:283–287. doi: 10.1016/j.ygyno.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 28.Bristow RE, Tomacruz RS, Armstrong DK, Trimble EL, Montz FJ. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. J Clin Oncol. 2002;20:1248–1259. doi: 10.1200/JCO.2002.20.5.1248. [DOI] [PubMed] [Google Scholar]
- 29.Chi DS, Eisenhauer EL, Lang J, Huh J, Haddad L, Abu-Rustum NR, Sonoda Y, Levine DA, Hensley M, Barakat RR. What is the optimal goal of primary cytoreductive surgery for bulky stage IIIC epithelial ovarian carcinoma (EOC)? Gynecol Oncol. 2006;103:559–564. doi: 10.1016/j.ygyno.2006.03.051. [DOI] [PubMed] [Google Scholar]
- 30.du Bois A, Reuss A, Pujade-Lauraine E, Harter P, Ray-Coquard I, Pfisterer J. Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: a combined exploratory analysis of 3 prospectively randomized phase 3 multi- center trials: by the Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruppe Ovarialkarzinom (AGO-OVAR) and the Groupe d’Investigateurs Nationaux Pour les Etudes des Cancers de l’Ovaire (GINECO) Cancer. 2009;115:1234–1244. doi: 10.1002/cncr.24149. [DOI] [PubMed] [Google Scholar]
- 31.Kavallaris M, Kuo DY, Burkhart CA, Regl DL, Norris MD, Haber M, Horwitz SB. Taxol-resistant epithelial ovarian tumors are associated with altered expression of specific beta-tubulin isotypes. J Clin Invest. 1997;100(5):1282–1293. doi: 10.1172/JCI119642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mozzetti S, Ferlini C, Concolino P, Filippetti, Raspaglio G, Prislei S, Gallo D, Martinelli E, Ranelletti FO, Ferrandina G, Scambia G. Class III beta-tubulin overexpression is a prominent mechanism of paclitaxel resistance in ovarian cancer patients. Clin Cancer Res. 2005;11(1):298–305. [PubMed] [Google Scholar]
- 33.Mariani M, Shahabi S, Sieber S, Scambia G, Ferlini C. Class III β-tubulin (TUBB3): more than a biomarker in solid tumors? Curr Mol Med. 2011;11:726–731. doi: 10.2174/156652411798062368. [DOI] [PubMed] [Google Scholar]
- 34.Thongwatchara P, Promwikorn W, Srisomsap C, Chokchaichamnankit D, Boonyaphiphat P, Thongsuksai P. Differential protein expression in primary breast cancer and match axillary node metastasis. Oncol Reports. 2011;26(1):185–9. doi: 10.3892/or.2011.1266. [DOI] [PubMed] [Google Scholar]
- 35.Kang CH, Jang BG, Kim D-W, Chung DH, Kim YT, Jheon S, Sung S-W, Hyun J. Differences in the expression profiles of excision repair cross complementation group 1, X-ray repair crosscomplementation group 1, and β-III tubulin between primary non-small cell lung cancer and metastatic lymph nodes and the significance in mid-term survival. J Thoracic Oncol. 2009;4(11):1307–1312. doi: 10.1097/JTO.0b013e3181b9f236. [DOI] [PubMed] [Google Scholar]
- 36.van Diest PJ, van Dam P, Henzen-Logmans SC, Berns E, van der Burg ME, Green J, Vergote I. A scoring system for immunohistochemical staining: concensus report of the task force for basic research of the EORTC-GCCG. J Clin Pathol. 1997;50:801–804. doi: 10.1136/jcp.50.10.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ferrandina G, Zannoni GF, Martinelli E, Paglia A, Gallotta V, Mozzetti S, Scambia G, Ferlini C. Class III β-Tubulin overexpression is a marker of poor clinical outcome in advanced ovarian cancer patients. Clin Cancer Res. 2006;12:2774–2789. doi: 10.1158/1078-0432.CCR-05-2715. [DOI] [PubMed] [Google Scholar]
- 38.Chefetz I, Alvero AB, Holberg JC, Lebowitz N, Craveiro V, Yang-Hatwich Y, Yin G, Squillace L, Sorteras M, Aldo P, Mor G. TLR2 enhances ovarian cancer stem cell self-renewal and promotes tumor repair and recurrence. Cell Cycle. 2013;12(3):511–521. doi: 10.4161/cc.23406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ferlini C, Raspaglio G, Mozzetti S, Cicchillitti L, Filippetti F, Gallo D, Fattorusso C, Campiani G, Scambia G. The seco-taxane IDN5390 is able to target class III β-tubulin and to overcome paclitaxel resistance. Cancer Res. 2005;65:2397–2405. doi: 10.1158/0008-5472.CAN-04-3065. [DOI] [PubMed] [Google Scholar]
- 40.Ranganathan S, Benetatos CA, Colarusso PJ, Dexter DW, Hudes GR. Altered β-tubulin isotype expression in paclitaxel-resistant human prostate carcinoma cells. Br J Cancer. 1998;77:562–566. doi: 10.1038/bjc.1998.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Del Carmen MG, Birrer M, Shorge JO. Uterine papillary serous cancer: a review of the literature. Gynecol Oncol. 2012;127:652–661. doi: 10.1016/j.ygyno.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 42.Moller KA, Gehrig PA, Van Le L, Secord AA, Schorge J. The role of optimal debulking in advanced stage serous carcinoma of the uterus. Gynecol Oncol. 2004;94(1):170–174. doi: 10.1016/j.ygyno.2004.03.040. [DOI] [PubMed] [Google Scholar]
- 43.Resnik E, Taxy JB. Neoadjuvant chemotherapy in uterine papillary serous carcinoma. Gynecol Oncol. 1996;62(1):123–127. doi: 10.1006/gyno.1996.0201. [DOI] [PubMed] [Google Scholar]
- 44.Le TD, Yamada SD, Rutgers JL, DiSaia PJ. Complete response of a stage IV uterine papillary serous carcinoma to neoadjuvant chemotherapy with Taxol and carboplatin. Gynecol Oncol. 1999;73(3):461–3. doi: 10.1006/gyno.1999.5361. [DOI] [PubMed] [Google Scholar]
- 45.Gan PP, Pasquier E, Kavallaris M. Class III tubulin mediates sensitivity to chemotherapeutic drugs in non-small cell lung cancer. Cancer Res. 2007;67:9356–936. doi: 10.1158/0008-5472.CAN-07-0509. [DOI] [PubMed] [Google Scholar]
- 46.Gan PP, McCarroll JA, Byrne FL, Garner J, Kavallaris M. Specific-b-III tubulin isotypes can functionally enhance or diminish epothilone B sensitivity in non-small cell lung cancer cells. PLOS One. 2011;6(6):e21717. doi: 10.1371/journal.pone.0021717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pusztai L. Markers predicting clinical benefit in breast cancer from microtubule-targeting agents. Ann Oncol Suppl. 2007;12:xii15–20. doi: 10.1093/annonc/mdm534. [DOI] [PubMed] [Google Scholar]
- 48.De Geest K, Blessing JA, Morris RT, Monk BJ, Zweizig SL, Matei D, Muller CY, Richards WE. Phase II clinical trial of ixabepilone in patients with recurrent or persistent platinum- and taxane-resistant ovarian or primary peritoneal cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2009;28:149–153. doi: 10.1200/JCO.2009.24.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dizon DS, Blessing JA, McMeekin DS, Sharma SK, DiSilvestro P, Alvarez RD. Phase II trial of ixabepilone as second-line treatment in advanced endometrial cancer: Gynecologic Oncology Group Trial 129-P. J Clin Oncol. 2009;27:3104–3108. doi: 10.1200/JCO.2008.20.6995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.NIH Clinical Trials. Evaluation of carboplatin/paclitaxel/bevacizumab in the treatment of advanced stage endometrial carcinoma. http://clinicaltrials.gov/ct2/show/NCT00513786.
- 51.Vansteenkiste J, Lara PN, Jr, Le Chevalier T, Breton JL, Bonomi P, Sandler AB, Socinski MA, Delbaldo C, McHenry B, Lebwohl D, Peck R, Edelman MJ. Phase II clinical trial of the epothilone B analog, ixabepilone, in patients with non-small cell lung caner whose tumors have failed first-line platinum-based chemotherapy. J Clin Oncol. 2007;25(23):3448–3455. doi: 10.1200/JCO.2006.09.7097. [DOI] [PubMed] [Google Scholar]
- 52.Spigel DR, Anthony Greco F, Waterhouse DM, Shipley DL, Zubkus JD, Bury MJ, Webb CD, Hart LL, Gian VG, Infante JR, Burris HA, 3rd, Hainsworth JD. Phase II trial of ixabepilone and carboplatin with or without bevacizumab in patients with previously untreated advanced non-small cell lung cancer. Lung Cancer. 2012;78(1):70–75. doi: 10.1016/j.lungcan.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 53.McMeekin S, Patel R, Verschraegen C, Celano P, Burke J, 2nd, Plaxe S, Ghatage P, Giurescu M, Stredder C, ang Y, Schmelter T. Phase I/II study of sagopilone (ZK-EPO) plus carboplatin in women with recurrent platinum-sensitive ovarian cancer. Br J Cancer. 2012;106(1):70–76. doi: 10.1038/bjc.2011.499. [DOI] [PMC free article] [PubMed] [Google Scholar]