Abstract
Although constitutional chromosome abnormalities have been recognized since the 1960s, clinical characterization and development of treatment options have been hampered by their obvious genetic complexity and relative rarity. Additionally, deletions of 18q are particularly heterogeneous, with no two people having the same breakpoints. We identified sixteen individuals with deletions that, despite unique breakpoints, encompass the same set of genes within a 17.6 Mb region. This group represents the most genotypically similar group yet identified with distal 18q deletions. As the deletion is of average size when compared with other 18q deletions, this group can serve as a reference point for the clinical and molecular description of this condition. We performed a thorough medical record review as well as a series of clinical evaluations on 14 of the 16 individuals. Common functional findings included developmental delays, hypotonia, growth hormone deficiency, and hearing loss. Structural anomalies included foot anomalies, ear canal atresia/stenosis, and hypospadias. The majority of individuals performed within the low normal range of cognitive ability but had more serious deficits in adaptive abilities. Of interest, the hemizygous region contains 38 known genes, 26 of which are sufficiently understood to tentatively determine dosage sensitivity. Published data suggest that 20 are unlikely to cause an abnormal phenotype in the hemizygous state and five are likely to be dosage sensitive: TNX3, NETO1, ZNF407, TSHZ1, and NFATC. A sixth gene, ATP9B, may be conditionally dosage sensitive. Not all distal 18q- phenotypes can be attributed to these six genes; however, this is an important advance in the molecular characterization of 18q deletions.
Keywords: 18q-, chromosome abnormality, dosage sensitive genes, haploinsufficiency
Introduction
One of the primary concerns of a family receiving a new genetic diagnosis is prognosis. In the case of a diagnosis of 18q-, providing prognostic information is particularly challenging. The term “ 18q-” encompasses a collection of unique deletions. High resolution aCGH of now over 290 people has shown that no two unrelated individuals with terminal or interstitial deletions of 18q have the same breakpoints (Heard et al. 2009). While the extreme genotypic variability is helpful in discovering genotype-phenotype correlations, this heterogeneity presents a unique challenge in the clinical description of this condition. Because 18q- is fundamentally defined by genotype and not by any clinical manifestations, it cannot be considered a “syndrome” in the classic sense of the word. With no common genotype, it is impossible to provide a description of the “typical” phenotypic effects of 18q deletions.
The challenge of providing a clinical description for large chromosomal rearrangements such as 18q- can be illustrated with the dysmyelination phenotype. This phenotype is a common feature in individuals with 18q-. We have completed MRI’s on 88 individuals with 18q-, and 90% have dysmyelination of the central nervous system (Cody et al. 2009). However, this does not mean that everyone with an 18q deletion has a 90% chance of having dysmyelination. In actuality, everyone with a hemizygous deletion that includes the dysmyelination critical region has this finding. Individuals not hemizygous for the critical region do not have dysmyelination. Any summary data about 18q- will be misleading, as only those missing the critical region for dysmyelination are at risk. Therefore any compilation of the phenotypic features must precisely specify the genotype in order to be useful to the clinician in providing a prognosis.
Work is ongoing to refine the term “18q-” by subdividing it into several genotypically-defined subgroups. First, there is a region in 18q21.1 (43,832,732-45,297,446; hg18) that has never been found to be hemizygous (Heard et al. 2009). This presumably hemizygous lethal region serves as a dividing point that creates two distinct groups of individuals with 18q-: proximal and distal deletions (Cody et al. 2007).
We have further subdivided the distal 18q- description based on the inclusion or exclusion of TCF4 in the deletion. We have previously published data regarding the consequences of TCF4 hemizygosity on the 18q- phenotype (Hasi et al. 2011). Individuals with deletions inclusive of TCF4 have several features overlapping with those of Pitt-Hopkins syndrome. Thus, TCF4 status is an additional datapoint that aids in the stratification of 18q- phenotypes. However, even within the TCF4 +/+ group, there is significant variability, suggesting that there are additional genes that discriminate between phenotypic outcomes. This degree of phenotypic variability is not surprising, given that members of the TCF4+/+ groups have hemizygous deletions ranging from 700 kb to 20 Mb.
Despite the significant degree of genotypic variation, there is a small subset of sixteen individuals with similar deletions. These individuals have unique breakpoints; however, in terms of the genes involved, they are identical, as their breakpoints are between the same two functional elements. Thus, the extent of the genetic hemizygosity and its location is the same within this group. In relation to our entire cohort of nearly 300 people with distal 18q deletions, this hemizygous deletion is larger than about 40% of distal 18q deletions and smaller than about 60% of deletions, meaning that it may be considered an “average” terminal deletion.
Here, we describe the physical and behavioral characteristics of this group, which we call the Distal 18q- Reference Group. Our goal is to create a description of the physical and developmental characteristics of this group. In addition to providing guidance for individuals with deletions involving the same genes, this will also be useful as a reference point for clinical characterization of the broader group with distal 18q-.
Methods
Participants were enrolled as a part of the Chromosome 18 Clinical Research Center longitudinal study of individuals with chromosome 18 abnormalities. This study was approved by the University of Texas Health Science Center at San Antonio Institutional Review Board and all subjects have participated in a documented informed consent process.
Genotyping was performed on DNA from a peripheral blood samples by microarray comparative genomic hybridization (aCGH) using the Agilent system as previously described (Heard et al., 2009). Parental origin of the abnormal chromosome was also determined as previously described (Heard et al., 2009).
Medical records were gathered and abstracted on all 16 participants in this cohort. Fourteen of the participants were evaluated on-site by our team of investigators; several more than once. The expense of overseas travel prevented two of the participants from participating in the on-site evaluation. The evaluations included audiometric assessment, a dysmorphology exam, and a thorough endocrine evaluation including bone age films, provocative growth hormone stimulation testing, and thyroid and sex hormone levels, when appropriate. In addition, an MRI of the brain was obtained. The details of these assessments have been described previously (Cody et al. 2009).
Age appropriate cognitive and behavioral assessments were performed and the instruments used are listed in Supplemental Table 1. The intellectual scores utilized were obtained using one of the following tests with instrument choice dependent upon the age and ability of the person being tested: Bayley Scales of Infant Development, Second Edition – BISD-II (Bayley 1993) or Bayley Scales of Infant and Toddler Development-Third Edition - Bayley-III (Bayley 2006), The Differential Abilities Scales – DAS (Elliott 1990) or Differential Abilities Scale-Second Edition - DAS-II (Elliott 2006), or the Wechsler Adult Intelligence Scale-Third Edition – WAIS-III (Wechsler 1997) or Fourth Edition – WAIS-IV (Wechsler 2008). All measures of cognitive ability are rigorously standardized instruments with excellent psychometric properties. Internal and test-retest reliabilities for the summary cognitive indices used in this study typically range from high 80′s to high 90′s with all instruments having demonstrated clinical validity with special populations including those with developmental delay and cognitive impairment. Executive functioning or metacognition was examined using the Behavior Rating Inventory of Executive Function, Parent Form – BRIEF (Gioia et al. 2000) or the Behavior Rating Inventory of Executive Function – Adult Version, Informant Report – BRIEF-A (Roth et al. 2005).
To obtain a measure of adaptive behavior functioning and social emotional development parents completed standardized behavioral questionnaire(s). The choice of behavioral questionnaire was based on the chronological age of the child and included one or more of the following instruments: Vineland Adaptive Behavior Scales (Sparrow et al. 1984) or the Vineland Adaptive Behavior Scales - Second Edition (Sparrow et al. 2005). Additionally, to investigate the prevalence of maladaptive behavior, parents also completed the Behavioral Assessment System for Children First Edition – BASC (Reynolds and Kamphaus, 1992) or Second Edition – BASC-2 (Reynolds and Kamphaus, 2004). All of the behavioral questionnaires chosen are well-normed instruments with demonstrated reliability and validity information provided by the test publishers and by post-publication validation studies.
The probability of autism was assessed by parental report using either the Gilliam Autism Rating Scale - GARS (Gilliam 1995) or the Gilliam Autism Rating Scale-Second Edition – GARS-2 (Gilliam 2006). On the GARS/GARS-2 the following domains are evaluated: presence of stereotyped behaviors, social interaction problems, developmental delay (present only in the GARS), and communication difficulties. On the GARS, coefficient alpha estimates range from 0.88 for developmental delay to 0.96 for the overall Autism Quotient.
Results
Study Sample
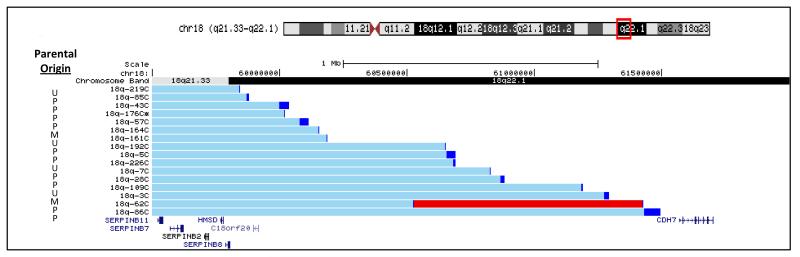
Within our cohort of over 290 individuals with chromosome 18q hemizygosity, we identified sixteen unrelated participants who had terminal deletions of approximately 17.6 Mb with breakpoints between two consecutive genes: SERPINB8 and CDH7 (59,807,588 – 61,568,468/hg18). Figure 1 shows the region of the chromosome that includes each of their breakpoints. One individual (18q-62C) within this reference group has a duplication proximal to the breakpoint. This is a common feature of 18q deletion breakpoints and since the duplicated region did not contain any genes, we did not eliminate this person from the reference group (Heard et al. 2009).
Fig 1.
Participant genotypes shown as custom tracks on the UCSC Genome Browser. The box (red) on the chromosome 18 ideogram at the top of the figure indicates the region depicted below. The bars (light blue) to the right of the participant study numbers indicate the region of chromosome 18 present in 2 copies with the breakpoint region indicated by the darker bar (dark blue). The darker (red) bar for participant 18q-62C indicates the region present in 3 copies. The locations of the UCSC genes are shown across the bottom of the figure. To the left of each individual’s genotype is the parental origin of the chromosome with the deletion. M = maternal chromosome, P = paternal chromosome and U = unknown
This reference group included thirteen females and three males. Their ages ranged from 2.8 years to 27.4 years with nine of the individuals over the age of eighteen at the time of the most recent assessment while two were under the age of five years.
The parental origin of the abnormal chromosome in this reference group was similar to the larger cohort with 17% (2/12) having a deletion of maternal origin in the reference group (Figure 1) compared to 12% in the larger cohort (Heard et al. 2009). In four individuals, parental origin was not determined for the entire cohort as DNA from both parents was not available.
Clinical Findings
The clinical findings of the reference group are listed by individual in Table 1. There are several features identified in more than half of the individuals, including delayed myelination of the brain, foot anomalies, atretic or stenotic ear canals, hypospadias in the males, tapered fingers, flat mid-face, proximally placed thumbs, and congenital heart abnormalities. The foot abnormalities included pes planus/pes cavus (64%); abnormal toe placement (71%); and metatarsus adductus (29%). The heart abnormalities included valvular heart disease (21%); atrial septal defects (36%); and one individual with total anomalous pulmonary venous return. Autoimmune disorders occurred in 31% of this cohort. Diagnoses included myalgia, arthritis, and hypothyroidism. These autoimmune diagnoses were only identified in female participants. The majority of participants had hearing loss. Twenty-eight percent had a conductive loss; 7% had sensorineural loss; and 36% had a mixed hearing deficit. Palatal abnormalities included one person with a cleft lip and palate, one with a high arched palate, four with a sub mucosal cleft palate, and two with an isolated bifid uvula. The neonatal complications included jaundice, apnea, respiratory difficulties, and meconium aspiration. All neonatal complications resolved after the newborn period. Gastroesophageal reflux was also common in this group with 56% with the diagnosis.
Table 1.
| Study ID | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||||||||
| Feature | Evalu ated |
Affec ted |
% | 219 | 85 | 43 | 176 | 57 | 164 | 161 | 192 | 5 | 226 | 7 | 28 | 109 | 3 | 62 | 86 |
| Gender | F | F | M | M | F | F | F | F | F | F | F | F | F | F | F | M | |||
| Age in yrs at the visit | 18 | 26.5 | 18.2 | 8.6 | 8.6 | n/a | 27.4 | 18.7 | 18.7 | 6.11 | 2.9 | 24.7 | 5.1 | 24.8 | 19.5 | 2.8 | |||
| Full Scale IQ | 67 | n/a | 85 | 89 | 77 | n/a | 75 | 50 | n/a | 83 | 50 | 89 | 59 | 75 | 69 | 116 | |||
| Verbal IQ | 66 | n/a | 95 | 87 | 77 | n/a | 85 | n/a | n/a | 98 | n/a | 93 | 64 | 91 | 73 | n/a | |||
| Nonverbal/ performance IQ | 84 | n/a | 96 | 100 | 89 | n/a | 84 | n/a | 85 | 88 | n/a | 97 | 63 | 79 | 70 | n/a | |||
| Birth weight z score | −1.32 | n/a | −0.66 | −0.82 | −0.51 | n/a | −0.68 | 0.60 | 0.54 | 0.12 | −0.16 | 1.65 | −2.54 | −0.84 | 0.54 | 0.85 | |||
| Birth Length z score | n/a | n/a | −0.1 | −0.46 | −0.98 | n/a | 0.09 | 0.59 | 1.50 | 1.05 | 1.50 | 1.00 | −1.57 | 1.07 | n/a | 2.25 | |||
| Delayed myelination | 11 | 11 | 100 | + | + | + | + | + | n/a | n/a | + | n/a | + | n/a | n/a | + | + | + | + |
| Motor development delay | 13 | 13 | 100 | + | n/a | + | + | + | n/a | + | + | + | n/a | + | + | + | + | + | + |
| Hypotonia | 14 | 13 | 93 | + | n/a | + | − | + | n/a | + | + | + | + | + | + | + | + | + | + |
| Narrow ear canals | 13 | 10 | 77 | + | n/a | + | − | − | n/a | + | + | + | + | n/a | + | + | + | + | − |
| Neonatal complications | 13 | 10 | 77 | + | n/a | + | − | + | + | + | − | − | + | − | + | + | + | n/a | + |
| Atopic Disorders | 16 | 12 | 75 | + | + | + | + | + | + | − | + | − | − | + | + | + | + | − | + |
| Food/ drug allergies | 16 | 9 | 56 | + | + | + | + | − | + | − | + | − | − | + | − | + | + | − | − |
| Airborne allergies | 16 | 9 | 56 | + | + | + | + | + | − | − | − | − | − | − | + | + | + | − | + |
| Eczema | 16 | 7 | 44 | + | + | + | + | + | + | − | − | − | − | − | − | − | − | − | + |
| High frequency sensorineural hearing loss | 4 | 3 | 75 | + | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | − | n/a | + | n/a | n/a |
| Growth hormone deficiency | 14 | 10 | 71 | − | n/a | + | + | + | n/a | + | − | − | + | + | + | + | + | − | + |
| Conductive hearing loss | 13 | 9 | 69 | + | n/a | + | − | n/a | n/a | + | + | − | + | + | + | − | + | + | − |
| Recurrent ear infections | 13 | 9 | 69 | + | n/a | + | − | + | n/a | − | − | + | + | + | n/a | + | + | − | + |
| Hypospadias (boys) | 3 | 2 | 67 | / | / | + | − | / | / | / | / | / | / | / | / | / | / | / | + |
| Complication during pregnancy (mother) | 15 | 10 | 67 | − | n/a | + | − | + | − | + | + | + | − | − | + | + | + | + | + |
| Tapered fingers | 14 | 9 | 64 | + | n/a | − | − | + | n/a | + | + | + | − | − | + | − | + | + | + |
| Midface hypoplasia | 10 | 6 | 60 | n/a | n/a | + | + | − | n/a | n/a | − | − | n/a | n/a | + | − | + | + | + |
| Mood disorder | 5 | 3 | 60 | − | n/a | n/a | n/a | n/a | n/a | + | − | n/a | n/a | n/a | + | n/a | + | n/a | n/a |
| Proximal thumbs | 14 | 8 | 57 | − | n/a | + | − | + | n/a | + | + | − | − | − | + | − | + | + | + |
| Foot anomalies | 14 | 8 | 57 | − | n/a | + | − | − | n/a | + | − | − | + | − | + | + | + | + | + |
| Congenital heart abn. | 13 | 7 | 54 | + | n/a | − | − | + | n/a | − | + | − | + | + | − | + | + | n/a | − |
| Sinus infections | 14 | 7 | 50 | − | n/a | + | − | + | n/a | + | − | − | − | − | + | − | + | + | + |
| Enuresis (occasional) | 10 | 5 | 50 | n/a | n/a | + | − | − | n/a | + | + | − | n/a | n/a | + | + | − | − | n/a |
| Heart murmur | 14 | 7 | 50 | + | n/a | − | − | + | n/a | − | + | + | + | + | − | − | − | + | − |
| Palate abnormality | 14 | 7 | 50 | − | n/a | + | − | − | n/a | + | − | − | − | + | + | − | + | + | + |
| Strabismus | 13 | 5 | 38 | n/a | n/a | + | + | − | n/a | − | + | − | − | + | − | − | − | + | − |
| Flat feet | 14 | 5 | 36 | − | n/a | − | − | + | n/a | − | − | + | − | − | + | + | − | + | − |
| Pneumonia | 14 | 5 | 36 | − | n/a | + | + | + | n/a | + | − | + | − | − | − | − | − | − | − |
| IgA deficiency | 9 | 3 | 33 | n/a | n/a | − | n/a | + | n/a | − | − | n/a | − | + | + | n/a | − | − | n/a |
| Scoliosis | 12 | 4 | 33 | n/a | n/a | + | − | − | n/a | + | − | − | − | n/a | − | − | + | + | − |
| Umbilical hernia | 15 | 5 | 33 | − | + | − | + | − | n/a | − | − | + | − | − | + | + | − | − | − |
| Autoimmune problems | 16 | 5 | 31 | M | M, A | C | − | n/a | M,H | − | − | − | -. | − | − | − | H | − | |
| Thyroid abnormality | 13 | 4 | 31 | + | n/a | − | − | + | n/a | + | − | − | − | n/a | − | − | − | + | − |
| Constipation | 14 | 4 | 29 | − | n/a | − | − | − | n/a | − | − | + | − | + | + | − | + | − | − |
| Kidney malformations | 8 | 2 | 25 | n/a | n/a | − | − | + | n/a | n/a | n/a | n/a | + | − | − | n/a | − | − | n/a |
| Hyper flexibility of joints | 14 | 3 | 21 | − | n/a | − | − | − | n/a | − | − | + | − | + | + | − | − | − | − |
| Seizures | 15 | 3 | 20 | − | n/a | − | − | − | − | + | − | + | − | − | − | − | − | + | − |
+ = the feature is present
− = feature is not present
n/a = not assessed or not available
/ = not applicable
Autoimmune Abbreviations, M = myalgia, C = celiac disease, H = hyperthyrodism, A = arthritis
Parental Origin Abbreviations, P = paternal, M = Maternal, U = Undetermined
Atopic diseases as a group were very common with 12 of 16 (75%) participants exhibiting some type of atopic allergic disease. Recurrent ear infections we found in 69%, food allergies in 56%, allergic rhinitis in 50%, chronic sinusitis in 50%, eczema in 44% and asthma in 21%. IgA deficiency was only found in 33% of participants.
Congenital heart conditions, while common, were not life threatening for most individuals in this group. Four individuals had small atrial septral defects, one has aortic insufficiency and mitral insufficiency with arrhythmia, one has mild pulmonic stenosis and one had total anomalous pulmonary venous return.
Neuropsychological Findings
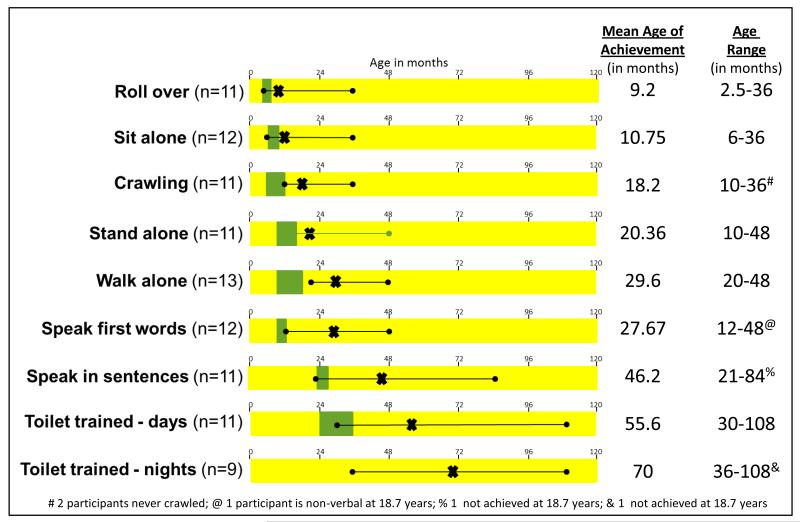
Timing of the acquisition of motor and language milestones for this cohort is illustrated in Figure 2. Although we received data from the parents of all sixteen individuals, there are some missing data despite review of medical records and subsequent data follow-up requests. When parents were unsure of exact milestone acquisition we chose to count this information as missing data rather than add potentially inaccurate information to our sample. The age range of skills achievement was very broad. While some individuals achieve developmental milestones within the typical age range of acquisition, on average the group was delayed across all motor and language milestones. One individual stood out from the rest of the group as having more severe cognitive deficits. At 18.7 years of age, this person had not achieved any speech or night-time toilet training. In addition, eleven of thirteen participants had nighttime enuresis after toilet training, but only one had nighttime encopresis after achieving toilet training.
Fig 2.
Developmental milestones of the study participants. The X indicates the mean age in months for the skill attainments the line with the ball points indicated the age range for each skill. The actual data for each is shown to the right of each scale. The typical age range of skill attainment in typically developing children is shown in green
We obtained cognitive data at our center for 13 of the 16 individuals as indicated in the Table. It should be noted that the cognitive data of two individuals who were under the age of 3 at assessment is difficult to compare to older individuals. The data for Verbal IQ, Nonverbal or Perceptual Reasoning IQ and Full Scale IQ are shown for each participant at the age at which they were evaluated. The mean Verbal IQ for the group was 82.9 and the mean Nonverbal IQ was 85. The mean full scale IQ score was 76.8. This number excludes the one nonverbal participant whose score was at the floor of the test (50 points). The majority of individuals scored within the low average or low normal range of cognitive abilities.
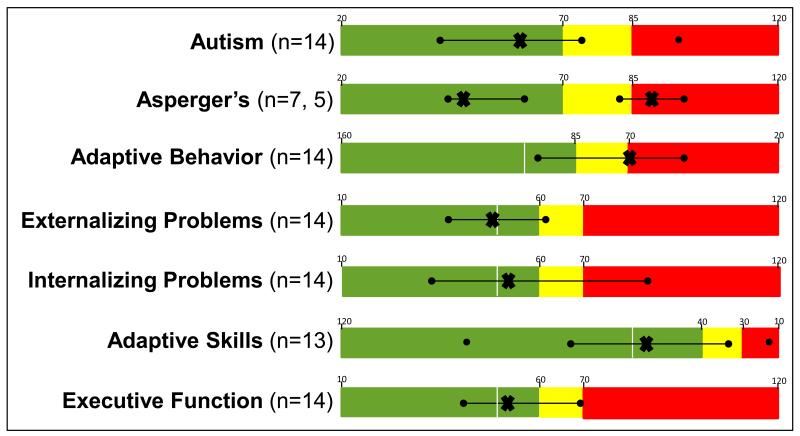
The results of the parental ratings of their children’s behaviors are shown in Figure 3. Within the broad continuum of autism spectrum disorders, the data on this group show a mixed pattern. On the Gilliam Autism Rating Scales, the same individual who was markedly developmentally delayed stood apart from the rest, scoring within the “high probability of autism” range, while the remainder of the participants fell into in the “very low risk” to the “possibly” range. The data from the Gilliam Asperger’s Disorder Scale (Gilliam 2001), which assesses for a mild form of autism, fell into two distinct groups; a group of 7 who scored well within the normal range and a group of 5 who scored in the “high probability” of Asperger’s range.
Fig 3.
Results of behavioral testing using seven different instruments. The scale for the results from each evaluation has been color coded for ease of comparison. Green indicates the normal range of scores. Yellow indicates the “at risk” range and red indicates the problematic range of scores
The data from the two measures of adaptive functioning (Vineland Adaptive Behavior Scales and the BASC-2 adaptive scales) revealed that the majority of participants have significant problems with activities of everyday life. These include activities related to communication, home living, daily self-care and managing social and leisure activities in ways similar to same age peers. It is however important to note that parental ratings of executive function skills such as impulse control, adapting to unexpected changes and planning and organizational skills (BRIEF) was rated as typically developing or only somewhat below expectancy. Additionally most individuals were not described by their parents as having significant difficulties with behavior regulation (BASC-2 externalizing problems) or problems with depression, anxiety or somatization (translation of anxiety into physical symptoms) although one individual has had several psychiatric hospitalizations with an eventual diagnosis of bipolar disorder.
Current Status
Ten of the study participants in the Reference Group are now adults and we have recent information on 9 of them. Five have graduated from high school and one has a high school completion certificate as their highest level of education. Three of these six are currently in college or have completed college. Seven live with their parents, one with a host family, and one is married and living with their spouse. Two have part-time paying jobs and one has a part-time volunteer job.
Discussion
The ultimate goal of the Chromosome 18 Clinical Research Center is to make the genomic disorders of chromosome 18 completely treatable. The approach we have taken follows two parallel but interdependent paths. The first path is to apply and evaluate current treatments, thereby defining standards of care for the symptoms in the context of the particular chromosome abnormality. The second is to understand the molecular underpinnings in order to design more targeted and hopefully more effective treatments.
In the case of distal 18q-, we have been challenged in the pursuit of both of these paths by the individual variability of the genotype within our patient population. Because there are no recurrent breakpoints or breakpoint regions on 18q, it is a uniquely heterogeneous genomic disorder. The consequences of this genomic heterogeneity lead to large phenotypic variability. Attempts to provide broad phenotypic summaries have, at best, limited clinical utility. In some cases, such as the dysmyelination example discussed above, phenotypic summaries of 18q- may be misleading.
Because this is a genotypically defined condition, we have begun to subdivide the broad group of people with 18q hemizygosity into subgroups based on genotype. Here we describe a group of 16 individuals who all have breakpoints between SERPINB8 and CDH7, even though, at a molecular level, their breakpoints span a 1.65 Mb region of 18q22.1. We are referring to this cohort as the “Distal 18q- Reference Group.”
The sixteen individuals described here are hemizygous for 38 known genes. We anticipate that the majority of these genes are unlikely to have a dosage effect (Cody and Hale 2011). Twelve of these genes have no data regarding dosage effects and most of these have no information on their biological function. Of the remaining 26 genes, we have classified 20 of them as haplosufficient based on a thorough literature review, meaning there are data suggesting these genes are not causative of an abnormal phenotype when hemizygous. Of the remaining six, five are associated with an abnormal phenotype when hemizygous, indicating that the phenotype is caused by haploinsufficiency. One gene is associated with a phenotype only when other factors are present and is therefore classified as conditional. The criteria for determining dosage sensitivity has been described previously (Cody et al. 2009) and the current Chromosome 18 Gene Dosage Map is found on our website (http://www.pediatrics.uthscsa.edu/centers/Chromosome18/dosage.asp). This map is continuously updated and designations revised as new information about gene function is published.
Characteristics associated with known genes
Specific genes have been identified for several of the features found in the Distal 18q- Reference Group. Aural atresia is caused by hemizygosity of the TSHZ1 gene (Feenstra et al., 2011). Hemizygosity of several genes have been implicated in cognitive abilities, ZNF407 (Ren et al., 2013) and NETO1 (Ng et al., 2009).
Characteristics associated with critical regions
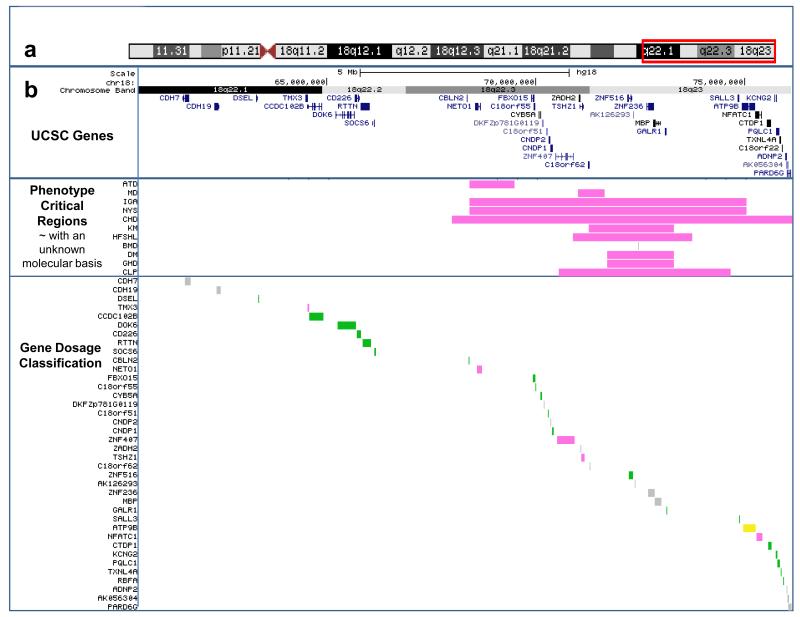
Several features do not have causative genes identified, but are associated with critical regions within which the causative gene likely resides. These regions are shown in pink in Figure 4 and are atopic disorders (Linnankivi et al., 2006), mood disorders (Daviss et al, in press), IgA deficiency (Linnankivi et al., 2006), congenital heart disease (van Trier et al., 2013), kidney malformations (Cody et al., 2009 and Margarit et al., 2012), high frequency sensorineural hearing loss (Perry et al., submitted), dysmyelination of the central nervous system (Cody et al., 2009), growth hormone deficiency (Cody et al., 2009), and cleft palate (Eudy et al., 2009).
Fig 4.
Phenotype critical regions and gene dosage designations for the Distal 18q- Reference Group region of hemizygosity. Panel a depicts the chromosome ideogram with the box (red) indicating the region of the chromosome shown in panel b which is taken from our custom tracks on the UCSC Genome Browser website at http://www.pediatrics.uthscsa.edu/centers/Chromosome18/dosage.asp. The top section of panel b shows the UCSC gene in this region. The next lower section indicates the phenotype critical regions. The abbreviation for the phenotype is shown to the left. ATD = Atopic Disorders; MD = Mood disorders; IGA = IgA deficiency; NYS = Nystagmus; CHD = Congenital Heart disease; KM = Kidney malformation; HFSNHL = High frequency sensorineural hearing loss; BMD = Bone mineral density; GHD = Growth hormone deficiency; CLP = Cleft palate. The color of the bar indicates the gene dosage category; green indicates that the mechanism of disease is not haploinsufficiency, grey means the mechanism is unknown and pink means that hemizygosity of this regions can cause the phenotype. The lower section indicates the gene dosage classification for each gene in the region. The classifications are the same as listed above, with the addition of yellow indicating a gene that does not cause a phenotype independently but only in conjunction with an additional gene mutation or environmental factor. Explanations and references for each element of the phenotype and gene tracks are accessible on the website by clicking on those elements to link to a details page
Chraracteristics without known critical regions or genes
Additionally, there are features found in this cohort for which there are neither genes nor critical regions yet identified. These include autoimmune disorders, though there are two candidate genes in the hemizygous region discussed above. These genes are CD226 and NFATC1. However evidence is inconclusive about the link between these genes and disease caused by haploinsufficiency. Additionally, hypospadias has not been linked to a specific gene or region, though a case could be made that hemizygosity of CYB5A gene could be responsible.
Conversely, there are several dosage sensitive genes or critical regions without a phenotype in this particular group, implying that the associated phenotype has very low penetrance. In additional to aural atresia, the TSHZ1 gene has also been associated with congenital vertical talus (Mark et al., 2013). Hemizygosity of the TMX3 gene causes micropthalmia in the heterozygous knock out mouse but with reduced penetrance (Chao et al., 2010).
A critical region for nystagmus has been identified however, no one in the Distal 18q- Reference group has exhibited this feature (Linnankivi et al., 2006). Genome wide association studies identified a locus at 72.4 Mb associated with bone mineral density (Estrada et al., 2012). However, GWAS studies do not rely on or implicate a molecular mechanisms. Thus, it is yet unknown if this has implications for individuals with hemizygosity of the region.
In the past we attributed the large variability in the phenotype to the variability in the genotype. In the case of the Distal 18q- Reference Group, however, we are able to eliminate the variability of the 18q deletion. Even with essentially the same deletion, there are many features with low penetrance. This implies that hemizygosity lowers the amount of gene product which then, depending on the pathway, is well below the threshold of insufficiency (100% penetrance); is close to the threshold of insufficiency (50% penetrance); or is still sufficient for normal function in the absence of other genetic influences (20% penetrance). This model acknowledges the effect of genetic background and the fact there are still two copies of more than 20,000 genes whose activity impacts the genes in hemizygosity on 18q. Additionally, those characteristics found at very low percentage in this cohort could also be caused by revealed recessive mutations of the genes in hemizygosity.
Clinical Recommendations
These data guide the development of clinical recommendations. Children diagnosed with a deletion that includes this region of 18q should;
- Be evaluated in the newborn period for
-
○hypospadias
-
○kidney, ureter and bladder abnormalities
-
○umbilical hernias
-
○foot anomalies
-
○congenital heart disease with a low threshold for referral to a cardiologist
-
○
Be evaluated by an ophthalmologist for strabismus
Be evaluated by an audiologist and otorhinolaryngologist for hearing loss on a periodic basis
Have linear growth monitored closely and early referral to a pediatric endocrinologist for slowing growth velocity.
Be enrolled in an early intervention program that includes, physical, occupational and speech therapy to reduce the delay in these areas as much as possible.
Given that these children are at high risk for developmental delay, it is critical to reduce as many barriers to optimal development as possible to maximize outcomes. Therefore, it is prudent to consider more vigilant monitoring and immediate treatment or interventions, when indicated. While typically developing children can often compensate and hide a single minor deficit in hearing or in vision, children with distal 18q- frequently have numerous deficiencies and a reduced capacity to commentate for challenges and attain skills on a typical schedule.
Care providers and parents should be aware of an increased risk to develop the following problems and seek evaluation if symptoms of these conditions arise:
Atopic disorders including food, drug, or seasonal allergies and eczema
Recurrent ear and sinus infections
disorders (depression and anxiety) in childhood and adulthood
Scoliosis
Thyroid abnormalities
Urinary tract issues resulting from a kidney malformation
Constipation
Seizures
Limitations
We assume that there may in fact be unappreciated functional genomic elements within the region of 18q22.1 that encompasses the reference group’s breakpoints. Therefore, this group may eventually be appreciated to be less genetically similar than we can recognize at this time. We will also be interrogating this region of the genome to determine if there are functional elements that could account for some of the differences within this group.
Conclusion
The delineation of this reference group serves several purposes. First, the phenotype of this group can be used as a reference for 18q deletions. In our cohort of nearly 200 participants with simple terminal deletions of distal 18q, 40% have deletions that are smaller and 60% have deletions that are larger. Therefore the phenotype of this group can be thought of as a “median” phenotype. Until the time when we can provide individualized prognoses based on genotype, this summary may be used as a starting point for families trying to understand the implications of an 18q deletion for their child. General clinical summaries typically include every abnormal phenotype ever reported in someone with the deletion. However, in the case of 18q-, it is probably that a large number of individuals are not at risk for these more rare features, inappropriately skewing most summaries of 18q-.
Lastly, the collective phenotype of this Reference Group can be used as a reference with which to compare other subgroups. We previously defined the effect of TCF4 deletions on those with 18q genomic deletions (Hasi et al. 2011), and we have previously defined several phenotype critical regions that are all within the hemizygous region in this group. Therefore the Reference Group data provide a reference point to identify new critical regions for phenotypes not observed in this group that are only present in those with deletions of proximal regions.
Supplementary Material
Acknowledgments
Foremost, the authors wish to thank the families who are participants in the Chromosome 18 Clinical Research Center, for their ongoing commitment to this work and to our shared vision of a smoother road for future families. This work was primarily funded by the Chromosome 18 Registry and Research Society and the MacDonald family. Additional support was provided through the UTHSCSA, Institute for the Integration of Medicine and Science (UL1TR000149 NCATS/NIH).
References
- Bayley N. Bayley scales of infant development. 2nd ed. The Psychological Corporation; San Antonio, TX: 1993. [Google Scholar]
- Bayley N. Bayley scales of Infant and toddler development. 3rd> ed. Harcourt Assessment, Inc.; San Antonio, TX: 2006. [Google Scholar]
- Chao R, Nevin L, Agarwal P, Riemer J, Bai X, Delaney A, Akana M, JimenezLopez N, Bardakjian T, Schneider A, Chassaing N, Schorderet DF, FitzPatrick D, Kwok PY, Ellgaard L, Gould DB, Zhang Y, Malicki J, Baier H, Slavotinek A. A male with unilateral microphthalmia reveals a role for TMX3 in eye development. PLoS One. 2010 May 11;5(5):e10565. doi: 10.1371/journal.pone.0010565. doi: 10.1371/journal.pone.0010565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cody JD, Ghidoni PD, DuPont BR, Hale DE, Hilsenbeck SG, Stratton RF, Hoffman DS, Muller S, Schaub RL, Leach RJ, Kaye CI. Congenital anomalies and anthropometry of 42 individuals with deletions of chromosome 18q. Am J Med Genet. 1999;85(5):455–62. doi: 10.1002/(sici)1096-8628(19990827)85:5<455::aid-ajmg5>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Cody JD, Sebold C, Malik A, Heard P, Carter E, Crandall A, Soileau B, Semrud-Clikeman M, Cody CM, Hardies LJ, Li J, Lancaster J, Fox PT, Stratton RF, Perry B, Hale DE. Recurrent interstitial deletions of proximal 18q: a new syndrome involving expressive speech delay. Am J Med Genet Part A. 2007;143:1181–1190. doi: 10.1002/ajmg.a.31729. [DOI] [PubMed] [Google Scholar]
- Cody JD, Heard P, Crandall AC, Carter EM, Li J, Hardies LJ, Lancaster J, Perry B, Stratton RF, Sebold C, Schaub RL, Soileau B, Hill A, Hasi M, Fox PT, Hale DE. Narrowing critical regions and determining penetrance for selected 18q- phenotypes. Am J Med Genet. 2009;149A:1421–1430. doi: 10.1002/ajmg.a.32899. doi: 10.1002/ajmg.a.32899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cody JD, Carter EM, Sebold C, Heard PL, Hale DE. A gene dosage map of Chromosome 18: a map with clinical utility. Genet Med. 2009;11(11):778–82. doi: 10.1097/GIM.0b013e3181b6573d. doi:10.1097/GIM.0b013e3181b6573d. [DOI] [PubMed] [Google Scholar]
- Cody JD, Hale DE. Linking chromosome abnormality and copy number variation. Am J Med Genet A. 2011;155A(3):469–75. doi: 10.1002/ajmg.a.33849. doi: 10.1002/ajmg.a.33849. [DOI] [PubMed] [Google Scholar]
- Elliott CD. Differential ability scales. The Psychological Corporation; San Antonio, TX: 1990. [Google Scholar]
- Elliott CD. Differential ability scales. 2nd ed. Harcourt Assessment, Inc.; San Antonio, TX: 2007. [Google Scholar]
- Estrada K, Styrkarsdottir U, Evangelou E, Hsu YH, Duncan EL, Ntzani EE, Oei L, Albagha OM, Amin N, Kemp JP, Koller DL, Li G, Liu CT, Minster RL, Moayyeri A, Vandenput L, Willner D, Xiao SM, Yerges-Armstrong LM, Zheng HF, Alonso N, Eriksson J, Kammerer CM, Kaptoge SK, Leo PJ, Thorleifsson G, Wilson SG, Wilson JF, Aalto V, Alen M, Aragaki AK, Aspelund T, Center JR, Dailiana Z, Duggan DJ, Garcia M, Garcia-Giralt N, Giroux S, Hallmans G, Hocking LJ, Husted LB, Jameson KA, Khusainova R, Kim GS, Kooperberg C, Koromila T, Kruk M, Laaksonen M, Lacroix AZ, Lee SH, Leung PC, Lewis JR, Masi L, Mencej-Bedrac S, Nguyen TV, Nogues X, Patel MS, Prezelj J, Rose LM, Scollen S, Siggeirsdottir K, Smith AV, Svensson O, Trompet S, Trummer O, van Schoor NM, Woo J, Zhu K, Balcells S, Brandi ML, Buckley BM, Cheng S, Christiansen C, Cooper C, Dedoussis G, Ford I, Frost M, Goltzman D, González-Macías J, Kähönen M, Karlsson M, Khusnutdinova E, Koh JM, Kollia P, Langdahl BL, Leslie WD, Lips P, Ljunggren Ö , Lorenc RS, Marc J, Mellström D, Obermayer-Pietsch B, Olmos JM, Pettersson-Kymmer U, Reid DM, Riancho JA, Ridker PM, Rousseau F, Slagboom PE, Tang NL, Urreizti R, Van Hul W, Viikari J, Zarrabeitia MT, Aulchenko YS, Castano-Betancourt M, Grundberg E, Herrera L, Ingvarsson T, Johannsdottir H, Kwan T, Li R, Luben R, Medina-Gómez C, Palsson ST, Reppe S, Rotter JI, Sigurdsson G, van Meurs JB, Verlaan D, Williams FM, Wood AR, Zhou Y, Gautvik KM, Pastinen T, Raychaudhuri S, Cauley JA, Chasman DI, Clark GR, Cummings SR, Danoy P, Dennison EM, Eastell R, Eisman JA, Gudnason V, Hofman A, Jackson RD, Jones G, Jukema JW, Khaw KT, Lehtimäki T, Liu Y, Lorentzon M, McCloskey E, Mitchell BD, Nandakumar K, Nicholson GC, Oostra BA, Peacock M, Pols HA, Prince RL, Raitakari O, Reid IR, Robbins J, Sambrook PN, Sham PC, Shuldiner AR, Tylavsky FA, van Duijn CM, Wareham NJ, Cupples LA, Econs MJ, Evans DM, Harris TB, Kung AW, Psaty BM, Reeve J, Spector TD, Streeten EA, Zillikens MC, Thorsteinsdottir U, Ohlsson C, Karasik D, Richards JB, Brown MA, Stefansson K, Uitterlinden AG, Ralston SH, Ioannidis JP, Kiel DP, Rivadeneira F. Genome-wide meta-analysis identifies 56 bone mineral density loci and reveals 14 loci associated with risk of fracture. Nature Genet. 2012;44:491–501. doi: 10.1038/ng.2249. doi: 10.1038/ng.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eudy JD, Pickering DL, Lutz R, Platt K, Dave BJ, Olney AH, Sanger WG. 18q22.3 > 18q23 deletion syndrome and cleft palate. Am J Med Genet Part A. 2010;152A:1046–1048. doi: 10.1002/ajmg.a.33336. doi: 10.1002/ajmg.a.33336. [DOI] [PubMed] [Google Scholar]
- Feenstra I, Vissers LELM, Pennings RJE, Nillessen W, Pfundt R, Kunst HP, Admiraal RJ, Veltman JA, van Ravenswaaij-Arts, Brunner HG, Cremers CWRJ. Disruption of teashirt zinc finger homeobox 1 is associated with congenital aural atresia in humans. An J Hum Genet. 2011;89:813–819. doi: 10.1016/j.ajhg.2011.11.008. doi: 10.1016/j.ajhg.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilliam JE. Gilliam autism rating scale. Pro-Ed, Inc.; Austin, TX: 1995. [Google Scholar]
- Gilliam JE. Gilliam asperger’s disorder scale. Pro-Ed, Inc.; Austin, TX: 2001. [Google Scholar]
- Gilliam JE. Gilliam autism rating scale. 2nd ed. Pro-Ed, Inc.; Austin, TX: 2006. [Google Scholar]
- Gioia GA, Isquith PK, Guy SC, Kenworthy L. Behavior rating inventory of executive function. Psychological Assessment Resources, Inc.; Lutz, FL: 2000. [Google Scholar]
- Hale DE, Cody JD, Baillargeon J, Schaub R, Danney MM, Leach RJ. The spectrum of growth abnormalities in children with 18q deletions. J Clin Endocrinol Metab. 2000;85(12):4450–4. doi: 10.1210/jcem.85.12.7016. [DOI] [PubMed] [Google Scholar]
- Hasi M, Soileau BT, Sebold C, Hill A, Hale DE, O’Donnell L, Cody JD. The role of the TCF4 gene in the phenotype of individuals with 18q segmental deletions. Hum Genet. 2011;130:777–787. doi: 10.1007/s00439-011-1020-y. doi: 10.1007/s00439-011-1020-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heard PL, Carter EM, Crandall AC, Sebold C, Hale DE, Cody JD. High resolution genomic analysis of 18q- using oligo-microarray comparative genomic hybridization (aCGH) Am J Med Genet. 2009;149A:1431–1437. doi: 10.1002/ajmg.a.32900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnankivi T, Tienari P, Somer M, Kähkönen M, Lönnqvist T, Valanne L, Pihko H. 18q deletions: Clinical, molecular, and brain MRI findings of 14 individuals. AM J Med Genet. 2006;140A:331–339. doi: 10.1002/ajmg.a.31072. [DOI] [PubMed] [Google Scholar]
- Margarit E, Morales C, Rodriguez L, Monne R, Badenas C, Soler A, Clusellas N, Mademont I, Sanchez A. Familial 4.8 Mb deletion on 18q23 associated with growth hormone insufficiency and phenotypic variability. Am J Med Genet 158A:611-616. 2012 doi: 10.1002/ajmg.a.34221. doi: 10.1002/ajmg.a.34221. [DOI] [PubMed] [Google Scholar]
- Mark PR, Radlinski BC, Core N, Fryer A, Kirk EP, Haldeman-Englert CR. Narrowing the critical region for congenital vertical talus in patients with interstitial 18q deletions. Am J Med Genet A. 2013;161(5):1117–21. doi: 10.1002/ajmg.a.35791. doi: 10.1002/ajmg.a.35791. [DOI] [PubMed] [Google Scholar]
- Martin M, Hiltner TD, Wood JC, Fraser SE, Jacobs RE, Readhead C. Myelin deficiencies visualized in vivo: visually evoked potentials and T2-weighted magnetic resonance images of shiverer mutant and wild-type mice. J Neurosci Res. 2006;84(8):1716–26. doi: 10.1002/jnr.21086. [DOI] [PubMed] [Google Scholar]
- Ng D, Pitcher GM, Szilard RK, Sertié A, Kanisek M, Clapcote SJ, Lipina T, Kalia LV, Joo D, McKerlie C, Cortez M, Roder JC, Salter MW, McInnes RR. Neto1 is a novel CUB-domain NMDA receptor-interacting protein required for synaptic plasticity and learning. PLoS Biol. 2009;7(2):e41. doi: 10.1371/journal.pbio.1000041. doi: 10.1371/journal.pbio.1000041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto D, Pagnamenta AT, Klei L, Anney R, Merico D, Regan R, Conroy J, Magalhaes TR, Correia C, Abrahams BS, Almeida J, Bacchelli E, Bader GD, Bailey AJ, Baird G, Battaglia A, Berney T, Bolshakova N, Bölte S, Bolton PF, Bourgeron T, Brennan S, Brian J, Bryson SE, Carson AR, Casallo G, Casey J, Chung BH, Cochrane L, Corsello C, Crawford EL, Crossett A, Cytrynbaum C, Dawson G, de Jonge M, Delorme R, Drmic I, Duketis E, Duque F, Estes A, Farrar P, Fernandez BA, Folstein SE, Fombonne E, Freitag CM, Gilbert J, Gillberg C, Glessner JT, Goldberg J, Green A, Green J, Guter SJ, Hakonarson H, Heron EA, Hill M, Holt R, Howe JL, Hughes G, Hus V, Igliozzi R, Kim C, Klauck SM, Kolevzon A, Korvatska O, Kustanovich V, Lajonchere CM, Lamb JA, Laskawiec M, Leboyer M, Le Couteur A, Leventhal BL, Lionel AC, Liu XQ, Lord C, Lotspeich L, Lund SC, Maestrini E, Mahoney W, Mantoulan C, Marshall CR, McConachie H, McDougle CJ, McGrath J, McMahon WM, Merikangas A, Migita O, Minshew NJ, Mirza GK, Munson J, Nelson SF, Noakes C, Noor A, Nygren G, Oliveira G, Papanikolaou K, Parr JR, Parrini B, Paton T, Pickles A, Pilorge M, Piven J, Ponting CP, Posey DJ, Poustka A, Poustka F, Prasad A, Ragoussis J, Renshaw K, Rickaby J, Roberts W, Roeder K, Roge B, Rutter ML, Bierut LJ, Rice JP, Salt J, Sansom K, Sato D, Segurado R, Sequeira AF, Senman L, Shah N, Sheffield VC, Soorya L, Sousa I, Stein O, Sykes N, Stoppioni V, Strawbridge C, Tancredi R, Tansey K, Thiruvahindrapduram B, Thompson AP, Thomson S, Tryfon A, Tsiantis J, Van Engeland H, Vincent JB, Volkmar F, Wallace S, Wang K, Wang Z, Wassink TH, Webber C, Weksberg R, Wing K, Wittemeyer K, Wood S, Wu J, Yaspan BL, Zurawiecki D, Zwaigenbaum L, Buxbaum JD, Cantor RM, Cook EH, Coon H, Cuccaro ML, Devlin B, Ennis S, Gallagher L, Geschwind DH, Gill M, Haines JL, Hallmayer J, Miller J, Monaco AP, Nurnberger JI, Jr, Paterson AD, Pericak-Vance MA, Schellenberg GD, Szatmari P, Vicente AM, Vieland VJ, Wijsman EM, Scherer SW, Sutcliffe JS, Betancur C. Functional impact of global rare copy number variation in autism spectrum disorders Nature. 2010;466(7304):368–72. doi: 10.1038/nature09146. doi: 10.1038/nature09146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth RM, Isquith PK, Gioia GA. Behavior rating inventory of executive function – adult version. Psychological Assessment Resources, Inc.; Lutz, FL: 2005. [Google Scholar]
- Reynolds CR, Kamphaus RW. Behavior assessment system for children. American Guidance Service, Inc.; Circle Pines, MN: 1992. [Google Scholar]
- Reynolds CR, Kamphaus RW. Behavior assessment system for children. 2nd ed. AGS Publishing; Circle Pines, MN: 2004. [Google Scholar]
- Ren CM, Liang Y, Wei F, Zhang YN, Zhong SQ, Gu H, Dong XS, Huang YY, Ke H, Son XM, Tang D, Chen Z. Balanced translocation t(3;18)(p13;q22.3) and points mutation in the ZNF407 gene detected in patients with both moderate non-syndromic intellectual disability and autism. Biochim Biophys Acta. 2013;1832(3):431–438. doi: 10.1016/j.bbadis.2012.11.009. doi:10.1016/j.bbadis.2012.11.009. [DOI] [PubMed] [Google Scholar]
- Sparrow SS, Balla DA, Cicchetti DV. Vineland adaptive behavior scales. Circle Pines, MN; American Guidance Service, Inc.: 1984. [Google Scholar]
- Sparrow SS, Cicchetti DV, Balla DA. Vineland adaptive behavior scales. 2nd ed. AGS Publishing; Circle Pines, MN: 2005. [Google Scholar]
- van Trier DC, Feenstra I, Bot P, de Leeuw N, Draaisma JMTh. Cardiac anomalies in individuals with the 18q deletion syndrome. Eur J Med Genet. 2013 doi: 10.1016/j.ejmg.2013.05.002. doi: 10.1016/j.ejmg.2013.05.002. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler adult intelligence scale. 3rd ed. The Psychological Corporation; San Antonio, TX: 1997. [Google Scholar]
- Wechsler D. Wechsler adult intelligence scale. 4th ed. Pearson; San Antonio, TX: 2008. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.