Abstract
BACKGROUND
Non-surgical bleeding (NSB) is a major complication among heart failure (HF) patients supported by CF-LVADs. Understanding the hemostatic defects contributing to NSB after CF-LVAD implantation is crucial for prevention of this adverse event. The aim of this study was to examine the link between platelet GPIbα ectodomain shedding and NSB in CF-LVAD recipients and to identify a potential biomarker of NSB.
METHODS
Serial blood samples were collected from thirty five HF patients supported with CF-LVADs. Platelet function was evaluated by Platelet Function Analyzer 100® and thromboelastography (TEG). Platelet GPIbα shedding, von Villebrand factor (vWF) antigen and vWF collagen binding capacity were determined using enzyme-linked immunosorbent assays (ELISAs). The structural analysis of vWF was performed by gel electrophoresis. These platelet functional measures with vWF parameters of the patients who experienced NSB between 4 to 32 days after CF-LVAD implantation (bleeder) were analyzed against those without NSB (non-bleeder). Blood samples from seven healthy individuals were collected to obtain the healthy reference values for the laboratory assays.
RESULTS
Elevated GPIbα shedding was found to be a preexisting condition in all HF patients prior to CF-LVAD implantation. Post-operative level of GPIbα shedding increased and remained elevated in the bleeder group while a consistent decrease was found in the non-bleeder group. A receiver operating characteristic (ROC) analysis indicated that the level of GPIbα shedding has a predictive power of NSB in patients supported with CF-LVADs.
CONCLUSION
Platelet GPIbα ectodomain shedding which attenuates platelet reactivity is associated with NSB. Plasma GPIbα level may potentially be used to refine bleeding risk stratification in CF-LVAD patients.
Keywords: heart failure, left ventricular assist device, non-surgical bleeding, platelet GPIbα shedding
Introduction
Mechanical circulatory support (MCS) therapy has evolved into a standard therapy for patients with advanced HF,1,2 either as a destination therapy or a bridge to cardiac transplantation or a bridge to myocardial recovery.3,4 The number of patients receiving MCS therapy quadrupled over the last five years.5 Although the recent data suggest that approximately 90 and 85% of patients supported with CF-LVADs will survive at 6 and 12 months respectively,6,7 right ventricular failure, multi-organ failure, infection, and most importantly bleeding8–11 remain significant problems for the MCS therapy. The recent INTERMACS annual report showed that bleeding was the most frequent adverse event.5
The higher prevalence of bleeding in patients with CF-LVADs was reported by several groups, compared to bleeding in patients supported with the HeartMate XVE pulsatile LVAD.10,12 Other studies found that almost all patients with CF-LVADs were diagnosed with acquired von Willebrand syndrome with significant loss of high molecular weight von Willebrand Factor (HMW-vWF) multimers.13,14 Impaired platelet aggregation was suggested as a cause of bleeding in patients with CF-LVADs.15 Altered primary hemostasis is a concern because of patients’ medication regimens that include platelet inhibitors and anticoagulation.14 The interaction of platelets with vWF initiates primary hemostasis. Therefore, the defect of either platelet function or vWF could result in impaired primary hemostasis. The impaired platelet aggregation in LVAD recipients could be caused by either dysfunctional platelets or plasma coagulation factors of patients or both.15
CF-LVADs are made of artificial biomaterials and employ a high speed rotating impeller to draw blood from the left ventricle and to pump it to the aorta. Non-physiological high shear stresses exist in some region within these devices.16 Exposure of blood to elevated shear stresses can not only result in fragmentation of HMW-vWF,17 but also cause shear-induced platelet activation and receptor shedding.18,19 Platelet receptor shedding has been thought to be a key mechanism for platelet dysfunction.20 We examined the platelet GPIbα shedding in serially collected blood samples from patients supported with two CF-LVADs for their prospective usefulness in identifying NSB in patients.
Methods
All procedures involving collection of human blood were approved by the Institutional Review Board (IRB). All patients and volunteers gave their written informed consent and were informed about the aim of the study.
Patients
From 2008 to 2012, sixty two HF patients were implanted with LVADs either as a bridge to transplant (94%) or destination therapy (6%) at the University of Maryland Medical Center. Thirty five patients were enrolled in this study and were implanted with the HeartMate II CF-LVAD (n = 21) or the Jarvik 2000 CF-LVAD (n = 13). Among the thirty five patients, four patients received the Levitronix CentriMag for right ventricular support along with either the HeartMate II (n = 2) or the Jarvik 2000 (n = 2), one patient was implanted twice with the Jarvik 2000 CF-LVAD and one patient was initially implanted with a HeartMate II LVAD which was replaced with another HeartMate II CF-LVAD and followed by a Jarvik 2000 LVAD.
NSB in these patients was defined as any major bleeding requiring intervention and/or packed red blood cell transfusion after CF-LVAD implantation without an identified surgical source. NSB was identified with the evidence of guaiac-positive stool, melena, hematochezia, hemothorax not requiring re-operation or epistaxis. The gastrointestinal bleeding was identified by an endoscopic procedure that provides visualization of the upper part of the gastrointestinal tract up to the duodenum. It does not require an incision into one of the major body cavities and does not require any significant recovery after this procedure.
Anticoagulation was initiated with a titrated heparin dose with the goal for partial thromboplastin time of 40–45s once chest drainage was less than 30 mL/h for at least 4 hours, and then the goal was aimed to have an anti-Xa activity level of 0.1–0.15 U/mL. This was subsequently converted to warfarin with an international normalized ratio (INR) goal from 1.8 to 2.3 for the HeartMate II, and 2 to 3 for the Jarvik 2000. Antiplatelet agents were titrated using the Platelet Function Analyzer (PFA-100, Dade Behring, Inc, Deerfield, IL) and thrombelastogram (TEG) (TEG® 5000 Thrombelastograph® Hemostasis Analyzer System, Haemonetics Corporation, Braintree, MA). All the patients received pentoxifylline to improve red blood cell (RBC) deformability in the hope of mitigating shear-induced hemolysis.
Sample collection
EDTA anticoagulated whole blood samples were collected from the patients before CF-LVAD implant surgery (baseline) and multiple time point after CF-LVAD implantation up to 32 days. Microparticle free plasma samples from the HF patients and 7 healthy volunteers were prepared by centrifugation at 1500×g for 15 min at room temperature followed by microcentrifuge at 20,000×g for 30 min at 4°C. Then the plasma samples were stored at −80°C for further analysis. Plasma samples from healthy donors were analyzed to obtain normal values for laboratory assays.
Platelet Function Test
The platelet function test was performed by using the PFA-100. The closure time (CT) for the collagen/ADP (CADP) or collagen/epinephrine (CEPI) cartridge, which is dependent on platelet function, plasma von Willebrand Factor level, platelet number, and hematocrit (to some extent), was used to evaluate platelet dysfunction. We also used thromboelastography (TEG) to assess the platelet function. The TEG-maximum amplitude (TEG-MA), kinetic time (TEG-KT) and angle (TEG-Angle) were analyzed.
Measurement of plasma GPIbα
The level of platelet GPIbα shedding was determined by a newly developed ELISA. The presence of residual platelets and platelet vesicles may give a false high concentration of plasma GPIbα.21 To avoid the interference of platelet microparticles, the plasma GPIbα was determined using microparticle-free plasma. Two monoclonal antibodies were used to detect 45-kDa extracellular fragment of GPIbα. Nunc MaxiSorp® flat-bottom 96 well plate (Nunc, Rochester, NY) were coated with 0.75 μg/mL monoclonal anti-GPIbα antibody (Abcam, Cambridge, MA) in 1×PBS buffer overnight at 4°C. Wells were washed and blocked with 1% (v/v) BSA. Human recombinant GPIbα ectodomain protein (R&D Systems, Inc., Minneapolis, MN) was used to generate a standard curve. Fifty microliters of plasma was added to the coated plate for 2 hours at room temperature. After washing, biotinylated monoclonal antibody (clone 486805, R&D Systems, Inc., Minneapolis, MN) was added at 0.75 μg/mL and followed by incubating with streptavidin peroxidase (Pierce, Rockford, IL). The concentration of soluble GPIbα was determined by incubating with tetramethylbenzidine (TMB) (Pierce, Rockford, IL) as substrate, and the absorbance at 450 nm was measured using a spectrophotometer (SpectraMax Plus384 Microplate Reader, Molecular Device, Sunnyvale, CA).
Measurement of vWF Parameters
Out of 35 HF patients enrolled in the study, vWF parameters were measured only for 24 patients (15 bleeders and 9 non-bleeders) because there was not a sufficient volume of plasma from other patients. Plasma vWF antigen and vWF collagen binding capacity were determined by ELISA using commercially available kits [REAADS® vWF Antigen Test Kit (cat no. 034-001) and Collagen Binding Assay Kit (cat no. 11160) from Corgenix, Inc., Broomfield, CO]. Electrophoresis with SDS-agarose gel (0.6%) was used to display VWF multimers. The vWF multimers were detected by western blot with polyclonal rabbit anti-human-vWF-horseradish peroxidase antibody (Dako, Glostrup, Denmark) and visualized using ECL western blotting detection reagents (Amersham Life Science, NJ).
Data Analysis
Data are presented as mean ± SE unless otherwise indicated for all serial measurements. Statistical analysis was done using SPSS statistical software (Statistical Package for Social Sciences for Windows, release 10.0; SPSS Inc., Chicago, IL, USA). Statistical significance was assigned at p<0.05.
To describe an overall pattern of change in repeated measurements of plasma GPIbα and other platelet function tests over time, linear mixed effect models were built using penalized splines to discern the trend of the data. The log (natural) transformed data were used in the model generation. To investigate whether or not the plasma GPIbα can predict NSB in the future 7 days, a receiver operating characteristic (ROC) curve was constructed based on the approach of Liu et al.22,23 The summary statistics of the area under the ROC curve (AUC) was used to evaluate the predictive power of the plasma GPIbα. In this analysis, the generated linear mixed model was used to estimate the predicted probabilities of bleeding complication firstly and these estimated probabilities were then used to construct the ROC curve. Since each patient has multiple measurements, the observations within a given subject will no longer be independent. The intra-subject correlation and variation were introduced for the ROC generation and AUC evaluation.
Results
NSB and demography
Twenty two patients experienced at least one episode of NSB between 4 to 32 days during CF-LVAD support (bleeder group). Comparative analyses of demographic and clinical characteristics of the patients in the bleeder group and those who did not experience NSB (non-bleeder group) before CF-LVAD implantation were summarized in Table 1. There were no significant differences in these characteristics in the two groups. Demographic information of young healthy volunteers without any cardiac complications is also presented in Table 1 only to show the reference values.
Table 1.
Demographic and baseline clinical characteristics of HF patients before LVAD implantation
| Characteristics | Healthy volunteers (n = 7) | Pre-operative HF patients (n = 35)
|
|
|---|---|---|---|
| Non-bleeder group (n = 13) | Bleeder group (n = 22) | ||
| Demography | |||
| Age in years, median (range) | 26 (23 – 31) | 62 (44 – 68) | 61 (40 – 82) |
| Sex (% male) | 85.7 | 92.3 | 95.5 |
| Height in meter, median (range) | 1.80 (1.56 – 1.84) | 1.75 (1.68 – 1.85) | 1.76 (1.68 – 1.84) |
| Weight in kilograms, median (range) | 89.45 (62.30 – 107.05) | 94.40 (63.50 – 116.40) | 97.56 (63.80 – 115.96) |
| Body mass index (kg/m2), median (range) | 27.66 (24.56 – 32.91) | 28.96 (19.47 – 39.35) | 30.93 (19.05 – 39.01) |
| History of smoking | |||
| Smokers (%) | 14.29 | 69.23 | 72.73 |
| Non-smokers (%) | 85.71 | 30.77 | 27.27 |
| History of alcoholism | |||
| Alcoholic (%) | 0 | 76.92 | 77.27 |
| Non-alcoholic (%) | 100 | 23.08 | 22.73 |
| Vital signs | |||
| Systolic blood pressure (mmHg), mean ± SD | 126.29 ± 13.90 | 103.11 ± 19.53 | 108.6 ± 9.86 |
| Diastolic blood pressure (mmHg), mean ± SD | 74.86 ± 11.94 | 62.22 ± 13.39 | 60.86 ± 11.99 |
| Past medical history | |||
| Heart failure (%) | 0 | 100 | 100 |
| Diabetes mellitus (%) | 0 | 76.92 | 77.27 |
| Hypertension (%) | 0 | 61.54 | 63.64 |
| Etiology of heart disease | |||
| Ischemic cardiomyopathy (%) | 0 | 53.85 | 54.55 |
| Non-ischemic cardiomyopathy (%) | 0 | 46.15 | 45.45 |
| Echocardiographic parameters | |||
| Left ventricular end diastolic diameter (mm), mean ± SD | – | 63.70 ± 10.40 | 60.77 ±10.75 |
| Left ventricular ejection fraction, (%),mean ± SD | – | 15.00 ± 2.90 | 14.62 ± 1.39 |
Demographic and clinical parameters of non-bleeder versus bleeder groups of HF patients were statistically compared by Mann-Whitney ‘U’ test (for median values with range), χ2-test (for results presented as percentages) and Student’s t-test as applicable.
p<0.05 is considered significant.
A total of 30 bleeding episodes mostly from gastrointestinal tract (GI) were recognized in 22 patients during the 32-day study period; 1 patient had 4 episodes, 5 patients had 2 episodes and 16 patients had 1 episode of NSB after CF-LVAD implantation. The median time of the first NSB episode was 15 days and varied from 4 to 32 days. In the bleeder group, there were 14 patients supported with the HeartMate II and 8 patients with the Jarvik 2000. All the patients were transfused with either RBCs or fresh frozen plasma or platelets. Anti-coagulation regimen at the time of bleeding was clinically optimized individually for each patient. No thromboembolic events occurred.
Laboratory hematology and blood chemistry
The comparison of routine laboratory hematologic and blood chemistry tests between the non-bleeder and bleeder groups before and after CF-LVAD implantation is summarized in Table 2. There were no significant differences in hematology and blood chemistry parameters between the non-bleeder and bleeder groups before LVAD implantation. Erythrocytes, hemoglobin, hematocrit and bilirubin (total and direct) significantly decreased after LAVD implantation in both the groups. Blood urea nitrogen (BUN) and INR increased after implantation in both the groups. The increase in BUN was more prominent in the bleeder group. Creatinine decreased significantly in the bleeder group after CF-LVAD implantation. There were no significant differences in the other tests between the two groups.
Table 2.
Laboratory Hematology and blood chemistry of non-bleeder and bleeder group of HF patients at baseline (Pre-LVAD) and at post-operative month one (Post-LVAD)
| Variable | Reference value | HF patients (n= 35) | |||
|---|---|---|---|---|---|
| Pre-LVAD (n = 35) | Post-LVAD (n = 35) | ||||
|
| |||||
| I | II | III | IV | ||
|
| |||||
| Non-bleeder group (n = 13) | Bleeder group (n = 22) | Non-bleeder group (n = 13) | Bleeder group (n = 22) | ||
| Leukocytes (×103/μL) | 4.5–11.0 | 7.94 ± 3.11 | 8.03 ± 2.84 | 10.87 ± 3.10a | 11.04 ± 2.95b |
| Erythrocytes (×106/μL) | 4.0–5.7 | 4.34 ± 1.12 | 4.06 ± 1.10 | 3.21 ± 0.97 a | 2.14 ± 0.57b,c |
| Platelet (×103/μL) | 153–367 | 202.70 ± 71.70 | 207.23 ± 73.99 | 309 ± 215.05 | 231.50 ±121.96 |
| Hemoglobin (g/dL) | 12.6–17.4 | 10.75 ± 2.34 | 10.24 ± 1.71 | 9.03 ± 1.02 a | 8.39 ± 0.78b,c |
| Hematocrit (%) | 37–50 | 35.15 ± 7.59 | 34.68 ± 8.37 | 29.08 ± 3.11a | 27.72 ± 2.35b |
| BUN (mg/dL) | 9–20 | 28.35 ± 8.94 | 30.48 ± 10.31 | 37.46 ± 13.61 | 50.37 ± 19.82b,c |
| Creatinine (mg/dL) | 0.66–1.25 | 1.41 ± 0.83 | 1.44 ± 0.58 | 1.59 ± 1.01 | 1.09 ± 0.23b,c |
| Total bilirubin (mg/dL) | 0.2–1.3 | 2.51 ± 1.03 | 2.63 ± 1.11 | 1.29 ± 0.56 a | 1.52 ± 0.72b |
| Direct bilirubin (mg/dL) | 0.1–0.5 | 0.78 ± 0.21 | 0.92 ± 0.14 | 0.41 ± 0.12 a | 0.63 ± 0.23b,c |
| AST (U/L) | 17–59 | 49.38 ± 14.38 | 50.87 ± 17.51 | 44.19 ± 10.36 | 54.39 ± 21.28 |
| ALT (U/L) | 21–72 | 43.33 ± 19.61 | 47.63 ± 23.03 | 50.29 ± 18.81 | 52.09 ± 24.11 |
| INR | <3.9 | 1.25 ± 0.31 | 1.41 ± 0.43 | 1.97 ± 0.82 a | 2.24 ± 0.76b |
| PTT | 25–28 | 41.37 ± 8.12 | 42.09 ± 6.39 | 40.82 ± 7.91 | 44.07 ± 8.73 |
BUN, blood urea nitrogen; AST, aspartate aminotransferase; ALT, alanine aminotransferase; INR, international normalized ratio; PTT, partial thromboplastin time. Results are expressed as mean ± standard deviation and a, b, c, p<0.05 is considered significant in Student’s t-test. I, II, III and IV represents group category.
I vs. III group,
II vs. IV group,
III vs. IV group.
Platelet Function Tests
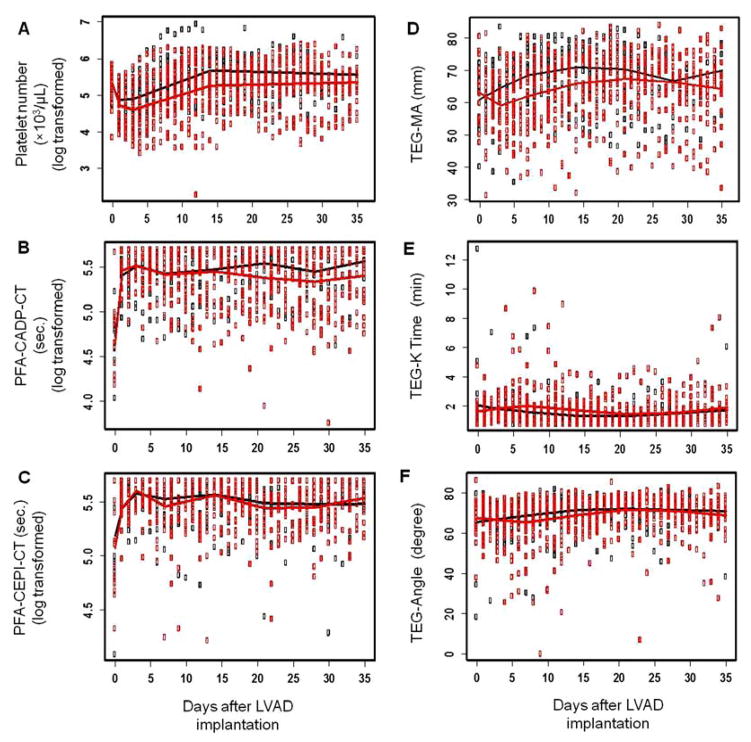
The platelet counts of the patients in the bleeder and non-bleeder groups are shown in Figure 1A. The platelet count dropped initially after CF-LVAD implantation and gradually returned to the baseline level after two weeks. The mean CTs for the CADP and CEPI cartridges before implantation were slightly higher than the normal range. The elevated CT for the CEPI cartridge might be related to intake of aspirin by 15 patients before implantation. The mean CTs for the CADP and CEPI cartridges in both the bleeder and non-bleeder groups increased immediately after implantation and remained at the elevated levels during CF-LVAD support (Figure 1B and 1C). These elevations were associated with the anticoagulation treatment. There was no significant difference in the severity of the primary hemostatic defect indicated by the PFA tests between the non-bleeder and bleeder groups.
Figure 1.
The thromboelastogram data (TEG-MA, TEG-K Time and TEG-Angle) between the bleeder and non-bleeder groups are shown in Figure 1D–1F. The mean values of the TEG-MA and TEG-K times of the two groups were in the normal range between 50 and 70 and between 1 and 3 min, respectively. To compare the TEG-MA (platelet aggregation) level between the bleeder and non-bleeder groups, linear mixed effect models for the TEG-MA data of the two groups were built using penalized splines. The fitted line for the TEG-MA data was found to be a piecewise liner model with knots at day 3, 7, 14, 21 and 28 for both the bleeder and non-bleeder groups, respectively (Figure 1D). Although the mean value of the TEG-MA of the bleeder group is lower than that of the non-bleeder group, there was no significant difference between the two groups (P = 0.382). The TEG-K time and TEG angle were almost the same between the two groups throughout the study period (Figure 1E and 1F).
Platelet GPIbα ectodomain shedding
The level of platelet GPIbα shedding in the reference group was rarely detectable using the custom ELISA. However, the patients from both the non-bleeder and bleeder groups exhibited much higher levels of GPIbα shedding in their baseline blood samples. The mean values of plasma GPIbα were 10.82 ± 3.07 ng/mL and 34.45 ± 15.14 ng/mL for the non-bleeder and bleeder groups, respectively, indicating that platelet GPIbα shedding was a preexisting condition in all the HF patients in this study (Figure 2A). In particular, the baseline plasma GPIbα in three patients from the bleeder group exceeded 100 ng/mL. Although GPIbα shedding was more pronounced in the bleeder group compared with that in the non-bleeder group before implantation, there was no significant difference (p=0.2964) between the two groups. After excluding the three patients with extremely high plasma GPIbα in the bleeder group, the bleeder and non-bleeder groups had almost the same value of the baseline plasma GPIbα (10.39±3.21 ng/mL vs. 10.82±3.07 ng/mL, p=0.9306).
Figure 2.
The level of plasma GPIbα decreased in the non-bleeder group after implantation, but increased at the 1st and 2nd weeks in the bleeder group. One patient in the bleeder group had a consistently high level of plasma GPIbα (>100 ng/mL). To compare the levels of plasma GPIbα between the non-bleeder and bleeder groups, a liner mixed effect model was utilized to discern the trend of the curve using penalized splines. The fitted lines of the plasma GPIbα is a linear model for the non-bleeder group and is a linear model with both linear and quadratic terms for the bleeder group. The test of the difference between the two fitted lines is statistically significant at the level of 0.05 (Figure 2B). We noticed more than 5-fold increase in the plasma GPIbα in the bleeder group compared to the non-bleeder group (45.15±8.77 vs. 8.46±2.66 ng/mL, p<0.001 in Students t-test) at 5th week after implantation even after excluding one patient with extremely high plasma GPIbα.
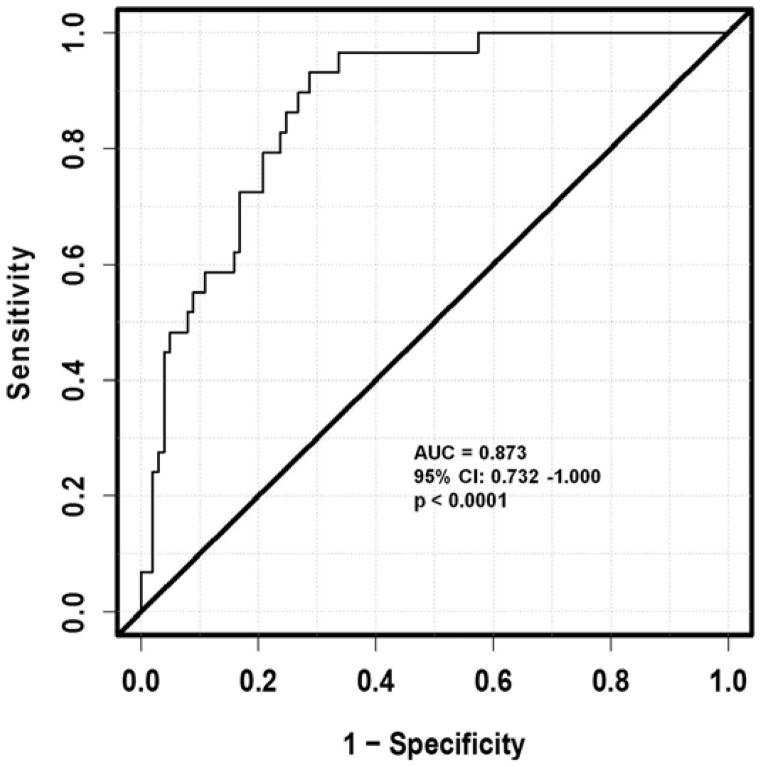
Estimation of ROC Curves for Repeated Measures Design
The fitted ROC curve for the plasma GPIbα is shown in Figure 3. The estimated AUC is 0.873 (95% CI: 0.732 – 1.000). This statistic indicates that if we randomly select a patient who is bleeding and a patient who is not bleeding on some specific days, the probability of bleeding (as predicted by the measured plasma GPIbα while controlling patient characteristics) being greater for the bleeding patient than for the non-bleeding patient is 0.873. To test whether the AUC is significantly different from 0.5, a t-statistic of 5.18 was obtained (using the estimated standard error of 0.072 from 300 replicates with bootstrapping), which yields a p-value of less than 0.0001, significant at a type I error rate of 5%. This means that the discriminating power of the bio-marker of the predicted probability for bleeding by the GPIbα measurement from the repeated measures model is significantly larger than that of chance alone. Thus the measured plasma GPIbα as a potential biomarker has a predictive power for NSB.
Figure 3.
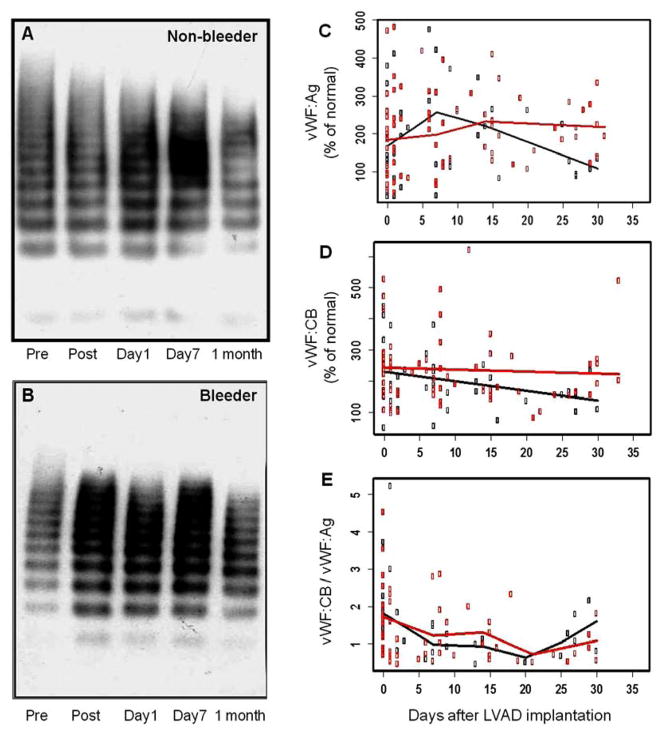
vWF analysis
As shown in Figure 4A and 4B, there was a reduction or absence of HMW-vWF multimers in the western blots after CF-LVAD implantation for both the bleeder and non-bleeder groups. These results, consistent with the previous observation13, suggested that the loss of HMW-vWF could not well explain the bleeding complication associated with CF-LVAD implantation. To compare vWF:Ag, vWF:CB and vWF:CB/vWF:Ag levels between the bleeder and non-bleeder groups, liner mixed effect models were generated (Figure 4C–4E). The fitted line for vWF:Ag was found to be a piecewise linear model with knots at day 7 and 14 for the bleeder group and with a knot at day 7 for the non-bleeder group, respectively. The same piecewise linear model was found for vWF:CB/vWF:Ag with knots at day 7,14 and 21 for both the bleeder and non-bleeder groups. A linear model without knots was found for vWF:CB for both the bleeder and non-bleeder groups. Although the mean values of vWF:Ag and vWF:CB in the bleeder group were higher than the non-bleeder group at the end of the study period, there were no significant differences between the two groups (P >0.05).
Figure 4.
Discussion
Previously, NSB was not recognized as a significant adverse event of postoperative morbidity in the REMATCH trial of the pulsatile LVAD.24–26 However, CF-LVADs have been associated with an increased incidence of NSB especially in the GI tract.11,27 Several possible explanations have been offered to explain the increased incidence of NSB associated with CF-LVADs.12,27,28–33 The possible mechanism of GI bleeding in patients with CF-LVADs is suggested to be the acquired von Willebrand disease which is manifested as a significant loss of HMW-vWF. Other studies suggested that the left ventricle of patients with CF-LVADs is relatively decompressed with minimal opening of the aortic valve, creating flow patterns similar to those observed with aortic stenosis, a condition that has been associated with GI bleeding.11,27,34–38 Primary hemostasis is initiated by surface-bound vWF which tethers platelets to a damaged vascular surface via the platelet GPIb-V-IX complex.39 Thus, the abnormalities of either platelet or vWF physiology could result in impaired primary hemostasis.
We examined the possible link between the platelet GPIbα shedding and NSB in patients with the two CF-LVADs. Platelet GPIbα is expressed as the ligand-binding subunit of the platelet GPIb-IX-V complex with vWF. The interaction produces transmembrane signaling and platelet activation. Platelet GPIbα shedding can release this ectodomain into the plasma pool as a soluble form. Since the loss of GPIbα molecules will impair receptor binding sites for vWF, GPIbα shedding potentially regulates GPIbα-dependent platelet function during thrombus formation.20,40 Circulating soluble GPIbα may also act as a potential inhibitor of platelet adhesion and aggregation to the injured vessel wall. An earlier study reported that the increased plasma GPIbα level is associated with the decreased ristocetin-induced platelet aggregation and results in the increase of bleeding risk.18 In the present study, Spearman rank correlation test did not established any significant association of GPIbα shedding with vWF parameters in either the non-bleeder group (ρ = 0.1548, p = 0.4503 for vWF:Ag and ρ = 0.3618, p = 0.0823 for vWF:CB) or bleeder group (ρ = −0.1159, p = 0.5345 for vWF:Ag and ρ = 0.2795, p = 0.1348).
Our results demonstrated that the GPIbα shedding was rarely detectable in the healthy subjects, but elevated significantly in all the HF patients. The results provide the first evidence that the GPIbα shedding is related to this advanced form of heart disease. This pre-existing GP1bα shedding together with CF-LVAD induced GPIbα elevation and acquired vWD may predispose the patient to bleeding complications. Although the difference in GPIbα shedding between the non-bleeder and bleeder groups before CF-LVAD implantation was not statistically significant, the GPIbα shedding exhibited different trends in the two groups after implantation. The level of GPIbα shedding decreased after the CF-LVAD implantation in the non-bleeder group while elevated further in the bleeder group in the first two weeks after implantation. The trends were statistically different although there were large variations in the level of GPIbα shedding in both the groups. This significantly high extent of proteolysis of GPIbα in the bleeder group might result in acquired platelet dysfunction and is associated with NSB during CF-LVAD support and may be a potential diagnostic biomarker for predicting NSB in patients with CF-LVADs. This observation also suggested that the response of platelets of the patients in the bleeder group to CF-LVAD support was different from that in the non-bleeder group.
We further verified the predictability of the measured GPIbα level for bleeding using the method designed for ROC analysis for the data from a repeated measures design. This approach has several advantages, including reducing the possible bias from one snapshot of data collected from each subject and providing the opportunity to analyze the intra-patient variation as well as the change over time.23 Being able to take these advantages is important because 6 patients in the bleeder group had developed recurrent NSB with 2 to 4 episodes. Based on this statistical analysis, the estimated AUC from the ROC curve of the measured GPIbα is 0.873 (95% CI: 0.732 ~ 1.000). According to the common statistical standard for the AUC, the level of plasma GPIbα would be a good biomarker as a diagnostic test for NSB in patients with CF-LVADs. In conclusion, this study provide first evidence that the platelet GPIbα ectodomain shedding which attenuates platelet reactivity is associated with NSB in patients supported with the current CF-LVADs.
Study limitation
This is a single-center study of a small number of patients who were screened for GPIBα shedding and NSB. Not all LVAD-supported patients were enrolled for this study. A larger multicenter study of CF-LVAD patients may be needed for accurate determination of cutoff value of plasma GPIbα for the diagnostic sensitivity and specificity of NSB and should be validated before this assay is marketed for clinical practice. The effects of antiplatelet drugs on GPIbα shedding may need to be explored.
Acknowledgments
The authors gratefully acknowledge Amelia C. Watkins and Shuqiong Niu for assistance in preparation of the manuscript.
This work was partially supported by the National Institutes of Health (Grant R01 HL 088100).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statement
The authors declare that there are no conflicts of interest.
References
- 1.Rose EA, Gelijns AC, Moskowitz AJ, et al. Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure (REMATCH) Study Group. Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med. 2001;345:1435–43. doi: 10.1056/NEJMoa012175. [DOI] [PubMed] [Google Scholar]
- 2.Slaughter MS, Rogers JG, Milano CA, et al. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med. 2009;361:2241–51. doi: 10.1056/NEJMoa0909938. [DOI] [PubMed] [Google Scholar]
- 3.Birks EJ, Yacoub MH, Banner NR, et al. The role of bridge to transplantation: should LVAD patients be transplanted? Curr Opin Cardiol. 2004;19:148–53. doi: 10.1097/00001573-200403000-00015. [DOI] [PubMed] [Google Scholar]
- 4.Birks EJ, Tansley PD, Hardy J, et al. Left ventricular assist device and drug therapy for the reversal of heart failure. N Engl J Med. 2006;355:1873–84. doi: 10.1056/NEJMoa053063. [DOI] [PubMed] [Google Scholar]
- 5.Kirklin JK, Naftel DC, Kormos RL, et al. Fifth INTERMACS annual report: Risk factor analysis from more than 6,000 mechanical circulatory support patients. J Heart Lung Transplant. 2013;32:141–56. doi: 10.1016/j.healun.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 6.Kirklin JK, Naftel DC, Kormos RL, et al. Third INTERMACS Annual Report: the evolution of destination therapy in the United States. J Heart Lung Transplant. 2011;30:115–23. doi: 10.1016/j.healun.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 7.Starling RC, Naka Y, Boyle AJ, et al. Results of the post-U.S. Food and Drug Administration-approval study with a continuous flow left ventricular assist device as a bridge to heart transplantation: a prospective study using the INTERMACS (Interagency Registry for Mechanically Assisted Circulatory Support) J Am Coll Cardiol. 2011;57:1890–98. doi: 10.1016/j.jacc.2010.10.062. [DOI] [PubMed] [Google Scholar]
- 8.Lietz K. Destination therapy: patient selection and current outcomes. J Card Surg. 2010;25:462–71. doi: 10.1111/j.1540-8191.2010.01050.x. [DOI] [PubMed] [Google Scholar]
- 9.Lahpor J, Khaghani A, Hetzer R, et al. European results with a continuous-flow ventricular assist device for advanced heart failure patients. Eur J Cardiothorac Surg. 2010;37:357–61. doi: 10.1016/j.ejcts.2009.05.043. [DOI] [PubMed] [Google Scholar]
- 10.Crow S, John R, Boyle A, et al. Gastrointestinal bleeding rates in recipients of nonpulsatile and pulsatile left ventricular assist devices. J Thorac Cardiovasc Surg. 2009;137:208–15. doi: 10.1016/j.jtcvs.2008.07.032. [DOI] [PubMed] [Google Scholar]
- 11.Stern DR, Kazam J, Edwards P, et al. Increased incidence of gastrointestinal bleeding following implantation of the HeartMate II LVAD. J Card Surg. 2010;25:352–6. doi: 10.1111/j.1540-8191.2010.01025.x. [DOI] [PubMed] [Google Scholar]
- 12.Uriel N, Pak SW, Jorde UP, et al. Acquired von Willebrand syndrome after continuous-flow mechanical device support contributes to a high prevalence of bleeding during long-term support and at the time of transplantation. J Am Coll Cardiol. 2010;56:1207–13. doi: 10.1016/j.jacc.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 13.Crow S, Chen D, Milano C, et al. Acquired von Willebrand syndrome in continuous-flow ventricular assist device recipients. Ann Thorac Surg. 2010;90:1263–9. doi: 10.1016/j.athoracsur.2010.04.099. [DOI] [PubMed] [Google Scholar]
- 14.Meyer AL, Malehsa D, Bara C, et al. Acquired von Willebrand syndrome in patients with an axial flow left ventricular assist device. Circ Heart Fail. 2010;3:675–81. doi: 10.1161/CIRCHEARTFAILURE.109.877597. [DOI] [PubMed] [Google Scholar]
- 15.Klovaite J, Gustafsson F, Mortensen SA, et al. Severely impaired von Willebrand factor-dependent platelet aggregation in patients with a continuous-flow left ventricular assist device (HeartMate II) J Am Coll Cardiol. 2009;53:2162–7. doi: 10.1016/j.jacc.2009.02.048. [DOI] [PubMed] [Google Scholar]
- 16.Fraser KH, Zhang T, Taskin ME, et al. A quantitative comparison of mechanical blood damage parameters in rotary ventricular assist devices: shear stress, exposure time and hemolysis index. J Biomech Eng. 2012;134:081002. doi: 10.1115/1.4007092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsai HM. Pathophysiology of thrombotic thrombocytopenic purpura. Int J Hematol. 2010;91:1–19. doi: 10.1007/s12185-009-0476-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Himmelfarb J, Nelson S, McMonagle E, et al. Elevated plasma glycocalicin levels and decreased ristocetin-induced platelet agglutination in hemodialysis patients. Am J Kidney Dis. 1998;32:132–8. doi: 10.1053/ajkd.1998.v32.pm9669434. [DOI] [PubMed] [Google Scholar]
- 19.Cheng H, Yan R, Li S, et al. Shear-induced interaction of platelets with von Willebrand factor results in glycoprotein Ibα shedding. Am J Physiol Heart Circ Physiol. 2009;297:H2128–35. doi: 10.1152/ajpheart.00107.2009. [DOI] [PubMed] [Google Scholar]
- 20.Andrews RK, Karunakaran D, Gardiner EE, et al. Platelet receptor proteolysis: a mechanism for downregulating platelet reactivity. Arterioscler Thromb Vasc Biol. 2007;27:1511–20. doi: 10.1161/ATVBAHA.107.141390. [DOI] [PubMed] [Google Scholar]
- 21.Beer JH, Buchi L, Steiner B. Glycocalicin: a new assay - the normal plasma levels and its potential usefulness in selected diseases. Blood. 1994;83:691–702. [PubMed] [Google Scholar]
- 22.Liu H, Wu TT. Estimating the area under a Receiver Operating Characteristic (ROC) curve for repeated measures design. J Stat Soft. 2003;8:1–18. [Google Scholar]
- 23.Liu H, Li G, Cumberland WG, et al. Testing statistical significance of the area under a receiving operating characteristics curve for repeated measures design with bootstrapping. J Data Sci. 2005;3:257–78. [Google Scholar]
- 24.Rose EA, Gelijns AC, Moskowitz AJ, et al. Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med. 2001;345:1435–43. doi: 10.1056/NEJMoa012175. [DOI] [PubMed] [Google Scholar]
- 25.Cannegieter SC, Rosendaal FR, Briet E. Thromboembolic and bleeding complications in patients with mechanical heart valve prostheses. Circulation. 1994;89:635–41. doi: 10.1161/01.cir.89.2.635. [DOI] [PubMed] [Google Scholar]
- 26.Levine MN, Raskob G, Beyth RJ, et al. Hemorrhagic complications of anticoagulant treatment: the seventh ACCP conference on antithrombotic and thrombolytic therapy. Chest. 2004;126:287–310. doi: 10.1378/chest.126.3_suppl.287S. [DOI] [PubMed] [Google Scholar]
- 27.Demirozu ZT, Radovancevic R, Hochman LF, et al. Arteriovenous malformation and gastrointestinal bleeding in patients with the HeartMate II left ventricular assist device. J Heart Lung Transplant. 2011;30:849–53. doi: 10.1016/j.healun.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 28.Schaffer JM, Arnaoutakis GJ, Allen JG, et al. Bleeding complications and blood product utilization with left ventricular assist device implantation. Ann Thorac Surg. 2011;91:740–9. doi: 10.1016/j.athoracsur.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 29.Slaughter MS, Sobieski MA, Gallagher C, et al. Fibrinolytic activation during long-term support with the HeartMate II left ventricular assist device. ASAIO J. 2008;54:115–9. doi: 10.1097/MAT.0b013e318161a987. [DOI] [PubMed] [Google Scholar]
- 30.Klovaite J, Gustafsson F, Mortensen SA, et al. Severely impaired von Willebrand factor-dependent platelet aggregation in patients with a continuous-flow left ventricular assist device (HeartMate II) J Am Coll Cardiol. 2009;53:2162–7. doi: 10.1016/j.jacc.2009.02.048. [DOI] [PubMed] [Google Scholar]
- 31.Letsou GV, Shah N, Gregoric ID, et al. Gastrointestinal bleeding from arteriovenous malformations in patients supported by the Jarvik 2000 axial-flow left ventricular assist device. J Heart Lung Transplant. 2005;24:105–9. doi: 10.1016/j.healun.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 32.Geisen U, Heilmann C, Beyersdorf F, et al. Nonsurgical bleeding in patients with ventricular assist devices could be explained by acquired von Willebrand disease. Eur J Cardiothorac Surg. 2008;33:679–84. doi: 10.1016/j.ejcts.2007.12.047. [DOI] [PubMed] [Google Scholar]
- 33.Boley SJ, Sammartano R, Adams A, et al. On the nature and etiology of vascular ectasias of the colon: degenerative lesions of aging. Gastroenterology. 1977;72:650–60. [PubMed] [Google Scholar]
- 34.Heyde EC. Gastrointestinal bleeding in aortic stenosis [letter to editor] N Engl J Med. 1958;259:196. [Google Scholar]
- 35.Shoenfeld Y, Eldar M, Bedazovsky B, et al. Aortic stenosis associated with gastrointestinal bleeding. A survey of 612 patients. Am Heart J. 1980;100:179–82. doi: 10.1016/0002-8703(80)90113-1. [DOI] [PubMed] [Google Scholar]
- 36.Greenstein RJ, McElhinney AJ, Reuben D, et al. Colonic vascular ectasias and aortic stenosis: coincidence or causal relationship? Am J Surg. 1986;151:347–51. doi: 10.1016/0002-9610(86)90465-4. [DOI] [PubMed] [Google Scholar]
- 37.Pate GE, Mulligan A. An epidemiological study of Heyde’s syndrome: an association between aortic stenosis and gastrointestinal bleeding. J Heart Valve Dis. 2004;13:713–6. [PubMed] [Google Scholar]
- 38.Vincentelli A, Susen S, Le Tourneau T, et al. Acquired von Willebrand’s syndrome in aortic stenosis. N Engl J Med. 2003;349:343–9. doi: 10.1056/NEJMoa022831. [DOI] [PubMed] [Google Scholar]
- 39.Ruggeri ZM, Mendolicchio GL. Adhesion mechanisms in platelet function. Circ Res. 2007;100:1673–85. doi: 10.1161/01.RES.0000267878.97021.ab. [DOI] [PubMed] [Google Scholar]
- 40.Qiao JL, Shen Y, Gardiner EE, et al. Proteolysis of platelet receptors in humans and other species. Biol Chem. 2010;391:893–900. doi: 10.1515/BC.2010.081. [DOI] [PubMed] [Google Scholar]