Abstract
Obesity, defined as an excessive increase in white adipose tissue (WAT), is a global health epidemic. In obesity, WAT expands by increased adipocyte size (hypertrophy) and number (hyperplasia). The location and cellular mechanisms of WAT expansion greatly affect the pathogenesis of obesity. However, the cellular and molecular mechanisms regulating adipocyte size, number and depot-dependent expansion in vivo remain largely unknown. This perspective summarizes previous work addressing adipocyte number in development and obesity and discusses recent advances in the methodologies, genetic tools, and characterization of in vivo adipocyte precursor cells allowing for directed study of hyperplastic WAT growth in vivo.
Introduction
White adipose tissue (WAT) is a highly plastic and dynamic tissue, accounting for less than 5% of body weight in some individuals to over 60% in others, with a capacity to change many fold in mass within the same individual (Drøyvold et al., 2006; Guo et al., 1999). Excessive accumulation of WAT is the defining feature of obesity, which is clinically defined as a body-mass-index (BMI = kg/m2) exceeding 30 (WHO, 2013). Over the last three decades the prevalence of obesity has risen dramatically throughout the world and now more than 35% of adults within the US are obese (Ogden et al., 2012; WHO, 2013). This worldwide increase in adiposity has become a major public health concern as obesity is a risk factor for several pathologies including type II diabetes, cardiovascular disease and some forms of cancer (WHO, 2013). For these and other reasons, the American Medical Association has recently classified obesity as a disease, further highlighting the importance of understanding WAT mass regulation in vivo.
The development of obesity has largely been attributed to overconsumption of food and decreased physical activity, as chronic positive energy balance results in the expansion of WAT due to the storage of excess energy as triglycerides within WAT (Spiegelman and Flier, 2001). It is well appreciated that regulation of food intake and energy expenditure is controlled by neuronal signaling within the hypothalamus and dysregulation of this signaling leads to energy imbalance and obesity (Friedman, 2004; Sternson et al., 2013). However, the WAT-intrinsic mechanisms that lead to the increase in tissue mass in response to positive energy imbalance are unknown. Even in cases of extreme positive energy balance such as the massively obese leptin-deficient ob/ob mouse, there must be molecular processes active within WAT that regulate tissue expansion.
Expansion of WAT occurs through both increased lipid filling within existing mature adipocytes to increase adipocyte size (hypertrophic WAT growth) and increased differentiation of adipocyte precursor cells to increase adipocyte number (hyperplastic WAT growth; also referred to as adipogenesis) (Joe et al., 2009; Wang et al., 2013). While genetics and obesogenic stimuli can affect the relative contribution of these mechanisms to the total accumulation of WAT in obesity (Jo et al., 2009), the WAT-intrinsic mechanisms involved in regulating adipocyte size and number in vivo are largely unknown.
The location of WAT accumulation can affect the pathogenesis of obesity. Specifically, WAT accumulates throughout the body in several distinct subcutaneous (SWAT) and visceral (VWAT) depots (Cinti, 2005). In humans the accumulation of VWAT and deep abdominal SWAT correlates with increased risk of diabetes, cardiovascular disease and mortality (Carey et al., 1997; Goodpaster et al., 1997; Kelley et al., 2000; Nicklas et al., 2006; Wang et al., 2005). Conversely, the accumulation of gluteofemoral SWAT, which is more prevalent in females, is associated with insulin sensitivity and decreased risk of diabetes and cardiovascular disease (Manolopoulos et al., 2010; Misra et al., 1997; Snijder et al., 2003; Tankó et al., 2003). In rodents, inguinal (posterior) SWAT is associated with improved metabolic parameters (Tran et al., 2008), similar to human gluteofemoral SWAT, while mouse perigonadal VWAT is associated with decreased insulin sensitivity (Foster et al., 2010). Therefore, determining how WAT accumulation affects metabolic disease requires understanding how WAT mass is regulated at specific anatomical sites in vivo.
Recent advances in the identification and isolation of in vivo adipocyte precursor cells now allows for directed study of the cellular and molecular mechanisms regulating adipocyte number in both development and obesity (Lee et al., 2012; Rodeheffer et al., 2008; Tang et al., 2008). Here we detail the current knowledge of adipocyte precursor cells including their developmental lineage, tissue localization, and abundance within WAT depots. We will also summarize previous work on the contribution of adipocyte number to the development and obesogenic expansion of WAT. Finally, we will discuss common approaches used to study these cells in vitro and in vivo, with a specific focus on genetic models useful for labeling and manipulating the adipocyte cellular lineage in vivo.
Establishment of Adipocyte Number during Development
It is established that WAT forms early in life, initially arising from highly vascularized ‘primitive organs’ that are observed at sites of WAT depot development (Birsoy et al., 2011; Clara, 1927a; Han et al., 2011; Wassermann, 1927, 1929). In humans, lipid filling of adipocyte precursors is first visible in loose connective tissue structures called ‘fat lobules’ during the second trimester (starting at 14 weeks) (Poissonnet et al., 1983). These primitive adipose tissue depots progressively increase in both size and number until gestational week 23 when fat lobule number begins to plateau. In the following weeks, fat lobule growth is almost exclusively accomplished through hypertrophy of existing adipocytes (Berg, 1911; Poissonnet et al., 1983; Poissonnet et al., 1984). Postnatally, the SWAT depot in non-obese children remains relatively quiescent until 10yrs of age when adipocyte number increases again through the teenage years before plateauing at the end of adolescence (Knittle, 1978; Knittle et al., 1979; Spalding et al., 2008). Less is known about the timing of VWAT establishment in humans, but there is little VWAT mass in non-obese individuals prior to puberty (Fox et al., 1993; Siegel et al., 2007) and at least some WAT within the abdominal cavity does not form until after birth (Wassermann, 1927).
In mice, SWAT primitive organs become populated by cells expressing adipogenic markers relatively late in embryogenesis, with overt lipid filling of white adipocytes being initiated at birth (Birsoy et al., 2011). The visceral perigonadal WAT (GWAT) depot forms after birth, with determined adipocyte precursors first appearing at postnatal day (P) 4 and lipid accumulation in developing GWAT evident at P7 (Han et al., 2011). The relative contribution of adipocyte size and number to murine WAT development is not well characterized, but studies in rats show increases in both adipocyte number and size early in life, followed by a plateau in adipocyte number by 3 months of age with WAT mass increases thereafter resulting from increased adipocyte size (Greenwood and Hirsch, 1974; Hirsch and Han, 1969; Johnson et al., 1971). A recent study using inducible indelible labeling specifically in mature adipocytes via the adiponectin promoter (termed AdipoChaser mice) supports these findings, showing that the vast majority of mature adipocytes in mouse SWAT are formed prenatally. Conversely, in VWAT adipocytes are formed during the first few weeks of life, with little formation of new adipocytes in early adulthood (Wang et al., 2013). Similar cellular mechanisms of WAT mass establishment have been reported in birds, ruminants and fish (Anderson and Kauffman, 1973; Flynn et al., 2009; Hood and Allen, 1973; Imrie and Sadler, 2010; Pfaff, 1975). This collection of studies indicates that there is an overarching framework of WAT development in which WAT depots are initially formed, both embryonically and postnatally, through a combination of hyperplastic and hypertrophic mechanisms that culminate in the stabilization of adipocyte number by the end of adolescence (Greenwood et al., 1979; Greenwood and Hirsch, 1974; Knittle and Hirsch, 1968), and the stabilization of adipocyte size in early adulthood (Hemmeryckx et al., 2010; Häger et al., 1977; Stiles et al., 1975).
In non-obese conditions, adipocyte number remains relatively constant in adult animals (Stiles et al., 1975), even during starvation and refeeding (Hirsch and Han, 1969; Yang et al., 1990), suggesting that the plateau in adipocyte number at the end of adolescence establishes an adipocyte number ‘set point’ in some conditions. In support of this idea, excision of inguinal SWAT from rats prior to the end of adolescence results in compensation by the remaining SWAT to generate the same total number of SWAT adipocytes as control animals (Faust et al., 1977a), a response not observed when WAT is excised from adult animals (Faust et al., 1977b; Kral, 1976). Additionally, indirect dietary manipulation through modulation of litter size during early development can lead to long-lasting decreases in adipocyte number (Knittle and Hirsch, 1968). These findings support that there is a critical developmental period for the establishment of adipocyte number and that there is an adipocyte number ‘set point’ under normal conditions. Thus, increased adipocyte number generated during adolescence may enhance the capacity to store lipid in adult life (Spalding et al., 2008). Additionally, it was recently shown that mature adipocytes turnover at a constant, albeit slow, rate in adulthood (Spalding et al., 2008; Wang et al., 2013), indicating that maintenance of the adipocyte number normally involves a tightly regulated balance of adipogenesis and adipocyte death. The mechanisms involved in the maintenance of adipocyte number throughout life are not known, but in obesity this balance can be at least temporarily disturbed, leading to increased adipocyte hyperplasia in many models of obesity.
Regulation of Adipocyte Number in Obesity
While adipocyte hypertrophy is a common feature of obesity, studies have shown varying contributions of increased adipocyte number to obesogenic increases in WAT mass. Some early studies in rodents and humans showed no increase in adipocyte number in obesity, indicating that the observed WAT mass accumulation was solely due to adipocyte hypertrophy (Hirsch and Han, 1969; Hirsch and Knittle, 1970; Johnson and Hirsch, 1972; Salans et al., 1973). Given the upper limit on adipocyte size (Clara, 1927b; Leonhardt et al., 1972), expansion by hypertrophy alone would limit WAT gain. Therefore in order to achieve the WAT mass increase seen in cases of extreme (morbid) obesity, increased adipocyte number must accompany adipocyte hypertrophy.
Many studies have since shown that hyperplasia and hypertrophy both contribute to WAT mass expansion in obesity. Some studies show that hyperplasia only occurs in early onset obesity, prior to the developmental establishment of adipocyte number (Lemonnier, 1972; Salans et al., 1973). Others have shown that increased adipocyte number can also contribute to WAT mass gain in adult onset obesity, including both genetic (Hirsch and Batchelor, 1976; Johnson and Hirsch, 1972; Johnson et al., 1971) and dietary induced obesity (Ellis et al., 1990; Faust et al., 1978; Faust et al., 1984; Klyde and Hirsch, 1979). It is also important to note that several of these studies show that hyperplastic WAT growth is not restricted to severe obesity. The studies referenced above, and numerous others confirm there is often an increase in adipocyte number in obesity, but the results from these studies have been varied and the precise contribution of increased adipocyte number to obesity in different contexts remains unclear.
Several potential explanations exist, both experimental and biological, for the disparate results with regard to adipocyte hyperplasia in obesity. Previous attempts to directly assess adipocyte production in vivo have taken two approaches. One is to digest WAT to single cells, float mature adipocytes and quantify the amount of newly synthesized DNA in the adipocyte fraction, usually via incorporation of thymidine analogs (Ellis et al., 1990; Miller et al., 1984). In this technique it has been shown that other cell types, such as macrophages, may contaminate the floating fraction to confound results (Tchoukalova et al., 2012). The alternative approach is to label newly synthesized DNA and then determine the location of the ‘new’ nuclei in situ, via autoradiography or immunohistochemistry (Joe et al., 2009; Miller et al., 1984). This technique is problematic as the majority of adipocytes within a single WAT section will not display a nucleus, due to the relatively large size of the cell compared to the nucleus. In addition, while adipocytes compose the vast majority of WAT mass/volume, they account for less than 20% of the total cells in WAT (Eto et al., 2009). Therefore, the majority of nuclei in a WAT section belong to non-adipocytes that lie in close proximity to the adipocyte lipid droplet. Due to these issues, the association of a labeled nucleus with a particular adipocyte can only be made after ruling out the presence of a plasma membrane between the nucleus and the adipocyte lipid droplet through electron microscopy or plasma membrane labeling (Figure 1).
Figure 1. Identification of mature adipocyte nuclei in situ.
Images of whole mounted white adipose tissue. The lipid is stained with LipidTox (green), the plasma membrane with Cell Mask Orange (red), and the nuclei with DAPI (blue). Most nuclei in WAT are in close proximity to lipid droplets. Therefore accurate identification of adipocyte nuclei (white arrowheads) from non-adipocyte nuclei (yellow arrowheads) requires staining of the plasma membrane. Nuclei that are not separated from the lipid droplets by a plasma membrane are the mature adipocyte nuclei. Scale bars = 25μm.
Other indirect methods have been used to assess adipocyte formation in adult animals in vivo. One approach is to determine adipocyte hyperplasia by extrapolating the total number of adipocytes from the average size of adipocytes - determined by direct measures of cell sizing or average diameter via histology - and the total weight/volume of the WAT depot (Hirsch and Han, 1969; Jo et al., 2009). A more recent genetic approach has used the AdipoChaser mouse to directly label all mature adipocytes and then assess the formation of non-labeled adipocytes (Wang et al., 2013). While these methods provide good estimates of adipocyte number and allow indirect tracing of adipocyte formation, respectively, they do not inform on the timing of the signals that initiate adipogenesis. Rather, they can only be used to measure adipocyte formation after differentiation has progressed enough to allow morphological characterization of the new adipocytes.
Recent studies of the depot-specific responses to HFD feeding highlight in the importance of the experimental approach taken to study hyperplasia in vivo. A study that assessed adipocyte hyperplasia via immunohistochemical analysis of incorporation of the thymidine analog bromodeoxyuridine (BrdU) concluded that SWAT undergoes more hyperplastic growth than VWAT (Joe et al., 2009). However, the study of hyperplasia in response to HFD with the AdipoChaser mouse demonstrated that a significant number of new adipocytes form in VWAT after 8 weeks of HFD feeding (Lee et al., 2012; Wang et al., 2013), but there is little to no contribution of new adipocytes to the increase in SWAT mass through at least 12 weeks of HFD feeding (Wang et al., 2013). While timing differences between these studies likely contribute to some of the discrepancies, these data suggest that despite increased cellular proliferation in SWAT, adipogenesis is restricted to VWAT at the onset of diet-induced obesity.
Although experimental limitations have made it difficult to accurately and reproducibly determine the precise contribution of hyperplastic WAT growth to different obesogenic contexts, the breadth of studies implicating increased adipocyte number as a contributor to WAT mass accumulation indicate that it is an important depot-dependent component of obesity that is influenced by a host of factors, including species/strain, sex, and obesogenic stimulus (genetic, dietary, etc.). However, the mechanisms that control adipogenesis in obesity are not clear. Whether increased adipocyte number is induced in adulthood or earlier, the similar turnover rates of adipocytes in obese and non-obese adults (Spalding et al., 2008) suggests that increases in adipocyte number results either from transient induction(s) of adipogenesis that alters the adipocyte set point, minimal increases in adipocyte formation over long periods, or both of these mechanisms. Several studies have suggested that new adipocytes form several weeks after the initiation of HFD feeding, as adipocytes begin to reach their maximal size (Faust et al., 1978; Joe et al., 2009; Wang et al., 2013). Based on this concept it has been hypothesized that an adipogenic signal is generated as mature adipocytes reach maximal size and can no longer expand to meet the increased demand for lipid storage (Faust et al., 1978). While the nature of this potential regulatory mechanism in obesity is not known, it has been shown that factors secreted by mature adipocytes can affect adipogenesis (Janke et al., 2002).
There are many different paths that can lead to obesity, with synergy between genetics and environment playing a pivotal role. It is important to understand how adipocyte number is regulated in WAT as adipocyte number and size are the two main contributors to WAT mass. While increased adipocyte size is associated with insulin resistance (Salans et al., 1968), the physiological ramifications of increased adipocyte number in obesity is not clear. Increased adipocyte generation has been postulated to be the mechanism that leads to increased insulin sensitivity with Thiazolidinedione (TZD) treatment (Lehmann et al., 1995), suggesting that having more adipocytes can be beneficial. However, the morbidly obese have increased adipocyte number and significantly worse health outcomes (Calle et al., 1999). In order to determine how increased adipocyte number impacts the development of obesity-associated disease we must understand the processes that regulate adipocyte number in vivo. However, the majority of our understanding of the process of adipogenesis has come from in vitro studies.
In Vitro Adipogenesis
Several approaches have been taken to study adipogenesis in cell culture. Rodbell pioneered the digestion of rodent WAT to single cells for study of isolated mature adipocytes in vitro (Rodbell, 1964). Subsequently, culturing of the remaining non-adipocyte cell fraction, referred to as the stromal-vascular fraction (SVF), resulted in the differentiation of adherent fibroblastic SVF human cells into lipid-laden cells after 2 months (Ng et al., 1971). These studies were the first to describe the presence of a WAT resident adipogenic stromal cell population appropriate for the study of human adipogenesis in cell culture. Today, the culture of whole SVF is routinely applied to tissue engineering (Lin et al., 2010; Zuk et al., 2001), where the resulting adherent cells are referred to as adipocyte-derived stem/stromal cells (ADSCs) or adipose stem cells (ASCs). Several studies have demonstrated that ADSC/ASC cultures can differentiate into many cell types in vitro, including adipocytes, osteoblasts, chondrocytes and skeletal myocytes (Gimble et al., 2007). However, analysis of cell surface marker expression indicates that ADSC/ASC cultures are a heterogeneous population of cells, even after several rounds of passage (Guilak et al., 2006; Mitchell et al., 2006; Zuk et al., 2002). Clonal analysis and colony formation studies have shown that most of the differentiation of ADSC/ASC cultures toward any particular lineage is provided by subpopulations of cells that are restricted to one or two fates, with very few cells displaying multipotency (Mitchell et al., 2006). Adherent cell populations are even more restricted in vivo, with mouse ADSCs readily forming chondrocytes and bone, but displaying little or no de novo adipogenic capacity when transplanted in mice (Zheng et al., 2006). Moreover, human ADSCs require prior stimulation of adipogenesis in vitro to form adipocytes after transplantation into SCID mice (Hong et al., 2006). These findings demonstrate that selection of WAT resident SVF cells by adherence alone results in a mixed population of cells with the majority of cells unable to form adipocytes in vitro, and no significant adipogenic activity in vivo. Regardless of whether this limited adipogenic capacity is the result of insufficient enrichment of adipocyte precursors or alterations in cell function during prolonged cell culture (Boquest et al., 2005), these results suggest that the study of adherent whole SVF provides little insight into the adipocyte cellular lineage in vivo.
In another approach to in vitro adipogenesis, adipogenic clones of the spontaneously immortalized Swiss mouse 3T3 fibroblast cell line, including 3T3L1 and 3T3-F442A, were isolated and shown to accumulate lipid after being maintained at confluence in culture (Green and Kehinde, 1975, 1976; Green and Meuth, 1974). Many factors have since been demonstrated to affect adipogenesis of these cells, but the addition of insulin (Green and Kehinde, 1975), 1-methyl-3-isobutyl xanthine (IBMX) (Russell and Ho, 1976) and glucocorticoid (e.g. dexamethasone) (Miller et al., 1978; Rubin et al., 1978), enhances adipogenesis, and these factors together are now considered the common adipogenic cocktail. However, it should be noted that while this adipogenic cocktail is effective in rodent models of adipogenesis, the differentiation of human primary cells and cell lines has required the addition of PPARγ agonists or indomethacin, which alters prostaglandin synthesis and, similar to PPARγ agonists, can drive lipid accumulation even in mesenchymal stem cells that are not committed to an adipogenic lineage (Styner et al., 2010). The requirement for these compounds limits the mechanistic studies of adipogenesis (Church et al., 2012), and suggests that improved culture models are required for human adipogenesis in vitro, either via improved enrichment of adipogenic cell populations, isolation procedures and/or identification of improved culture conditions.
Study of 3T3 cell lines has provided a wealth of knowledge on the molecular pathways that regulate adipogenesis. To summarize, a well-characterized transcription factor cascade leads to the induction of the transcription factor PPARγ2 (Tontonoz et al., 1994), which drives adipogenesis through the induction of several lipogenic genes (for review see (Rosen and MacDougald, 2006)). In addition to characterizing the adipogenic transcription factor cascade, cell culture studies have implicated a host of signaling factors as regulators of adipogenesis, including BMPs, WNTs, and IGFs (reviewed in (Cristancho and Lazar, 2011; Tang and Lane, 2012)). However, the study of adipogenesis in vitro does not inform if or when specific factors participate in adipogenesis in vivo. For example, the in vivo adipogenic role for the cyclic AMP and glucocorticoid-mediated signaling pathways, which are respectively stimulated by IBMX and dexamethasone during induction of adipogenesis in vitro, remains unclear. The gene expression program induced by the adipogenic cocktail in 3T3L1 cells mirrors that observed in developing murine SWAT (Birsoy et al., 2011), but there are a number of differences between 3T3L1 adipogenesis and the gene programs observed in adult mouse WAT (Soukas et al., 2001). Additionally, while overt lipid filling is observed within 48 hours during in vitro adipogenesis, rodent adipocyte precursors may take more than 35 days to fill with lipid in vivo (Greenwood and Hirsch, 1974; Wang et al., 2013). Based on these observations, it is possible that adipogenesis in vivo is regulated by unappreciated molecular pathways that are modulated well in advance of overt lipid filling. Therefore, understanding the timing of adipogenesis in various physiological contexts is necessary for focused study of the molecular mechanisms regulating adipocyte formation in vivo. Recent studies have identified in vivo adipocyte precursor cells as subpopulations of WAT SVF, allowing for directed study of the temporal, cellular and molecular mechanisms that regulate in vivo adipogenesis.
Adipocyte Precursors In Vivo
Several studies have now isolated different cell subpopulations from freshly isolated WAT SVF in effort to identify adipocyte precursors. Initial studies that used magnetic bead separation to isolate of cell subpopulations from human WAT SVF found the CD31-:CD34+ population is enriched for adipogenic capacity (Sengenès et al., 2005), but appropriate markers for significant further enrichment have not been clearly identified in human WAT.
Adipogenic cell populations have been characterized in greater detail from mice using flow cytometry and fluorescence-activated cell sorting (FACS) to isolate populations of live cells based on expression of cell surface proteins. Although this technology has long been used to characterize cell populations in stem cell fields and immunobiology, it has only recently been applied to WAT for identification of adipogenic SVF subpopulations through prospective analysis of adipogenesis both ex vivo in adipogenic cell culture conditions and in vivo following transplantation (Bénézech et al., 2012; Daquinag et al., 2011; Lee et al., 2012; Rodeheffer et al., 2008; Tang et al., 2008). In this approach, the SVF is labeled with fluor-conjugated antibodies recognizing cell-surface proteins to allow for separation and isolation of adipose stromal cell subpopulations. Using FACS, two stromal cell populations with in vitro adipogenic capacity were identified within murine subcutaneous WAT. These populations are negative for blood and endothelial specific markers (CD45, CD31, Ter119), positive for mesenchymal and stem-cell markers (CD29, CD34, Sca-1) and are separated based on their expression of CD24. One population, characterized by the cell surface marker profile CD45-;CD31-;Ter119-;CD29+;CD34+;Sca-1+;CD24-(CD24-), is highly adipogenic in culture, yet has restricted adipogenic capacity when transplanted into the underdeveloped peri-gonadal (VWAT) adipose tissue of lipodystrophic A/Zip mice. In contrast, the other population, characterized by the cell surface marker profile CD45-;CD31-;Ter119-;CD29+;CD34+;Sca-1+;CD24+ (CD24+), is highly adipogenic in culture and capable of considerable expansion prior to differentiation, leading to reconstitution of an entire functional WAT depot with correction of hyperglycemia and hyperinsulinemia when transplanted into fatless mice (Rodeheffer et al., 2008).
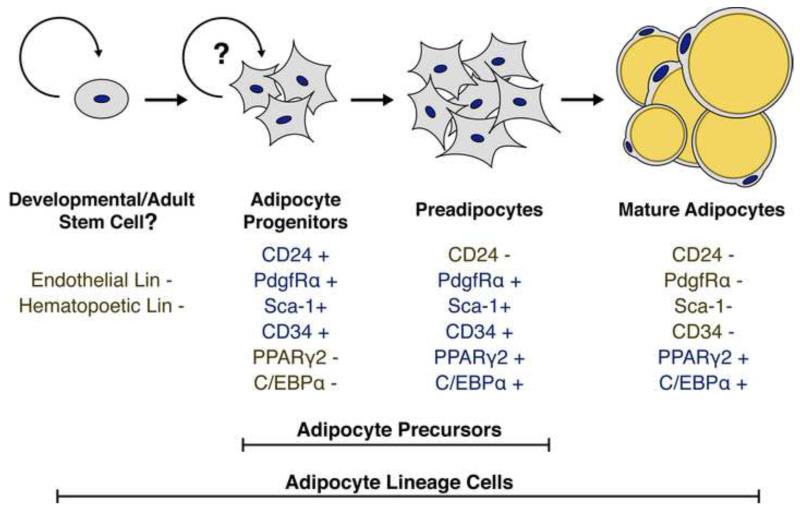
Through flow cytometry analysis of CD24+ cells following transplantation into A/Zip mice, it was demonstrated that the CD24+ population gives rise to the CD24− population in vivo, while the CD24− population does not produce CD24+ cells. When these populations are transplanted into a non-adipogenic tissue microenvironment, the CD24− population retains a low adipogenic capacity that fails to correct A/Zip hyperglycemia, while the CD24+ population is surprisingly not adipogenic (Berry and Rodeheffer, 2013). Based on these results, we have proposed the following model for in vivo adipogenesis (Figure 2). The CD24+ population contains early adipocyte progenitors that have high adipogenic capacity within the WAT microenvironment and are capable of extensive expansion prior to terminal differentiation, but require the WAT microenvironment for their adipogenic capacity. During adipogenesis, the CD24+ adipocyte progenitor loses CD24 expression to become CD24− preadipocytes. The CD24− preadipocyte population contains cells committed to the adipocyte lineage, which have low adipogenic capacity that is not dependent on the WAT microenvironment.
Figure 2. A model of in vivo adipogenesis.
A schematic representation of the adipocyte cellular lineage is shown. The defined lineage, cell surface, and transcriptional markers for each cell type are shown below each listed cell type. Positive markers are shown in blue while negative markers are shown in gold. The question marks highlight that it is currently unclear if the adipocyte progenitor population contains self-renewing adipocyte stem cells or if the adipocyte progenitors are repopulated by another, unidentified population of adipocyte stem cells in adults.
Consistent with adipogenic commitment, the CD24− cell population is enriched for expression of PPARγ2 and C/EBPα (Berry and Rodeheffer, 2013). These data suggest that the CD24+ and CD24− cell populations contain the adipocyte precursors, from early progenitors with substantial capacity to expand prior to differentiation to cells that are primed to fill with lipid to form mature adipocytes. Since mature adipocytes are post-mitotic, adipocyte precursors must exit the cell cycle at some point during adipogenesis. However, it is unclear precisely where in the in vivo adipocyte lineage cells become post-mitotic, and it may be difficult to determine given the rapid differentiation of CD24+ cells into CD24− cells in vivo (Berry and Rodeheffer, 2013). In vitro, cultured confluent preadipocytes undergo two rounds of synchronous cell division before permanently exiting the cell cycle in a process referred to as ‘mitotic clonal expansion’ (MCE) (Tang et al., 2003). However, it remains unclear if MCE is an important component of hyperplastic WAT growth in vivo. Given the relatively high abundance of CD24− adipocyte precursors in WAT depots (Figure 3), it is possible that post-mitotic adipocyte precursors contribute significantly to increased adipocyte number. At the other end of the lineage, it is unknown if the CD24+ population harbors bona fide adipocyte stem cells, capable of self-renewal and contributing mature adipocytes to maintain or expand WAT mass, or if the CD24+ population is maintained by another, currently unidentified adult stem cell population. Similar cell populations are found within all major WAT depots in mouse (Berry and Rodeheffer, 2013) (Figure 3), suggesting that these adipocyte precursors play a general role in the maintenance and expansion of WAT and that differential regulation of these cells leads to the depot-specific responses observed in obesity.
Figure 3. Adipocyte progenitors in WAT depots.
CD24+ adipocyte progenitor cells are a distinct population of WAT resident stromal cells. Shown are dot plots of the Lin-:CD29+:CD34+ cell populations from the indicated depots. The cells are present in levels as high at 2% in adult SWAT depots. Cells with an identical cell surface marker profile are present in VWAT depots, but are less abundant (peri-gonadal WAT (GWAT) is shown).
A benefit of using FACS and flow cytometry to isolate and analyze adipocyte precursors from WAT is that the technique is accessible, making use of commercially available reagents. Additionally, this approach can be applied to any mouse line or strain, as well as to other species, including human. CD34 has been shown to be expressed on adipocyte precursors in mice (Rodeheffer et al., 2008) and has also been used to enrich for adipogenic cells from human WAT SVF (Sengenès et al., 2005). However, at least one of the cell surface factors used for the isolation of mouse adipocyte precursors, Sca-1, does not have a clear human homolog (Holmes and Stanford, 2007), indicating that the human adipocyte precursors will likely require different markers for isolation. In addition, these markers do not have known functions in adipocyte precursor regulation in vivo. For example, while CD24 and Sca-1 are critical cell surface markers for identification of murine adipocyte progenitors, mice deficient in CD24 or Sca-1 do not display overt adipose phenotypes (Nielsen et al., 1997; Stanford et al., 1997). Furthermore, the use of several cell surface markers is currently required to significantly enrich for adipocyte precursors. A complimentary approach to studying adipogenesis in vivo is to develop tools based on functional markers of adipogenesis. Toward this end several genetic mouse models have been generated.
Genetic Approaches for Studying Adipogenesis in vivo
The most widely used genetic factor for studying WAT in vivo has been the aP2 promoter, which drives expression of Fatty Acid Binding Protein 4 (FABP4). However, in vitro studies have shown that aP2 is induced late in adipogenesis (Bernlohr et al., 1985) and A-Zip mice, which have a block in adipogenesis via expression of a dominant negative transcription factor from an aP2 promoter, accumulate more adipocyte precursors (Rodeheffer et al., 2008). This suggests that aP2 is not necessarily expressed in adipocyte precursor populations in vivo. In addition, the aP2 promoter is known to drive gene expression in many other tissues/cell lineages, including macrophages (Mullican et al., 2013). Therefore, other genetic strategies have been employed to characterize adipocyte precursor populations in vivo.
Pparγ was hypothesized to be a genetic marker of adipogenic cells in vivo, due to its expression pattern and role as the master regulator of adipogenesis in vitro. To identify Pparγ expressing cells in vivo, a mouse model was generated in which a tetracycline transactivator (tTA) gene was knocked into exon 2 of the Pparγ locus. This strain was crossed to reporter lines under control of tet-response elements to show that Pparγ expressing stromal cells undergo adipogenesis to form WAT during development and following transplantation into nude mice (Tang et al., 2008). Additionally, Tang et al concluded that adipocyte precursors are associated with adipose tissue vasculature as GFP labeled cells were found lining blood vessels in WAT of Pparγ-H2BGFP mice.
However, Pparγ expression is induced late in adipogenesis and therefore may not be appropriate for studying the regulation of early adipogenic events in vivo. A novel preadipocyte determination factor, Zfp423, was identified through transcriptome comparison of adipogenic and non-adipogenic clonal sublines of 3T3 Swiss fibroblasts prior to adipogenic expansion in vitro (Gupta et al., 2010). Zfp423 is enriched in adipogenic 3T3 sub-clones, and induces adipogenesis in non-adipogenic 3T3 sub-clones through induction of Pparγ2. To elucidate the function of Zfp423 on adipogenesis in vivo, a mouse was generated that expresses GFP from the Zfp423 locus (Gupta et al., 2012). Using these mice, it was demonstrated that FACS purification of GFP-expressing cells from whole SVF cultures enriched for cells with adipogenic capacity upon re-plating. While the role of Zfp423 in adipogenesis in vivo has yet to be assessed, the isolation of in vivo adipocyte precursors prior to expression of late adipogenic genes (PPARγ2, aP2, etc.) using an early functional marker, such as Zfp423, would facilitate the study of adipogenesis in vivo by simplifying the isolation and visualization strategies. However, the expression of Zfp423 in endothelial cells (Gupta et al., 2012) precludes the use of Zfp423 as a single marker of in vivo adipocyte precursors. Regardless, a combination of functional early and late stage adipocyte precursor markers would greatly facilitate the functional analysis of adipogenesis in vivo.
The Developmental Origins of White Adipose Tissue
Although flow cytometry and the previously mentioned genetic mouse models allow for study of adipocyte precursor populations, complete understanding of WAT formation requires understanding of the lineage(s) adipocytes are derived from during development. While adipocytes that form in depots in the head region have been shown to derive from the neural crest (Billon et al., 2007; Lemos et al., 2012), the developmental origin of adipocyte in major WAT depots has not been characterized. It has long been posited that adipocytes are of mesenchymal origin, based on their initial formation within connective tissue (Hammar, 1895), the morphology of adipocyte precursors (Napolitano, 1963) and the ability of mesenchymal cells to form adipocytes in culture (Taylor and Jones, 1979). In effort to further refine the developmental origin of adipocytes in the major WAT depots, recent studies have investigated if white adipocytes are derived from endothelial (Tran et al., 2012) or hematopoietic (Crossno et al., 2006; Sera et al., 2009) lineages.
There are many observations from early work on adipose tissue that showed adipocytes form in close proximity to vasculature (for review see (Wassermann, 1965)). Additionally, vascular propagation and adipogenesis are closely linked in vivo (Fukumura et al., 2003; Han et al., 2011; Rupnick et al., 2002). These observations have led to the hypothesis that adipocytes are derived from a vascular cell lineage. Recent studies have directly addressed whether adipocytes are derived from endothelial lineages through lineage tracing using the Cdh5 (VE-cadherin)-cre mouse model. Through analysis of X-gal stained WAT and BAT sections from Cdh5-cre:LacZ mice, it was concluded that mature brown and white adipocytes expressed the LacZ reporter and were therefore derived from Cdh5 expressing precursor cells (Tran et al., 2012). However, use of the LacZ reporter line for study of adipose tissue is confounded by the paucity of cytoplasm in mature adipocytes and the high vascularity of adipose tissue. In addition, neither the LacZ reporter or GFP reporter experiments in this study were combined with direct staining of endothelial cells to ensure the observed staining was not due to signal from capillaries in close association with mature adipocytes. To overcome the difficulties associated with reporters expressed in the adipocyte cytoplasm, we crossed the same Cdh5-cre mouse line to the fluorescent membrane dTomato/ membrane eGFP (mT/mG) reporter mouse. In mT/mG reporter mice, Cre mediated excision causes a permanent switch from expression of plasma membrane targeted dTomato to plasma membrane targeted eGFP (Muzumdar et al., 2007). The dual labeling of both non-cre expressing cells (red fluorescence) and Cre expressing cells (green fluorescence) not only permits clear distinction of Cre tracing via fluorescent microscopy, it also facilitates cre tracing of SVF cell populations via flow cytometry. Analysis of adipose depots from Cdh5-cre:mT/mG mice produced results that conflicted with data from the LacZ tracing, as all adipocytes and previously characterized adipocyte precursor populations in all major WAT and brown adipose depots remained dTomato+ and eGFP-, while vasculature and isolated CD31+ endothelial cells clearly expressed eGFP due to expression of cre-recombinase from the Cdh5 promoter. This result was found both for adipocytes generated during normal development and in diet induced obesity (DIO). These results from Cdh5-cre tracing, coupled with similar findings in another endothelial-driven Cre-recombinase model, the Tie2-cre mouse, indicate that adipocytes are not derived from cells of the vascular endothelium during development or early onset obesity (Berry and Rodeheffer, 2013). Both of the mature endothelial cell markers Cdh5 and Tie2 are initially expressed in angioblasts during embryonic vasculogenesis, and new endothelial cells are derived from mature endothelial cells during angiogenesis (Ferguson et al., 2005). Thus, the lack of mature adipocyte labeling in Cdh5-cre and Tie2-cre mice suggests that the close relationship between angiogenesis and adipogenesis in WAT does not result from regulation of a common progenitor, but is instead restricted to paracrine crosstalk between adipocyte lineage cells and vascular cells (Fukumura et al., 2003) to maintain proper vascularization and WAT morphology.
The hematopoietic lineage has also been proposed as a potential source of mature adipocytes (McCollough, 1944). Studies that followed the fate of transplanted bone marrow cells and genetically labeled myeloid-lineage cells found that up to 25% of mature adipocytes isolated through fractionation of whole WAT come from the hematopoietic lineage under certain conditions (Crossno et al., 2006; Majka et al., 2010; Majka et al., 2012). These studies also demonstrated that the floating myeloid lineage-derived cells were negative for macrophage markers, such as CD11b, and positive for the adipocyte markers perilipin and adiponectin. They also demonstrated that WAT was luminescent in HFD-fed mice that received bone marrow transplants from aP2-Cre;LSL-Luciferase mice (Majka et al., 2012). However, in a separate study, in situ analysis demonstrated that WAT-resident bone marrow transplant-derived cells expressed multiple macrophage markers and did not express markers of mature adipocytes (Koh et al., 2007). This study also showed that the bone marrow derived cells formed classic crown-like structures, indicative of macrophages responding to adipocyte death. Similar results were found in non-injury conditions by Vav1-Cre lineage tracing of hematopoietic stem cell-derived populations (Berry and Rodeheffer, 2013). The data from the different groups are contradictory with regard to the ability of the hematopoietic lineage to contribute to mature adipocytes. However, the case for a hematopoietic origin of mature adipocytes is largely based on the identification of hematopoetic lineage derived cells in the floating WAT fraction, which is often contaminated with macrophages (Tchoukalova et al., 2012). Furthermore, the additional evidence for a hematopoietic origin of adipocytes used the aP2-cre model, which has been shown to drive cre expression directly in macrophages as well as mature adipocytes (Mao et al., 2009).
Although adipocytes are not normally of endothelial or hematopoetic origin, cells with the morphology of differentiating preadipocytes are found to reside within the vascular compartment (Cinti et al., 1984; Wassermann, 1926). Additionally, vascular associated PPARγ-expressing cells have been shown to express the pericyte (mural cell) markers PdgfRβ, NG2 and αSMA (Tang et al., 2008). These observations have led to the hypothesis that a subpopulation of pericytes are adipocyte precursors (Cinti et al., 1984). In support of this theory, whole WAT depots stain positive for β-galactosidase expression in PdgfRβ-cre:R26R-LacZ mice and PDGFRβ+ cells can form adipocytes when transplanted into mice (Tang et al., 2008). The possibility that a subpopulation of pericytes function as adipocyte precursors is appealing given that the well-characterized interplay between pericytes and endothelial cells during vascular growth is consistent with the close relationship between vascular growth and adipogenesis in vivo (Fukumura et al., 2003; Rupnick et al., 2002).
Platelet-derived growth factor receptor alpha, PdgfRα, has also been implicated in the adipocyte lineage as it is expressed by adipogenic stromal cells from muscle and craniofacial adipose tissue that share several cell surface markers of CD24+ and CD24− adipocyte precursors (Joe et al., 2010; Lemos et al., 2012). Using PdgfRα-cre:mT/mG mice we showed that all adipocytes in all major WAT depots are derived from PdgfRα expressing precursors (Berry and Rodeheffer, 2013). This result supports the model for adipogenesis presented above as the CD24+ adipocyte progenitor cells and CD24− preadipocytes are the only adipogenic SVF populations that express PdgfRα (Berry and Rodeheffer, 2013). Additionally, WAT resident PdgfRα expressing cells have recently been shown to proliferate and differentiate into brown-like adipocytes in response to β-3 adrenergic agonist treatment and white adipocytes in response to high fat diet feeding (Lee et al., 2012). Taken together, these results indicate that mature adipocytes are derived from the CD24+ and CD24− adipocyte precursors in the formation, maintenance, expansion and remodeling of WAT. However, the tracing of mature adipocytes by PdgfRα does not inform on their developmental origin as PdgfRα is expressed in adipocyte precursors (Berry and Rodeheffer, 2013). Therefore the precise origin of white adipocytes remains uncharacterized.
Utilization of Mouse Models for Genetic Manipulation of Adipocyte Precursor Cells
The studies detailed above implicate PPARγ-tTA, PdgfRβ-cre, and PdgfRα-cre mice as candidate models for genetic targeting of adipocyte precursor cells in vivo. However, each of these models has inherent limitations. Primarily, none of these models are capable of specifically targeting adipocyte precursor cells. The PPARγ-tTA model lacks specificity as the tTA cassette was knocked into exon 2 of the PPARγ locus, which is shared by at least two isoforms of PPARγ (PPARγ1 and PPARγ2) that are generated through alternative splicing (Tang et al., 2008; Tontonoz et al., 1994; Zhu et al., 1995). Although PPARγ2 is expressed specifically in adipose tissue (Tontonoz et al., 1994), PPARγ1 is expressed in many cell types, including macrophages, colon epithelium, liver, heart, and adipose (Hevener et al., 2007). This lack of specificity limits the interpretation of adipocyte precursor cell function and localization using this model. Furthermore, Pparγ knock-in mice are heterozygous for Pparγ. As Pparγ heterozygosity is known to produce adipose phenotypes (Miles et al., 2000), data obtained from these mice need to be confirmed in wild type backgrounds.
Both PdgfRα-cre and PdgfRβ-cre mouse lines are also complicated by non-specificity. While PdgfRβ is a well-characterized marker of pericytes, it is only expressed on a subset of pericytes. More importantly, PdgfRβ+ pericytes are not specific to WAT as they are found throughout the body and have been well characterized as important neurovascular regulatory cells (Dore-Duffy, 2008). Through flow cytometry analysis of PdgfRα-cre:mT/mG mice, we found that PdgfRα-cre labels small populations of CD45+ and CD31+ cells in WAT (Berry and Rodeheffer, 2013). PdgfRα is also known to label glial lineage cells in the brain and retina (Rivers et al., 2008). Finally, the use of any model for genetic manipulation of adipocyte precursor cells must take into consideration that cells with similar cell surface marker profiles and functions have been located in brown adipose tissue (BAT) (Sanchez-Gurmaches et al., 2012), skeletal muscle (Birbrair et al., 2013; Joe et al., 2010; Uezumi et al., 2010), skin (Festa et al., 2011), and bone marrow (Morikawa et al., 2009) (Table 1).
Table 1. Cell surface markers of WAT resident adipocyte precursors and adipogenic cell populations found in other murine tissues.
| Tissue | Positive | Negative |
|---|---|---|
| White Adipose | CD341-5, Sca11-4, PdgfRα2,3, CD291,2, PdgfRβ4,5, ΔDecorin5, CD445, NG24, gp383, VCAM13, CD24*1-3 |
CD311-5, CD451-5, Ter1191,4, CD1051,4, CD1171,5, Mac14 |
| Brown Adipose6 | CD34, Sca1, CD29 | CD31, CD45 |
| Skeletal Muscle | CD347-9, Sca17-9, PdgrRa7-9, PdgfRp8,9, CD298, NG29, CD908, CD1469 |
CD317-9, CD457-9, α7-Integrin7,8, CD1178, SM/C-2.68 |
| Skin10 | CD34, Sca1, CD29, CD24* | CD31, CD45 |
| Bone Marrow11 | Sca1, PdgfRα | CD45, Ter119 |
CD24 is expressed by adipogenic cells in embryonic WAT, but is only expressed by the early adipocyte progenitors in adulthood, when they represent of rare subset of the adipocyte precursors.
References for Table 1: 1. (Rodeheffer et al., 2008) 2. (Berry and Rodeheffer, 2013) 3. (Bénézech et al., 2012) 4. (Tang et al., 2008) 5. (Daquinag et al., 2011) 6. (Sanchez-Gurmaches et al., 2012) 7. (Joe et al., 2009) 8. (Uezumi et al., 2010) 9. (Birbrair et al., 2013) 10. (Festa et al., 2011) 11. (Morikawa et al., 2009).
The lack of specificity of these genetic models complicates the targeting of white adipocyte precursor cells and highlights that care must be taken in interpreting studies performed with these genetic models. It is possible that estrogen responsive versions of PdgfR-cre mice, such as the recently published PdgfRα-ERCre mouse model may allow for less disruptive genetic manipulation (Lee et al., 2012). However, given the known effects of estrogen on WAT (Mattsson and Olsson, 2007), experiments involving tamoxifen have to be carefully considered. By using adipocyte precursor targeting models (PdgfRα-cre, PdgfRα-ERCre) in conjunction with mouse models specifically labeling mature adipocytes (Adiponectin-cre) (Eguchi et al., 2011), it will theoretically be possible to distinguish whether observed phenotypes are the result of gene function within adipocyte precursors or mature adipocytes. While the recent advances in tools and methodologies will surely prompt a variety of directed studies of in vivo adipogenesis, interpretation of data must consider possible limitations of the models that are used to study adipocyte precursor function in vivo.
Beige Adipocyte Formation
Although the majority of WAT mass is composed of classical lipid storing white adipocytes, studies have shown that increasing sympathetic tone through chronic cold exposure (Cousin et al., 1992) or direct stimulation of β3-adrenergic receptors via agonist treatment (Ghorbani and Himms-Hagen, 1997) results in the presence of lipolytic, multi-locular adipocytes within classical WAT depots in rodents. Due to their morphological and functional similarities to classical brown adipocytes and their location within WAT depots, these cells have been termed ‘beige’ or ‘brite’ adipocytes. Since genetic manipulations resulting in large increases in the number of beige adipocytes within WAT have been shown to protect against the development of obesity (Seale et al., 2011), beiging of WAT may be a potential treatment for obesity. However, the mechanisms of beige adipocyte formation are an area of current debate.
The two proposed mechanisms of beige adipocyte formation are trans-differentiation of mature white adipocytes to acquire a multi-locular lipolytic phenotype and de novo formation of beige adipocytes from adipocyte precursor cells (Lee and Cowan, 2013). A recent study using Ucp1-CreER:tdRFP (Ucp1-Tracer) mice to induce permanent fluorescent labeling of beige adipocytes provided evidence for the trans-differentiation of mature adipocytes. This study showed that labeling of beige adipocytes only during cold treatment resulted in RFP+ adipocytes expressing white adipocyte genes after several weeks of recovery. A second round of chronic cold treatment without re-labeling resulted in the presence of RFP+ beige adipocytes (Rosenwald et al., 2013). These experiments indicate that beige adipocytes can trans-differentiate to take on a white phenotype and that ‘whitened’ beige adipocytes can re-acquire their beige phenotype under beiging conditions. A separate study utilizing the AdipoChaser mouse concluded that the majority of beige adipocytes arise from de novo differentiation of adipocyte precursors. In these experiments, mature adipocytes in the AdipoChaser mice were permanently labeled prior to challenge with either cold exposure or β3-adrenergic receptor agonist treatment. Analysis of WAT depots revealed that most newly formed beige adipocytes were not labeled and were therefore not the result of trans-differentiation of previously labeled mature white adipocytes (Wang et al., 2013). While the latter study did not directly follow the differentiation of adipocyte precursors into beige adipocytes, transplantation studies have shown that FACS-isolated PDGFRα+ adipocyte precursors are capable of forming both white adipocytes in response to HFD and brown adipocytes in response to β3-adrenergic receptor agonist treatment (Lee et al., 2012), further supporting the model of de novo beige adipocyte formation from adipocyte precursors.
A major question that has yet to be addressed regarding the de novo formation of beige adipocytes is whether these cells arise from identical or distinct populations of PdgfRα expressing precursors residing within WAT. Tracing of mature WAT using Myf5-cre, which labels brown adipocytes (Seale et al., 2008), has revealed that a subpopulation of white adipocytes are also traced by this promoter (Sanchez-Gurmaches et al., 2012). This led to the hypothesis that beige adipocytes arise from Myf5-cre traced adipocyte precursors. However, β3-adrenergic receptor agonist treatment does not selectively increase the number of Myf5-cre traced adipocyte precursors in WAT (Sanchez-Gurmaches et al., 2012). Additionally, adipocytes derived from the differentiation of non-Myf5-cre traced precursors displayed gene expression profiles more similar to brown and beige cells than adipocytes derived from Myf5-cre traced precursors (Shan et al., 2013). While these results indicate that Myf5 does not distinguish a population of beige adipocyte precursors, it is unclear if there is a dedicated population of beige adipocyte precursors for which markers have yet to be identified, or if the white adipocyte precursors described above are bi-potent, capable of giving rise to beige and white adipocytes.
Conclusions
In the last 100 years, the field of adipose tissue biology has transitioned from gross observation of WAT and molecular characterization of adipogenesis in vitro to the identification and characterization of in vivo adipocyte lineage cells. This process has provided a wealth of knowledge about the temporal and cellular mechanisms of WAT accumulation. However, a lack of directed methodologies and genetic tools has limited our ability to definitively determine the WAT-intrinsic mechanisms that regulate adipocyte size and number in vivo. With the advent of new experimental models and the application of methodologies to the field of adipose tissue biology, we now have the opportunity to study the WAT-intrinsic molecular mechanisms regulating WAT mass in vivo.
While future studies will identify more specific markers of in vivo adipocyte precursors, the contributions of many groups over the last few decades have provided thorough characterization of many pro- and anti-adipogenic pathways regulating in vitro adipogenesis. The application of genetic mouse models now allows for the study of these pathways during WAT growth and remodeling in vivo. As the depot and cellular mechanisms of WAT accumulation greatly affect the pathogenesis of obesity, the study of WAT expansion in vivo has the potential to identify novel therapeutic targets for obesity. This would not only improve the health of those suffering from obesity and obesity associated pathologies, but would also alleviate the economic burden that is a result of this disease.
Acknowledgments
The authors thank Christopher D. Church for critical reading of the manuscript. This work was supported by American Diabetes Association Award 7-12-JF-46, DERC pilot project grant DK045735, Connecticut Innovations stem cell grant M140291 and NIDDK grant DK090489 to M.S.R.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson DB, Kauffman RG. Cellular and enzymatic changes in porcine adipose tissue during growth. J Lipid Res. 1973;14:160–168. [PubMed] [Google Scholar]
- Berg W. The development of human fat. Z. Morph. Anthrop. 1911;13 [Google Scholar]
- Bernlohr DA, Bolanowski MA, Kelly TJ, Lane MD. Evidence for an increase in transcription of specific mRNAs during differentiation of 3T3-L1 preadipocytes. J Biol Chem. 1985;260:5563–5567. [PubMed] [Google Scholar]
- Berry R, Rodeheffer MS. Characterization of the adipocyte cellular lineage in vivo. Nat Cell Biol. 2013;15:302–308. doi: 10.1038/ncb2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billon N, Iannarelli P, Monteiro MC, Glavieux-Pardanaud C, Richardson WD, Kessaris N, Dani C, Dupin E. The generation of adipocytes by the neural crest. Development. 2007;134:2283–2292. doi: 10.1242/dev.002642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birbrair A, Zhang T, Wang ZM, Messi ML, Enikolopov GN, Mintz A, Delbono O. Skeletal muscle pericyte subtypes differ in their differentiation potential. Stem Cell Res. 2013;10:67–84. doi: 10.1016/j.scr.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birsoy K, Berry R, Wang T, Ceyhan O, Tavazoie S, Friedman JM, Rodeheffer MS. Analysis of gene networks in white adipose tissue development reveals a role for ETS2 in adipogenesis. Development. 2011;138:4709–4719. doi: 10.1242/dev.067710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boquest AC, Shahdadfar A, Frønsdal K, Sigurjonsson O, Tunheim SH, Collas P, Brinchmann JE. Isolation and transcription profiling of purified uncultured human stromal stem cells: alteration of gene expression after in vitro cell culture. Mol Biol Cell. 2005;16:1131–1141. doi: 10.1091/mbc.E04-10-0949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bénézech C, Mader E, Desanti G, Khan M, Nakamura K, White A, Ware CF, Anderson G, Caamaño JH. Lymphotoxin-β receptor signaling through NF-κB2-RelB pathway reprograms adipocyte precursors as lymph node stromal cells. Immunity. 2012;37:721–734. doi: 10.1016/j.immuni.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calle EE, Thun MJ, Petrelli JM, Rodriguez C, Heath CW. Body-mass index and mortality in a prospective cohort of U.S. adults. N Engl J Med. 1999;341:1097–1105. doi: 10.1056/NEJM199910073411501. [DOI] [PubMed] [Google Scholar]
- Carey VJ, Walters EE, Colditz GA, Solomon CG, Willett WC, Rosner BA, Speizer FE, Manson JE. Body fat distribution and risk of non-insulin-dependent diabetes mellitus in women. The Nurses’ Health Study. Am J Epidemiol. 1997;145:614–619. doi: 10.1093/oxfordjournals.aje.a009158. [DOI] [PubMed] [Google Scholar]
- Church C, Horowitz M, Rodeheffer M. WAT is a functional adipocyte? Adipocyte. 2012;1:38–45. doi: 10.4161/adip.19132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinti S. The adipose organ. Prostaglandins Leukot Essent Fatty Acids. 2005;73:9–15. doi: 10.1016/j.plefa.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Cinti S, Cigolini M, Bosello O, Björntorp P. A morphological study of the adipocyte precursor. J Submicrosc Cytol. 1984;16:243–251. [PubMed] [Google Scholar]
- Clara M. Contribution to the study of the so-called adipose tissue of man. Monit. Zool. Ital. 1927a;38 [Google Scholar]
- Clara M. The adipose tissue of birds. Z. Anat. Entwicklungsgeschicte. 1927b;69 [Google Scholar]
- Cousin B, Cinti S, Morroni M, Raimbault S, Ricquier D, Pénicaud L, Casteilla L. Occurrence of brown adipocytes in rat white adipose tissue: molecular and morphological characterization. J Cell Sci. 1992;103(Pt 4):931–942. doi: 10.1242/jcs.103.4.931. [DOI] [PubMed] [Google Scholar]
- Cristancho AG, Lazar MA. Forming functional fat: a growing understanding of adipocyte differentiation. Nat Rev Mol Cell Biol. 2011;12:722–734. doi: 10.1038/nrm3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossno JT, Majka SM, Grazia T, Gill RG, Klemm DJ. Rosiglitazone promotes development of a novel adipocyte population from bone marrow-derived circulating progenitor cells. J Clin Invest. 2006;116:3220–3228. doi: 10.1172/JCI28510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daquinag AC, Zhang Y, Amaya-Manzanares F, Simmons PJ, Kolonin MG. An isoform of decorin is a resistin receptor on the surface of adipose progenitor cells. Cell Stem Cell. 2011;9:74–86. doi: 10.1016/j.stem.2011.05.017. [DOI] [PubMed] [Google Scholar]
- Dore-Duffy P. Pericytes: pluripotent cells of the blood brain barrier. Curr Pharm Des. 2008;14:1581–1593. doi: 10.2174/138161208784705469. [DOI] [PubMed] [Google Scholar]
- Drøyvold WB, Nilsen TI, Krüger O, Holmen TL, Krokstad S, Midthjell K, Holmen J. Change in height, weight and body mass index: Longitudinal data from the HUNT Study in Norway. Int J Obes (Lond) 2006;30:935–939. doi: 10.1038/sj.ijo.0803178. [DOI] [PubMed] [Google Scholar]
- Eguchi J, Wang X, Yu S, Kershaw EE, Chiu PC, Dushay J, Estall JL, Klein U, Maratos-Flier E, Rosen ED. Transcriptional control of adipose lipid handling by IRF4. Cell Metab. 2011;13:249–259. doi: 10.1016/j.cmet.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis JR, McDonald RB, Stern JS. A diet high in fat stimulates adipocyte proliferation in older (22 month) rats. Exp Gerontol. 1990;25:141–148. doi: 10.1016/0531-5565(90)90045-4. [DOI] [PubMed] [Google Scholar]
- Eto H, Suga H, Matsumoto D, Inoue K, Aoi N, Kato H, Araki J, Yoshimura K. Characterization of structure and cellular components of aspirated and excised adipose tissue. Plast Reconstr Surg. 2009;124:1087–1097. doi: 10.1097/PRS.0b013e3181b5a3f1. [DOI] [PubMed] [Google Scholar]
- Faust IM, Johnson PR, Hirsch J. Adipose tissue regeneration following lipectomy. Science. 1977a;197:391–393. doi: 10.1126/science.877563. [DOI] [PubMed] [Google Scholar]
- Faust IM, Johnson PR, Hirsch J. Surgical removal of adipose tissue alters feeding behavior and the development of obesity in rats. Science. 1977b;197:393–396. doi: 10.1126/science.877564. [DOI] [PubMed] [Google Scholar]
- Faust IM, Johnson PR, Stern JS, Hirsch J. Diet-induced adipocyte number increase in adult rats: a new model of obesity. Am J Physiol. 1978;235:E279–286. doi: 10.1152/ajpendo.1978.235.3.E279. [DOI] [PubMed] [Google Scholar]
- Faust IM, Miller WH, Sclafani A, Aravich PF, Triscari J, Sullivan AC. Diet-dependent hyperplastic growth of adipose tissue in hypothalamic obese rats. Am J Physiol. 1984;247:R1038–1046. doi: 10.1152/ajpregu.1984.247.6.R1038. [DOI] [PubMed] [Google Scholar]
- Ferguson JE, Kelley RW, Patterson C. Mechanisms of endothelial differentiation in embryonic vasculogenesis. Arterioscler Thromb Vasc Biol. 2005;25:2246–2254. doi: 10.1161/01.ATV.0000183609.55154.44. [DOI] [PubMed] [Google Scholar]
- Festa E, Fretz J, Berry R, Schmidt B, Rodeheffer M, Horowitz M, Horsley V. Adipocyte lineage cells contribute to the skin stem cell niche to drive hair cycling. Cell. 2011;146:761–771. doi: 10.1016/j.cell.2011.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn EJ, Trent CM, Rawls JF. Ontogeny and nutritional control of adipogenesis in zebrafish (Danio rerio) J Lipid Res. 2009;50:1641–1652. doi: 10.1194/jlr.M800590-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster MT, Shi H, Seeley RJ, Woods SC. Transplantation or removal of intra-abdominal adipose tissue prevents age-induced glucose insensitivity. Physiol Behav. 2010;101:282–288. doi: 10.1016/j.physbeh.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox K, Peters D, Armstrong N, Sharpe P, Bell M. Abdominal fat deposition in 11-year-old children. Int J Obes Relat Metab Disord. 1993;17:11–16. [PubMed] [Google Scholar]
- Friedman JM. Modern science versus the stigma of obesity. Nat Med. 2004;10:563–569. doi: 10.1038/nm0604-563. [DOI] [PubMed] [Google Scholar]
- Fukumura D, Ushiyama A, Duda DG, Xu L, Tam J, Krishna V, Chatterjee K, Garkavtsev I, Jain RK. Paracrine regulation of angiogenesis and adipocyte differentiation during in vivo adipogenesis. Circ Res. 2003;93:e88–97. doi: 10.1161/01.RES.0000099243.20096.FA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghorbani M, Himms-Hagen J. Appearance of brown adipocytes in white adipose tissue during CL 316,243-induced reversal of obesity and diabetes in Zucker fa/fa rats. Int J Obes Relat Metab Disord. 1997;21:465–475. doi: 10.1038/sj.ijo.0800432. [DOI] [PubMed] [Google Scholar]
- Gimble JM, Katz AJ, Bunnell BA. Adipose-derived stem cells for regenerative medicine. Circ Res. 2007;100:1249–1260. doi: 10.1161/01.RES.0000265074.83288.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodpaster BH, Thaete FL, Simoneau JA, Kelley DE. Subcutaneous abdominal fat and thigh muscle composition predict insulin sensitivity independently of visceral fat. Diabetes. 1997;46:1579–1585. doi: 10.2337/diacare.46.10.1579. [DOI] [PubMed] [Google Scholar]
- Green H, Kehinde O. An established preadipose cell line and its differentiation in culture. II. Factors affecting the adipose conversion. Cell. 1975;5:19–27. doi: 10.1016/0092-8674(75)90087-2. [DOI] [PubMed] [Google Scholar]
- Green H, Kehinde O. Spontaneous heritable changes leading to increased adipose conversion in 3T3 cells. Cell. 1976;7:105–113. doi: 10.1016/0092-8674(76)90260-9. [DOI] [PubMed] [Google Scholar]
- Green H, Meuth M. An established pre-adipose cell line and its differentiation in culture. Cell. 1974;3:127–133. doi: 10.1016/0092-8674(74)90116-0. [DOI] [PubMed] [Google Scholar]
- Greenwood MR, Cleary MP, Hirsch J. Genetic obesity in man and rodents. Curr Concepts Nutr. 1979;8:143–170. [PubMed] [Google Scholar]
- Greenwood MR, Hirsch J. Postnatal development of adipocyte cellularity in the normal rat. J Lipid Res. 1974;15:474–483. [PubMed] [Google Scholar]
- Guilak F, Lott KE, Awad HA, Cao Q, Hicok KC, Fermor B, Gimble JM. Clonal analysis of the differentiation potential of human adipose-derived adult stem cells. J Cell Physiol. 2006;206:229–237. doi: 10.1002/jcp.20463. [DOI] [PubMed] [Google Scholar]
- Guo SS, Zeller C, Chumlea WC, Siervogel RM. Aging, body composition, and lifestyle: the Fels Longitudinal Study. Am J Clin Nutr. 1999;70:405–411. doi: 10.1093/ajcn/70.3.405. [DOI] [PubMed] [Google Scholar]
- Gupta RK, Arany Z, Seale P, Mepani RJ, Ye L, Conroe HM, Roby YA, Kulaga H, Reed RR, Spiegelman BM. Transcriptional control of preadipocyte determination by Zfp423. Nature. 2010;464:619–623. doi: 10.1038/nature08816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta RK, Mepani RJ, Kleiner S, Lo JC, Khandekar MJ, Cohen P, Frontini A, Bhowmick DC, Ye L, Cinti S, et al. Zfp423 expression identifies committed preadipocytes and localizes to adipose endothelial and perivascular cells. Cell Metab. 2012;15:230–239. doi: 10.1016/j.cmet.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammar JA. Contribution to our knowledge of adipose tissue. Arch. Mikrosk. Anat. 1895;45:512. [Google Scholar]
- Han J, Lee JE, Jin J, Lim JS, Oh N, Kim K, Chang SI, Shibuya M, Kim H, Koh GY. The spatiotemporal development of adipose tissue. Development. 2011;138:5027–5037. doi: 10.1242/dev.067686. [DOI] [PubMed] [Google Scholar]
- Hemmeryckx B, Loeckx D, Dresselaers T, Himmelreich U, Hoylaerts MF, Lijnen HR. Age-associated adaptations in murine adipose tissues. Endocr J. 2010;57:925–930. doi: 10.1507/endocrj.k10e-179. [DOI] [PubMed] [Google Scholar]
- Hevener AL, Olefsky JM, Reichart D, Nguyen MT, Bandyopadyhay G, Leung HY, Watt MJ, Benner C, Febbraio MA, Nguyen AK, et al. Macrophage PPAR gamma is required for normal skeletal muscle and hepatic insulin sensitivity and full antidiabetic effects of thiazolidinediones. J Clin Invest. 2007;117:1658–1669. doi: 10.1172/JCI31561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch J, Batchelor B. Adipose tissue cellularity in human obesity. Clin Endocrinol Metab. 1976;5:299–311. doi: 10.1016/s0300-595x(76)80023-0. [DOI] [PubMed] [Google Scholar]
- Hirsch J, Han PW. Cellularity of rat adipose tissue: effects of growth, starvation, and obesity. J Lipid Res. 1969;10:77–82. [PubMed] [Google Scholar]
- Hirsch J, Knittle JL. Cellularity of obese and nonobese human adipose tissue. Fed Proc. 1970;29:1516–1521. [PubMed] [Google Scholar]
- Holmes C, Stanford WL. Concise review: stem cell antigen-1: expression, function, and enigma. Stem Cells. 2007;25:1339–1347. doi: 10.1634/stemcells.2006-0644. [DOI] [PubMed] [Google Scholar]
- Hong L, Peptan IA, Colpan A, Daw JL. Adipose tissue engineering by human adipose-derived stromal cells. Cells Tissues Organs. 2006;183:133–140. doi: 10.1159/000095987. [DOI] [PubMed] [Google Scholar]
- Hood RL, Allen CE. Cellularity of bovine adipose tissue. J Lipid Res. 1973;14:605–610. [PubMed] [Google Scholar]
- Häger A, Sjöstrm L, Arvidsson B, Björntorp P, Smith U. Body fat and adipose tissue cellularity in infants: a longitudinal study. Metabolism. 1977;26:607–614. doi: 10.1016/0026-0495(77)90082-8. [DOI] [PubMed] [Google Scholar]
- Imrie D, Sadler KC. White adipose tissue development in zebrafish is regulated by both developmental time and fish size. Dev Dyn. 2010;239:3013–3023. doi: 10.1002/dvdy.22443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janke J, Engeli S, Gorzelniak K, Luft FC, Sharma AM. Mature adipocytes inhibit in vitro differentiation of human preadipocytes via angiotensin type 1 receptors. Diabetes. 2002;51:1699–1707. doi: 10.2337/diabetes.51.6.1699. [DOI] [PubMed] [Google Scholar]
- Jo J, Gavrilova O, Pack S, Jou W, Mullen S, Sumner AE, Cushman SW, Periwal V. Hypertrophy and/or Hyperplasia: Dynamics of Adipose Tissue Growth. PLoS Comput Biol. 2009;5:e1000324. doi: 10.1371/journal.pcbi.1000324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joe AW, Yi L, Even Y, Vogl AW, Rossi FM. Depot-specific differences in adipogenic progenitor abundance and proliferative response to high-fat diet. Stem Cells. 2009;27:2563–2570. doi: 10.1002/stem.190. [DOI] [PubMed] [Google Scholar]
- Joe AW, Yi L, Natarajan A, Le Grand F, So L, Wang J, Rudnicki MA, Rossi FM. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol. 2010;12:153–163. doi: 10.1038/ncb2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PR, Hirsch J. Cellularity of adipose depots in six strains of genetically obese mice. J Lipid Res. 1972;13:2–11. [PubMed] [Google Scholar]
- Johnson PR, Zucker LM, Cruce JA, Hirsch J. Cellularity of adipose depots in the genetically obese Zucker rat. J Lipid Res. 1971;12:706–714. [PubMed] [Google Scholar]
- Kelley DE, Thaete FL, Troost F, Huwe T, Goodpaster BH. Subdivisions of subcutaneous abdominal adipose tissue and insulin resistance. Am J Physiol Endocrinol Metab. 2000;278:E941–948. doi: 10.1152/ajpendo.2000.278.5.E941. [DOI] [PubMed] [Google Scholar]
- Klyde BJ, Hirsch J. Increased cellular proliferation in adipose tissue of adult rats fed a high-fat diet. J Lipid Res. 1979;20:705–715. [PubMed] [Google Scholar]
- Knittle JL. Adipose tissue development in man. Plenum Press; New York: 1978. [Google Scholar]
- Knittle JL, Hirsch J. Effect of early nutrition on the development of rat epididymal fat pads: cellularity and metabolism. J Clin Invest. 1968;47:2091–2098. doi: 10.1172/JCI105894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knittle JL, Timmers K, Ginsberg-Fellner F, Brown RE, Katz DP. The growth of adipose tissue in children and adolescents. Cross-sectional and longitudinal studies of adipose cell number and size. J Clin Invest. 1979;63:239–246. doi: 10.1172/JCI109295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh YJ, Kang S, Lee HJ, Choi TS, Lee HS, Cho CH, Koh GY. Bone marrow-derived circulating progenitor cells fail to transdifferentiate into adipocytes in adult adipose tissues in mice. J Clin Invest. 2007;117:3684–3695. doi: 10.1172/JCI32504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kral JG. Surgical reduction of adipose tissue in the male Sprague-Dawley rat. Am J Physiol. 1976;231:1090–1096. doi: 10.1152/ajplegacy.1976.231.4.1090. [DOI] [PubMed] [Google Scholar]
- Lee YH, Petkova AP, Mottillo EP, Granneman JG. In vivo identification of bipotential adipocyte progenitors recruited by β3-adrenoceptor activation and high-fat feeding. Cell Metab. 2012;15:480–491. doi: 10.1016/j.cmet.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YK, Cowan CA. White to brite adipocyte transition and back again. Nat Cell Biol. 2013;15:568–569. doi: 10.1038/ncb2776. [DOI] [PubMed] [Google Scholar]
- Lehmann JM, Moore LB, Smith-Oliver TA, Wilkison WO, Willson TM, Kliewer SA. An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor gamma (PPAR gamma) J Biol Chem. 1995;270:12953–12956. doi: 10.1074/jbc.270.22.12953. [DOI] [PubMed] [Google Scholar]
- Lemonnier D. Effect of age, sex, and sites on the cellularity of the adipose tissue in mice and rats rendered obese by a high-fat diet. J Clin Invest. 1972;51:2907–2915. doi: 10.1172/JCI107115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemos DR, Paylor B, Chang C, Sampaio A, Underhill TM, Rossi FM. Functionally convergent white adipogenic progenitors of different lineages participate in a diffused system supporting tissue regeneration. Stem Cells. 2012;30:1152–1162. doi: 10.1002/stem.1082. [DOI] [PubMed] [Google Scholar]
- Leonhardt W, Hanefeld M, Schneider H, Haller H. Human adipocyte volumes: maximum size, and correlation to weight index in maturity onset-diabetes. Diabetologia. 1972;8:287–291. doi: 10.1007/BF01225573. [DOI] [PubMed] [Google Scholar]
- Lin CS, Xin ZC, Deng CH, Ning H, Lin G, Lue TF. Defining adipose tissue-derived stem cells in tissue and in culture. Histol Histopathol. 2010;25:807–815. doi: 10.14670/HH-25.807. [DOI] [PubMed] [Google Scholar]
- Majka SM, Fox KE, Psilas JC, Helm KM, Childs CR, Acosta AS, Janssen RC, Friedman JE, Woessner BT, Shade TR, et al. De novo generation of white adipocytes from the myeloid lineage via mesenchymal intermediates is age, adipose depot, and gender specific. Proc Natl Acad Sci U S A. 2010;107:14781–14786. doi: 10.1073/pnas.1003512107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majka SM, Miller HL, Sullivan T, Erickson PF, Kong R, Weiser-Evans M, Nemenoff R, Moldovan R, Morandi SA, Davis JA, et al. Adipose lineage specification of bone marrow-derived myeloid cells. Adipocyte. 2012;1:215–229. doi: 10.4161/adip.21496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manolopoulos KN, Karpe F, Frayn KN. Gluteofemoral body fat as a determinant of metabolic health. Int J Obes (Lond) 2010;34:949–959. doi: 10.1038/ijo.2009.286. [DOI] [PubMed] [Google Scholar]
- Mao J, Yang T, Gu Z, Heird WC, Finegold MJ, Lee B, Wakil SJ. aP2-Cre-mediated inactivation of acetyl-CoA carboxylase 1 causes growth retardation and reduced lipid accumulation in adipose tissues. Proc Natl Acad Sci U S A. 2009;106:17576–17581. doi: 10.1073/pnas.0909055106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattsson C, Olsson T. Estrogens and glucocorticoid hormones in adipose tissue metabolism. Curr Med Chem. 2007;14:2918–2924. doi: 10.2174/092986707782359972. [DOI] [PubMed] [Google Scholar]
- McCollough AW. Evidence of macrophagal origin of adipose cells in the white rat as shown by studies on starved animals. Journal of Morphology. 1944;75:193–201. [Google Scholar]
- Miles PD, Barak Y, He W, Evans RM, Olefsky JM. Improved insulin-sensitivity in mice heterozygous for PPAR-gamma deficiency. J Clin Invest. 2000;105:287–292. doi: 10.1172/JCI8538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RE, Hackenberg R, Gershman H. Regulation of glutamine synthetase in cultured 3T3-L1 cells by insulin, hydrocortisone, and dibutyryl cyclic AMP. Proc Natl Acad Sci U S A. 1978;75:1418–1422. doi: 10.1073/pnas.75.3.1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller WH, Faust IM, Hirsch J. Demonstration of de novo production of adipocytes in adult rats by biochemical and radioautographic techniques. J Lipid Res. 1984;25:336–347. [PubMed] [Google Scholar]
- Misra A, Garg A, Abate N, Peshock RM, Stray-Gundersen J, Grundy SM. Relationship of anterior and posterior subcutaneous abdominal fat to insulin sensitivity in nondiabetic men. Obes Res. 1997;5:93–99. doi: 10.1002/j.1550-8528.1997.tb00648.x. [DOI] [PubMed] [Google Scholar]
- Mitchell JB, McIntosh K, Zvonic S, Garrett S, Floyd ZE, Kloster A, Di Halvorsen Y, Storms RW, Goh B, Kilroy G, et al. Immunophenotype of human adipose-derived cells: temporal changes in stromal-associated and stem cell-associated markers. Stem Cells. 2006;24:376–385. doi: 10.1634/stemcells.2005-0234. [DOI] [PubMed] [Google Scholar]
- Morikawa S, Mabuchi Y, Kubota Y, Nagai Y, Niibe K, Hiratsu E, Suzuki S, Miyauchi-Hara C, Nagoshi N, Sunabori T, et al. Prospective identification, isolation, and systemic transplantation of multipotent mesenchymal stem cells in murine bone marrow. J Exp Med. 2009;206:2483–2496. doi: 10.1084/jem.20091046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullican SE, Tomaru T, Gaddis CA, Peed LC, Sundaram A, Lazar MA. A novel adipose-specific gene deletion model demonstrates potential pitfalls of existing methods. Mol Endocrinol. 2013;27:127–134. doi: 10.1210/me.2012-1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L. A global double-fluorescent Cre reporter mouse. Genesis. 2007;45:593–605. doi: 10.1002/dvg.20335. [DOI] [PubMed] [Google Scholar]
- Napolitano L. THE DIFFERENTIATION OF WHITE ADIPOSE CELLS. AN ELECTRON MICROSCOPE STUDY. J Cell Biol. 1963;18:663–679. doi: 10.1083/jcb.18.3.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng CW, Poznanski WJ, Borowiecki M, Reimer G. Differences in growth in vitro of adipose cells from normal and obese patients. Nature. 1971;231:445. doi: 10.1038/231445a0. [DOI] [PubMed] [Google Scholar]
- Nicklas BJ, Cesari M, Penninx BW, Kritchevsky SB, Ding J, Newman A, Kitzman DW, Kanaya AM, Pahor M, Harris TB. Abdominal obesity is an independent risk factor for chronic heart failure in older people. J Am Geriatr Soc. 2006;54:413–420. doi: 10.1111/j.1532-5415.2005.00624.x. [DOI] [PubMed] [Google Scholar]
- Nielsen PJ, Lorenz B, Müller AM, Wenger RH, Brombacher F, Simon M, von der Weid T, Langhorne WJ, Mossmann H, Köhler G. Altered erythrocytes and a leaky block in B-cell development in CD24/HSA-deficient mice. Blood. 1997;89:1058–1067. [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Fryar CD. NCHS data brief. 82. National Center for Health Sciences; Hyattsville, MD: 2012. Prevalence of obesity in the United States; pp. 2009–2010. [Google Scholar]
- Pfaff F.E.J.a.A.R.E. Influence of diet and development of the adbdominal fat pad in the pullet. Journal of Nutrition. 1975 [Google Scholar]
- Poissonnet CM, Burdi AR, Bookstein FL. Growth and development of human adipose tissue during early gestation. Early Hum Dev. 1983;8:1–11. doi: 10.1016/0378-3782(83)90028-2. [DOI] [PubMed] [Google Scholar]
- Poissonnet CM, Burdi AR, Garn SM. The chronology of adipose tissue appearance and distribution in the human fetus. Early Hum Dev. 1984;10:1–11. doi: 10.1016/0378-3782(84)90106-3. [DOI] [PubMed] [Google Scholar]
- Rivers LE, Young KM, Rizzi M, Jamen F, Psachoulia K, Wade A, Kessaris N, Richardson WD. PDGFRA/NG2 glia generate myelinating oligodendrocytes and piriform projection neurons in adult mice. Nat Neurosci. 2008;11:1392–1401. doi: 10.1038/nn.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodbell M. METABOLISM OF ISOLATED FAT CELLS. I. EFFECTS OF HORMONES ON GLUCOSE METABOLISM AND LIPOLYSIS. J Biol Chem. 1964;239:375–380. [PubMed] [Google Scholar]
- Rodeheffer MS, Birsoy K, Friedman JM. Identification of white adipocyte progenitor cells in vivo. Cell. 2008;135:240–249. doi: 10.1016/j.cell.2008.09.036. [DOI] [PubMed] [Google Scholar]
- Rosen ED, MacDougald OA. Adipocyte differentiation from the inside out. Nat Rev Mol Cell Biol. 2006;7:885–896. doi: 10.1038/nrm2066. [DOI] [PubMed] [Google Scholar]
- Rosenwald M, Perdikari A, Rülicke T, Wolfrum C. Bi-directional interconversion of brite and white adipocytes. Nat Cell Biol. 2013;15:659–667. doi: 10.1038/ncb2740. [DOI] [PubMed] [Google Scholar]
- Rubin CS, Hirsch A, Fung C, Rosen OM. Development of hormone receptors and hormonal responsiveness in vitro. Insulin receptors and insulin sensitivity in the preadipocyte and adipocyte forms of 3T3-L1 cells. J Biol Chem. 1978;253:7570–7578. [PubMed] [Google Scholar]
- Rupnick MA, Panigrahy D, Zhang CY, Dallabrida SM, Lowell BB, Langer R, Folkman MJ. Adipose tissue mass can be regulated through the vasculature. Proc Natl Acad Sci U S A. 2002;99:10730–10735. doi: 10.1073/pnas.162349799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell TR, Ho R. Conversion of 3T3 fibroblasts into adipose cells: triggering of differentiation by prostaglandin F2alpha and 1-methyl-3-isobutyl xanthine. Proc Natl Acad Sci U S A. 1976;73:4516–4520. doi: 10.1073/pnas.73.12.4516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salans LB, Cushman SW, Weismann RE. Studies of human adipose tissue. Adipose cell size and number in nonobese and obese patients. J Clin Invest. 1973;52:929–941. doi: 10.1172/JCI107258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salans LB, Knittle JL, Hirsch J. The role of adipose cell size and adipose tissue insulin sensitivity in the carbohydrate intolerance of human obesity. J Clin Invest. 1968;47:153–165. doi: 10.1172/JCI105705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Gurmaches J, Hung CM, Sparks CA, Tang Y, Li H, Guertin DA. PTEN loss in the Myf5 lineage redistributes body fat and reveals subsets of white adipocytes that arise from Myf5 precursors. Cell Metab. 2012;16:348–362. doi: 10.1016/j.cmet.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seale P, Bjork B, Yang W, Kajimura S, Chin S, Kuang S, Scimè A, Devarakonda S, Conroe HM, Erdjument-Bromage H, et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature. 2008;454:961–967. doi: 10.1038/nature07182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seale P, Conroe HM, Estall J, Kajimura S, Frontini A, Ishibashi J, Cohen P, Cinti S, Spiegelman BM. Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. J Clin Invest. 2011;121:96–105. doi: 10.1172/JCI44271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengenès C, Lolmède K, Zakaroff-Girard A, Busse R, Bouloumié A. Preadipocytes in the human subcutaneous adipose tissue display distinct features from the adult mesenchymal and hematopoietic stem cells. J Cell Physiol. 2005;205:114–122. doi: 10.1002/jcp.20381. [DOI] [PubMed] [Google Scholar]
- Sera Y, LaRue AC, Moussa O, Mehrotra M, Duncan JD, Williams CR, Nishimoto E, Schulte BA, Watson PM, Watson DK, et al. Hematopoietic stem cell origin of adipocytes. Exp Hematol. 2009;37:1108–1120. doi: 10.1016/j.exphem.2009.06.008. 1120.e1101-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan T, Liang X, Bi P, Zhang P, Liu W, Kuang S. Distinct populations of adipogenic and myogenic Myf5-lineage progenitors in white adipose tissues. J Lipid Res. 2013;54:2214–2224. doi: 10.1194/jlr.M038711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel MJ, Hildebolt CF, Bae KT, Hong C, White NH. Total and intraabdominal fat distribution in preadolescents and adolescents: measurement with MR imaging. Radiology. 2007;242:846–856. doi: 10.1148/radiol.2423060111. [DOI] [PubMed] [Google Scholar]
- Snijder MB, Dekker JM, Visser M, Yudkin JS, Stehouwer CD, Bouter LM, Heine RJ, Nijpels G, Seidell JC. Larger thigh and hip circumferences are associated with better glucose tolerance: the Hoorn study. Obes Res. 2003;11:104–111. doi: 10.1038/oby.2003.18. [DOI] [PubMed] [Google Scholar]
- Soukas A, Socci ND, Saatkamp BD, Novelli S, Friedman JM. Distinct transcriptional profiles of adipogenesis in vivo and in vitro. J Biol Chem. 2001;276:34167–34174. doi: 10.1074/jbc.M104421200. [DOI] [PubMed] [Google Scholar]
- Spalding KL, Arner E, Westermark PO, Bernard S, Buchholz BA, Bergmann O, Blomqvist L, Hoffstedt J, Näslund E, Britton T, et al. Dynamics of fat cell turnover in humans. Nature. 2008;453:783–787. doi: 10.1038/nature06902. [DOI] [PubMed] [Google Scholar]
- Spiegelman BM, Flier JS. Obesity and the regulation of energy balance. Cell. 2001;104:531–543. doi: 10.1016/s0092-8674(01)00240-9. [DOI] [PubMed] [Google Scholar]
- Stanford WL, Haque S, Alexander R, Liu X, Latour AM, Snodgrass HR, Koller BH, Flood PM. Altered proliferative response by T lymphocytes of Ly-6A (Sca-1) null mice. J Exp Med. 1997;186:705–717. doi: 10.1084/jem.186.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternson SM, Nicholas Betley J, Cao ZF. Neural circuits and motivational processes for hunger. Curr Opin Neurobiol. 2013;23:353–360. doi: 10.1016/j.conb.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiles JW, Francendese AA, Masoro EJ. Influence of age on size and number of fat cells in the epididymal depot. Am J Physiol. 1975;229:1561–1568. doi: 10.1152/ajplegacy.1975.229.6.1561. [DOI] [PubMed] [Google Scholar]
- Styner M, Sen B, Xie Z, Case N, Rubin J. Indomethacin promotes adipogenesis of mesenchymal stem cells through a cyclooxygenase independent mechanism. J Cell Biochem. 2010;111:1042–1050. doi: 10.1002/jcb.22793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang QQ, Lane MD. Adipogenesis: from stem cell to adipocyte. Annu Rev Biochem. 2012;81:715–736. doi: 10.1146/annurev-biochem-052110-115718. [DOI] [PubMed] [Google Scholar]
- Tang QQ, Otto TC, Lane MD. CCAAT/enhancer-binding protein beta is required for mitotic clonal expansion during adipogenesis; Proc Natl Acad Sci U S A (United States); 2003; pp. 850–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W, Zeve D, Suh JM, Bosnakovski D, Kyba M, Hammer RE, Tallquist MD, Graff JM. White fat progenitor cells reside in the adipose vasculature; Science (United States); 2008; pp. 583–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tankó LB, Bagger YZ, Alexandersen P, Larsen PJ, Christiansen C. Peripheral adiposity exhibits an independent dominant antiatherogenic effect in elderly women. Circulation. 2003;107:1626–1631. doi: 10.1161/01.CIR.0000057974.74060.68. [DOI] [PubMed] [Google Scholar]
- Taylor SM, Jones PA. Multiple new phenotypes induced in 10T1/2 and 3T3 cells treated with 5-azacytidine. Cell. 1979;17:771–779. doi: 10.1016/0092-8674(79)90317-9. [DOI] [PubMed] [Google Scholar]
- Tchoukalova YD, Fitch M, Rogers PM, Covington JD, Henagan TM, Ye J, Hellerstein MK, Ravussin E. In vivo adipogenesis in rats measured by cell kinetics in adipocytes and plastic-adherent stroma-vascular cells in response to high-fat diet and thiazolidinedione. Diabetes. 2012;61:137–144. doi: 10.2337/db10-1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tontonoz P, Hu E, Graves RA, Budavari AI, Spiegelman BM. mPPAR gamma 2: tissue-specific regulator of an adipocyte enhancer. Genes Dev. 1994;8:1224–1234. doi: 10.1101/gad.8.10.1224. [DOI] [PubMed] [Google Scholar]
- Tran KV, Gealekman O, Frontini A, Zingaretti MC, Morroni M, Giordano A, Smorlesi A, Perugini J, De Matteis R, Sbarbati A, et al. The vascular endothelium of the adipose tissue gives rise to both white and brown fat cells. Cell Metab. 2012;15:222–229. doi: 10.1016/j.cmet.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran TT, Yamamoto Y, Gesta S, Kahn CR. Beneficial effects of subcutaneous fat transplantation on metabolism. Cell Metab. 2008;7:410–420. doi: 10.1016/j.cmet.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uezumi A, Fukada S, Yamamoto N, Takeda S, Tsuchida K. Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nat Cell Biol. 2010;12:143–152. doi: 10.1038/ncb2014. [DOI] [PubMed] [Google Scholar]
- Wang QA, Gupta RK, Scherer PE. AdipoChaser, a novel model system to track adipogenesis during white adipose tissue development, expansion and regeneration. (Nature Medicine) 2013 doi: 10.1038/nm.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Rimm EB, Stampfer MJ, Willett WC, Hu FB. Comparison of abdominal adiposity and overall obesity in predicting risk of type 2 diabetes among men. Am J Clin Nutr. 2005;81:555–563. doi: 10.1093/ajcn/81.3.555. [DOI] [PubMed] [Google Scholar]
- Wassermann F. Die Fettorgane des Menchen. Entwicklung, Bau und systematische Stellung des sogenannten Fettgewebes. Zeit. f. Zellforsch. u. Mikr. Anat. 1926;3:325–329. [Google Scholar]
- Wassermann F. On the formation of appendices epiploicae in man, with consideration of the development of the fat organs therein. Ergeb. Anat. Anz. 1927;63 [Google Scholar]
- Wassermann F. On storage, mobilization, and renewed storage in fat organs. Ergeb. Anat. Anz. 1929;67 [Google Scholar]
- Wassermann F. The development of adipose tissue. In: Renold A.E.a.C.G.F., editor. Handbook of Physiology Section 5: Adipose Tissue. American Physiological Society; Washington D.C.: 1965. pp. 87–100. [Google Scholar]
- WHO . Obesity and overweight. WHO (World Health Organization); 2013. [Google Scholar]
- Yang MU, Presta E, Björntorp P. Refeeding after fasting in rats: effects of duration of starvation and refeeding on food efficiency in diet-induced obesity. Am J Clin Nutr. 1990;51:970–978. doi: 10.1093/ajcn/51.6.970. [DOI] [PubMed] [Google Scholar]
- Zheng B, Cao B, Li G, Huard J. Mouse adipose-derived stem cells undergo multilineage differentiation in vitro but primarily osteogenic and chondrogenic differentiation in vivo. Tissue Eng. 2006;12:1891–1901. doi: 10.1089/ten.2006.12.1891. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Qi C, Korenberg JR, Chen XN, Noya D, Rao MS, Reddy JK. Structural organization of mouse peroxisome proliferator-activated receptor gamma (mPPAR gamma) gene: alternative promoter use and different splicing yield two mPPAR gamma isoforms. Proc Natl Acad Sci U S A. 1995;92 doi: 10.1073/pnas.92.17.7921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]