Abstract
Background and Aims
Hospital admissions in cirrhotic patients are a source of significant health care expenditure. Most studies to date have focused on readmissions in patients with decompensated cirrhosis. We sought to describe predictors of hospital admissions in an ambulatory cirrhosis cohort consisting of both compensated and decompensated patients to identify patients who could benefit from intensified outpatient chronic disease management.
Methods
We performed a retrospective cohort study of 395 cirrhotic patients followed at an academic medical center liver clinic. Inclusion criteria were documented cirrhosis and longitudinal care at our center during 2006–2008. Patients were followed until December 2011, death, or liver transplantation. The primary outcomes were non-elective cirrhosis-related hospital admissions within one year and time to admission. The secondary outcome was two-year cirrhosis-related mortality. The study was approved by the Partners Human Research Committee (protocol 2012P001912).
Results
Seventy-eight patients (19.7%) had at least one cirrhosis-related hospital admission within one year. The following were significant predictors in the multivariable model: Model for End-Stage Liver Disease (MELD) score ≥ 15 (OR 2.22, 95% CI [1.21–4.07], p=0.01), diagnosis of hepatocellular carcinoma (HCC) (3.64 [1.42–9.35], 0.007), diuretic use (2.27 [1.23–4.17], 0.008), at least one cirrhosis-related admission during the baseline year (2.17 [1.21–3.89], 0.01), and being unmarried (1.92 [1.10–3.35], 0.02).
Conclusions
Advanced disease, diuretic use, and marital status were associated with cirrhosis-related hospital admissions in patients followed at an academic medical center liver clinic. Our findings suggest that patients with inadequately or overzealously treated ascites, as well as those with limited social supports, could benefit from intensified outpatient management.
Keywords: Liver cirrhosis, Ascites, Hospitalization, Quality Improvement
INTRODUCTION
Cirrhosis is the twelfth leading cause of death in the United States and a major cause of worldwide morbidity and mortality. According to the Centers for Disease Control and Prevention, cirrhosis and chronic liver disease were responsible for over 30,000 deaths in the United States in 2009, representing 1.3 percent of all deaths in the United States that year.1 When deaths attributed to complications of cirrhosis are added, there are over 44,000 deaths per year due to cirrhosis nearly as many as result from diabetes mellitus. These data likely underestimate the true impact of liver disease on mortality a recent study suggests that the actual toll (25.7 deaths per 100,000 people) may be double what is widely quoted (11.7 deaths per 100,000 people).2 Cirrhosis is the second leading cause of digestive disease related mortality, behind colorectal cancer.
Moreover, patients with decompensated cirrhosis fare worse than those with compensated cirrhosis. One study estimated that survival rates at 1 and 5 years after a first episode of decompensation are 81.8 and 50.8 percent, respectively.3 Furthermore, cirrhosis incurs a substantial financial burden on the health care system. The estimated cost of a single cirrhosis-related hospital admission is $15,000, and the total estimated cost of cirrhosis-related hospitalizations in the United States is $4 billion annually.4 This burden is predicted to significantly increase over the next decade due to an aging population with hepatitis C.
Despite the availability of evidence-based professional guidelines for the prevention of cirrhosis complications, the adherence to these guidelines by care providers and patients is suboptimal. In one study of patients with cirrhosis and ascites seen at Veterans Affairs Medical Centers, only 33.2 percent of patients received all recommended care as described in professional guidelines.5 Patients with cirrhosis are at high risk for hospital readmission once admitted for a cirrhosis-related complication. In a study of patients with decompensated cirrhosis, the median time to readmission after index hospitalization was 67 days. Within 30 days of discharge, 37% were re-admitted at a cost of over $20,000 per admission; 22% of these admissions were thought to be preventable. High Model for End-Stage Liver Disease (MELD) score, low serum sodium, and the presence of complications of cirrhosis were predictors of readmission. The most common causes for preventable readmissions in these patients were hepatic encephalopathy and hyper- or hypovolemia secondary to diuretics.6
Given the complexity of cirrhosis as a chronic disease, adopting a chronic disease management model has been identified as a potential strategy for coordinating the care of these patients. With such a strategy, cirrhosis patients would be followed by a well-coordinated, multidisciplinary team, and effective monitoring systems would be in place to ensure delivery of all indicated care.7 Ideally, the chronic disease management model would optimize patients’ quality of life between visits and minimize preventable hospital admissions.
To maximize the potential of the chronic care model, high-risk patients who would benefit most from the chronic disease management model should be identified. Existing literature has identified risk factors for hospital admission and readmission in decompensated cirrhosis patients5, for example higher MELD score and complications of cirrhosis as noted above. In addition, obese and overweight patients with cirrhosis have higher rates of hospitalization and death compared to non-obese patients.8 However, existing literature focuses on readmission rates in decompensated cirrhotics, who are already at high risk for hospital admission.9 A review of recent literature6–9 yielded no studies examining predictors of hospital admission and clinical deterioration in a more general cirrhosis population (both compensated and decompensated) receiving care in a liver clinic. By describing patient characteristics associated with hospitalization and death, we sought to identify patients who would benefit from a chronic disease management program.
At our center, patients with cirrhosis are managed in a multi-disciplinary Liver Clinic. The clinic is staffed by seven attending hepatologists, along with gastroenterology fellows. A nurse practitioner is involved in the care of patients who are listed for liver transplantation. This setting would be ideal for implementation of a chronic disease management initiative.
METHODS
Selection of cohort
The Research Patient Data Registry (RPDR) at Partners Healthcare is a database composed of 1 billion records from over 4.5 million patients from 1988 to the present. Data come from ten sources, including billing registries and electronic medical records, and contain patient encounters, laboratory test results, imaging, and other clinical data. We searched this database for International Classification of Diseases 9 (ICD-9) codes associated with cirrhosis (Supplemental Table 1). Patients ages 18 through 80 with at least two encounters for one of these ICD-9 codes during the inclusion window from January 1, 2006 until December 31, 2008 were included in the initial study cohort.
An additional inclusion criterion was evidence of an established relationship with our institution, as we sought patients who were likely to be admitted to our hospital. This criterion was defined by receipt of each of the following at our center during the inclusion window:
At least two office visits to our outpatient liver clinic separated by at least one month;
At least one upper gastrointestinal endoscopy;
At least one abdominal imaging study.
Medical records for patients meeting the above criteria were manually reviewed. Patients who did not have documented cirrhosis (by liver clinic notes, imaging, or liver biopsy), who had undergone prior liver transplantation, or who did not live in the region of our center (Northeastern United States) were excluded.
Collection of baseline data
We defined a start date for each patient, which was the date when the last inclusion criterion (second office visit, upper endoscopy, or imaging study) occurred during the 2006–2008 inclusion period. We then established a baseline period for each patient, defined as one calendar year before this start date. Baseline variables extracted from chart review and RPDR data included age, gender, race, etiology of cirrhosis as documented in liver clinic notes, MELD score, prior complications of liver disease, comorbidities, medications, laboratory test results, marital status, health insurance, smoking history and alcohol use. MELD score was calculated using the creatinine, total bilirubin, and International Normalized Ratio (INR) collected closest to the inclusion date. Because 77 patients (19.5%) had lab values used for MELD calculation that were not collected on the same date, we conducted an analysis of MELD scores with same-date lab values versus the most up to date values for each lab. The mean for both the up to date and same-date MELD scores was 10.3, with a mean difference between the values of 1.0. Twenty-two of these MELD scores could not be calculated using same-date values; the mean for these scores was 10.0. We therefore used most up to date values for those patients without same date values. The Mayo Clinic MELD calculator was used for all MELD calculations.10 Demographics, including gender, race, language, health insurance, and marital status were taken from RPDR data. The Charlson Comorbidity Index (CCI) was calculated for each patient using the Deyo adaptation.11
Cirrhosis-related hospital admissions and Emergency Department (ED) visits during the baseline year were identified by manual review of discharge summaries. An admission was deemed related to cirrhosis if cirrhosis was the primary factor leading to the admission, or if it contributed significantly to the admission. Elective admissions (e.g. transjugular intrahepatic portosystemic shunt revision, radiofrequency ablation) were excluded, as were admissions solely for liver transplantation. We did not include cirrhosis-related admissions to outside hospitals.
Follow-up data and end points
The follow up period was defined as the patient’s start date through December 31, 2011. The primary outcomes studied were one-year cirrhosis-related hospital admissions and time to first admission. Patients were censored at the time of liver transplantation or death if these events occurred prior to December 31, 2011.
The secondary outcome was two-year cirrhosis-related death. A death was considered cirrhosis-related if it resulted from complications of cirrhosis, or if cirrhosis contributed significantly to the patient’s demise. A subgroup analysis was performed for primary and secondary outcomes on patients who had no cirrhosis-related admissions during the baseline year.
Statistical analysis
Univariate analyses for candidate variables were performed using logistic regression models or cox proportional-hazards models where appropriate. Multivariable logistic regression was performed using candidate variables with p < 0.10 in univariate analyses. We observed that presence of ascites, diuretic use, and cirrhosis-related ED visits during the baseline year were colinear. We performed chi-square analysis on pairs of these variables and found that baseline ED visits and diuretic were associated (p = 0.06). Similarly, diuretic and presence of ascites were highly associated (p < 0.001). Because of their colinearity with diuretic use, presence of ascites and Emergency Department visits during the baseline year were excluded from each of the multivariable models. Two-sided p-values ≤ 0.05 were considered significant. All statistical analyses were performed using SAS version 9.2.
This study was approved by the Partners Human Research Committee (protocol 2012P001912). All authors had access to the study data, and all approved the final draft of this manuscript.
RESULTS
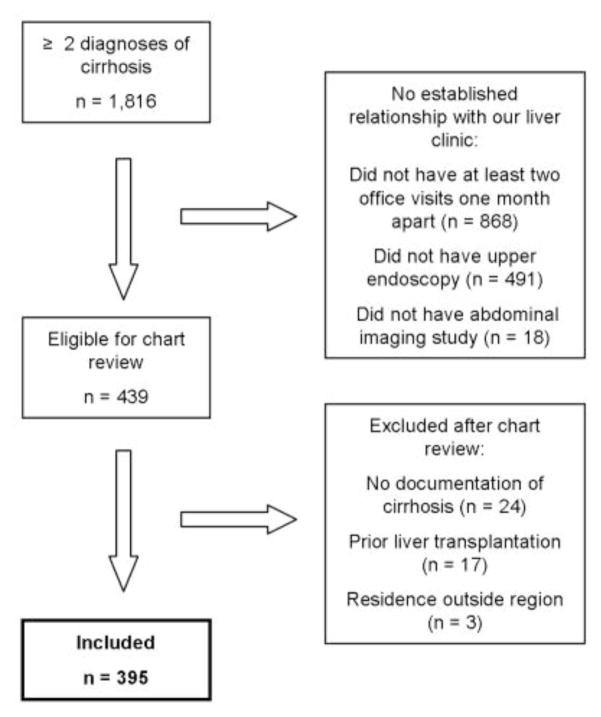
In the inclusion window from January 1, 2006 to December 31, 2008, 1,816 patients had at least two encounters coded from the Supplemental Table 1 diagnoses. Of these, 439 patients had at least two office visits, one upper gastrointestinal endoscopy and one abdominal imaging study. Seventeen patients had undergone prior liver transplantation and were excluded. An additional 24 patients were excluded who did not have documentation of cirrhosis in the medical record. Finally, three patients were excluded who lived outside the Northeastern United States (eligible states: Massachusetts, Maine, Rhode Island, New Hampshire, Vermont, New York, Connecticut), as these patients were unlikely to be admitted to our hospital. In total, 395 patients were included in our cohort for analysis (Figure 1). Baseline characteristics of the cohort are shown in Table 1.
Figure 1.
Selection of Cohort (during the inclusion window from January 1, 2006 through December 31, 2008)
Table 1.
Baseline characteristics of cohort
| Characteristic | Number of Patients (%) |
|---|---|
|
| |
| Age | Mean: 56 years |
| Range 20 – 85 years | |
|
| |
| Male | 254 (64.3) |
|
| |
| White | 307 (79.5) |
|
| |
| Married | 197 (51.3) |
|
| |
| MELD score ≥ 15 | 99 (28.7) |
|
| |
| Etiology of cirrhosis | |
| Hepatitis C | 163 (41.4) |
| Alcohol | 98 (24.9) |
| Other | 78 (19.8) |
| Hepatitis B | 28 (7.1) |
| NASH/NAFLD | 27 (6.9) |
|
| |
| Medical comorbidities | |
| Diabetes mellitus | 98 (24.8) |
| Congestive heart failure | 48 (12.2) |
|
| |
| Outpatient medications | |
| Beta blocker (any) | 189 (48.1) |
| Diuretic | 173 (44.0) |
| Lactulose or rifaximin | 93 (23.7) |
| SBP prophylaxis | 37 (9.4) |
| Methadone or suboxone | 22 (5.6) |
|
| |
| Complications of cirrhosis during the baseline year | |
| Ascites | 217 (54.9) |
| Variceal bleeding | 38 (9.6) |
| HCC | 24 (6.1) |
| Hepatic encephalopathy | 19 (4.8) |
| SBP | 13 (3.3) |
|
| |
| Charlson Comorbidity Index (CCI) | Mean: 4.8 |
|
| |
| Alcohol use | |
| Former heavy use | 184 (46.6) |
| No significant history | 120 (30.4) |
| Current heavy use | 31 (7.8) |
|
| |
| Smoking | |
| Never | 129 (32.7) |
| Former smoker | 91 (23.0) |
| Current smoker | 81 (20.5) |
|
| |
| Insurance status | |
| Private insurance | 179 (45.3) |
| Medicare | 154 (39.0) |
| Medicaid or Mass Health | 50 (12.7) |
| None (self pay/free care) | 12 (3.0) |
The median length of follow up was 3.70 years. During follow up, 21 patients (5.3%) developed HCC, 46 patients (11.6%) underwent liver transplantation, and 124 patients (31.4%) died from any cause. Of these deaths, 69 patients (55.6%) died due to cirrhosis-related complications, excluding those who died after liver transplantation. One hundred forty-two patients (35.9%) were admitted for cirrhosis-related causes at any point during follow up. Of these, 44 (31.0%) required Intensive Care Unit (ICU) admission. The most common cirrhosis-related complication leading to admission was hepatic encephalopathy, with 64 patients (16.2%) admitted during follow up.
Predictors of one-year admissions
For our first primary outcome, 78 patients (19.7%) had at least one cirrhosis-related hospital admission within one year of their start dates. The univariate and multivariable results are shown in Table 2. In the univariate analysis, the following were associated with one-year admissions: diagnosis of HCC, diuretic use, MELD score ≥15, being unmarried, at least one cirrhosis-related admission during the baseline year, at least one cirrhosis-related ICU admission during the baseline year, at least one cirrhosis-related Emergency Department visit during the baseline year, etiology of cirrhosis, CCI, diabetes mellitus, congestive heart failure, lactulose or rifaximin use, SBP prophylaxis use, and presence of ascites. In the multivariable analysis, MELD score ≥15, diagnosis of HCC, diuretic use, at least one cirrhosis-related admission during the baseline year, and being unmarried were associated with one-year hospital admissions.
Table 2.
Predictors of one-year admissions
| Variable | Univariate Analysis | Multivariable Analysis | ||
|---|---|---|---|---|
| Odds Ratio [95% CI] | p-value | Odds Ratio [95% CI] | p-value | |
| HCC | 2.97 [1.27–6.95] | 0.01 | 3.64 [1.42–9.35] | 0.007 |
| Diuretic | 3.57 [2.13–6.00] | < 0.001 | 2.27 [1.23–4.17] | 0.008 |
| MELD ≥ 15 | 3.49 [2.07–5.90] | < 0.001 | 2.22 [1.21–4.07] | 0.01 |
| Unmarried | 1.90 [1.15–3.15] | 0.01 | 1.92 [1.10–3.35] | 0.02 |
| Admission during baseline year | 4.09 [2.45–6.82] | < 0.001 | 2.17 [1.21–3.89] | 0.01 |
| ICU admission during baseline year | 2.04 [0.91–4.54] | 0.08 | -- | -- |
| Alcoholic cirrhosis (vs. hepatitis C-related cirrhosis) | 2.18 [1.23–3.84] | <.0001 | -- | -- |
| ED visit during baseline year | 6.66 [2.12–20.90] | 0.001 | -- | -- |
| CCI (per one point increase) | 1.17 [1.09–1.26] | < 0.001 | -- | -- |
| Diabetes mellitus | 1.67 [0.98–2.85] | 0.06 | -- | -- |
| Congestive heart failure | 1.90 [0.98–3.70] | 0.06 | -- | -- |
| Lactulose or rifaximin | 2.11 [1.24–3.59] | 0.006 | -- | -- |
| SBP prophylaxis | 2.58 [1.26–5.27] | 0.009 | -- | -- |
| Ascites | 7.38 [3.77–14.47] | < 0.001 | -- | -- |
Predictors of time to first admission
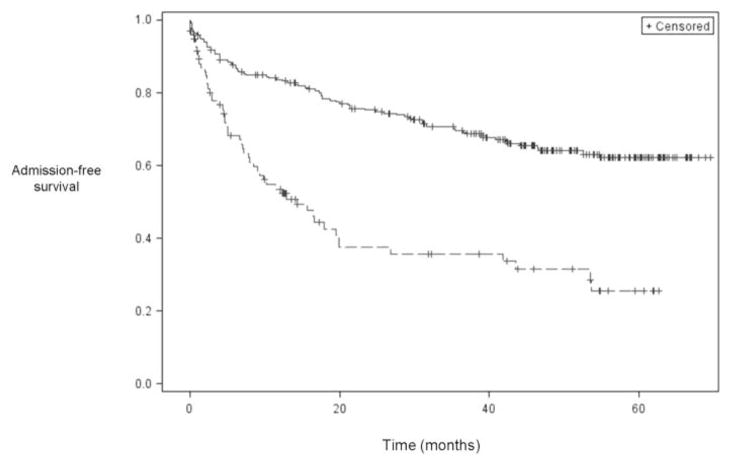
For those patients who were admitted during follow up, the median time to admission was 242 days. The results of the statistical analyses for time to admission, the second primary outcome, are shown in Table 3. In the univariate analysis, the following variables were associated with a shorter time to admission: congestive heart failure, lactulose or rifaximin use, SBP prophylaxis use, diuretic use, MELD score ≥15, diagnosis of HCC, presence of ascites, at least one cirrhosis-related Emergency Department visit during the baseline year, at least one cirrhosis-related hospital admission during the baseline year, at least one cirrhosis-related ICU admission during the baseline year, age ≥50, being unmarried, CCI, number of liver clinic visits per year during follow up, seeing a mid-level provider during at least half of the liver clinic visits (nurse practitioner (NP) or physician assistant (PA)), and number of outpatient medications. In the multivariable model, MELD score ≥15, diagnosis of HCC, age ≥50, at least one cirrhosis-related hospital admission during the baseline year, number of liver clinic visits per year during follow up, and being unmarried were associated with a shorter time to admission. Kaplan-Meier curves for time to admission stratified by MELD score and marital status are shown in Figures 2 and 3, respectively. Additional curves stratified by cirrhosis-related admissions during the baseline year as well as diuretic use are shown in Supplemental Figures 1 and 2, respectively.
Table 3.
Predictors of time to hospital admission
| Univariate Analysis | Multivariable Analysis | |||
|---|---|---|---|---|
| Variable | Hazard Ratio [95% CI] | p-value | Hazard Ratio [95% CI] | p-value |
| MELD ≥ 15 | 2.97 [2.10–4.20] | < 0.001 | 2.39 [1.64–3.48] | < 0.001 |
| HCC | 2.86 [1.66–4.90] | < 0.001 | 2.43 [1.41–4.19] | 0.002 |
| Age ≥ 50 | 1.54 [1.02–2.34] | < 0.001 | 1.56 [1.01–2.41] | 0.05 |
| Admission during baseline year | 2.71 [1.93–3.81] | < 0.001 | 1.70 [1.17–2.48] | 0.006 |
| Follow up liver clinic visits per year (per one visit increase) | 1.01 [1.00–1.02] | 0.004 | 1.009 [1.00–1.02] | 0.05 |
| Unmarried | 1.58 [1.13–2.22] | 0.008 | 1.59 [1.12–2.25] | 0.05 |
| Congestive heart failure | 1.75 [1.11–2.76] | 0.02 | -- | -- |
| Lactulose or rifaximin | 1.64 [1.13–2.38] | 0.009 | -- | -- |
| SBP prophylaxis | 1.91 [1.16–3.13] | 0.01 | -- | -- |
| Diuretic | 2.20 [1.58–3.07] | < 0.001 | -- | -- |
| ED visit during baseline year | 3.75 [2.02–6.96] | < 0.001 | -- | -- |
| ICU admission during baseline year | 1.62 [0.91–2.86] | 0.10 | -- | -- |
| Ascites | 2.89 [2.01–4.16] | 0.04 | -- | -- |
| CCI (per one point increase) | 1.11 [1.06–1.16] | < 0.001 | -- | -- |
| NP or PA seen on at least half of visits | 1.63 [0.99–2.68] | 0.05 | -- | -- |
| Number of medications (per one medication increase) | 1.05 [1.01–1.10] | 0.02 | -- | -- |
Figure 2.
Kaplan-Meier curve for time to admission stratified by MELD score. The top line represents patients with MELD score < 15, and the bottom line represents patients with MELD score ≥ 15.
Figure 3.
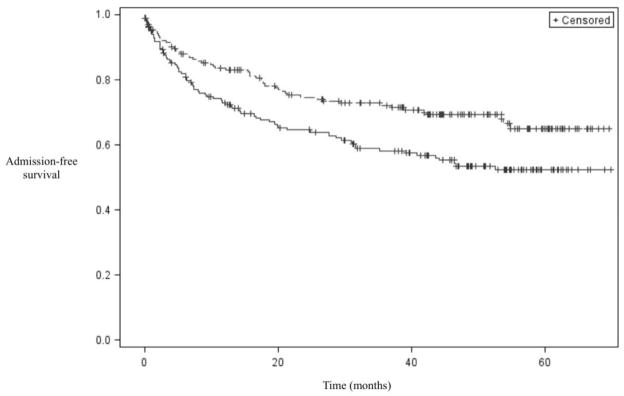
Kaplan-Meier curve for time to admission stratified by marital status. The top line represents married patients, and the bottom line represents unmarried patients.
Predictors of two-year cirrhosis-related death
Forty-four patients (11.1%) died from cirrhosis-related complications within two years of their start dates, excluding those who died after liver transplantation. In the univariate analysis, congestive heart failure, MELD score ≥15, diagnosis of HCC, being unmarried, presence of ascites, lactulose or rifaximin use, diuretic use, at least one cirrhosis-related Emergency Department visit during the baseline year, at least one cirrhosis-related hospital admission during the baseline year, at least one cirrhosis-related ICU admission during the baseline year, CCI, and the number of liver clinic visits during the baseline year were associated with two-year cirrhosis-related mortality, as shown in Table 4. In the multivariable model, congestive heart failure, MELD score ≥15, diagnosis of HCC, and being unmarried were associated with two-year cirrhosis-related death.
Table 4.
Predictors of two-year cirrhosis-related death
| Univariate Analysis | Multivariable Analysis | |||
|---|---|---|---|---|
| Variable | Odds Ratio [95% CI] | p-value | Odds Ratio [95% CI] | p-value |
| Congestive heart failure | 3.54 [1.70–7.34] | 0.001 | 3.40 [1.49–7.75] | 0.004 |
| MELD ≥ 15 | 4.26 [2.23–8.13] | < 0.001 | 4.44 [2.22–8.93] | < 0.001 |
| HCC | 3.51 [1.37–8.98] | 0.009 | 4.10 [1.43–11.76] | 0.009 |
| Unmarried | 2.08 [1.09–3.96] | 0.03 | 2.49 [1.21–5.11] | 0.01 |
| Ascites | 8.06 [3.11–20.88] | < 0.001 | -- | -- |
| Lactulose or rifaximin | 3.21 [1.70–6.06] | < 0.001 | -- | -- |
| Diuretic | 3.34 [1.72–6.48] | < 0.001 | -- | -- |
| ED visit during baseline year | 3.60 [1.06–12.20] | 0.04 | -- | -- |
| ICU admission during baseline year | 2.54 [1.03–6.31] | 0.04 | -- | -- |
| Admission during baseline year | 3.94 [2.10–7.41] | < 0.001 | -- | -- |
| CCI (per one point increase) | 1.16 [1.07–1.27] | 0.001 | -- | -- |
| Baseline liver clinic visits (per one visit increase) | 1.19[1.02–1.39] | 0.03 | -- | -- |
Complications of Diuretic Use
Although not a study outcome, we examined hospital admissions for complications of diuretic use, such as acute kidney injury or electrolyte disturbances. Twenty-nine patients (7.3%) were admitted for such complications at any point during follow up.
Subgroup analysis: patients without cirrhosis-related hospital admissions during the baseline year
To exclude the possibility that there were different sets of predictors for those patients with and without recent cirrhosis-related admissions, we performed a subgroup analysis on those patients who were not admitted during their baseline year. For one-year cirrhosis-related hospital admissions, diuretic use and the number of liver clinic visits per year during follow up were predictors (Supplemental Table 2). For time to first cirrhosis-related hospital admission, the following were predictors of a shorter time to admission in the multivariable model: MELD score ≥ 15, diuretic use, diagnosis of HCC, and the number of liver clinic visits per year during follow (Supplemental Table 3). For two-year cirrhosis-related mortality, diuretic use, being unmarried, and diagnosis of HCC emerged as significant in the multivariable model (Supplemental Table 4).
DISCUSSION
Although hospital readmissions have been the focus of increasing attention due to impending Medicare penalties, risk factors for hospital admissions in ambulatory cirrhosis patients, including compensated cirrhotic patients, have not been systematically studied. This is thus the first study that sought to identify ambulatory cirrhosis patients at higher risk for hospital admissions. We found that patients with cirrhosis followed in an ambulatory liver clinic are commonly admitted for cirrhosis-related complications. MELD score ≥ 15, diagnosis of HCC, and taking a diuretic were predictors for cirrhosis-related hospital admissions and cirrhosis-related death.
We found that more advanced cirrhosis, represented by higher MELD scores and diagnosis of HCC, is associated with higher risk for hospitalization. This association is consistent with the known increased morbidity and mortality experienced by patients with advanced disease.3 Cirrhosis-related admissions during the baseline year were associated with a shorter time to the next admission, suggesting that patients with recent admissions are at higher risk for future admissions and should be followed closely.
Diuretic use also emerged as strongly associated with cirrhosis-related hospital admissions. The colinearity between ascites and diuretics was expected, consistent with the practice of prescribing diuretics for ascites in cirrhotic patients. ED visits also displayed colinearity with these variables. However, in this study it was not possible to identify the precise nature of these relationships. Patients with ascites on diuretics may be more likely to both be seen in the ED and admitted to the hospital; of the 13 patients with cirrhosis-related ED visits during their baseline year, seven (53.8%) presented with ascites. In addition, our examination of the reasons for hospital admissions revealed that not only were patients on diuretics admitted for refractory ascites, they also experienced complications of diuresis. Although we did not include admissions for hepatic encephalopathy as “complications of diuresis,” it is likely that at least some of these were related to diuretic therapy. Given that hepatic encephalopathy was the most common cirrhosis-related complication experienced by patients in our cohort, finding preventable causes of these admissions would improve patient care. This is also consistent with previous studies suggesting that hypo- and hypervolemia in cirrhotic patients on diuretics are significant contributors to hospital admissions.5
These observations suggest that management of ascites with diuretics in cirrhotic patients is complex and requires close medical supervision. The American Association for the Study of Liver Diseases (AASLD) guidelines state that the first line treatment of ascites due to cirrhosis is salt restriction and diuretics.12 Given this recommendation, providers can expect many of their cirrhotic patients, particularly those with advanced disease, to be on diuretic therapy. Our results indicate that these patients should be targeted for more intensive management, as these admissions could possibly be prevented by better communication between physician and patient between liver clinic visits.
The factors associated with first hospital admission in patients who had not been admitted the previous year were similar to the factors for the entire cohort, based on our subgroup analysis. Disease severity as evidenced by MELD score and diagnosis of HCC was a predictor, as would be expected. Taking a diuretic was strongly associated with one-year admissions and time to first admission. The number of office visits per year during follow up emerged as a predictor for one-year admissions and two-year cirrhosis-related death; this likely displays high colinearity with disease severity. The subgroup analysis reveals that for ambulatory patients without recent cirrhosis-related hospital admissions, those taking diuretics should be followed carefully, in addition to those with more advanced disease.
Our analysis also suggests the importance of social factors in the risk for hospital admissions among cirrhosis patients. Existing literature suggests that marriage is associated with lower mortality in male patients hospitalized for pneumonia,13 and also with fewer hospitalizations related to medication errors in diabetes.14 In our study, being married was associated with fewer cirrhosis-related hospital admissions, a longer time to hospital admission, and a lower risk of cirrhosis-related death. It is not clear how this effect is mediated. Because we also accounted for methadone or suboxone use and tobacco and alcohol histories, we believe that marriage is not simply a proxy for other psychosocial factors. One hypothesis is that marriage promotes adherence to prescribed medications. This could explain the protective effect of marriage in a cohort in which diuretic use is associated with higher risk for admission. This hypothesis cannot be tested with our data set, but suggests that patients lacking social supports should be followed closely in the ambulatory setting, as they may be more likely to be admitted to the hospital. Our analysis did not account for domestic partnerships or other forms of cohabitation; this is an area that should be explored further.
The principal limitation of this study is its single institution design. Although we included only those patients who had an established relationship with our institution, it is possible that patients in our cohort were admitted to outside hospitals. This would lead us to underestimate the already high rate of admissions. An additional limitation is the retrospective nature of the study. The study was performed at a tertiary care center whose Liver Clinic cares for a complicated panel of patients. This could limit the generalizability to other settings such as community hospitals. Additionally, although we analyzed only cirrhosis-related admissions and deaths, we realize that cirrhosis has a profound impact on a patient’s overall health and therefore may have contributed to further hospital admissions or deaths that were not included in our analysis. Finally, our findings on the effects of marital status on hospital admissions are limited by the fact that we did not have access to information on patients’ living situations. The presence of non-spousal caregivers in the home could affect these conclusions.
Existing studies have focused on hospital readmissions in decompensated cirrhotic patients, a relevant and timely topic given upcoming changes to reimbursements and penalties for preventable readmissions. Known predictors for readmission in patients with decompensated cirrhosis include obesity and advanced liver disease. However, no study that we are aware of has focused on predictors of hospitalization in an ambulatory cohort that includes compensated cirrhotic patients. Our findings have relevance for hepatologists attempting to risk stratify cirrhotic patients and determine which patients merit closer follow up to prevent hospital admissions.
Our data form the foundation for the design of an outpatient intervention, now underway at our institution, to prevent hospital admissions in ambulatory cirrhotic patients. We will target cirrhotic patients with MELD score ≥15 who are on diuretics and who have limited social supports. Limited social support will be defined as living alone, lower socioeconomic class, and non-English as the primary language. Although we used marital status in this study because of its availability in our clinical research database, patients’ living situations might serve as a more accurate measure of social supports. Relationship status will also be considered, as patients who live alone and therefore do not have partners available to act as caregiver would be appropriate targets for the outpatient intervention. ZIP codes can be used to identify patients from areas of lower socioeconomic status. Non-English primary language can identify patients who may have trouble accessing social and health services due to language barriers. In Massachusetts, all patients are mandated to have insurance, thus insurance data might not be as instructive here as it would in other states.
The ability to study this group of high-risk patients prospectively will add valuable information to our existing predictors and help us better target those at the highest risk for hospital admissions.
Supplementary Material
Kaplan-Meier curve for time to admission stratified by recent admissions. The top line represents patients not admitted within one year of study start, and the bottom line represents patients admitted within one year of study start.
Kaplan-Meier curve for time to admission stratified by diuretic use. The top line represents patients not on diuretics, and the bottom line represents patients on diuretics.
Acknowledgments
The authors would like to thank Eric Braun for his technical assistance and Amy Nash for her administrative support.
Grant support: None
Abbreviations used in this manuscript
- HCC
Hepatocellular carcinoma
- RPDR
Research Patient Data Registry
- MELD
Model for End-Stage Liver Disease
- ICD-9
International Classification of Diseases 9
- CCI
Charlson Comorbidity Index
- INR
International Normalized Ratio
- NP
Nurse practitioner
- PA
Physician assistant
- ED
Emergency Department
- SBP
Spontaneous bacterial peritonitis
- ICU
Intensive Care Unit
Footnotes
Author contact information and contributions:
Kara B. Johnson, BA: Study concept and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript, and critical revision of the manuscript for important intellectual content.
Department of Medicine, Division of Gastroenterology, Massachusetts General Hospital, 55 Fruit Street, Boston, MA 02114, KARA_JOHNSON@HMS.HARVARD.EDU
Emily J. Campbell, MPH: Acquisition of data and critical revision of the manuscript for important intellectual content.
Department of Medicine, Division of Gastroenterology, Massachusetts General Hospital, 55 Fruit Street, Boston, MA 02114, EJCAMPBELL@PARTNERS.ORG
Heng Chi, BSc: Acquisition of data and analysis and interpretation of data.
Department of Gastroenterology & Hepatology, Erasmus MC, University Medical Center, ’s Gravendijkwal 230, Room Ca-411, 3015 CE Rotterdam, The Netherlands, H.CHI@ERASMUSMC.NL
Hui Zheng, PhD: Statistical analysis.
Biostatistics Center, Massachusetts General Hospital, 55 Fruit Street, Boston, MA 02114, HZHENG1@PARTNERS.ORG
Lindsay Y. King, MD: Analysis and interpretation of data and critical revision of the manuscript for important intellectual content.
Department of Medicine, Division of Gastroenterology, Massachusetts General Hospital, 55 Fruit Street, Boston, MA 02114, LYKING@PARTNERS.ORG
Ying Wu, MD: Acquisition of data, analysis and interpretation of data, and critical revision of the manuscript for important intellectual content.
Department of Medicine, Division of Gastroenterology, Massachusetts General Hospital, 55 Fruit Street, Boston, MA 02114, DYWU@PARTNERS.ORG
Andrew DeLemos, MD: Study concept and design.
Department of Medicine, Division of Gastroenterology, Massachusetts General Hospital, 55 Fruit Street, Boston, MA 02114, ADELEMOS@PARTNERS.ORG
Abu Hurairah, MD: Acquisition of data.
Department of Medicine, Mount Auburn Hospital, 330 Mount Auburn Street, Cambridge, MA 02138, AHURAIRAH@PARTNERS.ORG
Kathleen Corey, MD: Study concept and design, analysis and interpretation of data, and critical revision of the manuscript for important intellectual content.
Department of Medicine, Division of Gastroenterology, Massachusetts General Hospital, 55 Fruit Street, Boston, MA 02114, KCOREY@PARTNERS.ORG
James M. Richter, MD: Study concept and design, analysis and interpretation of data, critical revision of the manuscript for important intellectual content, and study supervision.
Department of Medicine, Division of Gastroenterology, Massachusetts General Hospital, 55 Fruit Street, Boston, MA 02114, JRICHTER@PARTNERS.ORG
Raymond T. Chung, MD: Study concept and design, analysis and interpretation of data, critical revision of the manuscript for important intellectual content, and study supervision.
The authors do not have any financial conflicts of interest to disclose.
References
- 1.Heron M. Deaths: leading causes for 2009. National Vital Statistics Reports. 2012:61. [PubMed] [Google Scholar]
- 2.Asrani SK, Larson JJ, Yawn B, et al. Underestimation of Liver-Related Mortality in the United States. Gastroenterology. 2013 doi: 10.1053/j.gastro.2013.04.005. in press. pii: S0016-5085(13)00498-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Planas R, Balleste B, Alvarez MA, et al. Natural history of decompensated hepatitis C virus-related cirrhosis. A study of 200 patients. J Hepatol. 2004;40:823–830. doi: 10.1016/j.jhep.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 4.Kim WR, Brown RS, Jr, Terrault NA, et al. Burden of liver disease in the United States: summary of a workshop. Hepatology. 2002;36:227–242. doi: 10.1053/jhep.2002.34734. [DOI] [PubMed] [Google Scholar]
- 5.Kanwal F, Kramer JR, Buchanan P, et al. The quality of care provided to patients with cirrhosis and ascites in the Department of Veterans Affairs. Gastroenterology. 2012;143:70–77. doi: 10.1053/j.gastro.2012.03.038. [DOI] [PubMed] [Google Scholar]
- 6.Volk ML, Tocco RS, Bazick J, et al. Hospital readmissions among patients with decompensated cirrhosis. Am J Gastroenterol. 2012;107:247–252. doi: 10.1038/ajg.2011.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Volk ML, Piette JD, Singal AS, et al. Chronic disease management for patients with cirrhosis. Gastroenterology. 2010;139:14–6.e1. doi: 10.1053/j.gastro.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ioannou GN, Weiss NS, Kowdley KV, Dominitz JA. Is obesity a risk factor for cirrhosis-related death or hospitalization? A population-based cohort study. Gastroenterology. 2003;125:1053–1059. doi: 10.1016/s0016-5085(03)01200-9. [DOI] [PubMed] [Google Scholar]
- 9.Berman K, Tandra S, Forssell K, et al. Incidence and predictors of 30-day readmission among patients hospitalized for advanced liver disease. Clin Gastroenterol Hepatol. 2011;9:254–259. doi: 10.1016/j.cgh.2010.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kamath PS, Kim WR. The model for end-stage liver disease (MELD) Hepatology. 2007;45:797–805. doi: 10.1002/hep.21563. [DOI] [PubMed] [Google Scholar]
- 11.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 12.Runyon BA AASLD Practice Guidelines Committee. Management of adult patients with ascites due to cirrhosis: an update. Hepatology. 2009;49:2087–107. doi: 10.1002/hep.22853. [DOI] [PubMed] [Google Scholar]
- 13.Metersky ML, Fine MJ, Mortensen EM. The effect of marital status on the presentation and outcomes of elderly male veterans hospitalized for pneumonia. Chest. 2012;142:982–7. doi: 10.1378/chest.11-3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Claydon-Platt K, Manias E, Dunning T. Medication-related problems occurring in people with diabetes during an admission to an adult teaching hospital: a retrospective cohort study. Diabetes Res Clin Pract. 2012;97:223–30. doi: 10.1016/j.diabres.2012.03.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Kaplan-Meier curve for time to admission stratified by recent admissions. The top line represents patients not admitted within one year of study start, and the bottom line represents patients admitted within one year of study start.
Kaplan-Meier curve for time to admission stratified by diuretic use. The top line represents patients not on diuretics, and the bottom line represents patients on diuretics.