Abstract
Backround
Inflammation is considered as a mechanism leading to depression, but the association between inflammatory dietary pattern and depression risk is unknown.
Methods
Using reduced-rank regression, we identified a dietary pattern that was related to plasma levels of inflammatory markers (C-reactive protein, interleukin-6, tumor necrosis factor α receptor 2), and we conducted a prospective analysis of the relationship of this pattern and depression risk among participants in the Nurses’ Health Study. A total of 43,685 women (aged 50–77) without depression at baseline (1996) were included and followed up until 2008. Diet information was obtained from food frequency questionnaires completed between 1984 through 2002 and computed as cumulative average of dietary intakes with a 2-year latency applied. We used a strict definition of depression that required both self-reported physician-diagnosed depression and use of antidepressants, and a broader definition that included women who reported either clinical diagnosis or antidepressant use.
Results
During the 12-year follow-up, we documented 2,594 incident cases of depression using the stricter definition and 6,446 using the broader definition. After adjustment for body mass index and other potential confounders, relative risks comparing extreme quintiles of the inflammatory dietary pattern were 1.41 (95% confidence interval [CI], 1.22, 1.63; P-trend <.001) for the strict definition and 1.29 (95% CI, 1.18, 1.41; P-trend <.001) for the broader definition of depression.
Conclusions
The inflammatory dietary pattern is associated with a higher depression risk. This finding suggests that chronic inflammation may underlie the association between diet and depression.
Keywords: depression, inflammatory markers, C-reactive protein, interleukin-6, tumor necrosis factor α receptor 2, diet pattern, cohort, reduced-rank regression, women
1. Introduction
Depression is a chronic and recurrent illness that affects more women than men, and about 20% of US women are affected during their lifetime (Kessler et al. 2003). Depression is associated with leading causes of morbidity and mortality, including cardiovascular disease (CVD), diabetes, and cancer (Musselman et al. 1998; Pan et al. 2011a; Spiegel and Giese-Davis, 2003). As indicated in a recent literature review (Sanchez-Villegas and Martínez-González, 2013), depression shares common mechanisms (e.g. insulin resistance, higher plasma homocysteine levels, and endothelial dysfunction, etc.) with cardiometabolic disorders that could explain the link between these diseases. Inflammatory processes have been also suggested as common link between depression and CVD, diabetes and cancer (Howren et al. 2009). Proinflammatory cytokines may have an adverse effect on neurotransmitters, and inflammatory status and endothelial dysfunction could impair the expression of brain-derived neurotrophic factor (BDNF) (Sanchez-Villegas and Martínez-González, 2013). Current evidence indicates that diet could stimulate chronic inflammatory diseases (Bosma-den Boer et al. 2012; Giugliano et al. 2006).
The traditional single-nutrient or food analysis in relation to chronic diseases is challenged by several conceptual and methodological limitations (Hu 2002). Dietary pattern analysis, which reflect different combinations of food intake, has emerged as a complementary approach that better reflects the complexity of the diet and its relationship with disease risk (Hu 2002). Therefore, it is also important to consider the overall diet relationship with depression risk, and not only isolated nutrients or foods. To date, only five cohort studies have evaluated the associations between patterns and depression risk. Sanchez-Villegas et al. (Sanchez-Villegas et al. 2009) noted a lower incidence of depression with higher scores on a Mediterranean diet score in the Seguimiento Universidad de Navarra (SUN) cohort. Through the principal component analysis (PCA) among the Australian Longitudinal Study on Women’s Health (ALSWH), Rienks et al. (Rienks et al. 2013) observed that a Mediterranean-style dietary pattern was associated with a lower incidence of depressive symptoms. Using PCA and data of the Whitehall II prospective cohort, Akbaraly et al. (Akbaraly et al. 2009) noted a higher odds of depressive symptoms with a “processed food pattern”. Using PCA and data of the GAZEL cohort, Le Port et al. (Le Port et al. 2012) reported that healthy and traditional patterns were associated with lower risk of depressive symptoms in women. However, in our recent analyses among women in the Nurses’ Health Study (NHS), no significant association was found between dietary Prudent (high in fruits, vegetables, legumes, fish, poultry, and whole grains) and Western pattern (high in red and processed meats, sweets and desserts, french fries, and refined grains) scores (using PCA) and depression risk (Chocano-Bedoya et al. 2013).
The reduced rank regression (RRR) is an alternative empirical approach to generating dietary patterns that may be better predictors of disease (Hoffmann et al. 2004). Unlike analyses using PCA or factor analysis, which derive dietary patterns based on covariance among foods, the RRR method uses information on biomarkers to derive dietary patterns. To our knowledge, no previous study has examined the association between inflammation prone dietary patterns (RRR derived) and depression risk. Here we first derived a dietary pattern that was associated with selected inflammatory biomarkers and then conducted a prospective analysis to determine the relationship of this inflammatory dietary pattern (IDP) with depression risk among participants of the NHS.
2. Methods and Materials
2.1 Study Population
The NHS is a prospective cohort of 121,700 U.S. female registered nurses aged 30 to 55 years at enrollment in 1976. Every 2 years, participants provide updated information, via mailed questionnaires, about lifestyle, medical history and newly-diagnosed medical illnesses. Women were first asked to report their use of antidepressants in 1996 and their history of physician-diagnosed depression in 2000. A total of 97,103 women completed one of the 1996, 1998, or 2000 questionnaires. To examine prospectively the relation of IDP to depression, we excluded from the analyses those women who could have had depression before 1996. This group included 36,225 women with an incomplete depression history (i.e. those who did not report their depressive status in 1996i.e. those who did not report their depressive status in 1998, or 2000, or did not return or answer the 1992 or 1996 Mental Health Index (MHI-5) questionnaires (Berwick et al. 1991; Yamazaki et al. 2005), a 5-item subscale of the Short-Form 36 Health Status Survey), as well as women who reported in 1996 using antidepressants (n=2,052) or had a physician-diagnosed episode of depression in 1996 or before (n=3,445), or with an unknown start date (n=198), or reported severe depressive symptoms (score ≤52) on the 1992 (n=2,374) or 1996 (n=2,271) MHI-5 questionnaire. Thus, a total of 50,538 women were considered depression-free in 1996, comprising the baseline population for the current analyses. Further excluding those who had missing values for IDP (N=6,853), the final 1996 baseline population included in the IDP and depression analyses comprised 43,685 women.
The study protocol was approved by the Institutional Review Boards of Brigham and Women’s Hospital and the Harvard School of Public Health.
2.2 Assessment of Exposure
The dietary variables were assessed using validated semiquantitative food-frequency questionnaires (FFQs) (Willett et al. 1985). In 1984, 1986 and every 4 years since, an expanded 131-item FFQ were sent to participants. Women were asked how often they had consumed a commonly used unit or portion size of each food on average during the previous year, with 9 possible frequency responses ranging from “never” to “more than 6 times a day.” Food items were aggregated into 39 food groups on the basis of nutrient profiles and culinary usage (Hu et al. 1999). We included each type of alcoholic beverage (wine, liquor, beer) separately as food groups in factor analysis. Vitamin and mineral supplements were not included in the patterns. The validity and reproducibility of the FFQ have been evaluated before in a subgroup of participants using four one-week long diet records completed during the previous year and repeated FFQs one year apart (Salvini et al. 1989; Willett et al. 1985).
2.3 Case Ascertainment
We used two definitions for depression, a strict definition that required both self-reported physician-diagnosed depression and regular antidepressant use (i.e. used regularly in the past 2 years), and a broader definition that included women who reported either clinical diagnosis or regular antidepressant use. In 2000, participants were asked to report the year of their first episode of physician-diagnosed depression (1996 or before, 1997 or before, 1998 or before, 1999, or 2000). Thereafter, this information was updated biennially through 2006. Regular antidepressant medication use was first asked in 1996 and then biennially updated through 2006. Hence, the 1996 questionnaire cycle was considered as baseline.
2.4 Covariate Assessment
Demographic, lifestyle, behavior, and comorbidity information were collected using the standardized questionnaires mailed to the participants. In the baseline questionnaire (1996), we requested information about age, weight and smoking, menopausal status and use of postmenopausal hormone therapy, and previously diagnosed medical conditions. This information has been updated in the biennial follow-up questionnaires. Marital status and retirement were obtained at baseline (1996) and updated in 2000 and 2004. Education levels of the nurses (registered nurses, bachelor, master and doctorate), and their husband (<high school, high school, college graduate school, graduate school), and ethnicity (White, Black, Amerindian and Hawaiian, Asian) were measured in 1992. Participants were asked to report the hours spent per week on moderate (e.g., brisk walking) and vigorous (e.g., strenuous sports and jogging) exercise, and then the total hours of metabolic equivalent tasks per week (METh/wk) were estimated on the basis of the MET score assigned to each activity. Mental health at baseline was assessed using the MHI-5 score, a subscale of the SF-36 Health Status Survey.
2.5 Laboratory Procedures
Between 1989 and 1990, 32,826 women free of diagnosed diabetes, ischemic heart disease, stroke, or cancer provided fasting blood samples in heparin-containing tubes. Women shipped their blood samples overnight in an icepack provided by the study and completed a questionnaires which included information of time of blood draw, weight, and medication use among others. Upon arrival, blood samples were aliquoted into plasma, white blood cells, and red blood cells components and stored in liquid nitrogen freezers with an electronic alarm system; the majority of samples arrived for processing within 24 hours of draw. Blood levels of the inflammatory markers C-reactive protein (CRP), interleukin-6 (IL-6), and tumor necrosis factor α (TNFα) receptor 2 have been measured in subsets of the population (overall 12,188 participants) for 16 nested case-control studies and in one validation study, where all cases occurred after 1990. From the aforementioned studies, we used the data of 4,692 participants who had complete measures on the three inflammatory biomarkers and dietary intakes in 1986–1990.
CRP concentrations were measured by use of a high-sensitivity latexenhanced immunonephelometric assay on a BN II analyzer (Dade Behring, Newark, DE). IL-6 was measured by a quantitative sandwich enzyme immunoassay technique (Quantikine HS Immunoassay kit; R&D Systems, Minneapolis). As most of the TNFα had likely degraded after shipping and processing, plasma concentrations of of soluble fractions of tumor necrosis factor α receptor 2 (sTNFR2) was measured instead of TNFα using a quantitative sandwich ELISA assay from R & D Systems. Levels of inflammatory markers were found to be reproducible within subjects over time (Pai et al. 2002; Pai et al. 2004). CRP and sTNFR2 stability was assessed in 17 fresh blood samples from the NHS, at baseline, and after a delay in shipping of 24 hours and 36 hours, and the intraclass correlation coefficient (ICC) was >75% for the comparison of 0 to 36 hours (Pai et al. 2002). The interassay coefficients of variation for each analyte were as follows: 1.6–3.0% for CRP, 0.07–6.1% for IL-6, and 4.9–11.6% for sTNFR2.
2.6 Statistical Analysis
2.6.1 Assessment of components of the IDP
To determine components of the IDP, we used the data of 4,692 participants who had complete measures on the three inflammatory biomarkers, in which we calculated the mean intake from the 1986 and 1990 FFQs for the 39 food groups to reduce random within-person variation and best represent long-term diet (Hu et al. 1999) (Supplementary Table 1). We subsequently applied the RRR to derive the IDP. The RRR identifies linear functions of predictors (e.g., the 39 food groups) that explain as much response (e.g., inflammatory biomarkers such as CRP, IL-6, sTNFR2) variation in as possible. The first factor obtained by RRR was retained for subsequent analyses because it explains the largest amount of variation among the biomarkers. A more detailed description of the RRR method, including the SAS code and its application in nutritional epidemiology, can be found elsewhere (Hoffmann et al. 2004). Thereafter, we identified the more important component foods of the RRR pattern by stepwise linear regression with a significance level of P < .05 for entry into and staying in the model. Finaly, the IDP score was then calculated as the linear combination of these standardized component food items (Schulze et al. 2003), where a higher score indicates a more inflammatory diet. Pearson correlation coefficients were used to evaluate associations between IDP score and associated food groups with inflammatory biomarkers.
2.6.2 Prospective analysis of the IDP score
To determine the relationship of the IDP score with depression risk, we conducted a prospective analysis among 43,685 women free from depression or severe depressive symptoms at baseline (Supplementary Table 1). To calculate the IDP score, we used the cumulative average of food items starting in 1984 up to a 2 to 4 year lag period before the follow-up period ended. For example, the cumulative average of intakes of 1984 through 1994 was used to predict depression episode in 1996–1998, consumptions of 1984 through 1998 for 2000–2002 follow-up period, and so on. For all women, the IDP score was calculated as the linear combination of standardized component food items identified in the subset of the population (n=4,692).
Cox proportional hazards models, stratified on age in months and questionnaire cycle, were used to estimate the relative risks (RR) and their 95% confidence intervals (95% CIs) of developing depression for each quintile of IDP score. In the multivariate model, we further adjusted for BMI (kg/m2), total energy intake (Kcal/day), smoking (never smoked, past smoker 1–24 cig./d, past smoker ≥25 cig./day, current smoker 1–24 cig./day, current smoker ≥25 cig./day), physical activity (MET-h/wk in quintiles), menopause status and hormone replacement therapy (HRT) (postmenopause never use HRT, postmenopause past use HRT, postmenopause current use HRT, postmenopause unknown use HRT, premenopause), marital status (married/partnership, widowed, separated/divorced/single), retired (yes/no), education (registered nurses, bachelor/master/doctorate), husband’s education (<high school, ≥high school), ethnicity (White, Black/Amerindian/Hawaiian/Asian), multivitamin use (yes/no), self-reported diagnosis of cancer, high blood pressure, hypercholesterolemia, heart disease (myocardial infarction or angina), and diabetes (all binary), and MHI-5 score at baseline (continuous). In the sensitivity analysis of multivariate model, we further adjusted for alcohol (g/day) and thereafter for caffeine (mg/day), because we previously reported a decrease depression risk with caffeine (Lucas et al. 2011). We used the cumulative average for the potential confounders BMI (since 1996), physical activity (since 1986), total energy intake, alcohol and caffeine intake (since 1984). Other dichotomous or categorical covariates were updated during follow-up by using the most recent data for each 2-y follow-up interval. The significance of linear trends across quintiles of the IDP score was tested by assigning each participant the median value for the quintile and modeling this value as a continuous variable. Finally, we assessed whether the relationship between IDP and depression varied by obesity (BMI <30 vs. ≥30), smoking status (never vs. ever), and presence (yes or not) of comorbidities (heart disease or diabetes) at baseline by including their multiplicative terms in the multivariable Cox models and applying the likelihood ratio test with a cutoff point .05.
3.1 Results
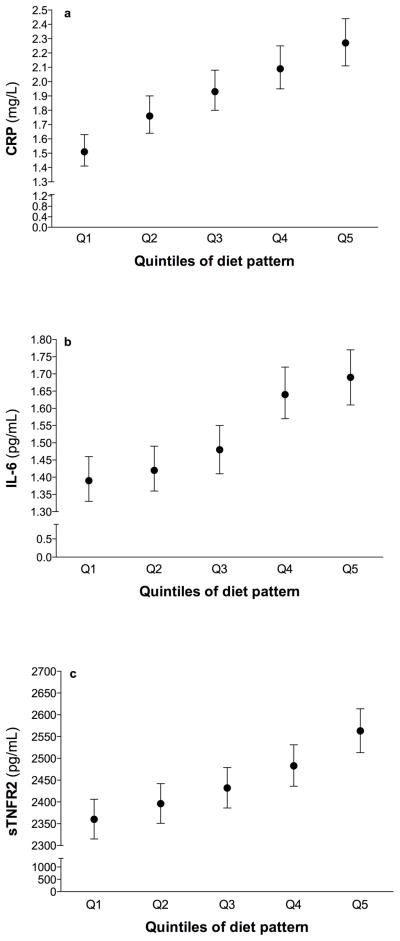
We identified a dietary pattern with the RRR method that was positively correlated with all inflammatory biomarkers (see Table 1). Pearson correlation coefficients for the IDP score were 0.15 for sTNFR2 (P<.001), 0.18 for IL-6 (P<.001) and 0.23 for CRP (P<.001). The pattern represented a diet relatively high in sugar-sweetened soft drinks, refined grains, red meat, diet soft drinks, margarine, other vegetables, and fish but low in wine, coffee, olive oil, green leafy and yellow vegetables. All food groups were significantly correlated with diet pattern score. With the exception of other vegetables and fish, all food groups were significantly correlated with at least two inflammatory markers. Geometric mean biomarker concentrations increased across the quintiles of diet pattern; the difference between the 5th and 1st quintiles was 203 pg/mL (8.6%) for sTNFR2, 0.3 pg/mL (21.2%) for IL-6, and 0.75 mg/L (49.7%) for CRP (Fig. 1).
Table 1.
Pearson correlations between diet pattern score and associated food groups with biomarkers of inflammation, Nurses’ Health Study (n=4,692)
| Pattern and food groups | Pearson correlation with diet pattern score | Pearson correlation with biomarkersa | ||
|---|---|---|---|---|
| sTNFR2 | IL-6 | CRP | ||
| Diet pattern score | 1.00 | 0.15*** | 0.18*** | 0.23*** |
| Food groupsb | ||||
| Positive associations | ||||
| Sugar-sweetened soft drinks | 0.39*** | 0.05*** | 0.09*** | 0.08*** |
| Refined grainsc | 0.36*** | 0.04** | 0.08*** | 0.08*** |
| Red meat | 0.33*** | 0.01 | 0.07*** | 0.09*** |
| Margarine | 0.26*** | 0.04* | 0.04** | 0.06*** |
| Diet soft drinks | 0.23*** | 0.01 | 0.05** | 0.06*** |
| Other vegetablesd | 0.10** | 0.01 | −0.001 | 0.04** |
| Fish | 0.06*** | 0.0003 | −0.02 | 0.04** |
| Negative associations | ||||
| Wine | −0.48*** | −0.10*** | −0.07*** | −0.10*** |
| Coffeee | −0.45*** | −0.08*** | −0.06*** | −0.10*** |
| Olive oil | −0.23*** | −0.04** | −0.05** | −0.04** |
| Green, leafy vegetablesf | −0.21*** | −0.05*** | −0.05*** | −0.02 |
| Yellow vegetablesg | −0.15*** | −0.03* | −0.05*** | −0.004 |
sTNFR2, soluble tumor necrosis factor α receptor 2; IL-6, interleukin 6; CRP, C-reactive protein.
Biomarkers were log transformed.
P < .05
P < .01
P < .001.
Food groups were identified through the use of stepwise regression with P < .05 for inclusion and exclusion and by modeling the biomarkers response score as the dependent variable.
Refined grains include white bread, English muffins, bagels or rolls, muffins or biscuits, white rice, pasta, pancakes or waffles.
Other vegetables include corn, celery, mushrooms, green pepper, eggplant, summer squash, and mixed vegetables.
Coffee includes caffeinated and decaffeinated coffee.
Green, leafy vegetables include Spinach, iceberg or head lettuce, romaine or leaf lettuce.
Yellow vegetables include carrots, yellow (winter) squash, yams.
Fig. 1.
Geometric mean concentrations and 95% CIs of C-reactive protein (CRP) (a), interleukin-6 (IL-6) (b), and soluble tumor necrosis factor α receptor 2 (sTNFR2) (c) by quintiles of diet pattern score adjusted for age and BMI (continuous).
Participant characteristics according to quintiles of IDP score are presented in Table 2. Compared with women in the lowest quintile, those in high scores of the IDP were more likely to be never smoker, nonwhite, to have a husband with less than high school-level education, and to have a higher BMI, total energy intake, and prevalence of high blood pressure, hypercholesterolemia, heart disease, and diabetes. Women with high scores on IDP were also less physically active, less frequent users of multivitamins, and had a lower intake of caffeine and alcohol.
Table 2.
Age-adjusted characteristics at baseline by quintile of Inflammatory Dietary Pattern (IDP) scorea
| Quintile of IDP scoreb | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Q1 (n=8,202) | Q2 (n=8,840) | Q3 (n=8,955) | Q4 (n=9,006) | Q5 (n=8,682) | P Trend | |
| Age, mean (SD), y | 63.4(6.7) | 63.5(6.9) | 62.9(6.9) | 62.2(7.1) | 60.9(7.1) | <.0001 |
| BMI, mean (SD), kg/m2 | 24.7(4.1) | 25.5(4.3) | 26.1(4.6) | 26.8(5.1) | 27.9(5.8) | <.0001 |
| Physical activity, mean (SD), MET/wk | 22.0(19.2) | 19.3(17.1) | 18.1(16.4) | 16.8(15.5) | 15.7(15.2) | <.0001 |
| MHI scorec, mean (SD) | 83.4(9.8) | 83.2(9.8) | 82.9(9.8) | 82.6(9.9) | 81.9(10.0) | <.0001 |
| Total energy intake, mean (SD), Kcal/day | 1608(404) | 1608(392) | 1681(388) | 1801(388) | 2032(431) | <.0001 |
| Alcohol intake, mean (SD), g/day | 10.0(11.6) | 6.3(8.7) | 5.4(8.0) | 4.5(7.5) | 3.9(7.3) | <.0001 |
| Caffeine intaked, mean (SD), mg/day | 335(204) | 296(190) | 265(182) | 252(176) | 245(175) | <.0001 |
| Menopausal status, % | 0.05 | |||||
| Premenopause | 4.0 | 4.9 | 5.2 | 5.7 | 6.1 | |
| Menopause, current use HRT | 46.0 | 45.2 | 44.0 | 43.0 | 39.7 | |
| Menopause, past use HRT | 19.7 | 19.9 | 19.7 | 20.2 | 20.4 | |
| Menopause, never use HRT | 27.3 | 26.8 | 28.0 | 28.0 | 30.4 | |
| Menopause, unknown use HRT | 0.6 | 0.7 | 0.7 | 0.5 | 0.7 | |
| Smoking status, % | <.0001 | |||||
| Never smoker | 37.2 | 43.9 | 46.9 | 49.9 | 51.5 | |
| Past smoker 1–24 cig./d | 42.8 | 38.3 | 36.6 | 34.1 | 31.3 | |
| Past smoker ≥25 cig./day | 7.4 | 6.9 | 6.4 | 6.5 | 6.3 | |
| Current smoker 1–24 cig./day | 10.3 | 8.8 | 8.3 | 7.6 | 8.5 | |
| Current smoker ≥25 cig./day | 1.4 | 1.2 | 1.1 | 1.1 | 1.7 | |
| Marital Status, % | 0.003 | |||||
| Married | 79.3 | 79.8 | 80.0 | 80.4 | 79.3 | |
| Widowed | 12.3 | 13.2 | 13.6 | 13.7 | 14.5 | |
| Separated/divorced/single | 8.3 | 6.9 | 6.3 | 5.8 | 6.1 | |
| Black/Amerindian/Hawaiian/Asian, % | 1.0 | 1.5 | 1.9 | 2.1 | 2.4 | <.0001 |
| Bachelor/Master/Doctorate degree, % | 7.8 | 6.3 | 5.9 | 5.3 | 5.4 | <.0001 |
| Husband education <high school, % | 0.9 | 1.3 | 1.4 | 1.7 | 2.4 | <.0001 |
| Retired, % | 48.6 | 49.4 | 50.0 | 49.6 | 49.7 | 0.08 |
| Cancer, % | 13.0 | 11.4 | 11.5 | 12.0 | 11.7 | 0.06 |
| High blood pressure, % | 32.4 | 36.0 | 39.3 | 42.6 | 46.9 | <.0001 |
| Hypercholesterolemia, % | 49.7 | 52.5 | 55.1 | 56.2 | 57.5 | <.0001 |
| Heart disease, % | 6.0 | 7.0 | 7.7 | 8.5 | 10.7 | <.0001 |
| Diabetes, % | 3.0 | 3.7 | 5.2 | 6.9 | 10.4 | <.0001 |
| Multivitamin use, % | 55.3 | 55.4 | 54.1 | 51.6 | 49.1 | <.0001 |
| Food intake, servings/day | ||||||
| Sugar-sweetened soft drinks | 0.1(.2) | 0.1(.2) | 0.2(.3) | 0.3(.3) | 0.5(.7) | <.0001 |
| Refined grains | 0.9(.5) | 1.0(.5) | 1.1(.6) | 1.4(.7) | 1.9(1.0) | <.0001 |
| Red meat | 0.4(.2) | 0.5(.2) | 0.5(.2) | 0.6(.3) | 0.8(.3) | <.0001 |
| Margarine | 0.5(.5) | 0.6(.5) | 0.8(.6) | 1.0(.7) | 1.4(1.0) | <.0001 |
| Diet soft drinks | 0.3(.5) | 0.4(.5) | 0.5(.6) | .6(.7) | 1.0(1.2) | <.0001 |
| Other vegetables | 0.9(.6) | 0.9(.5) | 0.9(.5) | 0.9(.5) | 1.0(.6) | <.0001 |
| Fish | 0.3(.2) | 0.3(.2) | 0.3(.2) | 0.3(.2) | 0.4(.3) | <.0001 |
| Wine | 0.3(.5) | 0.1(.2) | 0.1(.2) | 0.1(.1) | 0.1(.1) | <.0001 |
| Coffee | 3.1(1.7) | 2.6(1.5) | 2.2(1.5) | 2.0(1.4) | 1.7(1.5) | <.0001 |
| Olive oil | 0.4(.5) | 0.2(.2) | 0.1(.2) | 0.1(.2) | 0.1(.2) | <.0001 |
| Green, leafy vegetables | 1.1(.6) | 0.9(.5) | 0.8(.4) | 0.7(.4) | 0.7(.4) | <.0001 |
| Yellow vegetables | 0.6(.4) | 0.5(.3) | 0.4(.3) | 0.4(.3) | 0.4(.3) | <.0001 |
BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); HRT, hormone replacement therapy; IDP, inflammatory dietary pattern; MET, metabolic equivalent of task; MHI, Mental Health Index (a 5-item subscale of the 36-Item Short-Form Health Survey).
All characteristics are age-standardized with the exception of age. Percentages may not total 100% due to rounding and missing data.
Computed as the cumulative average intake from 1984 to 2002 FFQs for the 39 food groups (see Methods section).
MHI score measured in 1996. A higher score denotes a better mental health.
Caffeine was calculated from coffee and non-coffee sources (tea, soft drink, chocolate).
Among the 43,685 women who were free from clinical depression or severe depressive symptoms at baseline, we documented 2,594 incident cases of depression using the stricter definition and 6,446 using the broader definition, during the 12-year follow-up (1996–2008). A dose-response relationship between IDP and depression risk was noted for both stricter and broader definition with an age-adjusted model (P-trend <.001, see Table 3). These gradients became slightly attenuated after further adjustment for BMI and other all covariates. Similar gradients, but slightly lower, were noted when we ran our multivariate-model for the broader definition of depression. Findings remained essentially unchanged after further adjustment for alcohol and caffeine. The RRs comparing extreme quintiles of the IDP were 1.41 (95% CI, 1.22, 1.63; P-trend <.001) for the strict definition and 1.29 (95% CI, 1.18, 1.41; P-trend <.001) for the broader definition of depression. The effect of IDP score on depression risk (strict definition) was not modified by obesity (BMI <30 vs. ≥30) (P-interaction = .86), smoking status (never vs. ever) (P-interaction = .54), and diseases (heart disease, diabetes) (yes or no) (P-interaction = .45) (data not shown).
Table 3.
Relative Risks (95% CI) of depression by quintile of Inflammatory Dietary Pattern (IDP) score, Nurses’ Health Study 1996–2008.
| Quintile of IDP score | ||||||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | P for trend | |
| Strict definition of depression (diagnosis AND antidepressant use, nb. cases = 2,594) | ||||||
| No. of cases | 424 | 467 | 531 | 509 | 663 | |
| Person-years | 90,224 | 90,305 | 90,233 | 90,227 | 90,076 | |
| Age-adjusteda | 1.00 | 1.08 (0.94, 1.23) | 1.21 (1.07, 1.38) | 1.15 (1.01, 1.31) | 1.50 (1.33, 1.70) | <.001 |
| Multivariate modelb | 1.00 | 1.04 (0.91, 1.19) | 1.16 (1.02, 1.32) | 1.08 (0.94, 1.23) | 1.37 (1.20, 1.57) | <.001 |
| + alcoholc | 1.00 | 1.07 (0.93, 1.22) | 1.20 (1.05, 1.36) | 1.12 (0.97, 1.28) | 1.43 (1.25, 1.65) | <.001 |
| + caffeined | 1.00 | 1.06 (0.93, 1.22) | 1.19 (1.04, 1.35) | 1.10 (0.96, 1.27) | 1.41 (1.22, 1.63) | <.001 |
| Broad definition of depression (diagnosis AND/OR antidepressant use, nb. cases = 6,446) | ||||||
| No. of cases | 1,085 | 1,175 | 1,307 | 1,325 | 1,554 | |
| Person-years | 89,426 | 89,450 | 89,309 | 89,266 | 89,038 | |
| Age-adjusteda | 1.00 | 1.07 (0.99, 1.16) | 1.19 (1.10, 1.29) | 1.21 (1.12, 1.31) | 1.43 (1.32, 1.54) | <.001 |
| Multivariate modelb | 1.00 | 1.04 (0.96, 1.13) | 1.14 (1.05, 1.24) | 1.13 (1.04, 1.23) | 1.31 (1.20, 1.43) | <.001 |
| + alcoholc | 1.00 | 1.05 (0.96, 1.14) | 1.15 (1.06, 1.25) | 1.15 (1.05, 1.25) | 1.33 (1.22, 1.46) | <.001 |
| + caffeined | 1.00 | 1.04 (0.96, 1.13) | 1.13 (1.04, 1.23) | 1.12 (1.03, 1.22) | 1.29 (1.18, 1.41) | <.001 |
Abbreviations: HRT, hormone replacement therapy; IDP, inflammatory dietary pattern; MHI-5, Mental Health Index score-5.
Adjusted for age (continuous) and interval.
Further adjusted for BMI (kg/m2), total energy intake (Kcal/day), smoking (never smoked, past smoker 1–24 cig./d, past smoker ≥25 cig./day, current smoker 1–24 cig./day, current smoker ≥25 cig./day), physical activity (quintiles), menopause status and HRT (postmenopause never use HRT, postmenopause past use HRT, postmenopause current use HRT, postmenopause unknown use HRT, premenopause), marital status (married/partnership, widowed, separated/divorced/single), retired (yes/no), education (registered nurses, bachelor/master/doctorate), husband education (<high school, ≥high school), ethnicity (White, Black/Amerindian/Hawaiian/Asian), multivitamin use (yes/no), reported diagnosis of cancer, high blood pressure, hypercholesterolemia, heart disease (myocardial infarction or angina), and diabetes (all binary), and MHI-5 score at baseline (continuous).
Further adjusted for alcohol intake (g/day, continuous).
Further adjusted for caffeine intake (mg/day, continuous).
4. Discussion
In this large prospective cohort of middle-age and older women free from depression or severe depressive symptoms at baseline, we used an empirical statistical method to derive a diet pattern score that was associated with markers of inflammation, and observed that depression risk increases with increasing score of this IDP. These observations suggest that the association between dietary factors and depression may be mediated in part by inflammation. Our study is the first to investigate depression risk of a dietary pattern associated with inflammatory biomarkers.
Most previous studies that investigated the relationship between dietary pattern and depression were cross-sectional (Jacka et al. 2010; Kuczmarski et al. 2010; Nanri et al. 2010; Samieri et al. 2008) and thus were unable to determine whether a dietary pattern affects depression or vice-versa. To date, only five other prospective studies have analyzed the role of dietary patterns in depression (Akbaraly et al. 2009; Chocano-Bedoya et al. 2012; Rienks et al. 2013; Sanchez-Villegas et al. 2009). Through the use of “a priori” approach, a higher adherence to the Mediterranean dietary pattern was associated with a lower depression risk in the SUN cohort (Sanchez-Villegas et al. 2009). Using PCA among participants of the Whitehall II cohort, Akbaraly et al. (Akbaraly et al. 2009) noted that those in the highest tertile of processed food pattern had a higher risk of depressive symptoms (multivariate odds ratio (OR) = 1.69; 95% CI, 1.10, 2.60), compared to the lowest tertile. Using PCA among participants of the ALSWH, Rienks et al. (Rienks et al. 2013) observed that women in the highest quintile of the Mediterranean-style dietary pattern had a lower incidence of depressive symptoms (multivariate OR = 0.63; 95% CI, 0.47, 0.85), compared to the lowest quintile. After exclusion of participants with significant depressive symptoms at baseline, no significant associations between the healthy pattern and depressive symptoms were observed by Le Port et al. (Le Port et al. 2012), but the protective effect of the traditional pattern remained significant. Among NHS participants, we recently conducted analyses of PCA defined dietary patterns (Prudent and Western pattern) in relationship with depression risk (Chocano-Bedoya et al. 2013). Using the strict and broad definitions of depression, no significant association was found between the highest quintile of Prudent pattern (multivariate RR = 1.05; 95% CI, 0.91, 1.20) or Western pattern (multivariate RR = 1.05; 95% CI, 0.89, 1.23), compared to the lowest quintile of each dietary pattern score (Chocano-Bedoya et al. 2013). In contrast with these four previous cohort analyses, the present study uses information on inflammatory biomarkers to derive the dietary pattern. The advantage of the RRR approach as opposed to PCA approach is that the derived dietary pattern incorporates information on biological pathways instead of being driven by patterns of eating behavior (Hoffmann et al. 2004).
In our study, we selected inflammatory markers that have been previously associated with depression (Howren et al. 2009). Although pro-inflammatory cytokine secretion increases in depression, and exposure to cytokines induces depressive symptoms and some antidepressant drugs have anti-inflammatory properties (Dantzer et al. 1999), it is not clear whether the association of inflammation and depression is causative or not (Belmaker and Agam, 2008; Dantzer et al. 1999). However, a meta-analysis suggests that inflammation and depression relationship might be bidirectional (Howren et al. 2009). The etiology of depression is complex and involves several factors, and many mechanisms have been proposed (Belmaker and Agam, 2008; Krishnan and Nestler, 2008). Cytokines might contribute to the development of depression by several pathophysiological mechanisms (Miller et al. 2009). Inflammation is a common link that is shared among many chronic conditions such as CVD, obesity, diabetes and cancer (Aggarwal et al. 2006; Giugliano et al. 2006; Hotamisligil 2006; Hu et al. 2004), which are also risk factors for depression (Musselman et al. 1998; Pan et al. 2011a; Spiegel and Giese-Davis, 2003). However, the relationship of our dietary pattern with depression did not seem to be mediated through these chronic diseases because our results were not altered when we adjusted for the occurrence of diseases during follow-up.
Diet could stimulated chronic inflammatory diseases (Bosma-den Boer et al. 2012) and some foods that we identified have been associated with inflammation (Galland 2010; Giugliano et al. 2006). Indeed, we previously identified similar proinflammatory-diet pattern, which has been associated with an increased risk of diabetes among NHS and NHS2 (Schulze et al. 2005). Refined grains and sugar-sweetened soft drinks, which have been associated with obesity and diabetes risks, contribute importantly to glycemic load (Liu et al. 2002) and could increase susceptibility to the development of chronic inflammation (Bosma-den Boer et al. 2012). Red meat has been associated with biomarkers of inflammation (Azadbakht and Esmaillzadeh, 2009; Montonen et al. 2013), and increased risks of diabetes (Pan et al. 2011b), CVD and cancer mortality (Pan et al. 2012a), and depression (Sanchez-Villegas et al. 2009). In a previous analysis of NHS data, we did not find any significant relationship between fish, neither fatty fish nor long-chain omega-3 intake with depression (Lucas et al. 2011b). These findings are also consistent with a recently published meta-analysis of 13 randomized, double-blind, placebo-controlled trials, that concluded that long-chain omega-3 supplementation had neither statistically nor clinically significant impacts on depression symptom severity (Bloch and Hannestad, 2012). Moreover, a recent systematic review indicates that long-chain omega-3 (0.9 to 2 g/d) do not change inflammatory biomarkers in healthy subjects (Rangel-Huerta et al. 2012). It is also possible that fish or long-chain omega-3 intake of our population is insufficient to note substantial beneficial effect.
In a previous analysis of NHS, we noted that depression risk decreases with increasing caffeinated coffee or caffeine consumption, but not with decaffeinated coffee (Lucas et al. 2011a). In the present analysis, diet pattern relationship with depression does not seem to be mediated through caffeine intake because when we adjusted for caffeine intake our results were not altered. This might be explained by the fact that caffeinated coffee contribute to 82% of caffeine intake (Lucas et al. 2011a). However, studies on the relationship between coffee consumption and inflammatory biomarkers have yielded inconsistent results (Lopez-Garcia et al. 2006). Studies on the relationship between longitudinal assessment of total alcohol intake and depression are scarce, but recent observations from the SUN cohort indicate a U-shaped risk relationship among women only (Gea et al. 2012). Lower levels of systemic inflammatory markers have been observed in moderate alcohol drinkers, compared to non-drinkers and heavy drinkers (Imhof et al. 2004). Evidence also suggests that both ethanol (Imhof and Koenig, 2003) and nonalcoholic compounds (Sacanella et al. 2007) (e.g., polyphenols of wine) appear to contribute to the potential anti-inflammatory effects of alcoholic beverages. Olive oil, a major component of Mediterranean diet, could partly explain Mediterranean diet beneficial effect in depression (Kyrozis et al. 2009; Sanchez-Villegas et al. 2011). However, very few studies suggest that oleic acid, a monounsaturated fatty acid, has anti-inflammatory effects (Basu et al. 2006), but extra-virgin olive oil has phenolic compounds that has anti-inflammatory activities (Cicerale et al. 2012).
Obesity is known to induce a chronic low-grade inflammation state (Hotamisligil 2006). Excess body fat may explain the associations between food groups in our dietary pattern and inflammatory markers. Therefore, it is possible that weight gain is one potential pathway by which the dietary pattern is associated with inflammation and depression risk. However, in a previous analysis of NHS, we found that obesity was associated with a modestly increased depression risk and overweight was not significantly associated with depression, compared to normal weight women (Pan et al. 2012b). Moreover, the association between IDP and depression risk remained significant after adjustment for BMI.
The major strengths of this study include its large sample size, prospective design, updated covariates, and long term measurement of dietary intakes, which relied on the use of validated FFQ administered 6 times over a period of 18 years. Indeed, other cohorts (Akbaraly et al. 2009; Rienks et al. 2013; Sanchez-Villegas et al. 2009) completed only 1 baseline FFQ as a measure of exposure. This approach is less accurate than ours as it assumes that dietary intake measured once at baseline is representative of usual diet that remains unchanged for the entire follow-up period. Another advantage is the efficient use of measured biomarkers in a subset of the population to predict the IDP in a large number of nurses. This study also has limitations and the results should thus be interpreted with caution. First and foremost, because of the observational design, neither this nor previous investigations can prove that IDP reduces or increases depression risk, and it remains possible that individuals with higher scores on IDP have lower depression risk for reasons other than dietary component. Only the top quintile of the IDP is significantly associated with higher depression risk, which might suggest that these women have some unique characteristics (e.g., poorer health status) not related to their diet that may explain the association. It is also possible that IDP and inflammation relationship may be explained by uncontrolled confounders. Residual confounding cannot be ruled out despite adjustment. The major limitation of RRR approach is that it requires responses variables (biomarkers) which may not reflect the current state of knowledge. Indeed, many factors have been involved in the pathogenesis of the depression, and other pathways than inflammation may also be relevant in the evaluation of dietary pattern and depression risk (Belmaker and Agam, 2008; Krishnan and Nestler, 2008). Even after adjustment for social factors, such as ethnicity, marital status, and education, our results were not significantly altered. However, we can not exclude the possibility of co-occurrence of psychosocial characteristics with diet and depression.
Reverse causation is another concern in most epidemiological studies. To minimize bias from this source, we excluded, at baseline, 10,340 women with severe depressive symptoms at baseline, and we computed the cumulative average of dietary inatkes with at least 2-year latency; yet, we cannot exclude the possibility that presence of subsyndromal depressive symptoms were the common reason for some dietary consumption and incident depression. Therefore, we adjusted for continuous MHI-5 score at baseline, which importantly would incorporate symptoms scores of any participants with subclinical depressions; yet, associations were unchanged. Finally, because our analysis was realized among middle-age and older women, generalization of the results to younger women and male populations is limited.
In conclusion, our results among this large cohort of women indicate that the IDP was associated with increased risk of developing depression. These results suggest that chronic inflammation may underlie the relationship between diet and depression.
Supplementary Material
Research highlight.
Our study is the first to observe an association between an inflammatory prone dietary pattern (derived from reduced-rank regression) and depression risk.
Acknowledgments
We thank the Nurses’ Health Study participants for their continuing contributions.
Funding/Support: The study was supported by National Institutes of Health (NIH) Grant DK58845. Dr Ascherio received a grant from the National Alliance for Research on Schizophrenia & Depression (Project ID: 5048070–01).
Role of the Sponsors: The funding sources were not involved in the data collection, data analysis, manuscript writing, and publication.
Footnotes
Conflict of Interest Disclosures: None reported
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aggarwal BB, Shishodia S, Sandur SK, Pandey MK, Sethi G. Inflammation and cancer: how hot is the link? Biochem Pharmacol. 2006;72:1605–1621. doi: 10.1016/j.bcp.2006.06.029. [DOI] [PubMed] [Google Scholar]
- Akbaraly TN, Brunner EJ, Ferrie JE, Marmot MG, Kivimaki M, Singh-Manoux A. Dietary pattern and depressive symptoms in middle age. Br J Psychiatry. 2009;195:408–413. doi: 10.1192/bjp.bp.108.058925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azadbakht L, Esmaillzadeh A. Red meat intake is associated with metabolic syndrome and the plasma C-reactive protein concentration in women. J Nutr. 2009;139:335–339. doi: 10.3945/jn.108.096297. [DOI] [PubMed] [Google Scholar]
- Basu A, Devaraj S, Jialal I. Dietary factors that promote or retard inflammation. Arterioscler Thromb Vasc Biol. 2006;26:995–1001. doi: 10.1161/01.ATV.0000214295.86079.d1. [DOI] [PubMed] [Google Scholar]
- Belmaker RH, Agam G. Major depressive disorder. N Engl J Med. 2008;358:55–68. doi: 10.1056/NEJMra073096. [DOI] [PubMed] [Google Scholar]
- Berwick DM, Murphy JM, Goldman PA, Ware JE, Jr, Barsky AJ, Weinstein MC. Performance of a five-item mental health screening test. Med Care. 1991;29:169–176. doi: 10.1097/00005650-199102000-00008. [DOI] [PubMed] [Google Scholar]
- Bloch MH, Hannestad J. Omega-3 fatty acids for the treatment of depression: systematic review and meta-analysis. Mol Psychiatry. 2012;17:1272–1282. doi: 10.1038/mp.2011.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosma-den Boer MM, van Wetten ML, Pruimboom L. Chronic inflammatory diseases are stimulated by current lifestyle: how diet, stress levels and medication prevent our body from recovering. Nutr Metab (Lond) 2012;9:32. doi: 10.1186/1743-7075-9-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chocano-Bedoya PO, O’Reilly EJ, Lucas M, Mirzaei F, Okereke OI, Fung TT, Hu FB, Ascherio A. Prospective study on long-term dietary patterns and incident depression in middle-aged and older women. Am J Clin Nutr. 2013 doi: 10.3945/ajcn.112.052761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicerale S, Lucas LJ, Keast RS. Antimicrobial, antioxidant and anti-inflammatory phenolic activities in extra virgin olive oil. Curr Opin Biotechnol. 2012;23:129–135. doi: 10.1016/j.copbio.2011.09.006. [DOI] [PubMed] [Google Scholar]
- Dantzer R, Wollman E, Vitkovic L, Yirmiya R. Cytokines and depression: fortuitous or causative association? Mol Psychiatry. 1999;4:328–332. doi: 10.1038/sj.mp.4000572. [DOI] [PubMed] [Google Scholar]
- Galland L. Diet and inflammation. Nutr Clin Pract. 2010;25:634–640. doi: 10.1177/0884533610385703. [DOI] [PubMed] [Google Scholar]
- Gea A, Martinez-Gonzalez MA, Toledo E, Sanchez-Villegas A, Bes-Rastrollo M, Nunez-Cordoba JM, Sayon-Orea C, Beunza JJ. A longitudinal assessment of alcohol intake and incident depression: the SUN project. BMC Public Health. 2012;12:954. doi: 10.1186/1471-2458-12-954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giugliano D, Ceriello A, Esposito K. The effects of diet on inflammation: emphasis on the metabolic syndrome. J Am Coll Cardiol. 2006;48:677–685. doi: 10.1016/j.jacc.2006.03.052. [DOI] [PubMed] [Google Scholar]
- Hoffmann K, Schulze MB, Schienkiewitz A, Nothlings U, Boeing H. Application of a new statistical method to derive dietary patterns in nutritional epidemiology. Am J Epidemiol. 2004;159:935–944. doi: 10.1093/aje/kwh134. [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med. 2009;71:171–186. doi: 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- Hu FB. Dietary pattern analysis: a new direction in nutritional epidemiology. Curr Opin Lipidol. 2002;13:3–9. doi: 10.1097/00041433-200202000-00002. [DOI] [PubMed] [Google Scholar]
- Hu FB, Meigs JB, Li TY, Rifai N, Manson JE. Inflammatory markers and risk of developing type 2 diabetes in women. Diabetes. 2004;53:693–700. doi: 10.2337/diabetes.53.3.693. [DOI] [PubMed] [Google Scholar]
- Hu FB, Rimm E, Smith-Warner SA, Feskanich D, Stampfer MJ, Ascherio A, Sampson L, Willett WC. Reproducibility and validity of dietary patterns assessed with a food-frequency questionnaire. Am J Clin Nutr. 1999;69:243–249. doi: 10.1093/ajcn/69.2.243. [DOI] [PubMed] [Google Scholar]
- Imhof A, Koenig W. Alcohol inflammation and coronary heart disease. Addict Biol. 2003;8:271–277. doi: 10.1080/13556210310001602176. [DOI] [PubMed] [Google Scholar]
- Imhof A, Woodward M, Doering A, Helbecque N, Loewel H, Amouyel P, Lowe GD, Koenig W. Overall alcohol intake, beer, wine, and systemic markers of inflammation in western Europe: results from three MONICA samples (Augsburg, Glasgow, Lille) Eur Heart J. 2004;25:2092–2100. doi: 10.1016/j.ehj.2004.09.032. [DOI] [PubMed] [Google Scholar]
- Jacka FN, Pasco JA, Mykletun A, Williams LJ, Hodge AM, O’Reilly SL, Nicholson GC, Kotowicz MA, Berk M. Association of Western and traditional diets with depression and anxiety in women. Am J Psychiatry. 2010;167:305–311. doi: 10.1176/appi.ajp.2009.09060881. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, Rush AJ, Walters EE, Wang PS. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) JAMA. 2003;289:3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Nestler EJ. The molecular neurobiology of depression. Nature. 2008;455:894–902. doi: 10.1038/nature07455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuczmarski MF, Cremer Sees A, Hotchkiss L, Cotugna N, Evans MK, Zonderman AB. Higher Healthy Eating Index-2005 scores associated with reduced symptoms of depression in an urban population: findings from the Healthy Aging in Neighborhoods of Diversity Across the Life Span (HANDLS) study. J Am Diet Assoc. 2010;110:383–389. doi: 10.1016/j.jada.2009.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyrozis A, Psaltopoulou T, Stathopoulos P, Trichopoulos D, Vassilopoulos D, Trichopoulou A. Dietary lipids and geriatric depression scale score among elders: the EPIC-Greece cohort. J Psychiatr Res. 2009;43:763–769. doi: 10.1016/j.jpsychires.2008.09.003. [DOI] [PubMed] [Google Scholar]
- Le Port A, Gueguen A, Kesse-Guyot E, Melchior M, Lemogne C, Nabi H, Goldberg M, Zins M, Czernichow S. Association between dietary patterns and depressive symptoms over time: a 10-year follow-up study of the GAZEL cohort. PLoS One. 2012;7:e51593. doi: 10.1371/journal.pone.0051593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Manson JE, Buring JE, Stampfer MJ, Willett WC, Ridker PM. Relation between a diet with a high glycemic load and plasma concentrations of high-sensitivity C-reactive protein in middle-aged women. Am J Clin Nutr. 2002;75:492–498. doi: 10.1093/ajcn/75.3.492. [DOI] [PubMed] [Google Scholar]
- Lopez-Garcia E, van Dam RM, Qi L, Hu FB. Coffee consumption and markers of inflammation and endothelial dysfunction in healthy and diabetic women. Am J Clin Nutr. 2006;84:888–893. doi: 10.1093/ajcn/84.4.888. [DOI] [PubMed] [Google Scholar]
- Lucas M, Mirzaei F, Pan A, Okereke OI, Willett WC, O’Reilly EJ, Koenen K, Ascherio A. Coffee, caffeine, and risk of depression among women. Arch Intern Med. 2011a;171:1571–1578. doi: 10.1001/archinternmed.2011.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas M, Mirzaei F, O’Reilly EJ, Pan A, Willett WC, Kawachi I, Karestan K, Ascherio A. Dietary intake of n-3 and n-6 fatty acids and the risk of clinical depression in women: a 10-y prospective follow-up study. Am J Clin Nutr. 2011b;93:1337–1343. doi: 10.3945/ajcn.111.011817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65:732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montonen J, Boeing H, Fritsche A, Schleicher E, Joost HG, Schulze MB, Steffen A, Pischon T. Consumption of red meat and whole-grain bread in relation to biomarkers of obesity, inflammation, glucose metabolism and oxidative stress. Eur J Nutr. 2013;52:337–345. doi: 10.1007/s00394-012-0340-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musselman DL, Evans DL, Nemeroff CB. The relationship of depression to cardiovascular disease: epidemiology, biology, and treatment. Arch Gen Psychiatry. 1998;55:580–592. doi: 10.1001/archpsyc.55.7.580. [DOI] [PubMed] [Google Scholar]
- Nanri A, Kimura Y, Matsushita Y, Ohta M, Sato M, Mishima N, Sasaki S, Mizoue T. Dietary patterns and depressive symptoms among Japanese men and women. Eur J Clin Nutr. 2010;64:832–839. doi: 10.1038/ejcn.2010.86. [DOI] [PubMed] [Google Scholar]
- Pai JK, Curhan GC, Cannuscio CC, Rifai N, Ridker PM, Rimm EB. Stability of novel plasma markers associated with cardiovascular disease: processing within 36 hours of specimen collection. Clin Chem. 2002;48:1781–1784. [PubMed] [Google Scholar]
- Pai JK, Pischon T, Ma J, Manson JE, Hankinson SE, Joshipura K, Curhan GC, Rifai N, Cannuscio CC, Stampfer MJ, Rimm EB. Inflammatory markers and the risk of coronary heart disease in men and women. N Engl J Med. 2004;351:2599–2610. doi: 10.1056/NEJMoa040967. [DOI] [PubMed] [Google Scholar]
- Pan A, Lucas M, Sun Q, van Dam RM, Franco OH, Willett WC, Manson JE, Rexrode KM, Ascherio A, Hu FB. Increased mortality risk in women with depression and diabetes mellitus. Arch Gen Psychiatry. 2011a;68:42–50. doi: 10.1001/archgenpsychiatry.2010.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan A, Sun Q, Bernstein AM, Schulze MB, Manson JE, Willett WC, Hu FB. Red meat consumption and risk of type 2 diabetes: 3 cohorts of US adults and an updated meta-analysis. Am J Clin Nutr. 2011b;94:1088–1096. doi: 10.3945/ajcn.111.018978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan A, Sun Q, Bernstein AM, Schulze MB, Manson JE, Stampfer MJ, Willett WC, Hu FB. Red meat consumption and mortality: results from 2 prospective cohort studies. Arch Intern Med. 2012a;172:555–563. doi: 10.1001/archinternmed.2011.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan A, Sun Q, Czernichow S, Kivimaki M, Okereke OI, Lucas M, Manson JE, Ascherio A, Hu FB. Bidirectional association between depression and obesity in middle-aged and older women. Int J Obes (Lond) 2012b;36:595–602. doi: 10.1038/ijo.2011.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangel-Huerta OD, Aguilera CM, Mesa MD, Gil A. Omega-3 long-chain polyunsaturated fatty acids supplementation on inflammatory biomakers: a systematic review of randomised clinical trials. Br J Nutr. 2012;107:s159–s170. doi: 10.1017/S0007114512001559. [DOI] [PubMed] [Google Scholar]
- Rienks J, Dobson AJ, Mishra GD. Mediterranean dietary pattern and prevalence and incidence of depressive symptoms in mid-aged women: results from a large community-based prospective study. Eur J Clin Nutr. 2013;67:75–82. doi: 10.1038/ejcn.2012.193. [DOI] [PubMed] [Google Scholar]
- Sacanella E, Vazquez-Agell M, Mena MP, Antunez E, Fernandez-Sola J, Nicolas JM, Lamuela-Raventos RM, Ros E, Estruch R. Down-regulation of adhesion molecules and other inflammatory biomarkers after moderate wine consumption in healthy women: a randomized trial. Am J Clin Nutr. 2007;86:1463–1469. doi: 10.1093/ajcn/86.5.1463. [DOI] [PubMed] [Google Scholar]
- Salvini S, Hunter DJ, Sampson L, Stampfer MJ, Colditz GA, Rosner B, Willett WC. Food-based validation of a dietary questionnaire: the effects of week-to-week variation in food consumption. Int J Epidemiol. 1989;18:858–867. doi: 10.1093/ije/18.4.858. [DOI] [PubMed] [Google Scholar]
- Samieri C, Jutand MA, Feart C, Capuron L, Letenneur L, Barberger-Gateau P. Dietary patterns derived by hybrid clustering method in older people: association with cognition, mood, and self-rated health. J Am Diet Assoc. 2008;108:1461–1471. doi: 10.1016/j.jada.2008.06.437. [DOI] [PubMed] [Google Scholar]
- Sanchez-Villegas A, Delgado-Rodriguez M, Alonso A, Schlatter J, Lahortiga F, Majem LS, Martinez-Gonzalez MA. Association of the Mediterranean dietary pattern with the incidence of depression: the Seguimiento Universidad de Navarra/University of Navarra follow-up (SUN) cohort. Arch Gen Psychiatry. 2009;66:1090–1098. doi: 10.1001/archgenpsychiatry.2009.129. [DOI] [PubMed] [Google Scholar]
- Sanchez-Villegas A, Martinez-Gonzalez MA. Diet, a new target to prevent depression? BMC Med. 2013;11:3. doi: 10.1186/1741-7015-11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Villegas A, Verberne L, De Irala J, Ruiz-Canela M, Toledo E, Serra-Majem L, Martinez-Gonzalez MA. Dietary fat intake and the risk of depression: the SUN Project. PLoS One. 2011;6:e16268. doi: 10.1371/journal.pone.0016268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze MB, Hoffmann K, Kroke A, Boeing H. An approach to construct simplified measures of dietary patterns from exploratory factor analysis. Br J Nutr. 2003;89:409–419. doi: 10.1079/BJN2002778. [DOI] [PubMed] [Google Scholar]
- Schulze MB, Hoffmann K, Manson JE, Willett WC, Meigs JB, Weikert C, Heidemann C, Colditz GA, Hu FB. Dietary pattern, inflammation, and incidence of type 2 diabetes in women. Am J Clin Nutr. 2005;82:675–684. doi: 10.1093/ajcn.82.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel D, Giese-Davis J. Depression and cancer: mechanisms and disease progression. Biol Psychiatry. 2003;54:269–282. doi: 10.1016/s0006-3223(03)00566-3. [DOI] [PubMed] [Google Scholar]
- Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, Hennekens CH, Speizer FE. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122:51–65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
- Yamazaki S, Fukuhara S, Green J. Usefulness of five-item and three-item Mental Health Inventories to screen for depressive symptoms in the general population of Japan. Health Qual Life Outcomes. 2005;3:48. doi: 10.1186/1477-7525-3-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.