Abstract
Background
Current guidelines for aerobic activity require that adults carry out ≥150 minutes/week of moderate-intensity physical activity, with a large body of epidemiologic evidence showing this level of activity to decrease the incidence of many chronic diseases. Less is known about whether light-intensity activities also have such benefits, and whether sedentary behavior is an independent predictor of increased risks of these chronic diseases, as imprecise assessments of these behaviours and cross-sectional study designs have limited knowledge to date.
Methods
Recent technological advances in assessment methods have made the use of movement sensors, such as the accelerometer, feasible for use in longitudinal, large-scale epidemiologic studies. Several such studies are collecting sensor-assessed, objective measures of physical activity with the aim of relating these to the development of clinical endpoints. This is a relatively new area of research; thus, in this paper, we use the Women’s Health Study (WHS) as a case study to illustrate challenges related to data collection, data processing, and analyses of the vast amount of data collected.
Results
The WHS plans to collect 7 days of accelerometer-assessed physical activity and sedentary behavior in ~18,000 women aged ≥62 years. Several logistical challenges exist in collecting data; nonetheless as of 31 August 2013, 11,590 women have already provided some data. Additionally, the WHS experience on data reduction and data analyses can help inform other similar large-scale epidemiologic studies.
Conclusions
Important data on the health effects of light-intensity activity and sedentary behaviour will emerge from large-scale epidemiologic studies collecting objective assessments of these behaviours.
Keywords: accelerometer, epidemiology, physical activity, women
Introduction
Physical activity is medicine, as this special issue of the British Journal of Sports Medicine declares. A large body of epidemiologic literature, accumulated over more than 60 years, shows that individuals who are physically active have a better cardio-metabolic risk profile, lower rates of major non-communicable diseases, better physical and mental function, and live longer, compared with those inactive.[1-3] It is therefore unfortunate that approximately one-third of the world’s population does not get sufficient physical activity to meet current recommendations.[4] Indeed, this high prevalence of insufficient activity has been estimated to cause as many deaths worldwide each year as smoking:[5] although smoking increases the risk of dying for the individual more than does inactivity, the prevalence of smoking worldwide (about one-quarter) is less than that of inactivity,[6] resulting in comparable harm attributable to each behaviour at the population level.
Although we have learnt much about the health benefits of physical activity, there remain major gaps in knowledge. Some of these gaps have resulted from imprecise assessments of physical activity in research studies. In recent years, technological advances have allowed for better assessments via movement sensors such as the accelerometer, which has become increasingly feasible for use in research from the perspective of the subject (minimal inconvenience), as well as the investigator (reasonable cost).[7] In this paper, we address two specific gaps in knowledge related to physical activity and health and discuss how accelerometers can add information, as well as challenges related to employing these devices in large-scale epidemiologic studies, using the Women’s Health Study as an example.
Current Physical Activity Guidelines – Why No Prescription for Light-Intensity Activity?
In 2008, the United States (US) federal government published its first ever comprehensive physical activity guidelines for the population.[8] With regard to aerobic activities, the guidelines require that adults carry out at least 150 minutes per week of moderate-intensity physical activity (e.g., brisk walking, whether for leisure or commuting), or 75 minutes per week of vigorous-intensity activity (e.g., jogging), or some combination of activities from both intensities that expend an equivalent amount of energy. Similar guidelines were later adopted by other countries worldwide.[3]
Missing from these guidelines are any recommendations for light-intensity physical activities. Indeed, a table from the US guidelines (page 4 of the guidelines[8]) implied no health benefits from light-intensity activities: the overall health benefits from being inactive, or engaging in no physical activities beyond baseline activity, was stated as “none” (baseline activity was defined as[8] “light-intensity activities of daily life, such as standing, walking slowly, and lifting lightweight objects … people who do only baseline activity are considered to be inactive.”)
How did this transpire, and is it indeed true that light-intensity activities – those ubiquitous in activities of daily living – have no health benefits? To appreciate why light-intensity activities are absent from current guidelines, it is necessary to understand their genesis. The US guidelines based its recommendations on the findings from an extensive review of existing literature by an expert panel.[1] This panel emphasized data from studies that examined the relation between physical activity and the risks of developing hard clinical endpoints, such as all-cause mortality, cardiovascular disease, type 2 diabetes, and cancer, reviewing the data from individual studies in detail. The expert panel did consider data from studies of physical activity in relation to cardio-metabolic risk factors (e.g., blood pressure, lipid parameters, measures of glucose and insulin processing); however, these data were considered supporting information.
To date, information how physical activity influences the risks of developing hard clinical endpoints among generally healthy adults have, in large part, come from observational epidemiologic studies which used self-reports of physical activity by study participants.[1] This has occurred because such studies typically require thousands, and even hundreds of thousands, of subjects to possess sufficient statistical power for investigating associations of physical activity with the incidence of hard clinical endpoints (e.g., major non-communicable diseases), and self-report is a cost-effective method of assessment. Self-reports of physical activity are more reliable and valid for activities of moderate-to-vigorous intensity than light-intensity activities, which tend to be poorly reported.[9 10] Therefore, observational epidemiologic studies that rely on self-reports have tended to limit their assessments to activities of moderate- and vigorous-intensity only. As a consequence, current recommendations have no provision for light-intensity physical activity because few data are available, and not because existing data indicate no benefit. Indeed, the US guidelines acknowledge that “we don’t understand enough about whether doing more baseline activity results in health benefits”.[8] International physical activity guidelines have also employed similar reasoning for their recommendations.[3]
Sedentary Behaviour – An Independent Risk Factor?
A growing body of epidemiologic literature over the past 5-10 years has described associations between sedentary behaviour and increased risks of all-cause mortality and cardio-metabolic diseases,[11] with plausible biologic mechanisms (e.g., lipoprotein lipase regulation) proposed from animal studies.[12] In several studies, investigators reported on the persistence of these associations even among persons active enough to meet physical activity recommendations.[13-20] For example, in a recent study of almost one-quarter million US older adults (50-71 years) free from cardiovascular disease and cancer, more time spent on TV viewing was associated with higher all-cause mortality rates, regardless of physical activity level. Among persons reporting >7 hours a week of moderate-to-vigorous intensity activity, the risk of dying during follow-up was 50% higher among those declaring 7 or more hours of TV viewing a day, compared to <1 hour a day.[18]
One limitation of these studies on sedentary behaviour has been, as with studies of physical activity, reliance on self-reports since this method of assessment is most feasible for studies with large numbers of subjects. In a review of 48 longitudinal studies investigating sedentary behaviour in relation to subsequent health outcomes, 45 used participant self-reports, primarily of TV and screen viewing time.[21] It is unclear how well self-reported TV/screen time reflects total sedentary time. A study comparing self-reported TV viewing time with accelerometer-assessed sedentary time among a representative sample of US adults in the National Health and Nutrition Examination Survey yielded a correlation of only 0.22, indicating low agreement.[22] Imprecision in self-reports also may partly explain findings such as these: in a nationally representative sample of adults from the Health Survey for England, cross-sectional analyses showed consistent deleterious associations of self-reported sitting time with a panel of cardio-metabolic risk factors; however, when accelerometer measures of sedentary behaviour were analysed, little relation was observed.[23] Thus, one could argue that it may not be the sedentary behaviour per se but, rather, associated unhealthy behaviours – such as snacking while watching TV – that accounts for the observed adverse associations.
There do exist data from several studies that used accelerometers to objectively measure sedentary behavior, showing unfavourable associations with risk factors (e.g., the Australian AusDiab study,[24] the Proactiv UK trial,[25] the US National Health and Nutrition Examination Survey[26]). However, these studies are predominantly cross-sectional in design; thus, one cannot ascertain the direction of association (i.e., does being sedentary result in poor health? Or, does being in poor health predispose one towards being sedentary?). Additionally, observed improvements in risk factors do not always result in reduced rates of disease, as demonstrated by the Look AHEAD trial, a randomized controlled trial comparing an intensive lifestyle intervention with a traditional diabetes support and education program among overweight/obese type 2 diabetics.[27] In recently published results, the intensive intervention clearly improved cardio-metabolic risk factors; however, there was no difference in rates of cardiovascular events between groups after a median follow-up of 9.6 years, when the trial was stopped for futility.
Therefore, there is a need for longitudinal studies of sedentary behaviour employing objective assessments in relation to hard clinical endpoints, to complement and clarify existing data that may be limited by one or more of the following factors: self-reports of sedentary behaviour, cross-sectional design, ascertainment of only risk factors but not disease incidence.
Using Accelerometers in Large-Scale Epidemiologic Studies
One movement sensor capable of measuring light-intensity physical activity and sedentary behaviour objectively is the accelerometer. As its name suggests, the device measures accelerations. The first commercial use of the accelerometer occurred in the 1920’s, where they were used in bridges (to record vibrations) and aircraft (to measure accelerations).[28] It weighed about a pound and cost $420 in the 1930’s (some $6,000 today). Technological advances have made the device smaller and lighter (<1 oz), and less expensive (e.g., the Actigraph GT3X+ model, used in the case study of the Women’s Health Study (WHS) below, costs $200-$250) today. Thus, the accelerometer has now become feasible for use in large-scale studies, such as the examples shown in Table 1.
Table 1.
Examples of Cohort Studies of Cardio-Metabolic Diseases and Cancer Using Accelerometers to Assess Physical Activity and Sedentary Behaviour
| Study, Country | Approximate N, Age | Years and Method of Data Collection |
Device |
|---|---|---|---|
| European Prospective Investigation of Cancer (EPIC) – Norfolk Study, United Kingdom* |
3,900 men and women, 60-80y |
2008-2011; in person | Actigraph GT1M |
| Reasons for Geographic and Racial Differences in Stroke (REGARDS) Study, USA [29] |
9,400 men and women, 56+ y |
2009-2012; by mail | Actical |
| British Regional Heart Study, United Kingdom† |
2,500 men and women, 70-90 y |
2010-2012; by mail | Actigraph GT3X |
| Maastricht Study, Netherlands‡ | 10,000 men and women, 40-75 y |
2010 until sample reaches 10,000; in person |
ActivPal |
| Women’s Health Study (WHS), USA[30] |
18,000 women, 62+ y | 2011-2014; by mail | Actigraph GT3X+ |
| Women’s Health Initiative Study (WHI), USA[31] |
8,000 women, 72+ y | 2012-2013; in person and by |
Actigraph GT3X+ |
Ekelund, U. Personal communication, 2013.
Jefferis, B. Personal communication, 2013.
Koster, A; Personal communication, 2013.
While accelerometers provide objective measurements, it is important to note some limitations. They primarily measure locomotor activity when worn over the hip (typical placement position), and so miss upper body movement. Further, they cannot distinguish whether a person is carrying any weight (e.g., walking carrying a heavy bag expends more energy vs. walking with no load). Accelerometers do not inform on body posture; thus, they cannot distinguish between sitting and standing still. However, for some populations, these are not major limitations; for example, in the WHS below that includes older women, walking is the most common physical activity. Since participants are older, accelerometer indications of very low levels of activity over long periods during waking hours likely indicate sitting, and not standing with little movement.
A Case Study: Issues and Challenges
The Women’s Health Study (WHS)
The WHS is a completed randomized trial (1992-2004) testing aspirin and vitamin E for preventing cardiovascular disease and cancer among 39,876 healthy women, ≥45 years, living throughout the US.[32-34] When the trial ended, women were invited to continue in an observational study. Of those alive, 89% or 33,681 women consented, reporting on their health habits and medical history annually on questionnaires. In 2011, data collection began for an ancillary study, in which the main aim is to examine relations of accelerometer-measured physical activity and sedentary behavior with health outcomes. Women are asked to wear an accelerometer (ActiGraph GT3X+, ActiGraph Corporation, Pensacola, FL) on their hip during all waking hours for 7 days. Data collection will be completed at the end of 2014. Below, we discuss our experience and show some preliminary data.
Logistical challenges
When data collection for the WHS accelerometer study began, approximately 30,000 women were alive; thus, the most feasible method for collecting data was via mail. Because participants had already been in the main study for an average of almost 17 years, we believed it was possible to get good response rates and compliance with instructions. Typically, in the main study, we obtain ~80% response to the first mailing of study questionnaires, another ~10% to the second, and a further ~5% to the third, for a total response of ~95%. However, because of funding cuts, we chose initially to send only a single invitation to participate in the accelerometer study (and will consider a second request to achieve our goal of ~18,000 women). Invitations are sent on a rolling basis because of the limited number of accelerometers available.
When a participant agrees to participate, we mail her an accelerometer, a log sheet to record dates/times that the device is put on and taken off (further discussed below), and instructions. On receiving this packet, some women change their mind about participation, or request postponement to a more convenient time. Two issues result: first, in such a large study, it is challenging to keep track of the stage at which each individual participant is. Additionally, other sub-studies are occurring in the WHS (e.g., repeated yearly assessments in a subgroup; comparing wrist- and hip-worn devices in another subgroup), which further complicate tracking. Second, with dwindling participation at each stage, sample size can decrease substantially.
As of 31 August 2013, 23,266 women have been invited; 19,432 (84%) have responded, of whom 15,314 (79% of respondents) have agreed to participate (16% declined and 5% were not eligible because they cannot walk outside without assistance). Packets have been mailed to 14,073 women, of whom 11,590 have worn and returned their devices, 725 have requested participation at a later date (we assume some of these will eventually decline), and 1,087 have changed their mind about participation (the remaining 671 women were not due as of 31/8/13 to return their device). Of the ~30,000 total women, we expect 1320 (4.4%) to be non-eligible, and project participation by ~18,000 (~63%) of the remaining 28,680. This experience of dwindling participation is not unique; for example, in the mail-based REGARDS study (Table 1), 48% (10,863/22,195) of invited participants returned a device with some data.[29]
Participants do lose or fail to return their accelerometers; the rate of loss in the WHS is 2.1%. While this seems negligible, 2.1% of the ~20,000 whom we anticipate sending accelerometers translates to 420 units, at substantial cost. In the REGARDS study, investigators reported an 8% loss rate.[29]
Challenges related to data reduction
The technological capability for data capture using accelerometers has outpaced current knowledge on how to reduce and process these data. Further, while best practices and standards have been proposed,[35-37] no consensus has been reached. Thus, in the WHS, we keep multiple data files, processed using different conventions, for each participant: consequently, we anticipate storing a large – ~20 terabytes – amount of data when data collection ends.
To illustrate challenges in this area, we discuss two important decisions that have to be made. The first relates to determining actual wear time of the device by participants, and not mistaking movements in the mail process as participant physical activity. We ask women to record, daily, dates and times of wear on a log; ideally, these would not be required as they are onerous for both participant and investigator, and missing data may be an issue. Automated algorithms are available (e.g., the commonly used Troiano[38] and Choi[39] algorithms) for wear-time determination; however, these were developed for protocols where participants received the device directly from investigators (thus, “mail noise” is not applicable). We hope to use the WHS experience to develop guidelines for other large-scale studies using a mail protocol. Based on preliminary data, we have found that using logs only to record whether the device was “worn” or “not worn” for the day, but not to indicate times (minimizing participant burden and likelihood of missing data), and then applying the Choi algorithm best reflected actual wear-time as assessed by participant detailed logs.
A second decision relates to which band-pass filter to use. For the accelerometer used in the WHS, the manufacturer’s software allows a “normal” and “low-frequency extension” (LFE) option. Since the LFE filter may be more sensitive to detecting slower gait, the manufacturer recommends this option for older populations. Preliminary data in the WHS indicates that the LFE filter substantially overestimates steps taken per day (median of ~5,000 steps per day using the normal filter; ~13,000 steps per day using the LFE filter); thus, we do not recommend this option for assessing steps. Whether the LFE filter is preferable for other physical activity variables is unclear from the WHS; further exploration is needed.
Challenges related to data analyses
As with data reduction, current analytic methods for accelerometer-assessed data appear rudimentary when compared with the technology available for data collection. Most analyses to date integrate acceleration data from the vertical axis only into counts per user-specified time period (e.g., counts per minute, cpm) and utilize cutpoints for classifying time spent in sedentary behaviour or physical activity (higher cpm reflects higher intensity activities), which are then related to health outcomes.[24-26] However, there is no consensus on what the “correct” cutpoints might be, with several proposed in the literature based on calibration studies primarily carried out under laboratory settings and not in free-living conditions.[40 41] Further, cutpoints developed among middle-aged persons may not be applicable for older populations.[42] And, current prediction techniques that use cutpoints to estimate energy expenditure are limited, with both under- and over-estimations occurring.[43 44]
Newer accelerometer models (including the device used in the WHS) collect data not only from the vertical (up-and-down movements), but also antero-posterior (back-to-front) and lateral (side-to-side) axes. There are hardly any data for cutpoints developed using triaxial data.[45] Recent work indicates a strong correlation between counts from the vertical axis and counts combined from all three axes using vector magnitude; thus, the vector magnitude data may not provide much additional information beyond vertical axis counts.[46]
Finally, cutpoint-based analyses make only very limited use of the wealth of data available. Efforts are ongoing to better take advantage of the rich data, such as pattern recognition or machine learning data analytic approaches to identify specific activities carried out.[47 48]
Some preliminary data
Data collection in the WHS is anticipated to occur between 2011 and 2014. While the entire sample of eligible and willing women will be needed to provide sufficient statistical power for examining accelerometer-assessed sedentary behaviour and physical activity in relation to incidence of chronic diseases and all-cause mortality (primary aims of the study), a smaller sample size is adequate to address secondary aims, such as describing patterns of sedentary behaviour and physical activity.
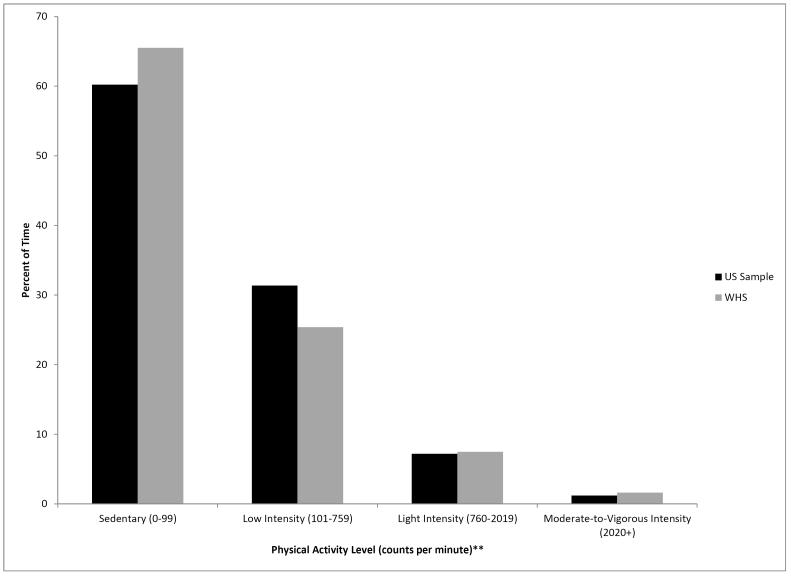
Thus, we decided a priori to create an interim, closed dataset that comprised all women who returned their accelerometer by 31 March 2013 (n = 8,373; approximately half the total anticipated sample of ~18,000). Women were of mean age 71 years, 20% were obese, and 4% were current smokers.[30] They complied well with instructions: 95% wore their device for ≥10 hours each day on ≥4 days (conventionally regarded as “valid” data[49]). Figure 1 shows their physical activity levels compared with a comparably aged US national sample (data are presented using commonly used cutpoints for defining different intensities of physical activity[18 38]). A detailed description of patterns of sedentary behaviour among women will be published elsewhere.[30]
Figure 1.
Physical Activity Levels, Women’s Health Study (WHS) 2011-2013, Compared With a US National Sample* (2003-2004)
* Women aged 60-75 years from the 2003-2004 US National Health and Nutrition Examination Survey
** Higher counts per minute reflect higher intensity activities
Conclusions
That we face the challenges discussed is a testament to how far the field of physical activity epidemiology has advanced. In the early days of research in this field, pioneers – led by Professors Jerry Morris and Ralph Paffenbarger, Jr.[50] – sought to convince others of the health benefits of physical activity. Today, with this clearly established, investigators have moved to answering other questions such as those related to the “dose” needed. In particular, given the low levels of physical activity around the world,[4] there is a great deal of interest on the low end of the physical activity spectrum, i.e., light-intensity activities and sedentary behaviour. Imprecise assessments of these behaviours and cross-sectional study designs have limited our knowledge. However, recent technological advances have made the use of movement sensors, such as the accelerometer, feasible for use in longitudinal, large-scale epidemiologic studies. Presently, several such studies, including the Women’s Health Study that we used as a case study, are collecting sensor-assessed, objective measures of physical activity with the aim of relating these to the development of hard clinical endpoints. While challenges exist, especially those related to processing and analyzing the vast amount of data collected, over the next 5 to 10 years much interesting data will emerge.
Summary Box – What are the new findings?
Recent technological advances have made the use of movement sensors, such as the accelerometer, feasible for use in longitudinal, large-scale epidemiologic studies that intend to investigate the associations of objectively-measured physical activity or sedentary behaviour with hard clinical outcomes.
Logistical challenges exist in collecting objectively-measured physical activity or sedentary behaviour data from large-scale epidemiologic studies, which result in a reduced number of participants available for investigation.
While large amounts of data can be collected using accelerometers, procedures to reduce and process these data are not well developed; thus, best practices and standards for accelerometer data reduction and processing are needed.
Summary Box – How might it impact on clinical practice in the near future?
Current physical activity guidelines recommend activities of at least moderate intensity, but there are no guidelines targeting activities of lower intensity or sedentary behaviour. Imprecise assessments of these behaviours and cross-sectional study designs have limited knowledge; however, recent technological advances have allowed for more precise assessments that are feasible in longitudinal, large-scale epidemiologic studies. Several such ongoing studies will provide information that can inform the development of guidelines related to light-intensity activity and sedentary behaviour.
Acknowledgements
We are grateful to Drs. Patty Freedson and Steven Hooker, as well as to the staff of the Women’s Health Study, particularly Ara Sarkissian, Bonnie Church, Colby Smith, and Jane Jones, for their assistance with the study.
Funding This study is supported by grant CA154647 from the US National Institutes of Health.
Footnotes
This paper is based on a presentation given at the 3rd International Conference on Ambulatory Monitoring of Physical Activity and Movement, June 2013, at Amherst, Massachusetts, USA.
Competing Interests Dr. Lee and Mr. Shiroma report no competing interests.
Contributor Information
I-Min Lee, Division of Preventive Medicine Brigham & Women’s Hospital, Harvard Medical School Department of Epidemiology Harvard School of Public Health 900 Commonwealth Ave, 3rd Floor Boston, MA USA 02215.
Eric J Shiroma, Division of Preventive Medicine Brigham & Women’s Hospital, Harvard Medical School Department of Epidemiology Harvard School of Public Health Boston, MA USA.
References
- 1.U.S. Department of Health & Human Services Physical Activity Guidelines Advisory Committee report. Secondary 2008 Physical Activity Guidelines Advisory Committee report. 2008 http://www.health.gov/paguidelines/
- 2.Warburton DE, Charlesworth S, Ivey A, et al. A systematic review of the evidence for Canada’s Physical Activity Guidelines for Adults. Int J Behav Nutr Phys Act. 2010;7:39. doi: 10.1186/1479-5868-7-39. doi: 10.1186/1479-5868-7-39 [published Online First: 2010/05/13] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization . Global recommendations on physical activity for health. WHO Press; Geneva, Switzerland: 2010. [PubMed] [Google Scholar]
- 4.Hallal PC, Andersen LB, Bull FC, et al. Global physical activity levels: surveillance progress, pitfalls, and prospects. Lancet. 2012;380(9838):247–57. doi: 10.1016/S0140-6736(12)60646-1. doi: 10.1016/S0140-6736(12)60646-1 [published Online First: 2012/07/24] [DOI] [PubMed] [Google Scholar]
- 5.Lee IM, Shiroma EJ, Lobelo F, et al. Effect of physical inactivity on major non-communicable diseases worldwide: an analysis of burden of disease and life expectancy. Lancet. 2012;380(9838):219–29. doi: 10.1016/S0140-6736(12)61031-9. doi: 10.1016/S0140-6736(12)61031-9 [published Online First: 2012/07/24] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wen CP, Wu X. Stressing harms of physical inactivity to promote exercise. Lancet. 2012;380(9838):192–3. doi: 10.1016/S0140-6736(12)60954-4. doi: 10.1016/S0140-6736(12)60954-4 [published Online First: 2012/07/24] [DOI] [PubMed] [Google Scholar]
- 7.Esliger DW, Tremblay MS. Physical activity and inactivity profiling: the next generation. Can J Public Health. 2007;98(Suppl 2):S195–207. [published Online First: 2008/01/25] [PubMed] [Google Scholar]
- 8.U.S. Department of Health and Human Services Physical Activity Guidelines for Americans. Secondary 2008 Physical Activity Guidelines for Americans. 2008 http://www.health.gov/paguidelines/
- 9.Strath SJ, Bassett DR, Jr., Swartz AM. Comparison of the college alumnus questionnaire physical activity index with objective monitoring. Ann Epidemiol. 2004;14(6):409–15. doi: 10.1016/j.annepidem.2003.07.001. doi: 10.1016/j.annepidem.2003.07.001 [published Online First: 2004/07/13] [DOI] [PubMed] [Google Scholar]
- 10.Bonnefoy M, Normand S, Pachiaudi C, et al. Simultaneous validation of ten physical activity questionnaires in older men: a doubly labeled water study. J Am Geriatr Soc. 2001;49(1):28–35. doi: 10.1046/j.1532-5415.2001.49006.x. [published Online First: 2001/02/24] [DOI] [PubMed] [Google Scholar]
- 11.Wilmot EG, Edwardson CL, Achana FA, et al. Sedentary time in adults and the association with diabetes, cardiovascular disease and death: systematic review and meta-analysis. Diabetologia. 2012;55(11):2895–905. doi: 10.1007/s00125-012-2677-z. doi: 10.1007/s00125-012-2677-z [published Online First: 2012/08/15] [DOI] [PubMed] [Google Scholar]
- 12.Hamilton MT, Hamilton DG, Zderic TW. Role of low energy expenditure and sitting in obesity, metabolic syndrome, type 2 diabetes, and cardiovascular disease. Diabetes. 2007;56(11):2655–67. doi: 10.2337/db07-0882. [published Online First] [DOI] [PubMed] [Google Scholar]
- 13.Hu FB, Leitzmann MF, Stampfer MJ, et al. Physical activity and television watching in relation to risk for type 2 diabetes mellitus in men. Arch Int Med. 2001;161(12):1542–8. doi: 10.1001/archinte.161.12.1542. [published Online First: 2001/06/28] [DOI] [PubMed] [Google Scholar]
- 14.Hu FB, Li TY, Colditz GA, et al. Television watching and other sedentary behaviors in relation to risk of obesity and type 2 diabetes mellitus in women. JAMA. 2003;289(14):1785–91. doi: 10.1001/jama.289.14.1785. doi: 10.1001/jama.289.14.1785 [published Online First: 2003/04/10] [DOI] [PubMed] [Google Scholar]
- 15.Katzmarzyk PT, Church TS, Craig CL, et al. Sitting time and mortality from all causes, cardiovascular disease, and cancer. Medicine and science in sports and exercise. 2009;41(5):998–1005. doi: 10.1249/MSS.0b013e3181930355. doi: 10.1249/MSS.0b013e3181930355 [published Online First: 2009/04/07] [DOI] [PubMed] [Google Scholar]
- 16.Krishnan S, Rosenberg L, Palmer JR. Physical activity and television watching in relation to risk of type 2 diabetes: the Black Women’s Health Study. Am J Epidemiol. 2009;169(4):428–34. doi: 10.1093/aje/kwn344. doi: 10.1093/aje/kwn344 [published Online First: 2008/12/06] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wijndaele K, Brage S, Besson H, et al. Television viewing time independently predicts all-cause and cardiovascular mortality: the EPIC Norfolk study. Int J Epidemiol. 2011;40(1):150–9. doi: 10.1093/ije/dyq105. doi: 10.1093/ije/dyq105 [published Online First: 2010/06/26] [DOI] [PubMed] [Google Scholar]
- 18.Matthews CE, George SM, Moore SC, et al. Amount of time spent in sedentary behaviors and cause-specific mortality in US adults. Am J Clin Nutr. 2012;95(2):437–45. doi: 10.3945/ajcn.111.019620. doi: 10.3945/ajcn.111.019620 [published Online First: 2012/01/06] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patel AV, Bernstein L, Deka A, et al. Leisure time spent sitting in relation to total mortality in a prospective cohort of US adults. Am J Epidemiol. 2010;172(4):419–29. doi: 10.1093/aje/kwq155. [published Online First] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van der Ploeg HP, Chey T, Korda RJ, et al. Sitting time and all-cause mortality risk in 222 497 Australian adults. Arch Intern Med. 2012;172(6):494–500. doi: 10.1001/archinternmed.2011.2174. doi: 10.1001/archinternmed.2011.2174 [published Online First: 2012/03/28] [DOI] [PubMed] [Google Scholar]
- 21.Thorp AA, Owen N, Neuhaus M, et al. Sedentary behaviors and subsequent health outcomes in adults a systematic review of longitudinal studies, 1996-2011. Am J Prev Med. 2011;41(2):207–15. doi: 10.1016/j.amepre.2011.05.004. doi: 10.1016/j.amepre.2011.05.004 [published Online First: 2011/07/20] [DOI] [PubMed] [Google Scholar]
- 22.Clark BK, Healy GN, Winkler EA, et al. Relationship of television time with accelerometer-derived sedentary time: NHANES. Med Sci Sports Exerc. 2011;43(5):822–8. doi: 10.1249/MSS.0b013e3182019510. doi: 10.1249/MSS.0b013e3182019510 [published Online First: 2010/10/29] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stamatakis E, Hamer M, Tilling K, et al. Sedentary time in relation to cardio-metabolic risk factors: differential associations for self-report vs accelerometry in working age adults. Int J Epidemiol. 2012;41(5):1328–37. doi: 10.1093/ije/dys077. doi: 10.1093/ije/dys077 [published Online First] [DOI] [PubMed] [Google Scholar]
- 24.Dunstan DW, Salmon J, Owen N, et al. Physical activity and television viewing in relation to risk of undiagnosed abnormal glucose metabolism in adults. Diabetes Care. 2004;27(11):2603–9. doi: 10.2337/diacare.27.11.2603. [published Online First: 2004/10/27] [DOI] [PubMed] [Google Scholar]
- 25.Ekelund U, Brage S, Griffin SJ, et al. Objectively measured moderate- and vigorous-intensity physical activity but not sedentary time predicts insulin resistance in high-risk individuals. Diabetes Care. 2009;32(6):1081–6. doi: 10.2337/dc08-1895. doi: 10.2337/dc08-1895 [published Online First: 2009/03/03] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gennuso KP, Gangnon RE, Matthews CE, et al. Sedentary behavior, physical activity, and markers of health in older adults. Med Sci Sports Exerc. 2013;45(8):1493–500. doi: 10.1249/MSS.0b013e318288a1e5. doi: 10.1249/MSS.0b013e318288a1e5 [published Online First: 2013/03/12] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wing RR, Bolin P, Brancati FL, et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. 2013;369(2):145–54. doi: 10.1056/NEJMoa1212914. doi: 10.1056/NEJMoa1212914 [published Online First: 2013/06/26] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walter PL. The history of the accelerometer: 1920s-1996 - prologue and epilogue 2006. Sound and Vibration. 2007 Jan;:84–92. [published Online First] [Google Scholar]
- 29.Howard VJ, Rhodes JD, Hutto B, et al. Successful use of telephone and mail for obtaining usable accelerometer data from a national cohort: The experience of the Reasons for Geographic and Racial Differences in Stroke (REGARDS) Study. Circulation. 2013;127:AP145. [published Online First] [Google Scholar]
- 30.Shiroma EJ, Freedson PS, Trost SG, et al. Patterns of accelerometer-assessed sedentary behavior in older women. JAMA. doi: 10.1001/jama.2013.278896. in press [published Online First] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Women’s Health Initiative Secondary. http://hpdp.unc.edu/research/projects/objective-physical-activity-and-cardiovascular-health-in-women-aged-80-and-older/
- 32.Cook NR, Lee IM, Gaziano JM, et al. Low-dose aspirin in the primary prevention of cancer: the Women’s Health Study: a randomized controlled trial. JAMA. 2005;294(1):47–55. doi: 10.1001/jama.294.1.47. doi: 10.1001/jama.294.1.47 [published Online First: 2005/07/07] [DOI] [PubMed] [Google Scholar]
- 33.Lee IM, Cook NR, Gaziano JM, et al. Vitamin E in the primary prevention of cardiovascular disease and cancer: the Women’s Health Study: a randomized controlled trial. JAMA. 2005;294(1):56–65. doi: 10.1001/jama.294.1.56. doi: 10.1001/jama.294.1.56 [published Online First: 2005/07/07] [DOI] [PubMed] [Google Scholar]
- 34.Ridker PM, Cook NR, Lee IM, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med. 2005;352(13):1293–304. doi: 10.1056/NEJMoa050613. [published Online First] [DOI] [PubMed] [Google Scholar]
- 35.Heil DP, Brage S, Rothney MP. Modeling physical activity outcomes from wearable monitors. Med Sci Sports Exerc. 2012;44(1 Suppl 1):S50–60. doi: 10.1249/MSS.0b013e3182399dcc. doi: 10.1249/MSS.0b013e3182399dcc [published Online First: 2011/12/23] [DOI] [PubMed] [Google Scholar]
- 36.Matthews CE, Hagstromer M, Pober DM, et al. Best practices for using physical activity monitors in population-based research. Med Sci Sports Exerc. 2012;44(1 Suppl 1):S68–76. doi: 10.1249/MSS.0b013e3182399e5b. doi: 10.1249/MSS.0b013e3182399e5b [published Online First: 2011/12/23] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Trost SG, McIver KL, Pate RR. Conducting accelerometer-based activity assessments in field-based research. Med Sci Sports Exerc. 2005;37(11 Suppl):S531–43. doi: 10.1249/01.mss.0000185657.86065.98. [published Online First: 2005/11/19] [DOI] [PubMed] [Google Scholar]
- 38.Troiano RP, Berrigan D, Dodd KW, et al. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40(1):181–8. doi: 10.1249/mss.0b013e31815a51b3. [published Online First] [DOI] [PubMed] [Google Scholar]
- 39.Choi L, Liu Z, Matthews CE, et al. Validation of accelerometer wear and nonwear time classification algorithm. Med Sci Sports Exerc. 2011;43(2):357–64. doi: 10.1249/MSS.0b013e3181ed61a3. doi: 10.1249/MSS.0b013e3181ed61a3 [published Online First: 2010/06/29] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matthew CE. Calibration of accelerometer output for adults. Med Sci Sports Exerc. 2005;37(11 Suppl):S512–22. doi: 10.1249/01.mss.0000185659.11982.3d. [published Online First: 2005/11/19] [DOI] [PubMed] [Google Scholar]
- 41.Hagstromer M, Troiano RP, Sjostrom M, et al. Levels and patterns of objectively assessed physical activity--a comparison between Sweden and the United States. Am J Epidemiol. 2010;171(10):1055–64. doi: 10.1093/aje/kwq069. doi: 10.1093/aje/kwq069 [published Online First: 2010/04/22] [DOI] [PubMed] [Google Scholar]
- 42.Evenson KR, Buchner DM, Morland KB. Objective measurement of physical activity and sedentary behavior among US adults aged 60 years or older. Prev Chronic Dis. 2012;9:E26. [published Online First] [PMC free article] [PubMed] [Google Scholar]
- 43.Lyden K, Kozey SL, Staudenmeyer JW, et al. A comprehensive evaluation of commonly used accelerometer energy expenditure and MET prediction equations. Eur J Appl Physiol. 2011;111(2):187–201. doi: 10.1007/s00421-010-1639-8. doi: 10.1007/s00421-010-1639-8 [published Online First] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rothney MP, Schaefer EV, Neumann MM, et al. Validity of physical activity intensity predictions by ActiGraph, Actical, and RT3 accelerometers. Obesity (Silver Spring) 2008;16(8):1946–52. doi: 10.1038/oby.2008.279. doi: 10.1038/oby.2008.279 [published Online First: 2008/06/07] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sasaki JE, John D, Freedson PS. Validation and comparison of ActiGraph activity monitors. J Sci Med Sport. 2011;14(5):411–6. doi: 10.1016/j.jsams.2011.04.003. doi: 10.1016/j.jsams.2011.04.003 [published Online First] [DOI] [PubMed] [Google Scholar]
- 46.Crouter SE, Horton M, Bassett DR., Jr Use of a two-regression model for estimating energy expenditure in children. Med Sci Sports Exerc. 2012;44(6):1177–85. doi: 10.1249/MSS.0b013e3182447825. doi: 10.1249/MSS.0b013e3182447825 [published Online First: 2011/12/07] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ruch N, Joss F, Jimmy G, et al. Neural network versus activity-specific prediction equations for energy expenditure estimation in children. J Appl Physiol. 2013 doi: 10.1152/japplphysiol.01443.2012. doi: 10.1152/japplphysiol.01443.2012 [published Online First: 2013/08/31] [DOI] [PubMed] [Google Scholar]
- 48.Trost SG, Wong WK, Pfeiffer KA, et al. Artificial neural networks to predict activity type and energy expenditure in youth. Med Sci Sports Exerc. 2012;44(9):1801–9. doi: 10.1249/MSS.0b013e318258ac11. doi: 10.1249/MSS.0b013e318258ac11 [published Online First: 2012/04/25] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tudor-Locke C, Camhi SM, Troiano RP. A catalog of rules, variables, and definitions applied to accelerometer data in the National Health and Nutrition Examination Survey, 2003-2006. Prev Chronic Dis. 2012;9:E113. doi: 10.5888/pcd9.110332. [published Online First] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee I-M, Paffenbarger RS., Jr . Design of present-day epidemiologic studies of physical activity and health. In: Lee I-M, editor. Epidemiologic methods in physical activity studies. Oxford University Press; New York, NY: 2009. pp. 100–23. [Google Scholar]