Abstract
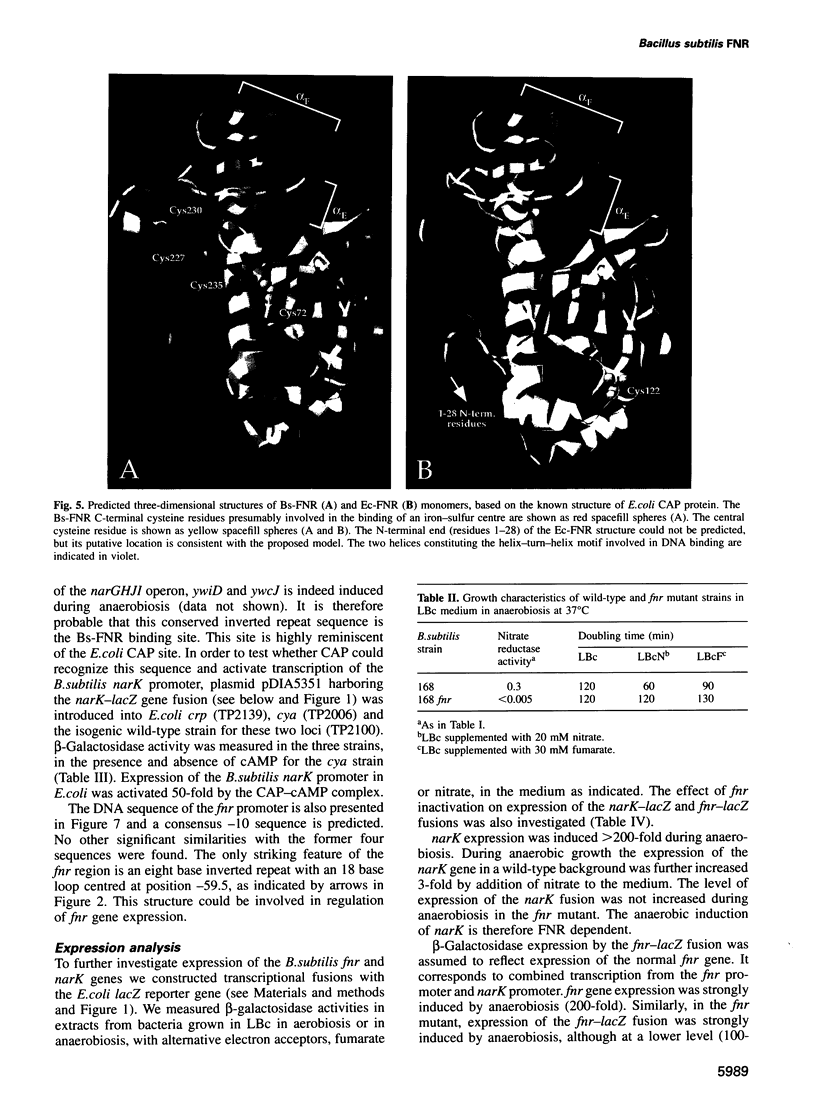
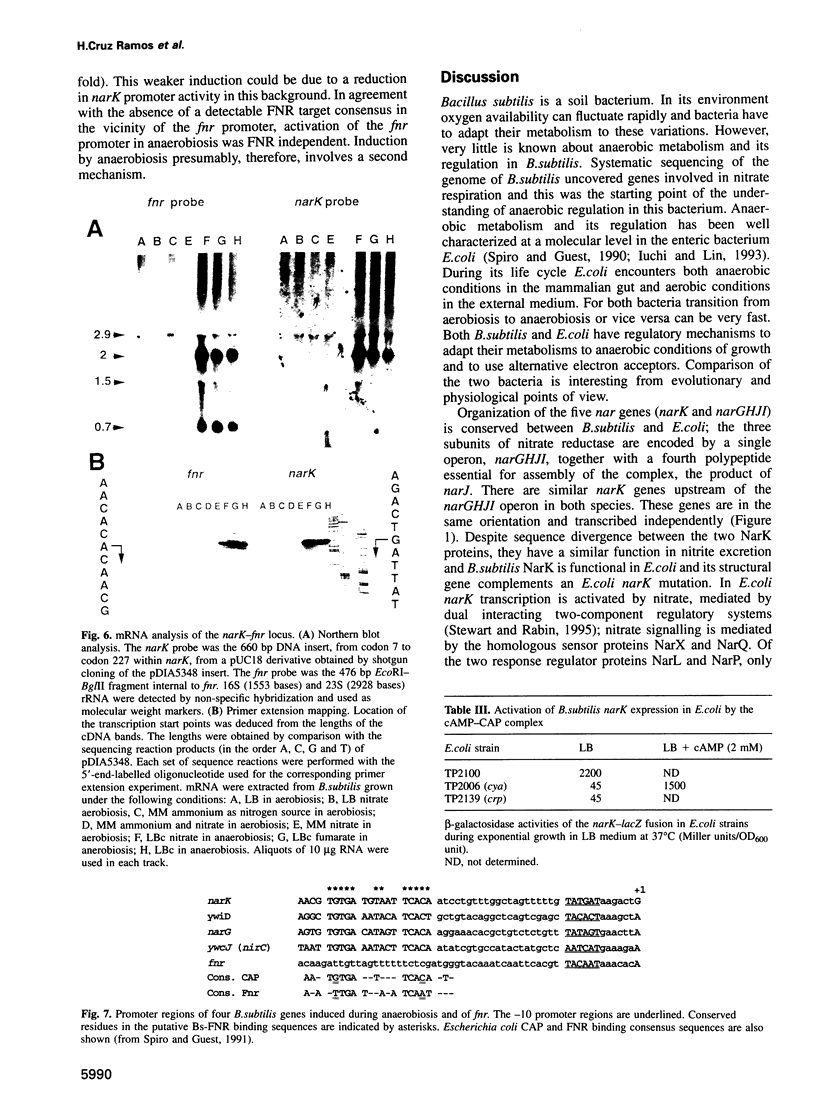
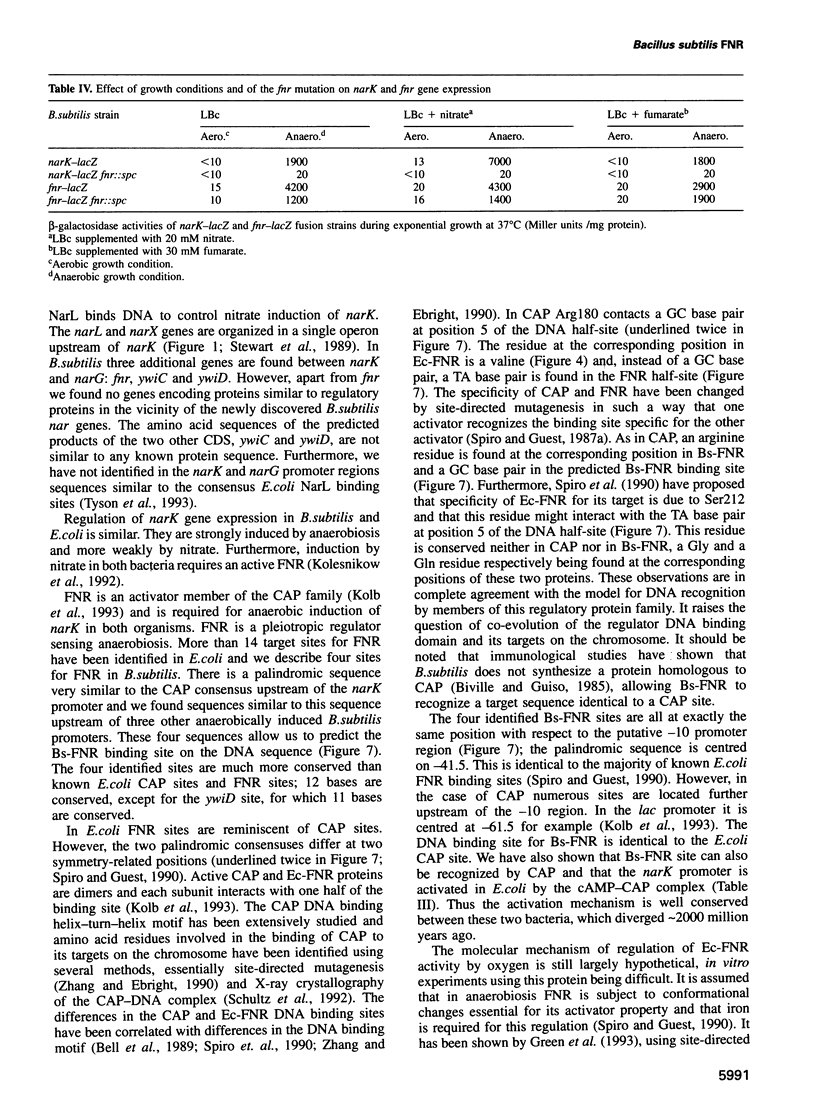
Bacillus subtilis is able to grow anaerobically using alternative electron acceptors, including nitrate or fumarate. We characterized an operon encoding the dissimilatory nitrate reductase subunits homologous to the Escherichia coli narGHJI operon and the narK gene encoding a protein with nitrite extrusion activity. Downstream from narK and co-transcribed with it a gene (fnr) encoding a protein homologous to E.coli FNR was found. Disruption of fnr abolished both nitrate and fumarate utilization as electron acceptors and anaerobic induction of narK. Four putative FNR binding sites were found in B.subtilis sequences. The consensus sequence, centred at position -41.5, is identical to the consensus for the DNA site for E.coli CAP. Bs-FNR contained a four cysteine residue cluster at its C-terminal end. This is in contrast to Ec-FNR, where a similar cluster is present at the N-terminal end. It is possible that oxygen modulates the activity of both activators by a similar mechanism involving iron. Unlike in E.coli, where fnr expression is weakly repressed by anaerobiosis, fnr gene expression in B.subtilis is strongly activated by anaerobiosis. We have identified in the narK-fnr intergenic region a promotor activated by anaerobiosis independently of FNR. Thus induction of genes involved in anaerobic respiration requires in B.subtilis at least two levels of regulation: activation of fnr transcription and activation of FNR to induce transcription of FNR-dependent promoters.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. Basic local alignment search tool. J Mol Biol. 1990 Oct 5;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Anagnostopoulos C., Spizizen J. REQUIREMENTS FOR TRANSFORMATION IN BACILLUS SUBTILIS. J Bacteriol. 1961 May;81(5):741–746. doi: 10.1128/jb.81.5.741-746.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell A. I., Gaston K. L., Cole J. A., Busby S. J. Cloning of binding sequences for the Escherichia coli transcription activators, FNR and CRP: location of bases involved in discrimination between FNR and CRP. Nucleic Acids Res. 1989 May 25;17(10):3865–3874. doi: 10.1093/nar/17.10.3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell A., Busby S. Location and orientation of an activating region in the Escherichia coli transcription factor, FNR. Mol Microbiol. 1994 Jan;11(2):383–390. doi: 10.1111/j.1365-2958.1994.tb00318.x. [DOI] [PubMed] [Google Scholar]
- Biville F., Guiso N. Evidence for the presence of cAMP, cAMP receptor and transcription termination factor rho in different gram-negative bacteria. J Gen Microbiol. 1985 Nov;131(11):2953–2960. doi: 10.1099/00221287-131-11-2953. [DOI] [PubMed] [Google Scholar]
- Blasco F., Iobbi C., Giordano G., Chippaux M., Bonnefoy V. Nitrate reductase of Escherichia coli: completion of the nucleotide sequence of the nar operon and reassessment of the role of the alpha and beta subunits in iron binding and electron transfer. Mol Gen Genet. 1989 Aug;218(2):249–256. doi: 10.1007/BF00331275. [DOI] [PubMed] [Google Scholar]
- Blasco F., Iobbi C., Ratouchniak J., Bonnefoy V., Chippaux M. Nitrate reductases of Escherichia coli: sequence of the second nitrate reductase and comparison with that encoded by the narGHJI operon. Mol Gen Genet. 1990 Jun;222(1):104–111. doi: 10.1007/BF00283030. [DOI] [PubMed] [Google Scholar]
- Calogero S., Gardan R., Glaser P., Schweizer J., Rapoport G., Debarbouille M. RocR, a novel regulatory protein controlling arginine utilization in Bacillus subtilis, belongs to the NtrC/NifA family of transcriptional activators. J Bacteriol. 1994 Mar;176(5):1234–1241. doi: 10.1128/jb.176.5.1234-1241.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung C. T., Miller R. H. A rapid and convenient method for the preparation and storage of competent bacterial cells. Nucleic Acids Res. 1988 Apr 25;16(8):3580–3580. doi: 10.1093/nar/16.8.3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossart P., Gicquel-Sanzey B. Cloning and sequence of the crp gene of Escherichia coli K 12. Nucleic Acids Res. 1982 Feb 25;10(4):1363–1378. doi: 10.1093/nar/10.4.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMoss J. A., Hsu P. Y. NarK enhances nitrate uptake and nitrite excretion in Escherichia coli. J Bacteriol. 1991 Jun;173(11):3303–3310. doi: 10.1128/jb.173.11.3303-3310.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dear S., Staden R. A sequence assembly and editing program for efficient management of large projects. Nucleic Acids Res. 1991 Jul 25;19(14):3907–3911. doi: 10.1093/nar/19.14.3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser P., Danchin A., Kunst F., Zuber P., Nakano M. M. Identification and isolation of a gene required for nitrate assimilation and anaerobic growth of Bacillus subtilis. J Bacteriol. 1995 Feb;177(4):1112–1115. doi: 10.1128/jb.177.4.1112-1115.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser P., Kunst F., Arnaud M., Coudart M. P., Gonzales W., Hullo M. F., Ionescu M., Lubochinsky B., Marcelino L., Moszer I. Bacillus subtilis genome project: cloning and sequencing of the 97 kb region from 325 degrees to 333 degrees. Mol Microbiol. 1993 Oct;10(2):371–384. [PubMed] [Google Scholar]
- Green J., Sharrocks A. D., Green B., Geisow M., Guest J. R. Properties of FNR proteins substituted at each of the five cysteine residues. Mol Microbiol. 1993 Apr;8(1):61–68. doi: 10.1111/j.1365-2958.1993.tb01203.x. [DOI] [PubMed] [Google Scholar]
- Hagen F. S., Young E. T. Effect of RNase III on efficiency of translation of bacteriophage T7 lysozyme mRNA. J Virol. 1978 Jun;26(3):793–804. doi: 10.1128/jvi.26.3.793-804.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Harborne N. R., Griffiths L., Busby S. J., Cole J. A. Transcriptional control, translation and function of the products of the five open reading frames of the Escherichia coli nir operon. Mol Microbiol. 1992 Oct;6(19):2805–2813. doi: 10.1111/j.1365-2958.1992.tb01460.x. [DOI] [PubMed] [Google Scholar]
- Higgins D. G., Bleasby A. J., Fuchs R. CLUSTAL V: improved software for multiple sequence alignment. Comput Appl Biosci. 1992 Apr;8(2):189–191. doi: 10.1093/bioinformatics/8.2.189. [DOI] [PubMed] [Google Scholar]
- Horinouchi S., Weisblum B. Nucleotide sequence and functional map of pC194, a plasmid that specifies inducible chloramphenicol resistance. J Bacteriol. 1982 May;150(2):815–825. doi: 10.1128/jb.150.2.815-825.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iuchi S., Lin E. C. Adaptation of Escherichia coli to redox environments by gene expression. Mol Microbiol. 1993 Jul;9(1):9–15. doi: 10.1111/j.1365-2958.1993.tb01664.x. [DOI] [PubMed] [Google Scholar]
- Khoroshilova N., Beinert H., Kiley P. J. Association of a polynuclear iron-sulfur center with a mutant FNR protein enhances DNA binding. Proc Natl Acad Sci U S A. 1995 Mar 28;92(7):2499–2503. doi: 10.1073/pnas.92.7.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb A., Busby S., Buc H., Garges S., Adhya S. Transcriptional regulation by cAMP and its receptor protein. Annu Rev Biochem. 1993;62:749–795. doi: 10.1146/annurev.bi.62.070193.003533. [DOI] [PubMed] [Google Scholar]
- Kolesnikow T., Schröder I., Gunsalus R. P. Regulation of narK gene expression in Escherichia coli in response to anaerobiosis, nitrate, iron, and molybdenum. J Bacteriol. 1992 Nov;174(22):7104–7111. doi: 10.1128/jb.174.22.7104-7111.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunst F., Rapoport G. Salt stress is an environmental signal affecting degradative enzyme synthesis in Bacillus subtilis. J Bacteriol. 1995 May;177(9):2403–2407. doi: 10.1128/jb.177.9.2403-2407.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marck C. 'DNA Strider': a 'C' program for the fast analysis of DNA and protein sequences on the Apple Macintosh family of computers. Nucleic Acids Res. 1988 Mar 11;16(5):1829–1836. doi: 10.1093/nar/16.5.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moszer I., Glaser P., Danchin A. Multiple IS insertion sequences near the replication terminus in Escherichia coli K-12. Biochimie. 1991 Nov;73(11):1361–1374. doi: 10.1016/0300-9084(91)90166-x. [DOI] [PubMed] [Google Scholar]
- Moszer I., Glaser P., Danchin A. SubtiList: a relational database for the Bacillus subtilis genome. Microbiology. 1995 Feb;141(Pt 2):261–268. doi: 10.1099/13500872-141-2-261. [DOI] [PubMed] [Google Scholar]
- Msadek T., Kunst F., Henner D., Klier A., Rapoport G., Dedonder R. Signal transduction pathway controlling synthesis of a class of degradative enzymes in Bacillus subtilis: expression of the regulatory genes and analysis of mutations in degS and degU. J Bacteriol. 1990 Feb;172(2):824–834. doi: 10.1128/jb.172.2.824-834.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy E. Nucleotide sequence of a spectinomycin adenyltransferase AAD(9) determinant from Staphylococcus aureus and its relationship to AAD(3") (9). Mol Gen Genet. 1985;200(1):33–39. doi: 10.1007/BF00383309. [DOI] [PubMed] [Google Scholar]
- Médigue C., Viari A., Hénaut A., Danchin A. Colibri: a functional data base for the Escherichia coli genome. Microbiol Rev. 1993 Sep;57(3):623–654. doi: 10.1128/mr.57.3.623-654.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano M. M., Yang F., Hardin P., Zuber P. Nitrogen regulation of nasA and the nasB operon, which encode genes required for nitrate assimilation in Bacillus subtilis. J Bacteriol. 1995 Feb;177(3):573–579. doi: 10.1128/jb.177.3.573-579.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noji S., Nohno T., Saito T., Taniguchi S. The narK gene product participates in nitrate transport induced in Escherichia coli nitrate-respiring cells. FEBS Lett. 1989 Jul 31;252(1-2):139–143. doi: 10.1016/0014-5793(89)80906-8. [DOI] [PubMed] [Google Scholar]
- Okamoto K., Hara S., Bhasin R., Freundlich M. Evidence in vivo for autogenous control of the cyclic AMP receptor protein gene (crp) in Escherichia coli by divergent RNA. J Bacteriol. 1988 Nov;170(11):5076–5079. doi: 10.1128/jb.170.11.5076-5079.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peakman T., Crouzet J., Mayaux J. F., Busby S., Mohan S., Harborne N., Wootton J., Nicolson R., Cole J. Nucleotide sequence, organisation and structural analysis of the products of genes in the nirB-cysG region of the Escherichia coli K-12 chromosome. Eur J Biochem. 1990 Jul 31;191(2):315–323. doi: 10.1111/j.1432-1033.1990.tb19125.x. [DOI] [PubMed] [Google Scholar]
- Peitsch M. C., Jongeneel C. V. A 3-D model for the CD40 ligand predicts that it is a compact trimer similar to the tumor necrosis factors. Int Immunol. 1993 Feb;5(2):233–238. doi: 10.1093/intimm/5.2.233. [DOI] [PubMed] [Google Scholar]
- Pikielny C. W., Rosbash M. mRNA splicing efficiency in yeast and the contribution of nonconserved sequences. Cell. 1985 May;41(1):119–126. doi: 10.1016/0092-8674(85)90066-2. [DOI] [PubMed] [Google Scholar]
- Rowe J. J., Ubbink-Kok T., Molenaar D., Konings W. N., Driessen A. J. NarK is a nitrite-extrusion system involved in anaerobic nitrate respiration by Escherichia coli. Mol Microbiol. 1994 May;12(4):579–586. doi: 10.1111/j.1365-2958.1994.tb01044.x. [DOI] [PubMed] [Google Scholar]
- Roy A., Danchin A. The cya locus of Escherichia coli K12: organization and gene products. Mol Gen Genet. 1982;188(3):465–471. doi: 10.1007/BF00330050. [DOI] [PubMed] [Google Scholar]
- Santana M., Ionescu M. S., Vertes A., Longin R., Kunst F., Danchin A., Glaser P. Bacillus subtilis F0F1 ATPase: DNA sequence of the atp operon and characterization of atp mutants. J Bacteriol. 1994 Nov;176(22):6802–6811. doi: 10.1128/jb.176.22.6802-6811.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawers R. G., Zehelein E., Böck A. Two-dimensional gel electrophoretic analysis of Escherichia coli proteins: influence of various anaerobic growth conditions and the fnr gene product on cellular protein composition. Arch Microbiol. 1988 Jan;149(3):240–244. doi: 10.1007/BF00422011. [DOI] [PubMed] [Google Scholar]
- Schaeffer P., Millet J., Aubert J. P. Catabolic repression of bacterial sporulation. Proc Natl Acad Sci U S A. 1965 Sep;54(3):704–711. doi: 10.1073/pnas.54.3.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz S. C., Shields G. C., Steitz T. A. Crystal structure of a CAP-DNA complex: the DNA is bent by 90 degrees. Science. 1991 Aug 30;253(5023):1001–1007. doi: 10.1126/science.1653449. [DOI] [PubMed] [Google Scholar]
- Spiro S., Gaston K. L., Bell A. I., Roberts R. E., Busby S. J., Guest J. R. Interconversion of the DNA-binding specificities of two related transcription regulators, CRP and FNR. Mol Microbiol. 1990 Nov;4(11):1831–1838. doi: 10.1111/j.1365-2958.1990.tb02031.x. [DOI] [PubMed] [Google Scholar]
- Spiro S., Guest J. R. Activation of the lac operon of Escherichia coli by a mutant FNR protein. Mol Microbiol. 1987 Jul;1(1):53–58. doi: 10.1111/j.1365-2958.1987.tb00526.x. [DOI] [PubMed] [Google Scholar]
- Spiro S., Guest J. R. Adaptive responses to oxygen limitation in Escherichia coli. Trends Biochem Sci. 1991 Aug;16(8):310–314. doi: 10.1016/0968-0004(91)90125-f. [DOI] [PubMed] [Google Scholar]
- Spiro S., Guest J. R. FNR and its role in oxygen-regulated gene expression in Escherichia coli. FEMS Microbiol Rev. 1990 Aug;6(4):399–428. doi: 10.1111/j.1574-6968.1990.tb04109.x. [DOI] [PubMed] [Google Scholar]
- Spiro S., Guest J. R. Regulation and over-expression of the fnr gene of Escherichia coli. J Gen Microbiol. 1987 Dec;133(12):3279–3288. doi: 10.1099/00221287-133-12-3279. [DOI] [PubMed] [Google Scholar]
- Stewart V., MacGregor C. H. Nitrate reductase in Escherichia coli K-12: involvement of chlC, chlE, and chlG loci. J Bacteriol. 1982 Aug;151(2):788–799. doi: 10.1128/jb.151.2.788-799.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart V., Parales J., Jr, Merkel S. M. Structure of genes narL and narX of the nar (nitrate reductase) locus in Escherichia coli K-12. J Bacteriol. 1989 Apr;171(4):2229–2234. doi: 10.1128/jb.171.4.2229-2234.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyson K. L., Bell A. I., Cole J. A., Busby S. J. Definition of nitrite and nitrate response elements at the anaerobically inducible Escherichia coli nirB promoter: interactions between FNR and NarL. Mol Microbiol. 1993 Jan;7(1):151–157. doi: 10.1111/j.1365-2958.1993.tb01106.x. [DOI] [PubMed] [Google Scholar]
- Weber I. T., Steitz T. A. Structure of a complex of catabolite gene activator protein and cyclic AMP refined at 2.5 A resolution. J Mol Biol. 1987 Nov 20;198(2):311–326. doi: 10.1016/0022-2836(87)90315-9. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Zhang X. P., Ebright R. H. Substitution of 2 base pairs (1 base pair per DNA half-site) within the Escherichia coli lac promoter DNA site for catabolite gene activator protein places the lac promoter in the FNR regulon. J Biol Chem. 1990 Jul 25;265(21):12400–12403. [PubMed] [Google Scholar]