Abstract
Pregnane X receptor (PXR) is a xenobiotic sensor regulating the expression of genes involved in xenobiotic detoxification and elimination. Phosphorylation plays an important role in modulating PXR activity and several phosphorylation sites have been predicted and characterized in in vitro experiments. Although PXR has been shown to be a phosphoprotein in vivo, the exact residues that are phosphorylated remain elusive. Using mass spectrometry, we identified for the first time S114, T133/135, S167, and S200 residues that are phosphorylated in PXR following an in vitro kinase assay using cyclin-dependent kinase 2. We further found that the phosphorylation at S114, T133, and T135 occurred in PXR isolated from cells. We tested the phosphodeficient and phosphomimetic mutants corresponding to all the sites identified and determined that phosphorylation at S114 attenuates the transcriptional activity of PXR, consistent with the observation that the S114D mutant displayed reduced association with the PXR-targeted DNA response element. Phosphomimetic mutations at either T133 or T135 did not show a significant change in transcriptional activity however, the dual phosphomimetic mutant T133D/T135D displayed reduced transcriptional activity. Subcellular localization studies showed a varied distribution of the mutants suggesting that the regulation of PXR is much more complex than what we can observe by just overexpressing the mutants. Thus, our results provide the first direct evidence that PXR is phosphorylated at specific residues and suggest that further investigation is warranted to fully understand the regulation of PXR by phosphorylation.
Keywords: PXR, drug metabolism, phosphorylation, phosphomimetic mutations
1. Introduction
Post-translation modification of proteins such as phosphorylation, ubiquitination, SUMOylation, methylation, glycosylation, and acetylation are mechanisms by which proteins regulate various biological processes. Phosphorylation is one such mechanism whereby a phosphate group is covalently attached to serine, threonine, or tyrosine residues by kinases to affect various aspects of protein functions such as subcellular localization, protein-protein interactions, cross-talk between pathways, and protein degradation. It is estimated that about 30% of proteins in the human cell are phosphorylated at any given time [1, 2].
The nuclear receptor pregnane X receptor (PXR) has been characterized as an important xenobiotic sensor and a regulator of genes encoding drug-metabolizing enzymes and transporters such as cytochrome P450 3A4 (CYP3A4) and multidrug resistance protein 1 (MDR1) [3]. The promoter regions of target genes are engaged by the zinc-finger containing DNA binding domain (DBD) of PXR, which is highly conserved across species and shares a similar domain structure to the other members of the nuclear receptor superfamily. The conserved ligand binding domain (LBD) of PXR is located in the carboxyl-terminal region and has a more flexible structure. PXR is activated when it associates with a wide range of structurally diverse compounds and endogenous metabolites leading to the upregulation of PXR target genes and thereby preventing the buildup of toxic byproducts in the liver [4].
PXR functions as a heterodimer with retinoid X receptor (RXR). The activity of the PXR-RXR dimer is either enhanced or downregulated via the association of PXR with co-activators such as steroid receptor coactivator-1 (SRC-1) or co-repressors such as nuclear receptor corepressor (NCoR) and silencing mediator for retinoid and thyroid receptors (SMRT) [5–7]. Several other proteins can associate with PXR to modulate transcription activity through direct binding. Transcription factors forkhead transcription receptor (FKHR/FOXO1) and p53 associate with PXR to augment or diminish PXR activity, respectively [8, 9]. To add another layer to its regulation, PXR has been predicted to undergo phosphorylation at multiple sites, and several kinases have been implicated in modulating PXR activity. In in vitro kinase assays, cAMP-dependent protein kinase (PKA), protein kinase C (PKC), glycogen synthase kinase 3 (GSK3), casein kinase II (CK2), cyclin-dependent kinase 5 (Cdk5), 70kDa ribosomal S6 kinase (p70 S6K), and cyclin-dependent kinase 2 (Cdk2) have all been shown to phosphorylate PXR [5, 10–12].
The PKA signaling pathway has been shown to modulate PXR activity in a species specific manner [10]. Overexpression of catalytically active PKA or treatment of cells with a PKA activator downregulates the ligand-induced activation of human PXR. Apart from PXR being a substrate for the kinase in vitro, the presence of PKA upregulated the phosphorylation at undefined threonine residues on PXR isolated from cells. Increasing association of PXR with the corepressor NCoR may be one potential mechanism responsible for PKA-mediated downregulation of PXR. Cdk5 was shown to attenuate both the basal and rifampicin-induced activity of wild-type PXR (PXR WT), while knockdown of Cdk5 using siRNA enhanced the activity [11]. Since Cdk5 can phosphorylate PXR in vitro, PXR may be a target for this kinase in vivo with modulation from various external stimuli such as the presence of flavonoids [13].
In the case of p70 S6K, T57 in the DBD of PXR has been predicted as the site of phosphorylation. The phosphomimetic mutant (T57D) of that site has been shown to be transcriptionally compromised with or without ligand stimulation and fails to bind to the target gene promoter [5]. While the PXR WT is predominantly present in the nucleus with a further concentration of PXR in the nuclear compartment with ligand activation, the T57D mutant displays a punctate distribution within the nucleus which may contribute to the downregulation in its activity. In another study, overexpression of Cdk2 attenuated PXR function in HepG2 cells, consistent with the observation that the phosphomimetic mutant of PXR at the putative Cdk2 site S350 (S350D) showed diminished activity [12]. The phosphodeficient mutant of S350 (S350A), on the other hand, showed partial resistance to the inhibitory effect of Cdk2 compared with PXR WT. The activity of the S350D mutant was further attenuated with the overexpression of Cdk2 kinase, suggesting that there may be additional Cdk2 mediated phosphorylation sites present that can further downregulate PXR activity once phosphorylated.
Using phosphorylation site prediction software, several other potential phosphorylation sites have been identified within the PXR protein. In a systematic approach of mutating predicted phosphorylation residues to the phosphomimetic mutant (mutating S or T to D), S8D, T90D, S208D, S305D, and T408D showed repressed basal activity, but only S8D, S208D, and T408D displayed compromised ligand-induced activation [14]. Additionally T408D displayed a punctate pattern not only in the nucleus in the presence of ligand, similar to the T57D mutant, but also within the cytoplasm, which is missing from the T57D mutant. Other phosphomimetic mutants such as Y249D and T422D have also been shown to attenuate the ligand-induced activity of PXR to varying extents, while T248D behaves as a constitutively active PXR mutant [15]. Although these in silico predicted phosphorylation sites confer a regulatory mechanism by which PXR can be modulated, the potential kinases that may target these sites have yet to be identified. While the functional significance of some of the predicted PXR phosphorylation sites has been investigated by using both the phosphomimetic and phosphodeficient mutants, the confirmation of phosphorylation at the specific amino acid residue, either in vitro or in vivo, has not been reported.
Immunopurified PXR from cells has been shown to have phosphorylated serine and threonine residues present, but the exact phosphorylation sites on PXR in vivo has remained elusive mainly due to the low levels of phospho-PXR present in the cells [10]. Furthermore, the physiological significance of the predicted sites characterized utilizing in vitro techniques has not been determined. Our goal was to identify specific PXR residues that are phosphorylated in the presence of Cdk2 and characterize the corresponding phosphomimetic mutants of those sites. Here we report several PXR phosphorylation sites that were identified for the first time using mass spectrometry (MS) from in vitro phosphorylated samples. We have also verified the presence of three of these sites in PXR protein samples prepared from cells, thus confirming that PXR does indeed exist within the cells as a phosphoprotein.
2. Materials and Methods
2.1 Materials
HEK293T and HepG2 cells were obtained from American Type Culture Collection (Manassas, VA). Rifampicin, EZ-View Red anti-Flag M2 affinity gel, histone H1 protein, Flag peptide, and anti-Flag M2 and anti-β-actin antibodies were obtained from Sigma-Aldrich (St. Louis, MO). Charcoal-dextran treated FBS was obtained from HyClone (Logan, UT); blocking buffer and anti-mouse IRDye secondary antibody were from LI-COR Biosciences (Lincoln, NE). Anti-PCNA antibody was obtained from Cell Signaling Technology (Danvers, MA), anti-PXR from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA), and anti-GAPDH from Life Technologies (Carlsbad, CA). Halt phosphatase inhibitor cocktail was obtained from Pierce Biotechnology (Rockford, IL) and protease inhibitor cocktail tablets were from Roche (Indianapolis, IN).
2.2 Cell Culture, plasmids, and transfection
All cells were maintained in a humidified environment at 37°C with 5% CO2. Cells were maintained in DMEM medium supplemented with 10% FBS, 100 units/ml penicillin, and 100 µg/ml streptomycin. pcDNA3.1 containing Flag-PXR or luciferase reporter gene under the control of the CYP3A4 promoter (CYP3A4-luc) were constructed as previously described [12, 16]. Standard molecular biology methods were used to generate various point-mutants of PXR. TK-Renilla luciferase plasmid was purchased from Promega (Madison, WI). All transfections were performed using Lipofectamine2000 (Life Technologies) according to the manufacturer’s recommendations.
2.3 In vitro kinase assay
His-tagged full length human PXR (His-PXR) was expressed in insect sf21 cells. The purified His-PXR was recovered at a low stock concentration (0.4 mg/ml) to ensure that the protein is in soluble state (Protein One, Rockville, MD). Different amount of His-PXR as indicated (1 µg or 2.5 µg) was incubated in kinase buffer with 20 ng Cdk2/cyclin E (EMD Millipore, Billerica, MA), 5 µCi [γ-32P]ATP (Perkin-Elmer, Santa Clara, CA), and 5 µM cold ATP. Kinase reaction was prepared in a final volume of 25 µl and incubated at 30°C for 30 min. His-PXR remained soluble in the kinase assays. The gel was then stained using SimplyBlue safe stain (Life Technologies) and desiccated. The dried gel was then exposed overnight in a Storage Phosphor Screen and images were attained using a Storm scanner (GE Healthcare, Pittsburgh, PA). For mass spectrometry analysis, [γ-32P]ATP was omitted, and the samples from the kinase reaction were resolved on a 4–12% gradient gel and stained using Sypro Ruby protein gel stain (Bio-Rad Life Science Research, Hercules, CA) and visualized using ImageQuant LAS 4000 (GE Healthcare).
2.4 Immunoprecipitation
HEK293T cells were transfected with either pcDNA3.1 Flag-PXR or pcDNA3.1-Flag vector using Lipofectamine2000 according to the manufacturer’s recommendation. After 48 h of incubation, cells were collected in lysis buffer containing 50 mM Tris-HCl (pH 7.4), 150 mM NaCl, 5 mM EDTA, 1% Triton X-100, protease inhibitor cocktail, and Halt phosphatase inhibitor cocktail and incubated for 20 min on ice. Lysates were centrifuged at 10,000 × g for 20 min at 4°C to remove cell debris, and the supernatant was incubated with 10 µl of EZview Red anti-Flag M2 affinity gel for 2 h at 4°C. The M2 beads with the bound proteins were then washed twice with lysis buffer, twice with TBS, and then eluted with 100 ng/µl Flag peptide in TBS for 1 h at 4°C to release the bound proteins.
2.5 Mass spectrometry analysis
The protein bands excised from Sypro Ruby gels were reduced with DTT to break disulfide bonds, and the cysteine (Cys) residues were alkylated by iodoacetamide to allow the recovery of Cys-containing peptides. The gel bands were then washed, dried down in a speed vacuum, and rehydrated with a buffer containing trypsin to allow the protease to enter the gel. Trypsin was used for overnight proteolysis. Resulting peptides were concentrated and analyzed by C18 capillary reverse-phase liquid chromatography coupled with tandem MS (LC-MS/MS) using an LTQ Orbitrap ELITE (Thermo Fisher Scientific Inc., San Jose, CA) under optimized conditions [17]. Database searches were performed using Sequest search engine in Thermo Proteome Discoverer 1.3 (v1.3.0.339) and an in-house SPIDERS software package. All matched MS/MS spectra were filtered by mass accuracy and matching scores to reduce the protein false discovery rate to ~1%. Modification sites were determined by dynamically assigning related mass addition to all possible amino acid residues during the database search. Phosphorylation site assignments were analyzed by Ascore and subjected to manual examination [18]. Modified residues were further validated by an independent manual sequencing of raw spectra and confirmed on the basis of the unambiguous assignment of characteristic site-specific fragment ions. In addition, the ion intensities of modified peptides and corresponding unmodified counterparts were compared to evaluate the stoichiometry of the modification events, assuming that the unmodified and modified peptide pairs have similar detection sensitivities.
2.6 Western blot analysis
Cells were harvested in lysis buffer and lysates were centrifuged at 10,000 × g at 4°C for 20 min to remove cell debris. The samples were boiled in sample loading buffer containing SDS, and equal amounts of samples were resolved on 4–12% SDS-PAGE gradient gel and then transferred onto a nitrocellulose membrane. The membrane was blocked and incubated with the indicated antibodies overnight at 4°C. All Western blot analyses and quantitation were performed on the Odyssey Infrared Imaging system (LI-COR Biosciences). One representative gel image was shown.
2.7 Transactivation assay
Cells were transfected with 0.2 µg of the indicated PXR expression construct or empty vector control along with 2.7 µg of CYP3A4-luc and 0.3 µg of vector expressing TK-Renilla luciferase as transfection control. For testing the effect of Cdk2 on PXR activity, either 1 µg of empty vector or 0.5 µg of Cdk2 along with 0.5 µg of cyclin E were transfected into the cells [12]. After 48 h of transfection, cells were plated in 96 -well plates containing either DMSO or the indicated amount of rifampicin in phenol-red free medium containing 10% charcoal-dextran treated FBS. The cells were incubated for 24 h before processing using the Dual-Glo Luciferase assay system (Promega). Data are reported in relative luciferase units (RLU) by normalizing the firefly luciferase activity to Renilla luciferase activity.
2.8 Electrophoretic Mobility Shift Assay (EMSA)
HEK293T cells were transfected with the indicated plasmid DNA for 48 h. Cells were collected and fractionated using the NE-PER kit (Thermo Fisher Scientific). The nuclear fractions were then incubated with biotinylated probe corresponding to the ER6 region of the CYP3A4 promoter [19]. The binding reactions were performed using the LightShift Chemiluminescent EMSA kit and using the manufacturer’s recommendations (Pierce Biotechnology). The samples were then resolved on a 6% DNA retardation gel and transferred onto a nylon membrane (Life Technologies). The membrane was exposed to 120 mJ/cm2 of UV light for 1 min to cross-link the DNA probe to the protein. The membrane was then incubated with blocking buffer followed by IRdye 680RD bound streptavidin (LI-COR Bioscience) and visualized and quantified using the Odyssey Infrared imager.
2.9 Immunofluorescence
HepG2 cells were transfected for 48 h with either Flag-tagged PXR WT or the indicated point mutants and cultured in glass-bottomed chamber slides (Lab-tek, Scotts Valley, CA). Cells were treated with either DMSO or 5µM rifampicin for 24 h. Cells were fixed using 4% paraformaldehyde solution (EMS, Hatfield, PA) followed by permeabilization with 0.5% Triton X-100 in PBS and incubated with the indicated primary antibodies overnight at 4°C. Cells were incubated with secondary antibodies for 1 h at room temperature. Cells were washed three times with PBS following each step. The slides were then mounted with coverslips using FluorSave (EMD Millipore) and imaged on Olympus IX-51. Images were analyzed using the Cell Analysis Tool (Cellomics vHCS Scan NucTrans Bioapplication) within the Cellomics vHCS Toolbox (Thermo Fisher Inc.). The ratio of the average intensity of the fluorescence signals in the nucleus and cytoplasm was determined, and the Mann-Whitney non-parametric analysis was performed using GraphPad Prism (GraphPad Software, La Jolla, CA).
3. Results
3.1 Mass spectrometric analysis of PXR reveals multiple phosphorylation sites in vitro and in cells
PXR is a substrate for Cdk2 in in vitro kinase assays (Fig. 1A); however, no specific residue on PXR has been previously confirmed by MS to be phosphorylated by protein kinases, including Cdk2 [12]. To this end, we wanted to identify phosphorylation sites that can modulate PXR activity. PXR WT was utilized in an in vitro kinase assay using Cdk2, cyclin E, and cold ATP, and the PXR was analyzed using MS to identify specific phosphorylation sites.
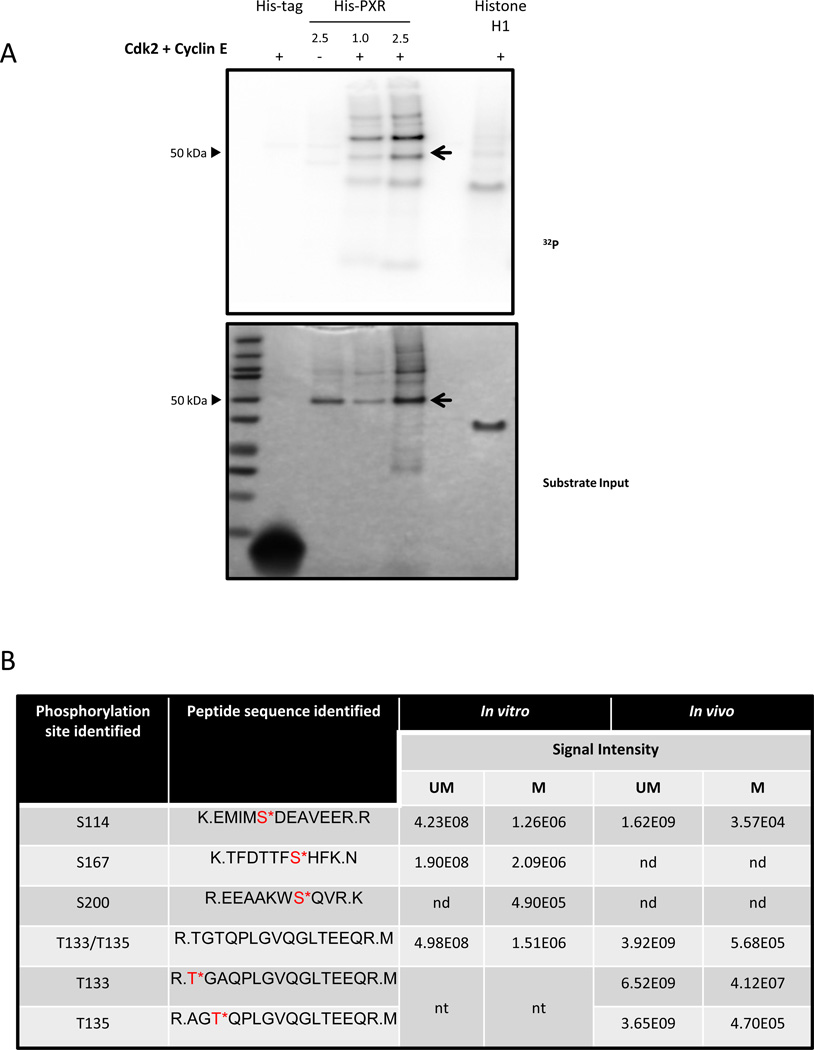
Figure 1. PXR is phosphorylated in vitro and in cells.
(A) His-PXR (1 or 2.5 µg) was incubated at 37°C for 30 min with Cdk2 and cyclin E along with [γ-32P]-ATP. Samples were resolved on a 4–12% gradient gel, and [γ-32P]-ATP incorporation was visualized using a phosphor screen (upper panel), and protein amounts in the samples were detected by SimplyBlue staining of the gel (lower panel). Histone H1 and His-tag were used as a positive and negative substrate control, respectively. The PXR band was indicated with an arrow. (B) Phosphorylation sites identified by using mass spectrometry analysis in His-PXR WT phosphorylated by Cdk2/cyclin E in vitro, and in Flag-PXR WT, Flag-PXR T133A, or Flag-PXR T135A immunoprecipitated from HEK293T cells transiently transfected with corresponding plasmid (in vivo). Serine or threonine residues followed by an asterisk (*) indicate phosphorylated residues; UM = unmodified peptide; M = phosphorylated peptide; nd = not detected; nt = not tested. Signal intensities are calculated from area under the curve for the detected precursor ions. (C) Anti-Flag immunoprecipitated samples prepared from HEK293T cells transiently overexpressing either Flag-PXR WT (lanes 1 & 2) or mutants Flag-PXR T133A (lanes 4 & 5) or Flag-PXR T135A (lanes 7 & 8) were resolved on gradient gel and stained using Sypro Ruby stain. (D) Modified peptide sequence TFDTTFS*HFK (asterisk indicating serine phosphorylation), was identified based on assignment of multiple product ions (b and y ions) in the MS/MS scan of the precursor ion at M/z 665.78. The phosphorylation of serine 167 was confirmed based on the assignment of characteristic “y-H3PO4” ions and other ions (based on a mass loss of 97.9769 Da). (E) Extracted-ion chromatography (XIC) of wild type and mutant PXR sequences showing elution times and signal intensities for the non-modified peptide as well as the singly phosphorylated peptide. Panel (a) and (b) are derived from the immunoprecipitated T133A sample and show the TGAQPLGVQGLTEEQR and T*GAQPLGVQGLTEEQR, respectively. Panel (c) and (d) are derived from the immunoprecipitated T135A sample and show the AGTQPLGVQGLTEEQR and AGT*QPLGVQGLTEEQR, respectively. Panel (e) and (f) are derived from the immunoprecipitated PXR WT sample and show the TGTQPLGVQGLTEEQR and T*GTQPLGVQGLTEEQR/ TGT*QPLGVQGLTEEQR, respectively. Relative abundance (RA) of the signals of the corresponding peptides is noted for each XIC.
From the in vitro phosphorylated PXR, we identified four phospho-peptides (Fig. 1B), of which three peptides unambiguously assigned as S114, S167, and S200 were identified as sites modified by Cdk2-mediated phosphorylation. On the fourth peptide, the specific phosphorylation site could not be distinguished between T133 and T135 due to the close proximity of these residues and the lack of detection of corresponding distinguishing characteristic fragment ions.
Cdk2 phosphorylates substrates at serine or threonine residues within the consensus sequence S/T-P-X-K/R [20, 21]. Interestingly, none of the sites identified in our assay were within the consensus sequences, possibly because the samples were prepared using an in vitro kinase assay and therefore led to non-specific phosphorylation by Cdk2 at sites that would not normally be targeted by this kinase within the cellular microenvironment, or because Cdk2 might phosphorylate sites outside the consensus sequences.
We next overexpressed Flag-tagged PXR WT or one of the PXR phospho-deficient mutants, where either T133 or T135 were mutated to alanine (T133A or T135A), in HEK293T cells and immunoprecipitated PXR using Flag M2 beads (Fig. 1C). HEK293T cells were utilized because PXR is functional in this cellular context and because of the efficiency of transfection and the high levels of expression of transfected plasmid in these cells [9]. We used the T133A and T135A point mutants to unequivocally determine which of the two threonine residues was phosphorylated. If T133 was phosphorylated, we expected to see the presence of phosphorylated peptide in samples immunoprecipitated from cells overexpressing T135A but not T133A and vice versa if T135 was phosphorylated.
Overall, the MS/MS spectra for the phosphorylated peptides were much weaker in signal than for the non-phosphorylated peptide. Since the MS/MS spectra generated from the in vitro phosphorylated PXR tended to produce stronger signals than the in vivo immunoprecipitated samples, these spectra were used to help with the manual sequencing and confirmation of MS/MS spectra from the weaker signals present in the in vivo samples. Figure 1D is a representation of the MS/MS spectra obtained for the peptide containing the S167 site, identified in the sample prepared using recombinant PXR following an in vitro kinase assay.
MS analysis of the immunoprecipitated PXR from cells (in vivo) revealed the presence of peptide containing phosphorylated S114 (Fig. 1B). To our surprise, phosphorylated peptides containing either T133/T135A (T*GAQPLGVQGLTEEQR) or T133A/T135 (AGT*QPLGVQGLTEEQR) were detected in samples from cells overexpressing either T135A or T133A, respectively, indicating that either T133 or T135 can be phosphorylated (Fig. 1B and Fig. 1E). Similar to the results obtained from the in vitro phosphorylated PXR, in the PXR WT overexpressing sample, singly, but not doubly phosphorylated peptides corresponding to the wild type T133/T135 sequence (TGTQPLGVQGLTEEQR) were detected and phosphorylation modification could be assigned to the N-terminal threonine residues (either T133 or T135) in the peptide (Fig. 1B and Fig. 1E); however, characteristic fragment ions that would allow discrimination between those two sites were not detected.
The inability to detect doubly phosphorylated peptides in the PXR WT samples does not preclude the existence of simultaneous phosphorylation at both T133 and T135 in vitro or in cells. Among other factors, detection of phospho-peptides may be limited due to low stoichiometry of phosphorylation modifications, as indicated by lower signal intensities of the phosphorylated versions of the peptides (at least two orders of magnitude lower) than of the non-phosphorylated versions (Fig. 1E and Fig. 1B). Together, the MS analysis for the first time showed that S114, S167, S200, and T133/T135 are residues phosphorylated in vitro by Cdk2, and the phosphorylation at S114, T133, and T135 was confirmed from PXR purified from cells. Whether both T133 and T135 are simultaneously phosphorylated, remains undetermined. These results also indicate that Cdk2 might phosphorylate sites outside the consensus sequences.
3.2 Phosphomimetic mutations at S114 and S167 attenuate PXR activity
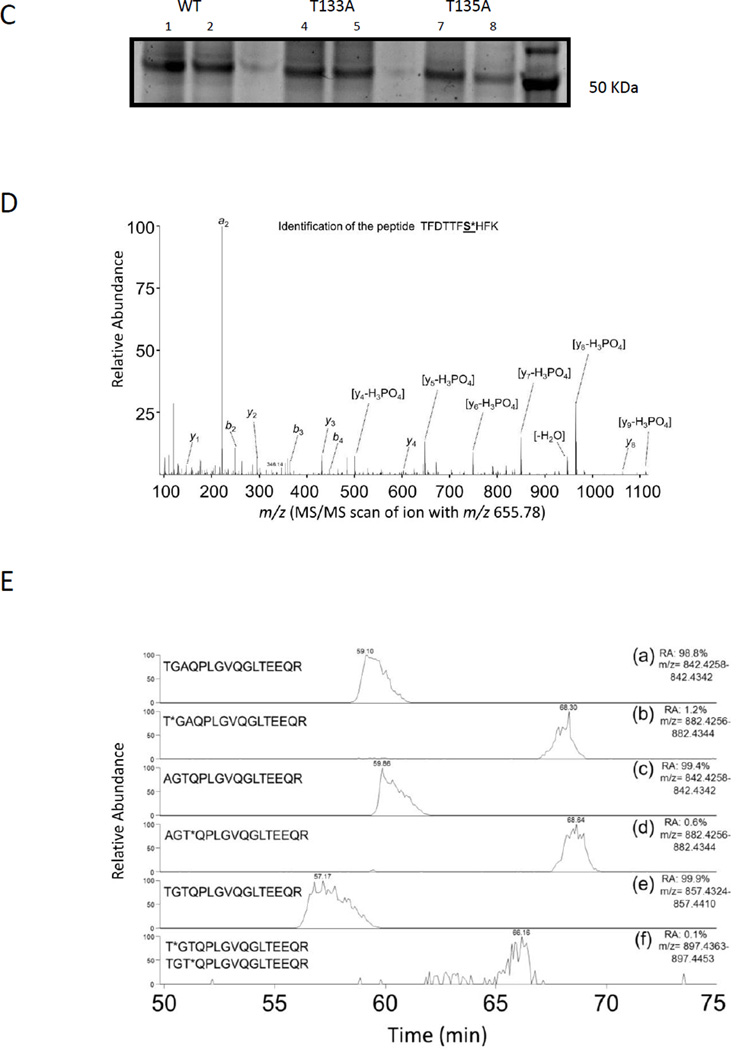
We next wanted to test the functional significance of phosphorylation by first evaluating the effect of phosphomimetic mutations of the identified phosphorylation sites on the transactivation of PXR. PXR phosphomimetic mutants for the sites identified were generated by substituting the S/T residue for the negatively charged Asp (D). Additionally, we generated phosphodeficient [S/T mutated to Ala (A)] mutants and used them as reference in a reporter assay using the activity of the PXR-regulated CYP3A4-luciferase reporter gene in HepG2 cells. The expression levels of the mutants were similar to that of PXR WT (Fig. 2A). Among the phosphodeficient mutants, S200A showed significantly higher basal activity than PXR WT but was induced with agonist to the same level as WT (Fig. 2B). All the A mutants were activated by PXR agonist rifampicin to a similar level as the PXR WT except for S114A, which was significantly downregulated, and T133A, which was significantly upregulated compared with PXR WT (Fig. 2B). Figure 2 shows a representative set of data, and the t-test was performed using data from three sets of independent experiments.
Figure 2. PXR phosphomimetic mutants show altered transcriptional activity.
(A) Expression levels of Flag-PXR point mutants utilized in reporter assays are indicated using anti-Flag antibody, and equal loading of samples is indicated using anti-β-actin antibody. Specificity of the Flag antibody is indicated in the left panel. (B–F) HepG2 cells were transiently transfected with TK-Renilla luciferase, CYP3A4-luc, and either empty vector control (EV), PXR WT, or the indicated PXR point mutant for 48 h. Cells were treated with either DMSO or the indicated concentration of rifampicin (Rif) for 24 h prior to analysis with the Dual-Glo luciferase detection system. Additionally, cells were transfected with either empty vector or Cdk2 and cyclin E in (D). Luciferase signal was normalized to corresponding Renilla signal output and reported as relative luciferase units (RLU). Error bars indicate standard errors of measurement (SEM). Results are expressed as mean ± SEM of at least 3 independent experiments, and statistical significance between samples (each PXR mutant was compared to PXR WT at each corresponding treatment condition: DMSO, 2.5 and 5 µM Rif) was determined using a Student’s t-test where * indicates statistical significance at p ≤ 0.05, ** indicates p ≤ 0.01, and *** indicates p ≤ 0.005
Among the phosphomimetic mutants, S114D and S167D both showed attenuated basal and induced activity compared with PXR WT (Fig. 2C); however, the attenuation of transcriptional activity for the S167D mutant was insignificant when treated with 5 µM rifampicin, suggesting that the attenuation of the S167D mutant might be dependent on the levels of agonists. For S114D, both the basal and rifampicin-induced activities were attenuated compared with PXR WT; however, the fold change induced by rifampicin was not attenuated (21 or 13 fold for S114D and 14 or 11 fold for PXR WT at 5 or 2.5 µM rifampicin, respectively), suggesting that the ligand binding ability of PXR LBD is unlikely attenuated as a result of the phosphomimetic mutation at S114. The extents to which S114 and S167 are downregulated by the phosphomimetic mutation were much less than that of S350D (Fig. 2C) [12, 22]. We previously showed that the reduced transcriptional activity of the S350D mutant can be further attenuated by the overexpression of Cdk2/cyclin E, suggesting that Cdk2 may phosphorylate PXR at sites other than S350 (Fig. 2D) [12]. Since S114 and S167 were both identified as in vitro phosphorylation sites by Cdk2, we wanted to test whether phosphomimetic mutation at these two residues can further attenuate the function of the S350D mutant. As shown in Figure 2C, both the double mutants S114D/S350D and S167D/S350D downregulated the activity of PXR compared with PXR WT; however, only the S114D/S350D mutant, but not S167D/S350D, showed significant attenuation in activity compared with S350D (p < 0.05).
Overexpression of Cdk2/cyclin E further attenuated the activity of all the mutants tested in Figure 2C, including the double mutants S114D/S350D and S167D/S350D, suggesting the existence of one or more unidentified Cdk2-mediated phosphorylation sites (Fig. 2D).
The phosphomimetic mutations at T133 and T135 did not alter the activity of PXR significantly; however, the double mutant T133D/S135D showed significant loss of induced activity (Fig. 2E). S200D was the only phosphomimetic mutant that exhibited a gain of activity, both at the basal level and with ligand treatment (Fig. 2F). As expected, the transcriptional activities of T133, T135D, and S200D were also negatively affected by Cdk2/cyclin E overexpression (data not shown).
3.3 PXR phosphomimetic mutants S114D, T133D, and T135D alter association with its DNA response element
As a ligand activated transcription factor, PXR engages specific response elements in the promoter regions of its target genes upon ligand binding to initiate gene expression. Studies have identified the everted repeats separated by 6 nucleotides (ER6) and direct repeats separated by 3 nucleotides (DR3) within the promoter regions of PXR target genes as PXR response elements [3, 4, 19]. Mutations within the DBD such as T57D can adversely affect DNA binding and hence the transactivation capacity of PXR [5, 14].
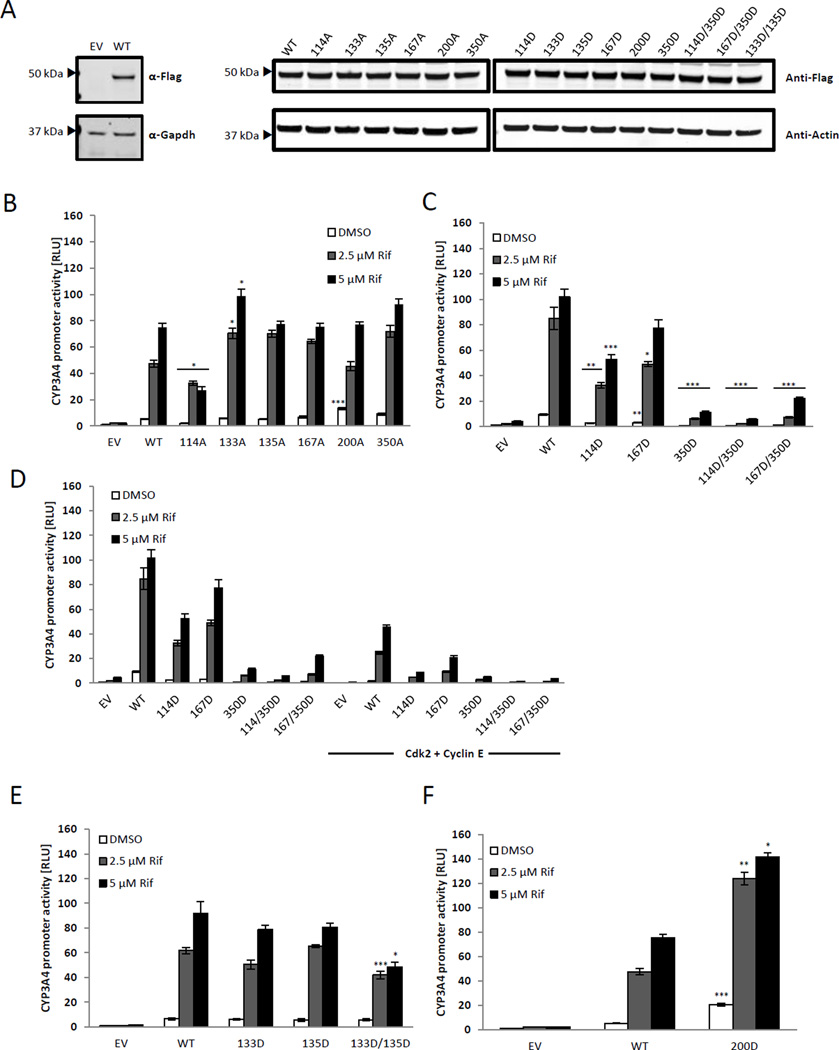
Although the LBD of PXR is mainly involved in ligand and coregulator binding, phosphomimetic mutations within the LBD at T422 and T248 have also been shown to alter association with DNA elements [15]. The phosphorylation sites we identified are within the LBD and we wanted to investigate whether phosphorylation of these sites can alter the DNA binding ability of PXR thus resulting in altered transcriptional activity. Nuclear extracts from HEK293T cells expressing one of the five phosphomimetic mutants (as indicated), PXR WT, or S350D were incubated with a biotin-labeled probe corresponding to the ER6 region of the CYP3A4 promoter. Extracts from cells expressing only the Flag-tag vector or PXR WT were used as negative and positive controls, respectively (Fig. 3A, first and second lanes). In the presence of an excess amount of the unlabeled probe, the association of PXR WT with the biotin-labeled ER6 probe was diminished, showing that PXR associates with the ER6 probe specifically (Fig. 3A, last lane). Among the mutants, the S114D, T133D, and T135D mutants exhibited decreased association with the response element compared with the WT. In the case of S114D, the attenuated association with the response element (Fig. 3A) is in agreement with its attenuated basal transcriptional activity (Fig. 2C). Since the DNA binding assay was performed in the absence of ligand treatment, it is unlikely that the attenuated association of S114D with the response element is due to the lack of ligand binding as a result of the phosphomimetic mutation. On the other hand, the S167D and S200D mutants showed a marginally decreased DNA binding compared with PXR WT.
Figure 3. Phosphomimetic mutants show differential binding to ER6.
(A) Nuclear extracts prepared from HEK293T cells expressing PXR WT or the indicated PXR mutants were incubated with biotinylated probe corresponding to the ER6 sequence (Biotin-CYP3A4-ER6). The reaction mixture was run on a native gel and the protein-probe complex was visualized using the Odyssey imager following incubation with streptavidin-IR dye. Relative intensity of the area representing the PXR-oligo complex above the free probe for each PXR mutant was normalized to the value obtained for the PXR WT. (B) Western blot analysis of the nuclear extracts prepared from HEK293T using anti-PXR antibody (α-PXR) and anti- PCNA (α-PCNA) antibody as loading control. The ratio of signal intensities of PXR to PCNA is indicated below each sample.
The T133D and T135D mutants displayed opposite behavior compared with S350D. Despite the severe impairment in its ability to activate target gene promoters, the S350D mutant could still bind to the promoter region (Fig. 3A) [12, 14, 22]. The ability of S350D to maintain DNA binding but display attenuated transcriptional activity suggests that this mutant is probably defective in other aspects of PXR function, such as a failure to interact with co-activators [14]. Both the T133D and T135D mutants show decreased association with the response element although the activities of these mutants are comparable Page 15 of 31 to that of the PXR WT (Fig. 2E). Figure 3B shows the expression levels of PXR WT and the mutant proteins in the nuclear extracts used for the EMSA.
3.4 PXR mutants show altered translocation
Many nuclear receptors shuttle between the cytoplasmic and nuclear compartments to mediate transcriptional activation of their target genes [23–25]. This dynamic movement of transcription factors, including nuclear receptors, can be regulated via phosphorylation. In the case of glucocorticoid receptors, several phosphorylation events have to occur in a sequential manner for the import of glucocorticoid receptors into the nucleus, export to the cytoplasm, and eventual degradation [24]. Several studies have shown PXR to be predominantly located in the nucleus in a variety of cell lines [5, 14, 26].
We wanted to determine whether the phosphomimetic or phosphodeficient mutants of the sites identified would affect the sub-cellular localization of PXR once activated with a known ligand, rifampicin. For this purpose, HepG2 cells were transfected with constructs expressing Flag-tagged PXR WT or mutants and treated with either DMSO or 5 µM rifampicin for 24 h. Sub-cellular localization of PXR WT and the mutants were visualized using fluorescence microscopy after Flag antibody staining (Fig. 4A). As others have reported before in different cell lines, PXR WT is localized mainly in the nucleus with or without rifampicin treatment, although we did observe some expression in the cytoplasm (Fig. 4A) [5, 14]. To determine the distribution of the various PXR mutants within the cell, the intensities of the Flag-PXR staining in the nuclear and cytoplasmic compartments were calculated and the ratios of intensities (nuclear PXR/cytoplasmic PXR) were compared with that of PXR WT (Fig. 4B).
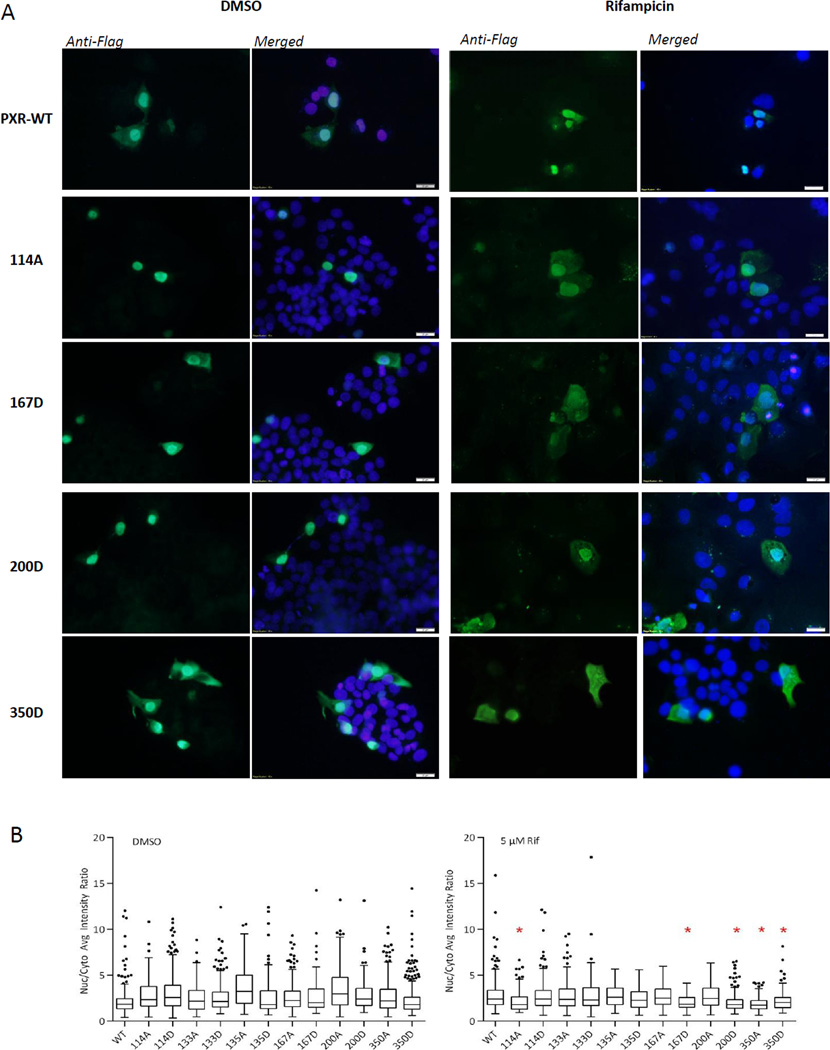
Figure 4. S114A, S167D, S200D, and S350D show cytoplasmic localization.
(A) HepG2 cells were transfected with the indicated PXR point mutant for 48 h and treated with either DMSO or 5 µM rifampicin (Rif) for 24 h. Cells were fixed, permeabilized, blocked, and then incubated with anti-Flag M2 antibody overnight. Fluorescence images were obtained at 40× magnification for detection of anti-Flag staining (green) and DAPI nuclear marker (blue). (B) The average fluorescence intensities of Flag-PXR in the nuclear and cytoplasmic compartments were calculated using the Cell Analysis Tool from Cellomics vHCS Toolbox, and the ratios of intensities (nuclear PXR/cytoplasmic PXR), represented on the y-axis, were compared with the PXR WT sample in each treatment group. Statistical analysis was performed using the Mann-Whitney non-parametric analysis, and * denotes statistical significance at p ≤ 0.05.
Without any ligand activation, the localization of most mutants is similar to PXR WT (Fig. 4B, left panel). Upon ligand activation, however, PXR phosphomimetic mutants S167D, S200D, and S350D tended to localize in the cytoplasmic compartment more than rifampicin-activated PXR WT (Figs. 4A and 4B). Compared with the transcriptional activity of PXR WT, the activity of S167D and S200D in cells treated with 5µM rifampicin was either not significantly downregulated (S167D) or induced to levels even higher than in WT (S200D). The subcellular localization patterns of S167D and S200D suggest that Page 16 of 31 the amount of protein present in the nucleus is more than sufficient for induction of PXR target genes. An interesting observation was that both the S350D and S350A mutants displayed more cytoplasmic distribution than PXR WT in the presence of rifampicin, although their transactivation activities were dramatically different (Figs. 2B and 2C). Apparently, nuclear translocation is not the roadblock for the S350A and S350D mutants, suggesting that a finer regulation, other than subcellular localization, is at play, which would be responsible for the differences in the activity of these two mutants (Figs. 4A and 4B).
PXR mutants S114D, T133D, and T135D displayed no difference in sub-cellular distribution from the WT (Fig. 4B and data not shown). However the S114A showed more localization to the cytoplasm with ligand treatment than PXR WT (Figs. 4A and 4B). This might be one reason S114A exhibits attenuated transcriptional activity with ligand treatment (Fig. 2B).
4. Discussion
Phosphorylation of certain nuclear receptors has been intensely studied as a mechanism of regulation. Glucocorticoid receptor, androgen receptor, and constitutive androstane receptor are a few examples of nuclear receptors that have been shown to be phosphorylated, which in turn affects the subcellular localization and activity of the nuclear receptor [24, 27, 28]. Detection of phosphorylation in vivo, however, is a challenging undertaking due to the low levels of phosphorylation present at a given time [29]. This has especially been true in the case of PXR, where the stoichiometry of the phosphorylated form of the endogenous protein in cells remains almost undetectable. Several groups, including ours, have undertaken in vitro approaches to identify phosphorylation sites on PXR and potential kinases or signaling pathways that may be involved in modulating PXR activity. One such kinase that has been shown to phosphorylate and attenuate PXR activity is Cdk2 [12]. Moreover, S350 a proline-directed residue, has been proposed as a site of Cdk2-dependent phosphorylation. The transcriptional activity of the phosphomimetic mutant of S350 (S350D) was further downregulated by Cdk2, suggesting the presence of additional sites that may be targeted by Cdk2, which together with S350 work to further the attenuation of PXR activity.
In the current study, our aim was to identify all sites that were phosphorylated in the presence of Cdk2 in an in vitro setting. For this purpose, we turned to MS as our tool of choice since MS has proven to be a more sensitive approach to studying low levels of phosphorylation and identifying specific phosphorylation sites [30]. For the first time, we identified five phosphorylation sites using purified PXR phosphorylated by Cdk2 in vitro. Interestingly, none of these sites were within the classical Cdk2 consensus sites. This is not surprising, however, as kinases can phosphorylate non-specifically under in vitro conditions since the regulatory mechanisms present in a cellular environment are absent, and properties of the recombinant proteins may be different from those of the proteins in cells.
In the follow-up experiment, PXR isolated from mammalian cells was analyzed using MS, and in this case three of the five sites identified from the in vitro sample were confirmed to be present in the cells. It must be noted here that the failure to detect other phosphorylated peptides in the MS analysis does not indicate that these residues are not phosphorylated. Although MS is a sensitive technique, there are several factors that might hinder its ability to detect phosphorylation, mainly the low levels of phospho-peptides present at a given time or altogether the absence of the peptide containing phosphorylated residues.
In this study, trypsin was utilized to digest PXR to generate peptides for MS analysis due to the high efficiency of digestion of this protease. In silico analysis of potential peptides generated from the trypsin-digested PXR protein, assuming 100% efficiency of digestion, revealed that the tryptic peptides containing the S350 residue were too large for efficient detection. To enhance the detection sensitivity of the S350-containing peptide, an alternative protease such as chymotrypsin may need to be used for further study. In these cases however, the amount of material used has to be greatly increased due to the low efficiency of digestion of the other proteases such as chymotrypsin.
Signals from phospho-peptides of interest can also be masked by peptides that are more abundant or cannot be detected due to incompatible LC-MS properties of the peptides such as low ionization efficiency or hydrophobicity which can affect column retention and elution. This is especially true in the case of PXR immunoprecipitated from cells. At any given time, a variety of cellular factors may influence the phosphorylation status of a protein thereby giving rise to multiple forms of the phosphorylated protein being present. Hence, it is not surprising that all the sites identified from the in vitro sample were not all detected in the sample prepared from cells. We can also assume that there are other sites in both the in vitro sample and the immunoprecipitated PXR that remained undetected due to the limitations of the detection method used here.
Of the five sites that were identified, S200D showed a gain of activity under both basal and induced conditions compared with PXR WT. Yet S200D showed no difference in localization under basal conditions and surprisingly a more cytoplasmic distribution in the presence of ligand than PXR WT. This suggests that the amount of S200D that is present within the nucleus might be constitutively more active than PXR WT and that same amount of protein is sufficient for further induction in the presence of ligand, even more so than PXR WT. A similar scenario may play out in the case of the S167D mutant. While the transcriptional activity of S167D was lower at basal levels, which is in agreement with its slightly reduced association with the PXR response element under these conditions, downregulation of S167D activity can be overcome in the presence of higher levels of ligand. Yet S167D is localized significantly more in the cytoplasmic compartment under these conditions. Hence, the amount of S167D present within the nucleus, while not basally active, is sufficient for ligand activation although higher concentrations of ligand is needed for S167D than for S200D to achieve the same level of activation. On the other hand, mutation at the S114 residue showed downregulated basal and induced activity, and although S114D did not alter its subcellular localization, its binding to the PXR response element under basal conditions was significantly reduced. The attenuated binding of S114D to the PXR response element is unlikely caused by the lack of ligand binding since the DNA binding assay was performed in the absence of ligand treatment. In addition, although both the basal and rifampicin-induced activities of S114D were attenuated compared with PXR WT, the fold change induced by rifampicin was not attenuated, suggesting that the ligand binding ability of S114D is unlikely attenuated as a result of the phosphomimetic mutation. The transcriptional activity of the S350D mutant was severely compromised, although it did associate with the PXR response element in a manner similar to the PXR WT. However, S350D showed a more cytoplasmic presence when induced with rifampicin, which is in line with its lower transcriptional activity observed under similar conditions. These cases present opposing properties and highlight the nuances of regulation through phosphorylation and hence the difficulty in understanding the contribution of multiple phosphorylation sites in a setting that is designed to study one or a few specific phosphorylated sites. The analysis of the T133 and T135 sites presents another example of this complex regulation of PXR by phosphorylation.
The basal activity of T133D and T135D were previously shown to be not significantly different from that of the PXR WT, similar to what we observed in our experiments [14]. We also did not observe much difference in the induced activity of these two mutants from that of PXR WT, although both T133D and T135D bind to the PXR response element at a level lower than that of PXR WT. However, the subcellular localization of T133D and T135D was not significantly different from that of PXR WT with or without ligand treatment. In the MS analysis of in vitro phosphorylated PXR WT, the peptide containing both the T133 and T135 residues was detected as singly phosphorylated and since these two sites were too close to each other, the assignment of phosphorylation to one or the other threonine residue was not possible. Interestingly, the corresponding singly phosphorylated peptides were detected in samples prepared from cells expressing either T133A or T135A. This suggests that either T135 or T133 can be phosphorylated, and although we did not detect the doubly phosphorylated form of the peptide, it is possible that this form does exist within the cell and was not detected in the MS analysis due to low abundance. The T133D-T135D mutant showed significantly lower induced activity than the PXR WT but did not show any altered subcellular localization (data not shown). Hence, subcellular localization did not contribute greatly to the attenuation of transcriptional properties of the T133D-T135D mutant, suggesting that other mechanisms might be responsible for its reduced transcriptional activity.
The observation that all the single and double mutants investigated in this study showed significantly more attenuated transcriptional activity when Cdk2 is overexpressed indicates that other unidentified Cdk2-mediated phosphorylation sites exist. Further work needs to be undertaken to identify all phosphorylation sites to fully understand the regulation of PXR by Cdk2. In addition, it is likely that the activity of PXR is altered by the introduction of either phosphomimetic or phosphodeficient mutations in a phosphorylation-independent manner, as observed for the S114A mutant (Fig. 2B).
In summary, our current MS analysis, for the first time, provides evidence that PXR can be phosphorylated at specific sites both in vitro and in cells. Our results also indicate that other Cdk2-mediated phosphorylation sites exist. These observations suggest that further studies are warranted to fully understand the intricate interplay between multiple phosphorylation sites in the cells in regulating PXR functions and the regulation of PXR by Cdk2 and other protein kinases.
Acknowledgment
This work was supported by the American Lebanese Syrian Associated Charities (ALSAC), St. Jude Children’s Research Hospital, National Institutes of Health National Institute of General Medical Sciences [Grant GM086415], and National Institutes of Health National Cancer Institute [Grant P30- CA21765]. The authors would like to thank the members of the Chen research laboratory and St. Jude Proteomics Facility for valuable discussions, and David Galloway (Department of Scientific Editing, St. Jude) for editing of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors have no conflicts of interest to declare.
References
- 1.Cohen P. The regulation of protein function by multisite phosphorylation--a 25 year update. Trends in biochemical sciences. 2000;25:596–601. doi: 10.1016/s0968-0004(00)01712-6. [DOI] [PubMed] [Google Scholar]
- 2.Trevino LS, Weigel NL. Phosphorylation: a fundamental regulator of steroid receptor action. Trends in endocrinology and metabolism: TEM. 2013;24:515–524. doi: 10.1016/j.tem.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kliewer SA, Moore JT, Wade L, Staudinger JL, Watson MA, Jones SA, et al. An orphan nuclear receptor activated by pregnanes defines a novel steroid signaling pathway. Cell. 1998;92:73–82. doi: 10.1016/s0092-8674(00)80900-9. [DOI] [PubMed] [Google Scholar]
- 4.Lehmann JM, McKee DD, Watson MA, Willson TM, Moore JT, Kliewer SA. The human orphan nuclear receptor PXR is activated by compounds that regulate CYP3A4 gene expression and cause drug interactions. The Journal of clinical investigation. 1998;102:1016–1023. doi: 10.1172/JCI3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pondugula SR, Brimer-Cline C, Wu J, Schuetz EG, Tyagi RK, Chen T. A phosphomimetic mutation at threonine-57 abolishes transactivation activity and alters nuclear localization pattern of human pregnane x receptor. Drug metabolism and disposition: the biological fate of chemicals. 2009;37:719–730. doi: 10.1124/dmd.108.024695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ding X, Staudinger JL. Repression of PXR-mediated induction of hepatic CYP3A gene expression by protein kinase C. Biochemical pharmacology. 2005;69:867–873. doi: 10.1016/j.bcp.2004.11.025. [DOI] [PubMed] [Google Scholar]
- 7.Johnson DR, Li CW, Chen LY, Ghosh JC, Chen JD. Regulation and binding of pregnane X receptor by nuclear receptor corepressor silencing mediator of retinoid and thyroid hormone receptors (SMRT) Molecular pharmacology. 2006;69:99–108. doi: 10.1124/mol.105.013375. [DOI] [PubMed] [Google Scholar]
- 8.Kodama S, Koike C, Negishi M, Yamamoto Y. Nuclear receptors CAR and PXR cross talk with FOXO1 to regulate genes that encode drug-metabolizing and gluconeogenic enzymes. Molecular and cellular biology. 2004;24:7931–7940. doi: 10.1128/MCB.24.18.7931-7940.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elias A, Wu J, Chen T. Tumor suppressor protein p53 negatively regulates human pregnane X receptor activity. Molecular pharmacology. 2013;83:1229–1236. doi: 10.1124/mol.113.085092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lichti-Kaiser K, Xu C, Staudinger JL. Cyclic AMP-dependent protein kinase signaling modulates pregnane x receptor activity in a species-specific manner. The Journal of biological chemistry. 2009;284:6639–6649. doi: 10.1074/jbc.M807426200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dong H, Lin W, Wu J, Chen T. Flavonoids activate pregnane x receptor-mediated CYP3A4 gene expression by inhibiting cyclin-dependent kinases in HepG2 liver carcinoma cells. BMC biochemistry. 2010;11:23. doi: 10.1186/1471-2091-11-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin W, Wu J, Dong H, Bouck D, Zeng FY, Chen T. Cyclin-dependent kinase 2 negatively regulates human pregnane X receptor-mediated CYP3A4 gene expression in HepG2 liver carcinoma cells. The Journal of biological chemistry. 2008;283:30650–30657. doi: 10.1074/jbc.M806132200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zapata-Torres G, Opazo F, Salgado C, Munoz JP, Krautwurst H, Mascayano C, et al. Effects of natural flavones and flavonols on the kinase activity of Cdk5. Journal of natural products. 2004;67:416–420. doi: 10.1021/np034011s. [DOI] [PubMed] [Google Scholar]
- 14.Lichti-Kaiser K, Brobst D, Xu C, Staudinger JL. A systematic analysis of predicted phosphorylation sites within the human pregnane X receptor protein. The Journal of pharmacology and experimental therapeutics. 2009;331:65–76. doi: 10.1124/jpet.109.157180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doricakova A, Novotna A, Vrzal R, Pavek P, Dvorak Z. The role of residues T248, Y249 and T422 in the function of human pregnane X receptor. Archives of toxicology. 2013;87:291–301. doi: 10.1007/s00204-012-0937-9. [DOI] [PubMed] [Google Scholar]
- 16.Luo G, Cunningham M, Kim S, Burn T, Lin J, Sinz M, et al. CYP3A4 induction by drugs: correlation between a pregnane X receptor reporter gene assay and CYP3A4 expression in human hepatocytes. Drug metabolism and disposition: the biological fate of chemicals. 2002;30:795–804. doi: 10.1124/dmd.30.7.795. [DOI] [PubMed] [Google Scholar]
- 17.Xu P, Duong DM, Peng J. Systematical optimization of reverse-phase chromatography for shotgun proteomics. Journal of proteome research. 2009;8:3944–3950. doi: 10.1021/pr900251d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beausoleil SA, Villen J, Gerber SA, Rush J, Gygi SP. A probability-based approach for highthroughput protein phosphorylation analysis and site localization. Nature biotechnology. 2006;24:1285–1292. doi: 10.1038/nbt1240. [DOI] [PubMed] [Google Scholar]
- 19.Goodwin B, Redinbo MR, Kliewer SA. Regulation of cyp3a gene transcription by the pregnane x receptor. Annual review of pharmacology and toxicology. 2002;42:1–23. doi: 10.1146/annurev.pharmtox.42.111901.111051. [DOI] [PubMed] [Google Scholar]
- 20.Nigg EA. Cellular substrates of p34(cdc2) and its companion cyclin-dependent kinases. Trends in cell biology. 1993;3:296–301. doi: 10.1016/0962-8924(93)90011-o. [DOI] [PubMed] [Google Scholar]
- 21.Holmes JK, Solomon MJ. A predictive scale for evaluating cyclin-dependent kinase substrates. A comparison of p34cdc2 and p33cdk2. The Journal of biological chemistry. 1996;271:25240–25246. doi: 10.1074/jbc.271.41.25240. [DOI] [PubMed] [Google Scholar]
- 22.Sugatani J, Uchida T, Kurosawa M, Yamaguchi M, Yamazaki Y, Ikari A, et al. Regulation of pregnane X receptor (PXR) function and UGT1A1 gene expression by posttranslational modification of PXR protein. Drug metabolism and disposition: the biological fate of chemicals. 2012;40:2031–2040. doi: 10.1124/dmd.112.046748. [DOI] [PubMed] [Google Scholar]
- 23.Maglich JM, Parks DJ, Moore LB, Collins JL, Goodwin B, Billin AN, et al. Identification of a novel human constitutive androstane receptor (CAR) agonist and its use in the identification of CAR target genes. The Journal of biological chemistry. 2003;278:17277–17283. doi: 10.1074/jbc.M300138200. [DOI] [PubMed] [Google Scholar]
- 24.Wang Z, Frederick J, Garabedian MJ. Deciphering the phosphorylation "code" of the glucocorticoid receptor in vivo. The Journal of biological chemistry. 2002;277:26573–26580. doi: 10.1074/jbc.M110530200. [DOI] [PubMed] [Google Scholar]
- 25.Lu Q, Ebling H, Mittler J, Baur WE, Karas RH. MAP kinase mediates growth factor-induced nuclear translocation of estrogen receptor alpha. FEBS letters. 2002;516:1–8. doi: 10.1016/s0014-5793(02)02432-8. [DOI] [PubMed] [Google Scholar]
- 26.Saradhi M, Sengupta A, Mukhopadhyay G, Tyagi RK. Pregnane and Xenobiotic Receptor (PXR/SXR) resides predominantly in the nuclear compartment of the interphase cell and associates with the condensed chromosomes during mitosis. Biochimica et biophysica acta. 2005;1746:85–94. doi: 10.1016/j.bbamcr.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 27.Chen S, Gulla S, Cai C, Balk SP. Androgen receptor serine 81 phosphorylation mediates chromatin binding and transcriptional activation. The Journal of biological chemistry. 2012;287:8571–8583. doi: 10.1074/jbc.M111.325290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Osabe M, Negishi M. Active ERK1/2 protein interacts with the phosphorylated nuclear constitutive active/androstane receptor (CAR; NR1I3), repressing dephosphorylation and sequestering CAR in the cytoplasm. The Journal of biological chemistry. 2011;286:35763–35769. doi: 10.1074/jbc.M111.284596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Steen H, Jebanathirajah JA, Rush J, Morrice N, Kirschner MW. Phosphorylation analysis by mass spectrometry: myths, facts, and the consequences for qualitative and quantitative measurements. Molecular & cellular proteomics : MCP. 2006;5:172–181. doi: 10.1074/mcp.M500135-MCP200. [DOI] [PubMed] [Google Scholar]
- 30.Wu R, Haas W, Dephoure N, Huttlin EL, Zhai B, Sowa ME, et al. A large-scale method to measure absolute protein phosphorylation stoichiometries. Nature methods. 2011;8:677–683. doi: 10.1038/nmeth.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]