Abstract
Background
Females exhibit more rapid escalation of cocaine use and enhanced cocaine-taking behavior as compared to males. While ovarian hormones likely play a role in this increased vulnerability, research has yet to examine the role of estradiol in affecting the behavioral and neurological response to cocaine in a brain region- and sex-specific way.
Methods
First, we examined stereotypy and locomotor sensitization after repeated cocaine administration (10 mg/kg i.p.) in intact (SHAM) and castrated (CAST) males, and ovariectomized (OVX) females treated with 5 μg estradiol benzoate (EB) or vehicle (OIL). Next, we used in vivo microdialysis to examine the effects of acute EB treatment on cocaine-induced DA in the regions mediating the display of these behaviors (i.e., the dorsolateral striatum, DLS; and the nucleus accumbens, NAc; respectively).
Results
We find that EB enhances sensitization of cocaine-induced stereotypy in OVX females after 12 days of cocaine treatment, and after a 10-day withdrawal. Similarly, the OVX/EB females show enhanced locomotor sensitization compared to the other three groups on the same days. Using in vivo microdialysis to assess the neurochemical response, we find that EB rapidly enhances cocaine-induced DA in DLS dialysate of OVX females but not CAST males, and has no effect in NAc of either sex.
Conclusions
With these experiments, we show that there are sex differences in the effects of estradiol to preferentially enhance the response to cocaine in the DLS over the NAc in females, which may contribute to the preferential sensitization of stereotypy in females.
Keywords: dopamine, microdialysis, behavioral sensitization, drug abuse, sex differences
1. INTRODUCTION
A variety of factors contribute to an individual’s risk for developing drug addiction or compulsive drug taking in animal models. High impulsivity or novelty-seeking predisposes an individual towards an addictive phenotype, whereas individuals demonstrating low impulsivity and novelty-seeking are at reduced risk for developing addiction (Belin et al., 2008, Cummings et al., 2011). There are also striking sex differences in drug taking: women escalate through the stages of addiction more rapidly (Kosten et al., 1993), have shorter periods of abstinence (Lynch et al., 2002), and are more responsive to cocaine cues as compared to men (Robbins et al., 1999). This sex difference is also widely noted in laboratory rodents, with females acquiring drug taking behavior more rapidly and demonstrating an enhanced motivation for cocaine compared to males (Hu et al., 2004, Jackson et al., 2006, Larson et al., 2005, Roberts et al., 1989, Zhao and Becker, 2010).
In both clinical and preclinical settings, gonadal hormones have been found to play a key role in this sexual dimorphism. Women report an increased “high” with smoked cocaine during the follicular phase of the menstrual cycle when estradiol is elevated (Sofuoglu et al., 1999), and female rats work harder to obtain an infusion of cocaine and acquire cocaine-taking more rapidly when estradiol is high (Becker and Hu, 2008, Hu et al., 2004, Roberts et al., 1989). Together, these data demonstrate the importance of estradiol in the development of drug dependence and compulsive drug taking in females.
The nucleus accumbens (NAc) and dorsolateral striatum (DLS) are thought to be crucial sites in the transition from drug taking to compulsive drug seeking, where the NAc is important for acquisition and escalation, and the DLS is necessary for compulsive or habitual drug taking (Belin-Rauscent et al., 2012, Willuhn et al., 2012). When drug intake transitions from casual use to compulsive drug abuse, DA transmission becomes enhanced in the DLS and reduced in the NAc (Willuhn et al., 2012). Importantly, ovarian hormones can modulate the neurochemical response to psychomotor stimulants by enhancing DA neurotransmission in reward-associated brain regions. Pretreatment of ovariectomized (OVX) female rats with estradiol 30 min prior to the psychostimulant amphetamine enhances DA in dialysate of DLS (Becker, 1990, Castner et al., 1993), and administration of cocaine to intact (i.e., cycling) females results in increased DA release and reduced DA clearance in dorsal striatum compared to OVX females (Walker et al., 2012). While it is clear from these data that ovarian hormones enhance psychostimulant-induced DA neurotransmission in DLS, it has yet to be determined whether it is estradiol alone that induces rapid changes in cocaine-induced DA in DLS, or how estradiol affects DA neurotransmission in the NAc—a phenomenon that we hypothesize is integral for expression of sex differences in the progression from drug use to abuse.
Cocaine sensitization is the escalation in behavioral response exhibited by an animal after repeated exposures to the same dose of the drug (Robinson and Becker, 1986), and is thought to be indicative of cocaine-induced neural changes that are significant in the development of drug abuse, addiction, and craving (Robinson and Berridge, 1993). The behaviors that are expressed during psychostimulant-induced sensitization (e.g., rotational behavior, locomotor hyperactivity, and stereotyped behavior) tap into the sensitization of DA neurotransmission in different regions of the mesotelencephalic dopaminergic circuitry; while dopaminergic activity in NAc and DLS drive locomotor behavior (Delfs et al., 1990), it is DA action in the DLS that is primarily responsible for the expression of psychostimulant-induced stereotypy (Dickson et al., 1994, Perrot et al., 2009). The influence of ovarian hormones on various aspects of psychomotor-induced sensitization has been studied (Hu and Becker, 2003, Sell et al., 2000, Walker et al., 2012), but again the role of estradiol in modulating these DLS- and NAc-driven behaviors in the context of cocaine-sensitization had not been studied in a sex-specific way.
We hypothesize that a female’s vulnerability for drug addiction is enhanced due in part to estradiol’s selective action in the female DLS, which can rapidly enhance DA neurotransmission and facilitate the transition from recreational to compulsive drug use by altering behavioral outcomes. To this end, we separately examined the two facets of behavioral sensitization (i.e., locomotor and stereotyped behavior) that are modulated primarily by the NAc and DLS respectively, and found that estradiol’s preferential enhancement of DLS DA is reflected in enhanced cocaine-induced stereotyped behavior as opposed to locomotor behavior in females. To determine if EB has selective, sex-specific effects in the DLS that may contribute to these changes in behavior, we used in vivo microdialysis in gonadectomized male and female rats to demonstrate that acute EB selectively affects the cocaine-induced increase in dialysate DA from the DLS, but not the NAc, in female but not male rats.
2. METHODS
2.1. Animals
Male and female Sprague Dawley rats (Charles River Breeding Laboratory; Portage, MI) 50-55 days of age were maintained on a 14:10 L:D cycle (lights on at 8:00 AM), housed in same-sex pairs in standard laboratory cages, and allowed free access to water and phytoestrogen free rat chow (2014 Teklad Global, 14% protein rodent maintenance diet, Harlan rat chow; Harlan Teklad, Madison, WI). All procedures were carried out in accordance with the National Institutes of Health guidelines on laboratory animal use and care, using a protocol approved by the University Committee on Use and Care of Animals.
2.2. Experiment 1: Cocaine sensitization
Fifty-three females were ovariectomized (OVX) and 57 males were either castrated (CAST) or received a sham (SHAM) operation under 2% isoflurane anesthesia. Briefly, OVX was performed via a single dorsal incision along the midline below the ribs, where the ovary from each side is externalized and then removed. For CAST, the testes are removed via a ventral approach in which the scrotal sac is opened and the testes are visualized and removed. Wounds are closed via 11 mm wound clips, and animals are given one week to recover. Additional details regarding surgeries are published in Hu and Becker, 2003.
On test days, animals were placed in testing chambers (heavy circular plastic tubs (20W x 42L x 21H cm) where all testing was videotaped. After a 30 min habituation they received either 5 μg estradiol benzoate ((EB), Sigma Aldrich, MO, in 0.1 ml peanut oil) or 0.1 ml peanut oil only (OIL), followed 30 min later by either cocaine (10 mg/kg, i.p., in 0.9% sterile saline) or saline vehicle. Testing concluded 1 hr after cocaine/saline administration. Cocaine exposure and EB/OIL treatment continued 4 days a week for 3 consecutive weeks for a total of 12 days followed by a 10 day withdrawal from drug and hormone treatments. After withdrawal, animals were placed in the testing chambers for Challenge Day, when all animals (cocaine and saline) received OIL, and 30 min later, cocaine (10 mg/kg, i.p.).
Behavior was scored from the videotapes that were analyzed for individual stereotyped movements and horizontal locomotion (the cage was divided into quadrants on the video screen; when the animal’s hindquarters crossed the line a quadrant crossing was scored). Tapes were scored for 60 min on Day 1, Day 12, and Challenge Day. Locomotor behavior (i.e., number of quadrants crossings) and stereotypy (i.e., total number of stereotyped headbobs and forelimb movements/hr) were analyzed by observers blind to animal treatments. All testing was conducted between 12:00 and 3:00PM (during the light phase).
Previous experiments have not found an effect of acute EB on locomotor behavior and stereotypy in castrated males (Becker et al., 2001, Castner et al., 1993), and no difference between CAST and intact males on cocaine-induced rotational behavior (Hu and Becker, 2003) so we did not include a CAST group treated with acute EB. Instead, we chose to include intact males that received a sham operation in addition to the CAST group. All males received OIL. The final group assignments and numbers were: OVX/EB coc (n = 18), OVX/OIL coc (n = 15), CAST/OIL coc (n = 17), SHAM/OIL coc (n = 19), OVX/EB sal (n = 10), OVX/OIL sal (n = 10), CAST/OIL sal (n = 11), SHAM/OIL sal (n = 10).
2.3. Experiment 2: In vivo microdialysis
Twenty females were OVX and 20 males were CAST as described above. Two weeks later animals were anesthetized using ketamine (40 mg/kg i.p.; Henry Schein Animal Health, Dublin, OH) and dexmedetomidine (0.3 mg/kg i.p.; Henry Schein Animal Health, Dublin, OH) and guide cannulae (CMA/Microdialysis AB, Chelmsford, MA) were implanted bilaterally: one was aimed at NAc (8 mm length; coordinates from bregma: 1.8 AP, +/- 1.4 ML, from bottom of skull: -1.0 DV), and one was aimed at DLS (5 mm length; 0.2 AP, +/- 3.0 ML, -1.2 DV) on the contralateral side. The cannulae were fixed in place using skull screws and dental cement, and a stylet was inserted to maintain patency. Dialysis probes (CMA/11, 2 mm and 4 mm; CMA/Microdialysis AB, Chelmsford, MA) were tested for in vitro recovery <1 week prior to the experiment, as described previously (Becker and Rudick, 1999). Only probes with greater than 10% (for 2 mm probes) and 20% (for 4 mm probes) recovery were used.
Eighteen hr prior to testing, probes were inserted into the guide cannulae as described previously (Jenkins and Becker, 2003). Samples were collected every 10 min. After three baseline samples, animals received an s.c. injection of either 5 μg EB or OIL. Collection continued for 30 min, after which animals received an i.p. injection of 10 mg/kg cocaine in 0.9% saline. Sample collection continued for an additional 90 min. DA in dialysate was determined by HPLC-EC (Becker and Rudick, 1999).
2.4. Histology
After microdialysis, animals received an overdose of FatalPlus (Vortech Pharmaceuticals, Dearborn, MI) and were decapitated. Brains were fixed in 4% paraformaldehyde, sectioned at 60 μm, and stained with cresyl violet to determine probe placement. Data from animals with probe placements outside the NAc or DLS were excluded. Probes were considered in the DLS if they were >2.5 mm lateral from midline. Data from the striatum of one animal was excluded for having a probe too medial. Due to the size and placement of the NAc probes, measurements from NAc included readings from the core and shell simultaneously. Data were excluded for four animals because of a leak in the probe or trouble with the equipment on the day of testing. The final numbers in each group were as follows, for DLS: CAST/EB, n = 8; CAST/OIL, n = 8; OVX/EB, n = 7; OVX/OIL, n = 7; and for NAc: CAST/EB, n = 9; CAST/OIL, n = 7; OVX/EB, n = 7; OVX/OIL, n = 7.
2.5. Statistics
Statistical analyses were performed using IBM SPSS Statistics 19. Data were analyzed using repeated measures ANOVA. Post hoc tests with Bonferroni correction were performed when significant main effects were present.
3. RESULTS
3.1. Effect of gonadal hormones on cocaine-induced stereotypy
A repeated measures ANOVA was conducted for stereotyped behavior to examine the effect of group and cocaine treatment on the display of stereotypy over days. There was a main effect of day [F (2, 180) = 102.51; p < 0.001], and a significant interaction between day and group [F (6,180) = 2.321; p = 0.035], and day and cocaine treatment [F (2, 180) = 9.343; p = 0.001]. On Day 1, there were no differences in stereotypy among the cocaine-treated groups upon their initial exposure to cocaine. Additionally, as illustrated in Figure 1, all groups exhibited greater stereotyped behavior on Day 12 than on Day 1 (Fig 1A), with the OVX/EB group exhibiting enhanced sensitization to cocaine compared to the other three groups (OVX/OIL, p = 0.014; CAST/OIL, p = 0.043; SHAM/OIL, p = 0.008). As expected, the saline-treated groups did not show significant changes in stereotypy from Day 1 to Day 12 (Figure 1A, dashed lines).
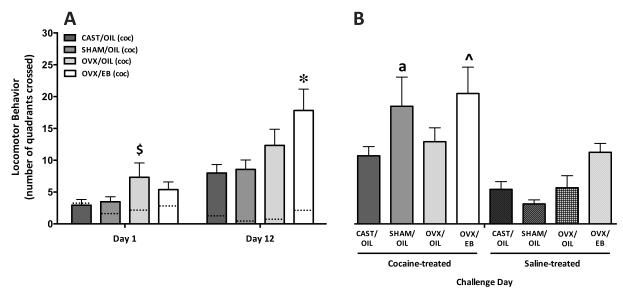
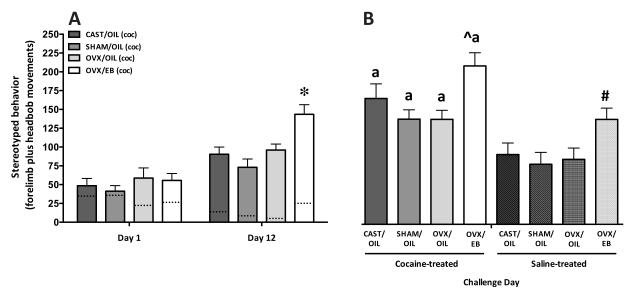
Figure 1. The effect of hormones on and sex differences in the number of stereotyped forelimb movements plus headbobs after 10 mg/kg cocaine.
A) The mean number of stereotyped behaviors (+/−SEM) exhibited in the 60 min following cocaine administration, after 1 (left) and 12 (right) days of cocaine exposure. (The dashed lines within each bar represent the means for control groups receiving saline only.) On Day 1, there are no differences in the number of stereotyped behaviors exhibited by the cocaine or saline treated groups. Sensitization (stereotyped behaviors on Day 12 minus Day 1) was seen in all cocaine-treated groups and the OVX/EB females exhibited enhanced stereotypy compared to the other three cocaine-treated groups (* p < 0.05). B) Challenge Day. The number of stereotyped behaviors exhibited after a challenge dose of cocaine by animals receiving 12 days of cocaine (left side; solid bars) or saline (right side; notched bars). The cocaine-treated animals exhibited significantly more stereotyped behaviors than did their saline-treated counterparts and in comparison to their behavior on Day 12 (a; p < 0.05). Among the animals previously treated with cocaine, OVX/EB females showed the greatest increase in stereotypy compared to the other cocaine-treated groups (^ p < 0.05). When comparing the display of stereotyped behaviors within the saline-treated animals that received cocaine for the first time on Challenge Day, OVX/EB animals showed more stereotyped behaviors than SHAM/OIL and OVX/OIL (# p < 0.05).
On Challenge Day (Fig. 1B), all animals were pre-treated with OIL 30 min before cocaine. When stereotyped behaviors were examined on Challenge Day, there was a significant main effect of prior treatment (cocaine/saline) [F (1, 102) = 29.705, p < 0.001], and of group [F (3, 102) = 6.400, p = 0.001]. Post hoc analyses indicated that all four groups previously treated with cocaine exhibited significantly more stereotyped behaviors than did their respective saline-treated controls (OVX/EB, p = 0.003; OVX/OIL, p = 0.032; SHAM/OIL, p = 0.012 and CAST/OIL, p = 0.002), demonstrating sensitization of stereotypy independent of any environmental conditioning effects.
Interestingly, in females previously treated with saline only (and for whom Challenge Day was their first exposure to cocaine), prior EB treatment lead to an enhanced behavioral response to cocaine on Challenge Day without additional EB treatment (Fig. 1B, right), with OVX/EB (sal) females showing a greater stereotyped response compared to SHAM/OIL (sal) and OVX/OIL (sal) (p = 0.029 and p = 0.05, respectively). Furthermore, on Challenge Day, cocaine produced a greater stereotyped response in all cocaine-treated groups relative to their respective responses on Day 12 (p < 0.01 for all groups), demonstrating an incubation effect on the stereotyped response to cocaine.
3.2. Effect of gonadal hormones on cocaine-induced locomotion
On Day 1, OVX/OIL females demonstrated significantly more locomotor behavior (Fig. 2A, left) than did CAST/OIL males (p = 0.004) and SHAM/OIL males (p = 0.01). The repeated measures ANOVA for locomotor behavior shows a main effect of day [F (2, 180) = 16.085; p < 0.001] and a significant day X cocaine treatment interaction [F (2, 180) = 5.443, p = 0.005]. When examining the degree of locomotor sensitization in the cocaine-treated groups (Day 12 minus Day 1), as with stereotyped behaviors, the OVX/EB females sensitized to a greater extent than the other three groups (OVX/OIL, p = 0.025; CAST/OIL, p = 0.042; SHAM/OIL, p = 0.038), as demonstrated by a greater increase in quadrant crosses after repeated cocaine treatment (Figure 2). The saline groups did not sensitize or show any group differences on these days (dashed lines Figure 2A).
Figure 2. The effect of hormones on and sex differences in locomotor behavior (quadrant crossings) after 10 mg/kg cocaine.
A) The mean number of quadrants crossed (+/−SEM) in 60 min on Day 1 and Day 12 of treatment. (The dashed lines within the bars represent the means for the saline-treated groups.) On Day 1, OVX/OIL females exhibited higher locomotor activity compared to CAST/OIL and SHAM/OIL males ($ p < 0.05). Sensitization (stereotyped behaviors on Day 12 minus Day 1) was seen in all cocaine-treated groups. The OVX/EB females show a greater increase in locomotor behavior than the other three groups (* p < 0.05). B) The number of quadrants crossed after a challenge dose of cocaine by animals receiving cocaine (left side; solid bars) or saline treatment (right side; notched bars) for the first 12 days. On Challenge Day only the SHAM/OIL males previously treated with cocaine exhibited sensitization relative to saline-treated counterparts (a, p < 0.05). Within the animals previously treated with cocaine, OVX/EB females showed the greatest increase in locomotion compared to the CAST group (^ p < 0.05). There were no differences in Challenge Day locomotion among the saline-treated groups.
When locomotor behavior was compared on Challenge Day, as shown in Figure 2B, on average, the groups previously treated with cocaine showed significantly greater locomotor behavior than the saline-treated groups [F (1, 102) = 15.3, p < 0.001]. On individual comparisons, however, only the SHAM/OIL males were different from their saline-treated counterparts (p = 0.001). These results suggest that sensitization of locomotor behavior in the other groups was dependent on environmental conditioning effects, rather than cocaine-specific effects.
Within cocaine-treated animals on Challenge Day (Fig. 2B, left), OVX/EB females demonstrated greater locomotor activity than the CAST/OIL group only (p = 0.018). Among the saline treated animals that received their first exposure to cocaine on the Challenge Day (Fig. 2B, right), there were no significant differences among the four groups. For the groups previously treated with cocaine (Fig. 2B left), the challenge dose of cocaine produced enhanced locomotion on Challenge Day compared to Day 12 in SHAM/OIL males only (p < 0.001).
3.3. Neurochemical Responses
We saw robust sensitization of stereotyped behaviors that was enhanced by EB in OVX females, but the effect of EB on locomotor behavior in the same animals was related to non-specific conditioning effects, since there was no difference from the saline-treated controls. We reasoned that this might be due to differential effects of EB in the DLS vs. NAc in female animals. So, as an initial test of this hypothesis, we compared the acute dopaminergic response to cocaine in gonadectomized males and females treated acutely with EB or OIL.
3.3.1. Basal DA in DLS and NAc
As shown in Table 1, there were group differences in basal DA. First, EB-treated OVX females had higher basal DA in DLS than females treated with OIL. Importantly, this occurred prior to any treatment and even though the animals were randomly assigned to groups prior to microdialysis. Second, the male groups had higher basal DA in DLS and NAc than did the female groups (p < 0.05). Since there were basal differences in DA in both brain regions, the responses to cocaine are expressed as the increase from baseline, rather than as a percent of baseline.
Table 1.
DA concentrations during baseline collection and after injecting with OIL or EB.
| Dorsolateral Striatum (DLS) | Nucleus Accumbens (NAc) | ||||
|---|---|---|---|---|---|
| Baseline | OIL/EB | Baseline | OIL/EB | ||
| Male | Oil | 5.72 ± 0.66 | 7.07 ± 0.96 | 9.05 ± 1.67 | 10.58 ± 2.88 |
| EB | 7.78 ± 1.08 | 8.40 ± 1.41 | 6.67 ± 1.52 | 5.83 ± 0.41 | |
| Female | Oil | 3.09 ± 0.46 | 4.47 ± 0.72 | 3.84 ± 0.82** | 6.25 ± 1.97 |
| EB | 7.30 ± 1.32* | 9.24 ± 2.16 | 5.41 ± 1.73 | 5.42 ± 0.95 | |
Values shown are the mean of 3 samples ± SEM in picograms per 10 min collection adjusted for recoveries.
indicates significant main effect of group in the basal and post OIL/EB injection periods in the DLS prior to cocaine treatment in; post hoc tests indicate a significant difference between OIL and EB females in 2 of the 3 time points (p < 0.05).
indicates a significant main effect of sex in the baseline DA levels in the DLS and the NAc, with post hoc tests indicating a significant difference between males and females in the oil groups in all 3 time points (p < 0.05).
3.3.2. Effect of estradiol on cocaine-induced DA in DLS
Cocaine induced a greater increase in DLS DA in dialysate in EB-treated animals than in animals receiving OIL (main effect of treatment [F (1,26) = 6.260; p = 0.019], time X treatment [F (8,208) = 2.418; p = 0.016]). As indicated in Figure 3, post hoc tests showed that this was due to the effect of EB in DLS of females at time points 50, 60, 70, and 120 (Figure 3A; p = 0.05, 0.034, 0.04, 0.031, respectively). There was no effect of EB in DLS of males (Figure 3B).
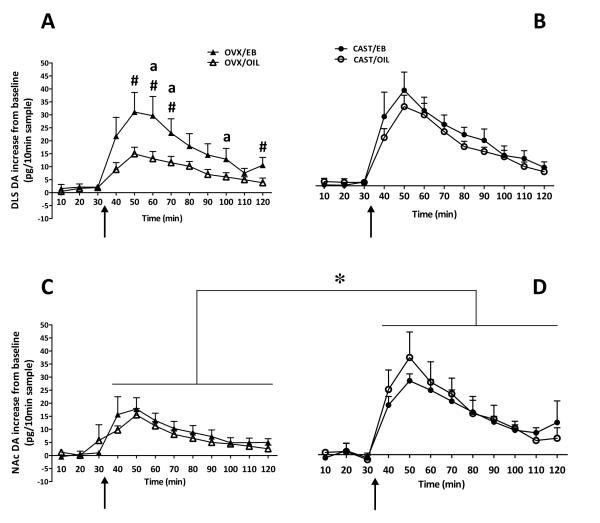
Figure 3. Cocaine-induced increase in DA dialysate from (A) female DLS, (B) male DLS, (C) female NAc, and (D) male NAc.
Data are presented as mean (+ SEM) DA increase from baseline during each 10-min sample collection (pg/10 min). Collections taken at 10, 20, and 30 min are after a subcutaneous injection of EB or OIL (5μg in 0.1ml oil, or oil alone); collections from 40 through 120 min are following cocaine administration (10 mg/kg, i.p.; indicated by the arrow). ‘a’ indicates that DA in DLS of EB females is significantly greater than NAc EB females (p<0.05); ‘#’ indicates that DA is higher in DLS in EB females compared to OIL females (p<0.05); * indicates a significant main effect of sex in NAc, with DA being higher in males than in females, regardless of treatment (p < 0.05). There was no effect of EB in the males in either brain regions.
3.3.3. Effect of estradiol on cocaine-induced DA in NAc
By contrast, when examining the effect of EB on cocaine-induced DA in NAc dialysate, there was a significant main effect of sex [F (1,26) = 5.622; p = 0.025], with no effect of EB/OIL treatment and no interaction. As can be seen in Figure 3C and 3D, cocaine treatment resulted in a greater increase in DA in dialysate for males as compared to females, regardless of treatment with EB or OIL (p < 0.05).
3.3.4. DLS vs. NAc
When the cocaine-induced increase in DA in dialysate for the DLS was compared to that in the NAc within treatment group, the EB-treated females showed significantly greater increases in DA in the DLS than the NAc (p < 0.05; Fig. 3A vs. 3C). There were no regional differences in the cocaine-induced increase in DA in dialysate for the other groups.
4. DISCUSSION
The results of these experiments demonstrate, for the first time and in the same animals, that estradiol enhances the cocaine-induced increase in DA in dialysate from the DLS but not the NAc in females but not male rats. Furthermore, in females treated with estradiol, sensitization of the behavioral response to cocaine was greater for behaviors primarily mediated by DLS, while gonadal hormones did not affect behavioral sensitization in male rats. These results suggest that in females estradiol acting in the DLS may play a role in the sex differences observed in sensitization of the behavioral responses to cocaine, and may underlie sex differences in cocaine-taking behavior.
Sensitization of stereotypy and locomotor behavior is a complex phenomenon and the underlying neurobiological mechanisms have not been systematically studied. We know that there are sex differences in sensitization (Hu and Becker, 2003), as well as effects of experience and genetics on whether animals express sensitization of stereotypy or locomotion (Leith and Kuczenski, 1982). Our finding that EB treatment enhances the display of stereotyped behaviors in females is in agreement with previous research examining the effect of estradiol on behavioral sensitization using rotational behavior as the outcome measure after unilateral striatal DA depletion (Hu and Becker, 2003). These results, along with our finding that acute EB did not alter the display of locomotion or stereotypy on Day 1, suggest that estradiol’s enhancement of DA in dialysate after cocaine treatment is more important for long term neural changes in response to psychomotor stimulants rather than the acute behavioral response to the drug. The neural processes involved in sensitization have been proposed as a mechanism for the transition to addiction (Robinson and Berridge, 1993, 2000), and the results presented here suggest that estradiol enhances these processes in females, which may facilitate the transition to addiction.
The role of dopaminergic neurotransmission in DLS vs. NAc in drug-seeking and compulsive drug-taking remains an important topic of investigation. Some investigators maintain that the DLS is just as important as the NAc in the reinforcing effects of cocaine in early stages of cocaine addiction (Veeneman et al., 2012), while others have postulated that the progression from voluntary to compulsive drug use involves a transition from prefrontal cortical and NAc control to mediation by the dorsal striatum (Dalley et al., 2011, Everitt and Robbins, 2005). The results presented here demonstrate that estradiol can rapidly enhance cocaine’s neurobiological effects in DLS in females but not males, as has been previously reported for amphetamine (Castner et al., 1993). It has also been previously reported that estradiol enhances acquisition of and motivation to take cocaine in female rats (Becker and Hu, 2008, Hu et al., 2004, Roberts et al., 1989). Thus, the magnitude of the DA response in the DLS is likely to be an important factor in early drug use as well as in the transition to dependence in females.
Intriguingly, we found a significant effect of sex on the DA response to cocaine in the NAc, with males showing higher cocaine-induced DA in dialysate compared to females, regardless of hormone treatment. It is interesting to note that even though levels of DA in NAc dialysate are higher in males than in females upon initial cocaine exposure, the locomotor response of the male groups was lower than that of OVX females. This suggests that males are less sensitive to the behavioral effects of DA than are OVX females.
We have previously reported sex differences in basal DA in the DLS (Castner et al., 1993, Xiao and Becker, 1994), and an ability of acute EB to enhance amphetamine-induced DA in striatum (Castner et al., 1993). In NAc, others have reported cocaine-induced increases in DA in males and females, without finding a sex difference in basal DA or the response to cocaine (Holly et al., 2012). In the latter study, no sex difference in the overall cocaine-induced percent increase in DA from the NAc shell was found, although females were found to have a greater percent increase in DA during the collection interval 35-45 min post-cocaine than did males (Holly et al., 2012). When looking at the percent increase from baseline in our animals, we did not see a significant difference between males and females. This could be due to differences between the studies in probe placement, as our probes are more lateral and include both core and shell of the NAc. Future studies will investigate whether EB affects the DA response specifically in NAc core vs. shell.
Enhanced DA in dialysate can occur via an increase in DA release (e.g., number or magnitude of release events; Aragona et al., 2008) or a reduction in DA clearance by inhibiting the action of the DA transporter (DAT; Koob and Bloom, 1988). Cocaine acts to increase DA in dialysate primarily by blocking its reuptake via competitive inhibition of DAT (Giros et al., 1996). There are sex differences in DAT activity in dorsal striatum with females having greater concentrations of DAT and enhanced DAT activity compared to males (Dluzen and McDermott, 2008, Rivest et al., 1995, Walker et al., 2000), and estradiol reduces DAT activity in females but not males (Attali et al., 1997). Therefore, estradiol’s modulation of DLS DAT activity to alter DA neurotransmission may be a mechanism by which females are rendered more vulnerable to the effects of psychoactive substances, particularly as estradiol’s actions are sex-specific and occur in reward-related brain regions, as shown here. Additionally, DA uptake rates are higher in DLS as compared to NAc (Calipari et al., 2012), supporting the notion that estradiol’s actions are greatest in DLS and may explain why we did not see an effect of EB on NAc DA in dialysate.
The finding that males did not respond to acute estradiol with a change in stimulated DA is in agreement with previous results. For example, we do not see an effect of CAST on cocaine sensitization using rotational behavior as the measure of sensitization (Hu and Becker, 2003), nor did we find an effect of estradiol on cocaine self-administration in CAST male rats (while there is an effect in females; Jackson et al., 2006). Additionally, EB treatment does not enhance amphetamine-stimulated DA in DLS of CAST male rats (Castner et al., 1993). Finally, we did not see any differences between CAST and SHAM males in any of the behaviors measured, just as we have not seen any difference between CAST and SHAM in cocaine self-administration behavior or sensitization of rotational behavior (Hu and Becker, 2003, Hu et al., 2004, Jackson et al., 2006). Therefore, gonadal hormones do not appear to play a large role in modulating the behavioral or neurochemical effects of cocaine in male rats.
Evolutionarily, it is adaptive for females to modify their behavior depending on their hormonal condition. Appropriate and rapid changes in motivational state and associated behaviors are important for obtaining food, engaging in sex, and caring for young to increase a female’s reproductive fitness. It stands to reason, then, that females would be more sensitive or responsive to estradiol than are males, and that this would impact the motivational systems of the brain. The findings reported here suggest that the effects of estradiol impact the DLS selectively in the female and have important implications for our understanding of the neural mechanisms mediating sex differences in drug abuse.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Aragona BJ, Cleaveland NA, Stuber GD, Day JJ, Carelli RM, Wightman RM. Preferential enhancement of dopamine transmission within the nucleus accumbens shell by cocaine is attributable to a direct increase in phasic dopamine release events. J. Neurosci. 2008;28:8821–8831. doi: 10.1523/JNEUROSCI.2225-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attali G, Weizman A, GilAd I, Rehavi M. Opposite modulatory effects of ovarian hormones on rat brain dopamine and serotonin transporters. Brain Res. 1997;756:153–159. doi: 10.1016/s0006-8993(97)00136-4. [DOI] [PubMed] [Google Scholar]
- Becker JB. Direct effect of 17 beta-estradiol on striatum: sex differences in dopamine release. Synapse. 1990;5:157–164. doi: 10.1002/syn.890050211. [DOI] [PubMed] [Google Scholar]
- Becker JB, Hu M. Sex differences in drug abuse. Front. Neuroendocrinol. 2008;29:36–47. doi: 10.1016/j.yfrne.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Molenda H, Hummer DL. Gender differences in the behavioral responses to cocaine and amphetamine - implications for mechanisms mediating gender differences in drug abuse. Ann. N.Y. Acad. Sci. 2001;937:172–187. doi: 10.1111/j.1749-6632.2001.tb03564.x. [DOI] [PubMed] [Google Scholar]
- Becker JB, Rudick CN. Rapid effects of estrogen or progesterone on the amphetamine-induced increase in striatal dopamine are enhanced by estrogen priming: a microdialysis study. Pharmacol. Biochemem. Behav. 1999;64:53–57. doi: 10.1016/s0091-3057(99)00091-x. [DOI] [PubMed] [Google Scholar]
- Belin D, Mar AC, Dalley JW, Robbins TW, Everitt BJ. High impulsivity predicts the switch to compulsive cocaine-taking. Science. 2008;320:1352–1355. doi: 10.1126/science.1158136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin-Rauscent A, Everitt BJ, Belin D. Intrastriatal shifts mediate the transition from drug-seeking actions to habits. Biol. Psychiatry. 2012;72:343–345. doi: 10.1016/j.biopsych.2012.07.001. [DOI] [PubMed] [Google Scholar]
- Calipari ES, Huggins KN, Mathews TA, Jones SR. Conserved dorsal ventral gradient of dopamine release and uptake rate in mice, rats and rhesus macaques. Neurochem. Intl. 2012;61:986–991. doi: 10.1016/j.neuint.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castner SA, Xiao L, Becker JB. Sex differences in striatal dopamine: in vivo microdialysis and behavioral studies. Brain Res. 1993;610:127–134. doi: 10.1016/0006-8993(93)91225-h. [DOI] [PubMed] [Google Scholar]
- Cummings JA, Gowl BA, Westenbroek C, Clinton SM, Akil H, Becker JB. Effects of a selectively bred novelty-seeking phenotype on the motivation to take cocaine in male and female rats. Biol. Sex Differ. 2011;2:3. doi: 10.1186/2042-6410-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Everitt BJ, Robbins TW. Impulsivity, compulsivity, and top-down cognitive control. Neuron. 2011;69:680–694. doi: 10.1016/j.neuron.2011.01.020. [DOI] [PubMed] [Google Scholar]
- Delfs JM, Schreiber L, Kelley AE. Microinjection of cocaine into the nucleus accubmens elicits locomotor activation in the rat. J. Neurosci. 1990;10:303–310. doi: 10.1523/JNEUROSCI.10-01-00303.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson PR, Lang CG, Hinton SC, Kelley AE. Oral stereotypy induced by amphetamine microinjection into striatum - an anatomical mapping study. Neuroscience. 1994;61:81–91. doi: 10.1016/0306-4522(94)90062-0. [DOI] [PubMed] [Google Scholar]
- Dluzen DE, McDermott JL. Sex differences in dopamine- and vesicular monoamine-transporter functions implications for methamphetamine use and neurotoxicity. In: Kuhar MJ, editor. Drug addiction: Research Frontiers and Treatment Advances. Blackwell Publishing; Oxford: 2008. pp. 140–150. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat. Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Giros B, Jaber M, Jones SR, Wightman RM, Caron MG. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature. 1996;379:606–612. doi: 10.1038/379606a0. [DOI] [PubMed] [Google Scholar]
- Holly EN, Shimamoto A, Debold JF, Miczek KA. Sex differences in behavioral and neural cross-sensitization and escalated cocaine taking as a result of episodic social defeat stress in rats. Psychopharmacology (Berl.) 2012;224:179–188. doi: 10.1007/s00213-012-2846-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu M, Becker JB. Effects of sex and estrogen on behavioral sensitization to cocaine in rats. J. Neurosci. 2003;23:693–699. doi: 10.1523/JNEUROSCI.23-02-00693.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu M, Crombag HS, Robinson TE, Becker JB. Biological basis of sex differences in the propensity to self-administer cocaine. Neuropsychopharmacology. 2004;29:81–85. doi: 10.1038/sj.npp.1300301. [DOI] [PubMed] [Google Scholar]
- Jackson LR, Robinson TE, Becker JB. Sex differences and hormonal influences on acquisition of cocaine self-administration in rats. Neuropsychopharmacology. 2006;31:129–138. doi: 10.1038/sj.npp.1300778. [DOI] [PubMed] [Google Scholar]
- Jenkins WJ, Becker JB. Dynamic increases in dopamine during paced copulation in the female rat. Eur. J. Neurosci. 2003;18:1997–2001. doi: 10.1046/j.1460-9568.2003.02923.x. [DOI] [PubMed] [Google Scholar]
- Koob GF, Bloom FE. Cellular and molecular mechanisms of drug-dependence. Science. 1988;242:715–723. doi: 10.1126/science.2903550. [DOI] [PubMed] [Google Scholar]
- Kosten TA, Gawin FH, Kosten TR, Rounsaville BJ. Gender differences in cocaine use and treatment response. J. Subst. Abuse Treat. 1993;10:63–66. doi: 10.1016/0740-5472(93)90100-g. [DOI] [PubMed] [Google Scholar]
- Larson EB, Roth ME, Anker JJ, Carroll ME. Effect of short- vs. long-term estrogen on reinstatement of cocaine-seeking behavior in female rats. Pharmacol. Biochem. Behav. 2005;82:98–108. doi: 10.1016/j.pbb.2005.07.015. [DOI] [PubMed] [Google Scholar]
- Leith NJ, Kuczenski R. Two dissociable components of behavioral sensitization following repeated amphetamine administration. Psychopharmacology (Berl.) 1982;76:310–315. doi: 10.1007/BF00449116. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Roth ME, Carroll ME. Biological basis of sex differences in drug abuse: preclinical and clinical studies. Psychopharmacology. 2002;164:121–137. doi: 10.1007/s00213-002-1183-2. [DOI] [PubMed] [Google Scholar]
- Perrot O, Laroche D, Pozzo T, Marie C. Quantitative assessment of stereotyped and challenged locomotion after lesion of the striatum: a 3d kinematic study in rats. Plos One. 2009;4:e7616. doi: 10.1371/journal.pone.0007616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivest R, Falardeau P, Dipaolo T. Brain dopamine transporter - gender differences and effect of chronic haloperidol. Brain Res. 1995;692:269–272. doi: 10.1016/0006-8993(95)00611-s. [DOI] [PubMed] [Google Scholar]
- Robbins SJ, Ehrman RN, Childress AR, O’Brien CP. Comparing levels of cocaine cue reactivity in male and female outpatients. Drug Alcohol Depend. 1999;53:223–230. doi: 10.1016/s0376-8716(98)00135-5. [DOI] [PubMed] [Google Scholar]
- Roberts DCS, Bennett SAL, Vickers GJ. The estrous-cycle affects cocaine self-administration on a progressive ratio schedule in rats. Psychopharmacology. 1989;98:408–411. doi: 10.1007/BF00451696. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Becker JB. Enduring changes in brain and behavior produced by chronic amphetamine administration - a review and evaluation of animal-models of amphetamine psychosis. Brain Res. Rev. 1986;11:157–198. doi: 10.1016/s0006-8993(86)80193-7. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving - an incentive-salience theory of addiction. Brain Res. Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The psychology and neurobiology of addiction: an incentive-sensitization view. Addiction. 2000;95:S91–S117. doi: 10.1080/09652140050111681. [DOI] [PubMed] [Google Scholar]
- Sell SL, Scalzitti JM, Thomas ML, Cunningham KA. Influence of ovarian hormones and estrous cycle on the behavioral response to cocaine in female rats. J. Pharmacol. Exp. Ther. 2000;293:879–886. [PubMed] [Google Scholar]
- Sofuoglu M, Dudish-Poulsen S, Nelson D, Pentel PR, Hatsukami DK. Sex and menstrual cycle differences in the subjective effects from smoked cocaine in humans. Exp. Clin. Psychopharmacol. 1999;7:274–283. doi: 10.1037//1064-1297.7.3.274. [DOI] [PubMed] [Google Scholar]
- Veeneman MM, van Ast M, Broekhoven MH, Limpens JH, Vanderschuren LJ. Seeking-taking chain schedules of cocaine and sucrose self-administration: effects of reward size, reward omission, and alpha-flupenthixol. Psychopharmacology. 2012;220:771–785. doi: 10.1007/s00213-011-2525-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker QD, Johnson ML, Van Swearingen AED, Arrant AE, Caster JM, Kuhn CM. Individual differences in psychostimulant responses of female rats are associated with ovarian hormones and dopamine neuroanatomy. Neuropharmacology. 2012;62:2267–2277. doi: 10.1016/j.neuropharm.2012.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker QD, Rooney MB, Wightman RM, Kuhn CM. Dopamine release and uptake are greater in female than male rat striatum as measured by fast cyclic voltammetry. Neuroscience. 2000;95:1061–1070. doi: 10.1016/s0306-4522(99)00500-x. [DOI] [PubMed] [Google Scholar]
- Willuhn I, Burgeno LM, Everitt BJ, Phillips PEM. Hierarchical recruitment of phasic dopamine signaling in the striatum during the progression of cocaine use. Proc. Natl. Acad. Sci. U.S.A. 2012;109:20703–20708. doi: 10.1073/pnas.1213460109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao L, Becker JB. Quantitative microdialysis determination of extracellular striatal dopamine concentration in male and female rats: effects of estrous cycle and gonadectomy. Neurosci. Lett. 1994;180:155–158. doi: 10.1016/0304-3940(94)90510-x. [DOI] [PubMed] [Google Scholar]
- Zhao W, Becker JB. Sensitization enhances acquisition of cocaine self-administration in female rats: estradiol further enhances cocaine intake after acquisition. Horm. Behav. 2010;58:8–12. doi: 10.1016/j.yhbeh.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]