Abstract
Purpose
Brachytherapy after lumpectomy is an increasingly popular breast cancer treatment, but data concerning its effectiveness are conflicting. Recently proposed “suitability” criteria guiding patient selection for brachytherapy have never been empirically validated.
Methods
Using the Surveillance, Epidemiology, and End Results–Medicare linked database, we compared women aged 66 years or older with invasive breast cancer (n=28,718) or ductal carcinoma in situ (n=7229) diagnosed from 2002 to 2007, treated with lumpectomy alone, brachytherapy, or external beam radiation therapy (EBRT). The likelihood of breast preservation, measured by subsequent mastectomy risk, was compared by use of multivariate proportional hazards, further stratified by American Society for Radiation Oncology (ASTRO) brachytherapy suitability groups. We compared 1-year postoperative complications using the χ2 test and 5-year local toxicities using the log-rank test.
Results
For patients with invasive cancer, the 5-year subsequent mastectomy risk was 4.7% after lumpectomy alone (95% confidence interval [CI], 4.1%–5.4%), 2.8% after brachytherapy (95% CI, 1.8%–4.3%), and 1.3% after EBRT (95% CI, 1.1%–1.5%) (P<.001). Compared with lumpectomy alone, brachytherapy achieved a more modest reduction in adjusted risk (hazard ratio [HR], 0.61; 95% CI, 0.40–0.94) than achieved with EBRT (HR, 0.22; 95% CI, 0.18–0.28). Relative risks did not differ when stratified by ASTRO suitability group (P=.84 for interaction), although ASTRO “suitable” patients did show a low absolute subsequent mastectomy risk, with a minimal absolute difference in risk after brachytherapy (1.6%; 95% CI, 0.7%–3.5%) versus EBRT (0.8%; 95% CI, 0.6%–1.1%). For patients with ductal carcinoma in situ, EBRT maintained a reduced risk of subsequent mastectomy (HR, 0.40; 95% CI, 0.28–0.55; P<.001), whereas the small number of patients treated with brachytherapy (n=179) precluded definitive comparison with lumpectomy alone. In all patients, brachytherapy showed a higher postoperative infection risk (16.5% vs 9.9% after lumpectomy alone vs 11.4% after EBRT, P<.001); higher incidence of breast pain (22.9% vs 11.2% vs 16.7%, P<.001); and higher incidence of fat necrosis (15.3% vs 5.3% vs 7.7%, P<.001).
Conclusions
In this study era, brachytherapy showed lesser breast-preservation benefit compared with EBRT. Suitability criteria predicted differential absolute, but not relative, benefit in patients with invasive cancer.
Introduction
Breast brachytherapy after lumpectomy for early breast cancer is an increasingly popular radiation treatment approach, increasingly used in lieu of standard whole-breast irradiation (1, 2). Brachytherapy limits radiation treatment to the tissue surrounding the lumpectomy cavity and conveniently accelerates treatment completion within 1 to 2 weeks (3). Yet controversy persists over whether this technique should be considered a standard of care, given the lack of clarity regarding which patient subgroups may derive the greatest benefit and which subgroups may incur potential harm from this treatment. One recent study found that older women with invasive breast cancer had decreased breast-preservation and higher complication rates after brachytherapy compared with standard radiation, but that analysis did not stratify by pathologic factors (1).
To guide clinicians, the American Society for Radiation Oncology (ASTRO) proposed “suitability” criteria to guide brachytherapy use in everyday practice, stratifying patients as “suitable,” “cautionary,” and “unsuitable” (3). The suitable group identifies low-risk patients with favorable local control outcomes (4, 5), although National Comprehensive Cancer Network guidelines suggest that adjuvant radiation could be forgone in many such suitable patients (3–5). Importantly, ASTRO categories have not been empirically validated for the ability to distinguish who might benefit from brachytherapy, particularly in comparison with the alternatives of lumpectomy alone (4) or whole-breast irradiation.
Given the existing heterogeneous—and sometimes conflicting—recommendations, a direct comparison is warranted of outcomes after lumpectomy alone versus adjuvant brachytherapy versus adjuvant external beam radiation therapy (EBRT). Comparing risk-benefit profiles for these treatments is essential for prospectively stratifying patients’ risk and guiding treatment decisions. Accordingly, in a cohort of Medicare patients diagnosed with early invasive breast cancer or ductal carcinoma in situ (DCIS), we sought to compare the following among treatment groups: (1) the likelihood of breast preservation after treatment, (2) the validity of suitability categories for modifying the likelihood of breast preservation, and (3) the risks of postoperative complications and local toxicities.
Methods
From the Surveillance, Epidemiology, and End Results (SEER)–Medicare population-based dataset, we retrospectively identified 35,947 women aged 66 years or older treated with lumpectomy for incident breast cancer diagnosed from 2002 to 2007. Radiation treatment (no radiation [lumpectomy alone] vs brachytherapy vs EBRT) and patient/tumor covariates were determined based on SEER records and Medicare claims. Socioeconomic covariates were linked from the Area Resource File for 2002 to 2007.
Outcomes
Subsequent mastectomy was defined as a claim for mastectomy identified from 1 year after diagnosis to December 31, 2009 (last date of follow-up). Postoperative complications (infectious and noninfectious) were determined by claims reported within 1 year of diagnosis. Local toxicities (breast pain, rib fracture, fat necrosis, and radiation pneumonitis) were determined by claims reported between the diagnosis date and date of last follow-up.
ASTRO suitability groupings
Patients with invasive cancer were classified as suitable, cautionary, or unsuitable for brachytherapy, according to groupings adapted from ASTRO consensus criteria (3) and recently published methods (2). Suitable patients had estrogen receptor (ER)–positive tumors, 2 cm or less in size, with invasive ductal, mucinous, or tubular histology; no extensive intraductal component; and clinically and pathologically node-negative disease with documentation of surgical and/or pathologic assessment of axillary lymph nodes. On the basis of the study inclusion criterion of age 66 years or older, all patients in this sample met the ASTRO age criterion. In our sample the subset of patients aged 70 years or older was classified as “older suitable.”
Unsuitable patients included those with T3/T4 disease, tumor size greater than 3 cm, documented nodal involvement, or no nodal sampling for those with invasive disease. Cautionary patients included those without unsuitable features but who had invasive cancer with tumor size between 2.1 and 3.0 cm or any of the following features with total tumor size of 3.0 cm or less: extensive intraductal component, invasive lobular histology, or ER-negative disease. Candidate patients with DCIS are considered ASTRO cautionary. Patients not meeting any of these criteria were considered “unclassified.” Surgical margin status, lymphovascular space invasion, and multicentricity are unavailable in the SEER-Medicare dataset (2).
Statistical analysis
Univariate associations between type of radiation treatment (lumpectomy alone vs brachytherapy vs EBRT) and covariates were determined by use of the Pearson χ2 test. The Cochran-Armitage test for trend was used to test for temporal changes in treatment.
Subsequent mastectomy risk
To address the first objective, the cumulative incidence of subsequent mastectomy for each radiation treatment type was calculated with the Kaplan-Meier method for the overall cohort and separately for patients with invasive cancer and DCIS. The log-rank test compared all paired comparisons.
We used multivariate proportional hazards regression to test whether radiation treatment type was associated with subsequent mastectomy risk after adjusting for patient, tumor, and socio-demographic covariates. Interactions were tested with the likelihood ratio test. Because the interaction term between treatment group and tumor behavior (invasive vs DCIS) was statistically significant (P=.01 for interaction), analyses for invasive cancer versus DCIS were conducted separately.
A secondary validation model (for invasive cancer) used propensity score analysis with 1:1 matched cohorts: lumpectomy alone matched to EBRT, lumpectomy alone matched to brachytherapy, and EBRT matched to brachytherapy. We determined propensity scores using logistic modeling adjusting for baseline covariates (6). Proportional hazards regression stratified by matched pair was used to determine associations between radiation treatment type and subsequent mastectomy risk.
ASTRO-stratified analysis
To address the second objective, we tested associations between radiation type and subsequent mastectomy risk within each ASTRO risk group (suitable, cautionary, unsuitable, and unclassified) using the Kaplan-Meier method and log-rank test, as well as stratified multivariate models.
Postoperative complications and local toxicities
To address the third objective, the frequencies of soft tissue infection and noninfectious postoperative complications (shock, hemorrhage, hematoma, seroma, persistent postoperative fistula, or nonhealing surgical wound) within 1 year of diagnosis were compared by use of the χ2 test. Multivariate logistic regression was used to calculate adjusted odds of infectious and noninfectious complications. Risks of breast pain, fat necrosis, pneumonitis, and rib fracture were compared by use of the log-rank test. Analyses were 2 tailed (SAS software, version 9.2; SAS Institute, Cary, NC). The details are shown in Tables E1, E2, and E3.
Results
Baseline characteristics
Among the 35,947 patients, the median follow-up was 3.5 years (interquartile range, 2.7–4.8 years). The median age was 75 years (interquartile range, 70–80 years), 5.8% of patients (n=2089) were black, and 12.3% (n=4419) had moderate to severe comorbidity. A total of 79.9% (n=28,718) were diagnosed with invasive breast cancer and 20.1% (n=7229) with pure DCIS. Of the patients, 23% (n=8254) received lumpectomy alone, 3.6% (n=1310) received brachytherapy, and 73.4% (n=26,383) received EBRT.
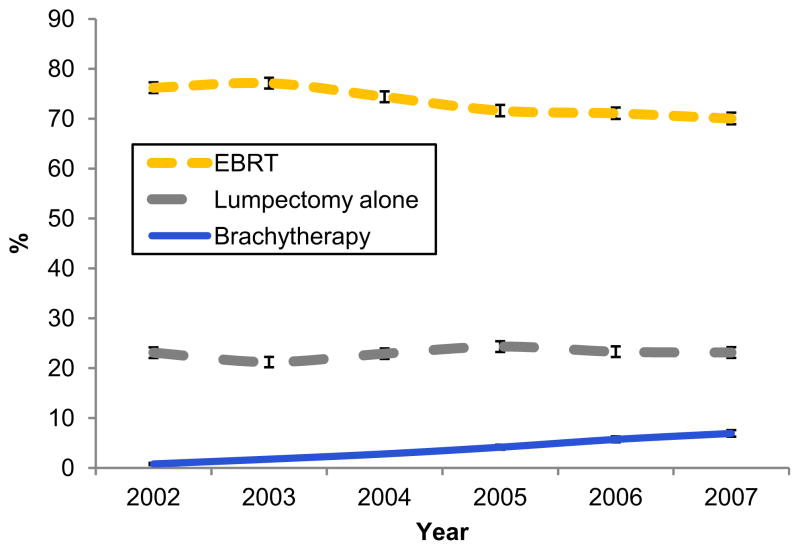
Brachytherapy use increased from 0.8% of patients (45 of 5959) in 2002 to 6.9% of patients (403 of 5850) in 2007 (P<.001 for trend), whereas EBRT use decreased (P<.001 for trend) and use of lumpectomy alone remained stable (P=.09 for trend) (Fig. 1). Patients treated with brachytherapy, in comparison with lumpectomy alone and EBRT, were more likely to have a tumor size of 2.0 cm or less, ER-positive receptor status, non–high-grade histology, and negative axillary lymph nodes and were more likely to undergo axillary surgery but were less likely to have DCIS (P<.001 for all comparisons) and less likely than patients treated with EBRT to receive chemotherapy (P<.001) (Table 1).
Fig. 1.
Time trends for use of external beam radiation therapy (EBRT), brachytherapy, and lumpectomy alone from 2002 to 2007. Brachytherapy increased whereas EBRT decreased (P<.001). Lumpectomy alone remained stable (P=.09).
Table 1.
Baseline characteristics for entire cohort and by radiation treatment group
| Entire cohort | No. of patients (%)
|
P value* | |||
|---|---|---|---|---|---|
| All patients (N=35,947) | Lumpectomy alone (n=8254) | EBRT (n=26,383) | Brachy (n=1310) | ||
| Patient factors | |||||
| Age | |||||
| 66–69 y | 8129 (22.6) | 887 (10.8) | 6898 (26.2) | 344 (26.3) | <.001 |
| 70–74 y | 9446 (26.3) | 1288 (15.6) | 7792 (29.5) | 366 (27.9) | |
| 75–79 y | 8577 (23.9) | 1631 (19.8) | 6627 (25.1) | 319 (24.4) | |
| ≥80 y | 9795 (27.3) | 4448 (53.9) | 5066 (19.2) | 281 (21.5) | |
| Race | |||||
| White | 32,089 (89.3) | 7332 (88.8) | 23,555 (89.3) | 1202 (91.8) | .01 |
| Black | 2089 (5.8) | 516 (6.3) | 1509 (5.7) | 64 (4.9) | |
| Other/unknown | 1769 (4.9) | 406 (4.9) | 1319 (5.0) | 44 (3.4) | |
| Charlson Comorbidity score | |||||
| 0 | 23,587 (65.6) | 4795 (58.1) | 17,924 (67.9) | 868 (66.3) | <.001 |
| 1 | 7941 (22.1) | 2024 (24.5) | 5615 (21.3) | 302 (23.1) | |
| ≥2 | 4419 (12.3) | 1435 (17.4) | 2844 (10.8) | 140 (10.7) | |
| Year of diagnosis | |||||
| 2002 | 5959 (16.6) | 1374 (16.7) | 4540 (17.2) | 45 (3.4) | <.001 |
| 2003 | 5942 (16.5) | 1259 (15.3) | 4581 (17.4) | 102 (7.8) | |
| 2004 | 6166 (17.2) | 1410 (17.1) | 4585 (17.4) | 171 (13.1) | |
| 2005 | 6049 (16.8) | 1469 (17.8) | 4331 (16.4) | 249 (19.0) | |
| 2006 | 5981 (16.6) | 1391 (16.9) | 4250 (16.1) | 340 (26.0) | |
| 2007 | 5850 (16.3) | 1351 (16.4) | 4096 (15.5) | 403 (30.8) | |
| Tumor factors | |||||
| Behavior | |||||
| Ductal carcinoma in situ | 7229 (20.1) | 2666 (32.3) | 4384 (16.6) | 179 (13.7) | <.001 |
| Invasive carcinoma | 28,718 (79.9) | 5588 (67.7) | 21,999 (83.4) | 1131 (86.3) | |
| Size | |||||
| 0–2.0 cm | 26,429 (73.5) | 5562 (67.4) | 19,758 (74.9) | 1109 (84.7) | <.001 |
| 2.1–5.0 cm | 5945 (16.5) | 1367 (16.6) | 4465 (16.9) | 113 (8.6) | |
| >5 cm | 382 (1.1) | 122 (1.5) | >240 (>0.9) | <11 (<0.9) | |
| Unknown | 3191 (8.9) | 1203 (14.6) | >1890 (>7.1) | <89 (<6.8) | |
| T stage | |||||
| Tis | 7229 (20.1) | 2666 (32.3) | 4384 (16.6) | 179 (13.7) | <.001 |
| T1 | 21,883 (60.9) | 3947 (47.8) | 16,947 (64.2) | 989 (75.5) | |
| T2 | 4912 (13.7) | 1023 (12.4) | 3787 (14.4) | 102 (7.8) | |
| T3 | 247 (0.7) | 71 (0.9) | >160 (>0.6) | <11 (<0.9) | |
| T4 | 831 (2.3) | 301 (3.7) | >510 (>1.9) | <11 (<0.9) | |
| Unknown | 845 (2.4) | 246 (3.0) | 569 (2.2) | 30 (2.3) | |
| Nodal status | |||||
| Pathologic N0 | 20,344 (56.6) | 2445 (29.6) | 16,878 (64.0) | 1021 (77.9) | <.001 |
| Clinical N0 | 4755 (13.2) | 2732 (33.1) | 1935 (7.3) | 88 (6.7) | |
| Pathologic N+ | 4625 (12.9) | 648 (7.9) | 3920 (14.9) | 57 (4.4) | |
| Unknown | 6223 (17.3) | 2429 (29.4) | 3650 (13.83) | 144 (11.0) | |
| Estrogen receptor status | |||||
| Positive | 25,916 (72.1) | 5301 (64.2) | 19,554 (74.1) | 1061 (81.0) | <.001 |
| Negative or borderline | 3958 (11.0) | 634 (7.7) | 3213 (12.2) | 111 (8.5) | |
| Unknown | 6073 (16.9) | 2319 (28.1) | 3616 (13.7) | 138 (10.5) | |
| Grade | |||||
| Low/intermediate grade | 24,137 (67.2) | 5569 (67.5) | 17,588 (66.7) | 980 (74.8) | <.001 |
| High grade | 8620 (24.0) | 1617 (19.6) | 6754 (25.6) | 249 (19.0) | |
| Missing | 3190 (8.9) | 1068 (12.9) | 2041 (7.7) | 81 (6.2) | |
| Extensive intraductal component† | |||||
| No | 28,420 (99.0) | 5541 (99.2) | >21,740 (>98.8) | >1120 (>99.1) | .077 |
| Yes | 298 (1.0) | 47 (0.8) | >230 (>1.0) | <11 (<0.9) | |
| Histology† | |||||
| Invasive ductal, mucinous, or tubular | 21,891 (76.2) | 4255 (76.2) | 16,701 (75.9) | 935 (82.7) | <.001 |
| Invasive lobular | 2361 (8.2) | 430 (7.7) | 1878 (8.5) | 53 (4.7) | |
| Other invasive | 4466 (15.6) | 903 (16.2) | 3420 (15.6) | 143 (12.6) | |
| Overlapping lesion‡ | |||||
| No | 31,787 (88.4) | 6899 (83.6) | 23,696 (89.8) | 1192 (91.0) | <.001 |
| Yes | 4160 (11.6) | 1355 (16.4) | 2687 (10.2) | 118 (9.0) | |
| Laterality | |||||
| Right | 17,611 (49.0) | 3929 (47.6) | 13,021 (49.4) | 661 (50.5) | .01 |
| Left | 18,336 (51.0) | 4325 (52.4) | 13,362 (50.7) | 649 (49.5) | |
| Treatment factors | |||||
| Receipt of chemotherapy | |||||
| No | 31,095 (86.5) | 7888 (95.6) | 22,005 (83.4) | 1202 (91.8) | <.001 |
| Yes | 4852 (13.5) | 366 (4.4) | 4378 (16.6) | 108 (8.2) | |
| Axillary surgery | |||||
| No | 11,533 (32.1) | 5464 (66.2) | 5832 (22.1) | 237 (18.1) | <.001 |
| Yes | 24,414 (67.9) | 2790 (33.8) | 20,551 (77.9) | 1073 (81.9) | |
| Overall patient classification (invasive patients only) | |||||
| ASTRO category† | |||||
| Suitable | 9966 (34.7) | 1175 (21.0) | 8164 (37.1) | 627 (5.4) | <.001 |
| Cautionary | 5059 (17.6) | 446 (8.0) | 4434 (20.2) | 179 (15.8) | |
| Unsuitable | 10,119 (35.2) | 3463 (62.0) | 6506 (29.6) | 150 (13.3) | |
| Unclassified | 3574 (12.5) | 504 (9.0) | 2895 (13.2) | 175 (15.4) | |
| Sociodemographic factors | |||||
| SEER registry | |||||
| Greater California | 12,395 (34.5) | 2953 (35.8) | 8920 (33.8) | 522 (39.9) | <.001 |
| Connecticut | 2965 (8.3) | 683 (8.3) | 2230 (8.5) | 52 (4.0) | |
| Detroit | 2490 (6.9) | 486 (5.9) | 1923 (7.3) | 81 (6.2) | |
| Hawaii | 524 (1.5) | 85 (1.0) | >420 (>1.6) | <11 (<0.9) | |
| Iowa | 2037 (5.7) | 471 (5.7) | >1520 (>5.7) | <28 (<2.1) | |
| New Mexico | 707 (2.0) | 179 (2.2) | 476 (1.8) | 52 (4.0) | |
| Seattle | 2345 (6.5) | 483 (5.9) | 1777 (6.7) | 85 (6.5) | |
| Utah | 851 (2.4) | 198 (2.4) | 603 (2.3) | 50 (3.8) | |
| Rural Georgia | 1228 (3.4) | 289 (3.5) | 812 (3.1) | 127 (9.7) | |
| Kentucky | 2350 (6.5) | 586 (7.1) | 1695 (6.4) | 69 (5.3) | |
| Louisiana | 1859 (5.2) | 418 (5.1) | 1327 (5.0) | 114 (8.7) | |
| New Jersey | 6196 (17.2) | 1423 (17.2) | 4646 (17.6) | 127 (9.7) | |
| Percent of adults in census tract or ZIP code with at least some college education§ | |||||
| 0–7.4 | 9023 (25.1) | 1898 (23.0) | 6727 (25.5) | 398 (30.4) | <.001 |
| 7.5–13.0 | 8956 (24.9) | 2003 (24.2) | 6631 (25.1) | 322 (24.6) | |
| 13.1–21.1 | 8987 (25.0) | 2090 (25.3) | 6599 (25.0) | 298 (22.8) | |
| 21.2–100 | 8981 (25.0) | 2263 (27.4) | 6426 (24.4) | 292 (22.3) | |
| Median income in census tract or | |||||
| ZIP code§ | |||||
| $0–$37,413 | 8990 (25.0) | 2299 (27.9) | 6394 (24.2) | 297 (22.7) | <.001 |
| $37,414–$50,066 | 8987 (25.0) | 2133 (25.8) | 6523 (24.7) | 331 (25.3) | |
| $50,067–$66,720 | 8986 (25.0) | 1932 (23.4) | 6716 (25.5) | 338 (25.8) | |
| $66,721–$200,008 | 8984 (25.0) | 1890 (22.9) | 6750 (25.6) | 344 (26.3) | |
| Urban/rural status | |||||
| Large metropolitan | 21,204 (59.0) | 4859 (58.9) | 15,472 (58.6) | 873 (66.6) | <.001 |
| Metropolitan | 10,377 (28.9) | 2289 (27.7) | 7749 (29.4) | 339 (25.9) | |
| Urban | 1979 (5.5) | 486 (5.9) | 1456 (5.5) | 37 (2.8) | |
| Less urban | 1983 (5.5) | 506 (6.1) | 1432 (5.4) | 45 (3.4) | |
| Rural | 404 (1.1) | 114 (1.4) | 274 (1.0) | 16 (1.2) | |
| Surgeon density in county of residence|| | |||||
| 0–8.3 | 9175 (25.5) | 2115 (25.6) | 6725 (25.5) | 335 (25.6) | <.001 |
| 8.4–11.6 | 8951 (24.9) | 2123 (25.7) | 6436 (24.4) | 392 (30.0) | |
| 11.7–15.4 | 8930 (24.8) | 2065 (25.0) | 6607 (25.0) | 258 (19.7) | |
| ≥15.5 | 8891 (24.7) | 1951 (23.6) | 6615 (25.1) | 325 (24.8) | |
| Radiation oncologist density in county of residence¶ | |||||
| 0–9.0 | 9095 (25.3) | 2232 (27.0) | 6503 (24.7) | 360 (27.5) | <.001 |
| 9.1–14.6 | 8826 (24.6) | 2025 (24.5) | 6419 (24.3) | 382 (29.2) | |
| 14.7–18.8 | 8955 (24.9) | 2051 (24.9) | 6645 (25.2) | 259 (19.8) | |
| ≥18.9 | 9071 (25.2) | 1946 (23.6) | 6816 (25.8) | 309 (23.6) | |
Abbreviations: ASTRO = American Society for Radiation Oncology; Brachy = brachytherapy; EBRT = external beam radiation therapy; SEER = Surveillance, Epidemiology, and End Results.
Cell sizes of less than 11 are reported with limited precision in accordance with the data-use agreement for this dataset. In some cases adjacent cells have also been reported with limited precision to prevent calculation of cell values of less than 11.
P value from χ2 test comparing type of treatment (external beam radiation therapy, brachytherapy, or no radiation with covariate).
Applies to invasive cases only.
Overlapping lesion refers to the coded site of involvement in the breast and has been used by other groups as a surrogate for multicentricity (2) but is not specific for this clinical-pathologic feature.
Fewer than 11 patients had missing values for these variables. Such patients were assumed to have a value for these variables equal to the median population value.
Per 100,000 persons.
Per 1,000,000 persons.
Subsequent mastectomy risk: invasive breast cancer
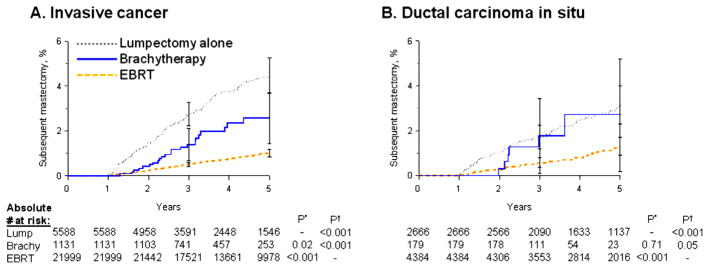
During follow-up, 1.8% of patients with invasive breast cancer (509 of 28,718) underwent subsequent mastectomy. The 5-year cumulative incidence of subsequent mastectomy was 4.7% (95% confidence interval [CI], 4.1%–5.4%) (number at risk, 5588) for those treated with lumpectomy alone, 2.8% (95% CI, 1.8%–4.3%) (number at risk, 1131) for brachytherapy, and 1.3% (95% CI, 1.1%–1.5%) (number at risk, 21,999) for EBRT (Table 2, Fig. 2A).
Table 2.
Subsequent mastectomy, postoperative complications, and local toxicities by radiation treatment
| Outcome | % (95% CI)
|
P value*
|
||||
|---|---|---|---|---|---|---|
| Lump | Brachy | EBRT | Lump vs Brachy | Lump vs EBRT | Brachy vs EBRT | |
| Subsequent mastectomy† | ||||||
| All patients (N=35,947) | .12 | <.001 | <.001 | |||
| 3 y | 2.6 (2.2–3.0) | 1.7 (1.1–2.7) | 0.7 (0.6–0.8) | |||
| 5 y | 4.2 (3.7–4.7) | 3.0 (2.0–4.4) | 1.4 (1.2–1.5) | |||
| Invasive patients (n=28,718) | .02 | <.001 | <.001 | |||
| 3 y | 2.9 (2.5–3.5) | 1.5 (0.9–2.5) | 0.6 (0.5–0.7) | |||
| 5 y | 4.7 (4.1–5.4) | 2.8 (1.8–4.3) | 1.3 (1.1–1.5) | |||
| DCIS patients (n=7229) | .39 | <.001 | .004 | |||
| 3 y | 1.9 (1.4–2.5) | 3.2 (1.3–7.7) | 0.8 (0.6–1.1) | |||
| 5 y | 3.2 (2.5–4.1) | 4.6 (2.0–10.5) | 1.6 (1.3–2.2) | |||
| Subsequent mastectomy† stratified by ASTRO risk group (invasive patients only) | ||||||
| Suitable (n=9966) | .20 | <.001 | .002 | |||
| 3 y | 2.6 (1.8–3.8) | 0.7 (0.3–1.9) | 0.3 (0.2–0.5) | |||
| 5 y | 3.7 (2.6–5.2) | 1.6 (0.7–3.5) | 0.8 (0.6–1.1) | |||
| Younger suitable (n=2516) | .91 | <.001 | <.001 | |||
| 3 y | 4.4 (1.7–11.5) | 1.2 (0.3–4.8) | 0.3 (0.2–0.7) | |||
| 5 y | 5.8 (2.4–13.6) | 3.1 (1.1–8.1) | 0.8 (0.5–1.4) | |||
| Older suitable (n=7450) | .07 | <.001 | .23 | |||
| 3 y | 2.4 (1.6–3.6) | 0.5 (0.1–2.1) | 0.3 (0.2–0.5) | |||
| 5 y | 3.5 (2.4–5.0) | 1.0 (0.3–3.4) | 0.8 (0.6–1.1) | |||
| Cautionary (n=5509) | .38 | <.001 | .006 | |||
| 3 y | 4.5 (2.8–7.0) | 3.0 (1.3–7.2) | 0.8 (0.6–1.2) | |||
| 5 y | 6.7 (4.4–10.2) | 5.4 (2.5–11.6) | 2.2 (1.7–2.8) | |||
| Unsuitable (n=10,119) | .43 | <.001 | .14 | |||
| 3 y | 3.2 (2.6–3.9) | 2.4 (0.8–7.2) | 1.0 (0.8–1.3) | |||
| 5 y | 5.0 (4.1–6.0) | 3.6 (1.3–9.4) | 1.6 (1.3–2.0) | |||
| Unclassified (n=3574) | .97 | <.001 | .02 | |||
| 3 y | 1.0 (0.4–2.4) | 2.0 (0.6–6.3) | 0.3 (0.1–0.6) | |||
| 5 y | 3.6 (2.0–6.4) | 3.5 (1.2–9.6) | 0.7 (0.4–1.1) | |||
| Postoperative complications in all patients (N=35,947)‡ | ||||||
| Infectious complication | ||||||
| 1 y | 9.9 (9.2–10.5) | 16.5 (14.5–18.5) | 11.4 (11.0–11.8) | <.001 | <.001 | <.001 |
| Noninfectious complication§ | ||||||
| 1 y | 6.0 (5.5–6.5) | 18.7 (16.6–20.8) | 9.5 (9.1–9.8) | <.001 | <.001 | <.001 |
| Local toxicities in all patients (N=35,947)|| | ||||||
| Rib fracture | .07 | <.001 | .93 | |||
| 1 y | 1.2 (1.0–1.5) | 0.7 (0.4–1.3) | 0.8 (0.7–0.9) | |||
| 3 y | 3.4 (3.0–3.8) | 2.3 (1.6–3.3) | 2.4 (2.2–2.6) | |||
| 5 y | 5.2 (4.6–5.8) | 4.2 (3.0–5.9) | 4.0 (3.7–4.3) | |||
| Fat necrosis | <.001 | <.001 | <.001 | |||
| 1 y | 4.2 (3.8–4.7) | 6.8 (5.6–8.3) | 4.4 (4.2–4.7) | |||
| 3 y | 4.9 (4.5–5.4) | 11.2 (9.5–13.0) | 6.3 (6.0–6.6) | |||
| 5 y | 5.3 (4.8–5.8) | 15.3 (13.0–17.9) | 7.7 (7.3–8.0) | |||
| Breast pain | <.001 | <.001 | <.001 | |||
| 1 y | 4.9 (4.5–5.4) | 11.0 (9.4–12.8) | 7.1 (6.8–7.4) | |||
| 3 y | 8.9 (8.3–9.5) | 18.2 (16.2–20.5) | 13.3 (12.9–13.7) | |||
| 5 y | 11.2 (10.5–12.0) | 22.9 (20.2–25.7) | 16.7 (16.2–17.2) | |||
| Radiation pneumonitis | .07 | <.001 | .08 | |||
| 1 y | 0.0 (0.00–0.1) | 0.2 (0.01–0.7) | 0.5 (0.4–0.6) | |||
| 3 y | 0.2 (0.01–0.3) | 0.4 (0.2–0.9) | 0.8 (0.7–0.9) | |||
| 5 y | 0.2 (0.1–0.4) | 0.4 (0.2–0.9) | 0.9 (0.8–1.0) | |||
Abbreviations: ASTRO = American Society for Radiation Oncology; Brachy = brachytherapy; DCIS = ductal carcinoma in situ; Lump = lumpectomy alone.
P value from log-rank test for all outcomes except postoperative complications, where P value is from Pearson χ2 test.
The time interval for this outcome begins 1 year after diagnosis (because treatment rendered within the first year of diagnosis is considered initial treatment and not an outcome).
These outcomes include any event that occurred within 1 year of diagnosis.
This outcome includes codes for postoperative shock, hematoma, hemorrhage, seroma, fistula, non-healing surgical wound, other specified postoperative complication, and postoperative complication not otherwise specified.
The time interval for these outcomes begins at diagnosis and continues through the time points specified.
Fig. 2.
Adjusted subsequent mastectomy risk (cumulative incidence) in invasive breast cancer patients (A) and ductal carcinoma in situ (DCIS) patients (B). P* indicates adjusted P value from proportional hazards regression with lumpectomy alone (Lump) as referent; P† indicates adjusted P value with EBRT as referent. Brachy = brachytherapy; EBRT = external beam radiation therapy.
On adjusted analysis, compared with lumpectomy alone as the referent (hazard ratio [HR], 1.00), both brachytherapy (HR, 0.61; 95% CI, 0.40–0.94; P=.02) and EBRT (HR, 0.22; 95% CI, 0.18–0.28; P<.001) were associated with lower subsequent mastectomy risks. However, compared with brachytherapy as the referent, EBRT maintained the lowest subsequent mastectomy risk (HR, 0.37; 95% CI, 0.24–0.55; P<.001). Other predictors of subsequent mastectomy included ER-negative status, large tumor size, T4 stage, and high grade (Table 3).
Table 3.
Proportional hazards regression: Predictors of adjusted subsequent mastectomy risk (N=35,947)
| HR | 95% CI | P value | |
|---|---|---|---|
| Treatment factors | |||
| For invasive cancers, type of radiation* | |||
| Lumpectomy alone | 1 | — | — |
| Brachytherapy | 0.61 | 0.40–0.94 | .02 |
| EBRT | 0.22 | 0.18–0.28 | <.001 |
| For DCIS, type of radiation* | |||
| Lumpectomy alone | 1 | — | — |
| Brachytherapy | 1.18 | 0.51–2.73 | .71 |
| EBRT | 0.40 | 0.28–0.55 | <.001 |
| Receipt of chemotherapy | |||
| No | 1 | — | — |
| Yes | 1.00 | 0.78–1.27 | .98 |
| Patient factors | |||
| Age | |||
| 66–69 y | 1 | — | — |
| 70–74 y | 0.82 | 0.66–1.03 | .08 |
| 75–79 y | 0.84 | 0.67–1.05 | .13 |
| ≥80 y | 0.68 | 0.54–0.85 | .001 |
| Race | |||
| White | 1 | — | — |
| Black | 1.37 | 1.03–1.82 | .03 |
| Other/unknown | 0.66 | 0.41–1.06 | .08 |
| Charlson Comorbidity score | |||
| 0 | 1 | — | — |
| 1 | 1.11 | 0.92–1.34 | .28 |
| ≥2 | 1.21 | 0.97–1.52 | .10 |
| Year of diagnosis | |||
| 2002 | 1 | — | — |
| 2003 | 0.95 | 0.75–1.20 | .66 |
| 2004 | 0.99 | 0.77–1.27 | .94 |
| 2005 | 1.08 | 0.83–1.40 | .57 |
| 2006 | 0.99 | 0.74–1.33 | .96 |
| 2007 | 1.19 | 0.86–1.66 | .30 |
| Tumor factors | |||
| Size | |||
| 0–2.0 cm | 1 | — | — |
| 2.1–5.0 cm | 1.30 | 1.06–1.59 | .01 |
| >5 cm | 2.14 | 1.28–3.56 | .004 |
| Unknown | 1.22 | 0.93–1.61 | .14 |
| T4 involvement | |||
| No | 1 | — | — |
| Yes | 1.65 | 1.10–2.48 | .02 |
| Nodal status | |||
| Pathologic N0 | 1 | — | — |
| Clinical N0 | 0.90 | 0.70–1.16 | .40 |
| Pathologic N+ | 1.26 | 0.98–1.61 | .08 |
| Unknown | 0.83 | 0.54–1.29 | .41 |
| Estrogen receptor status | |||
| Positive | 1 | — | — |
| Negative or borderline | 2.65 | 2.15–3.26 | <.001 |
| Unknown | 1.14 | 0.91–1.44 | .25 |
| Grade | |||
| Low/intermediate grade | 1 | — | — |
| High grade | 1.63 | 1.36–1.96 | <.001 |
| Missing | 1.15 | 0.88–1.51 | .31 |
| Tumor behavior in lumpectomy-alone patients | |||
| DCIS | 1 | — | — |
| Invasive cancer | 1.35 | 0.82–2.21 | .24 |
| Tumor behavior in brachytherapy patients | |||
| DCIS | 1 | — | — |
| Invasive cancer | 0.70 | 0.27–1.82 | .46 |
| Tumor behavior in EBRT patients | |||
| DCIS | 1 | — | — |
| Invasive cancer | 0.76 | 0.48–1.20 | .24 |
| Overlapping lesion | |||
| No | 1 | — | — |
| Yes | 1.10 | 0.88–1.37 | .42 |
| Laterality | |||
| Right | 1 | — | — |
| Left | 1.11 | 0.95–1.30 | .18 |
| Sociodemographic factors | |||
| SEER registry | |||
| Greater California | 1 | — | — |
| Connecticut | 0.98 | 0.63–1.53 | .93 |
| Detroit | 0.98 | 0.71–1.37 | .92 |
| Hawaii | 1.25 | 0.90–1.74 | .19 |
| Iowa | 0.98 | 0.44–2.22 | .97 |
| New Mexico | 0.97 | 0.64–1.48 | .89 |
| Seattle | 1.10 | 0.65–1.88 | .72 |
| Utah | 0.77 | 0.52–1.13 | .18 |
| Rural Georgia | 1.11 | 0.69–1.81 | .67 |
| Kentucky | 1.23 | 0.87–1.74 | .24 |
| Louisiana | 1.77 | 1.28–2.44 | <.001 |
| New Jersey | 0.93 | 0.71–1.20 | .57 |
| Percent of adults in census tract or ZIP code with at least some college education | |||
| 0–7.4 | 1 | — | — |
| 7.5–13.0 | 0.95 | 0.75–1.21 | .69 |
| 13.1–21.1 | 0.75 | 0.57–0.99 | .04 |
| 21.2–100 | 0.86 | 0.63–1.18 | .36 |
| Median income in census tract or ZIP code | |||
| $0–$37,413 | 1 | — | — |
| $37,414–$50,066 | 1.22 | 0.96–1.54 | .11 |
| $50,067–$66,720 | 1.04 | 0.77–1.39 | .81 |
| $66,721–$200,008 | 0.98 | 0.70–1.39 | .92 |
| Urban/rural status | |||
| Large metropolitan | 1 | — | — |
| Metropolitan | 1.11 | 0.89–1.37 | .35 |
| Urban | 1.28 | 0.90–1.84 | .17 |
| Less urban | 0.91 | 0.60–1.38 | .67 |
| Rural | 1.24 | 0.61–2.55 | .55 |
| Surgeon density in county of residence† | |||
| 0–8.3 | 1 | — | — |
| 8.4–11.6 | 0.79 | 0.63–0.99 | .04 |
| 11.7–15.4 | 0.93 | 0.74–1.17 | .54 |
| ≥15.5 | 0.86 | 0.67–1.09 | .21 |
Abbreviations: CI = confidence interval; DCIS = ductal carcinoma in situ; EBRT = external beam radiation therapy; HR = hazard ratio; SEER = Surveillance, Epidemiology, and End Results.
Interaction of type of treatment with tumor behavior was significant at P=.01.
Per 100,000 persons in county of residence.
Propensity score analysis
Propensity score matching balanced covariates in treatment groups (Table E4), with 4210 matched pairs treated with EBRT versus lumpectomy alone, 1131 pairs with EBRT versus brachytherapy, and 963 pairs with brachytherapy versus lumpectomy alone (Hosmer-Lemeshow P=.57, P=.50, and P=.10, respectively). Compared with lumpectomy alone, brachytherapy maintained a nonsignificant intermediate subsequent mastectomy risk (HR, 0.63; 95% CI, 0.34–1.16; P=.14). EBRT maintained the lowest risk (HR, 0.16; 95% CI, 0.11–0.26; P<.001) as well as compared with brachytherapy (HR, 0.29; 95% CI, 0.12–0.71; P=.007).
Stratified ASTRO suitability analysis
Among patients with invasive cancer, 34.7% (n=9966) were categorized as suitable, 17.6% (n=5059) as cautionary, 35.2% (n=10,119) as unsuitable, and 12.5% (n=3574) as unclassified. Actual brachytherapy use was correlated with ASTRO group (brachytherapy in 6.3% [627 of 9966], 3.2% [179 of 5509], 1.5% [150 of 10,119], and 4.9% [175 of 3574] of patients in each group, respectively; P<.001). Of all brachytherapy-treated patients, 55% (n=627) were categorized as suitable at presentation (Table 1). When we included all invasive patients regardless of treatment, the subsequent mastectomy risk was increased in the cautionary group (HR, 1.61; 95% CI, 1.22–2.13; P<.001) and unsuitable group (HR, 1.34; 95% CI, 1.04–1.72; P=.03) compared with the referent suitable group (HR, 1.00).
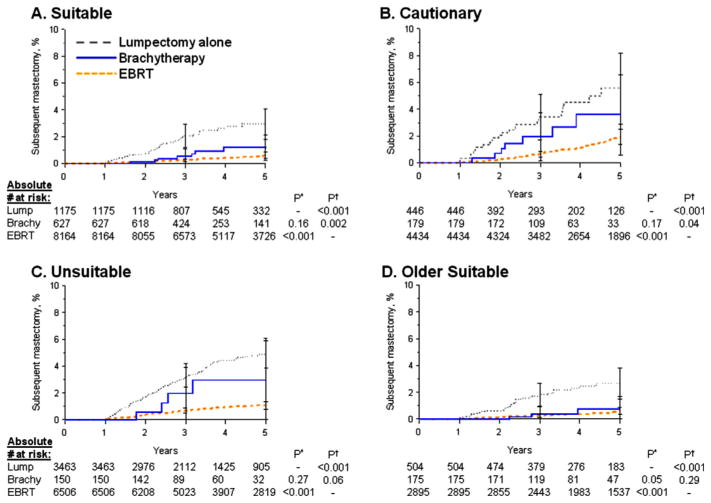
Within each ASTRO group, EBRT consistently showed the lowest subsequent mastectomy risks versus lumpectomy alone, whereas brachytherapy consistently showed intermediate subsequent mastectomy risks versus lumpectomy alone (Fig. 3A–C). Similar magnitudes of relative risks (RRs) within each ASTRO group were confirmed by the nonsignificant interaction term between treatment group and ASTRO group (P=.84 for interaction). Comparing brachytherapy with lumpectomy alone, we found similar risk reductions for suitable (adjusted HR, 0.60; 95% CI, 0.30–1.22; P=.16), cautionary (adjusted HR, 0.55; 95% CI, 0.23–1.29; P=.17), and unsuitable (adjusted HR, 0.57; 95% CI, 0.21–1.56; P=.27) patients, although were not statistically significant.
Fig. 3.
Subsequent mastectomy risk: patients with invasive breast cancer stratified by American Society for Radiation Oncology (ASTRO) suitability groups. P* indicates adjusted P value from proportional hazards regression with lumpectomy alone (Lump) as referent; P† indicates adjusted P value with external beam radiation therapy (EBRT) as referent. Brachy = brachytherapy.
Although ASTRO groups failed to show a differential relative benefit from brachytherapy, the ASTRO suitable group did identify patients with the lowest baseline risk of subsequent mastectomy. Accordingly, within the suitable group, absolute risks after treatment with brachytherapy compared with EBRT were most similar. Specifically, the 5-year cumulative incidence of subsequent mastectomy was 1.6% (95% CI, 0.7%–3.5%) for brachytherapy versus 0.8% (95% CI, 0.6%–1.1%) for EBRT in suitable patients, as compared with 5.4% (95% CI, 2.5%–11.6%) versus 2.2% (95% CI, 1.7%–2.8%) in cautionary patients and with 3.6% (95% CI, 1.3%–9.4%) versus 1.6% (95% CI, 1.3%–2.0%) in unsuitable patients (Table 2).
Brachytherapy outcomes were particularly favorable in older suitable patients (n=7450) (Table 2, PFig. 3D), with a 5-year cumulative incidence of subsequent mastectomy of 3.5% (95% CI, 2.4%–5.0%) for lumpectomy alone, 1.0% (95% CI, 0.3%–3.4%) for brachytherapy, and 0.8% (95% CI, 0.6%–1.1%) for EBRT. The adjusted subsequent mastectomy reduction for brachytherapy versus lumpectomy alone was marginally significant (HR, 0.39; 95% CI, 0.15–1.02; =.05).
Subsequent mastectomy risk: DCIS
During follow-up, 2.2% of DCIS patients (156 of 7229) underwent subsequent mastectomy. The 5-year cumulative incidence of subsequent mastectomy was 3.2% (95% CI, 2.5%–4.1%) (number at risk, 2666) for those treated with lumpectomy alone, 4.6% (95% CI, 2.0%–10.5%) (number at risk, 179) for brachytherapy, and 1.6% (95% CI, 1.3%–2.2%) (number at risk, 4384) for EBRT (Table 2, PFig. 2B). Compared with lumpectomy alone, EBRT showed a lower risk of subsequent mastectomy (HR, 0.40; 95% CI, 0.28–0.55; <.001) (Table 3).
Postoperative complications (all patients)
Within 1 year of diagnosis, the frequency of postoperative infections was 9.9% (95% CI, 9.2%–10.5%) for lumpectomy alone, 16.5% (95% CI, 14.5%–18.5%) for brachytherapy, and 11.4% (95% CI, 11.0%–11.8%) for EBRT (P<.001 for each pair-wise comparison) (Table 3). On adjusted analysis, compared with lumpectomy alone as the referent, brachytherapy was associated with an increased postoperative infection risk (RR, 1.54; 95% CI, 1.33–1.77; P<.001), but EBRT showed no difference (RR, 1.03; 95% CI, 0.94–1.11; P<.001) (Table E5).
The frequency of noninfectious postoperative complications was 6.0% (95% CI, 5.5%–6.5%) for lumpectomy alone, 18.7% (95% CI, 16.6%–20.8%) for brachytherapy, and 9.5% (95% CI, 9.1%–9.8%) for EBRT (P<.001 for each pair-wise comparison) (Table 3). After adjustment, compared with lumpectomy alone as the referent, brachytherapy was associated with an increased noninfectious complication risk (RR, 2.81; 95% CI, 2.43–3.24; P<.001), as was EBRT (RR, 1.37; 95% CI, 1.24–1.52; P<.001) (Table E5).
Local toxicities (all patients)
Brachytherapy was associated with a higher 5-year cumulative incidence of breast pain (22.9%; 95% CI, 20.2%–25.7%) compared with lumpectomy alone (11.2%; 95% CI, 10.5%–12.0%) or EBRT (16.7%; 95% CI, 16.2%–17.2%) (P<.001 for both comparisons). Brachytherapy was associated with a higher cumulative incidence of fat necrosis (15.3%; 95% CI, 13.0%–17.9%) compared with lumpectomy alone (5.3%; 95% CI, 4.8%–5.8%) or EBRT (7.7%; 95% CI, 7.3%–8.0%) (P<.001 for both comparisons). In contrast, brachytherapy—compared with lumpectomy alone and compared with EBRT—yielded no difference in the cumulative incidence of rib fracture (P=.93 and P=.07, respectively) or radiation pneumonitis (P=.08 and P=.07, respectively) (Table 2).
Discussion
In this cohort of older patients, for invasive breast cancer, brachytherapy was associated with an improved likelihood of breast preservation compared with lumpectomy alone but with a lesser magnitude of benefit compared with EBRT. Specifically, we found a 39% adjusted relative benefit for brachytherapy after lumpectomy versus a 78% adjusted relative benefit for EBRT. As practiced in this era, brachytherapy was associated with increased postoperative and local toxicity risks. This analysis adds to the literature a direct comparison of contemporary breast brachytherapy results against both a negative control group (lumpectomy alone) and a positive control group (EBRT) when applied in everyday US practice during the study era.
Previous randomized and population-based studies established a 70% local control benefit for adjuvant EBRT compared with lumpectomy alone for invasive breast cancer (7), consistent with the magnitude of subsequent mastectomy benefit for EBRT reported in our analysis. In contrast, the magnitude of relative improvement in local control for adjuvant brachytherapy compared with lumpectomy alone has, to date, been unclear. In the United States, the use of brachytherapy, a category 2a treatment option, has increased steadily whereas the use of lumpectomy alone, a category 1 treatment option, has remained static (8). This apparent discordance between care patterns and the best evidence underscores the timeliness of our detailed evaluation of brachytherapy versus other treatment options.
Unlike the intermediate breast-preservation benefit associated with brachytherapy in our study, historical comparisons of brachytherapy versus EBRT showed equivalent tumor control outcomes. Patients in these studies were treated by use of interstitial brachytherapy techniques to radiate a 1.5- to 3.0-cm rim of tissue surrounding the lumpectomy cavity (9–11). In contrast, in the vast majority of US patients treated in our study era, single-entry catheters were used, which radiate a smaller volume (1.0-cm rim of tissue) (3, 12). This difference could contribute to our contrasting findings and underscores the need for continued evaluation of modern breast brachytherapy outcomes.
No prior study has shown brachytherapy suitability selection criteria to be valid for identifying patients with a differential treatment benefit after brachytherapy versus EBRT. After stratification by suitability criteria in our study, we found that suitable patients were least likely to undergo subsequent mastectomy and had the smallest absolute difference in mastectomy risk (<1% absolute difference at 5 years) when treated with brachytherapy versus EBRT. This observation suggests that ASTRO criteria are most informative for predicting patients’ baseline risk of mastectomy without radiation and therefore for quantifying absolute gains in breast preservation attributable to radiation, consistent with another recent study evaluating ASTRO criteria (13). However, our analysis showed that the proposed ASTRO groups did not show differential relative treatment benefits, with the adjusted relative benefits of brachytherapy within each ASTRO group nearly identical. Suitable patients aged 70 years or older derived adjuvant benefit from brachytherapy (HR, 0.39) that nearly approached the benefit derived from EBRT (HR, 0.22) (0.2% absolute difference at 5 years).
Limitations
Despite covariate adjustment as well as the validating propensity score model analysis to account for imbalances between treatment groups, residual confounding may still exist in this observational analysis, for example, with factors such as compliance with adjuvant hormonal therapy and margin status, which were unavailable in our data. The outcome of subsequent mastectomy, though clinically relevant (4, 14), is only a proxy for local recurrence, although nevertheless, it is also known to be associated with recurrence risk factors (ER negativity, tumor stage/grade, radiation delay, and incompletion). The low number of patients with DCIS treated with brachytherapy in this study precluded definitive comparison of the effectiveness of brachytherapy in this patient population. Furthermore, we focused our evaluation on ASTRO guidelines; other breast brachytherapy patient selection guidelines exist, and future studies may seek to validate other selection algorithms (15–17).
Absolute risk differences by treatment group were small, and therefore, when placed in a clinical context, counseling of patients about the relative tradeoffs between lumpectomy alone versus either brachytherapy or EBRTrequires discussion of the clinical significance of both absolute and relative treatment effects—particularly in the older, most favorable low-risk patients. Future studies, with longer follow-up will be important, however, because recurrence and mastectomy risks increase with time. Importantly, the complication profile of brachytherapy may have continued improving since2007, as provider experience has increased or newer catheters have provided greater dosimetric flexibility. The generalizability of our findings requires validation in patients treated more recently and in centers that favor intraoperative or interstitial brachytherapy, for whom outcomes could substantially vary because of differing treatment techniques (11, 13, 18, 19). Improved technique could potentially impact breast-preservation rates, decreasing subsequent mastectomies because of treatment toxicity alone. Finally, it is important to acknowledge that whole-breast radiation is not without late toxicities, such as cardiac events and second malignancies, and although such outcomes are difficult to ascertain given the median follow-up of this study, they should not be neglected when one is evaluating the comparative effectiveness and toxicities of different radiation treatment options.
Conclusions
For older women with invasive breast cancer, brachytherapy generally offered a lesser breast-preservation benefit compared with standard EBRT, although absolute differences were minimal in suitable-risk patients. Future studies may seek to identify additional criteria to select optimal patient subgroups in which treatment benefits of brachytherapy are equivalent to EBRT.
Supplementary Material
Summary.
To improve patient selection for breast brachytherapy, “suitability” criteria are proposed but still require empiric validation. In this cohort of older breast cancer patients, there was an increased risk of subsequent mastectomy after breast brachytherapy compared with external beam radiation therapy. Suitability criteria identified patients with the lowest absolute, but not relative, risk of mastectomy.
Acknowledgments
This work was supported by several sources, including research grants from Varian Medical Systems (SR2011-00034954RG 01), the Cancer Prevention & Research Institute of Texas (RP101207), and the National Cancer Institute (grants CA16672 and T32CA77050). The funding sources played no role in any aspect of this study, including no role in data collection, analysis, or interpretation; trial design; patient recruitment; writing of the manuscript; or the decision to submit it for publication.
The interpretation and reporting of our data are the sole responsibility of the authors. The authors acknowledge the efforts of the Applied Research Program, NCI; the Office of Research, Development and Information, CMS; Information Management Services; and the Surveillance, Epidemiology, and End Results Program tumor registries in the creation of the Surveillance, Epidemiology, and End Results–Medicare database.
Footnotes
None of the authors were paid to write the manuscript.
Conflict of interest: none.
Supplementary material for this article can be found at www.redjournal.org.
References
- 1.Smith GL, Xu Y, Buchholz TA, et al. Association between treatment with brachytherapy vs whole-breast irradiation and subsequent mastectomy, complications, and survival among older women with invasive breast cancer. JAMA. 2012;307:1827–1837. doi: 10.1001/jama.2012.3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hattangadi JA, Taback N, Neville BA, et al. Accelerated partial breast irradiation using brachytherapy for breast cancer: patterns in utilization and guideline concordance. J Natl Cancer Inst. 2012;104:29–41. doi: 10.1093/jnci/djr495. [DOI] [PubMed] [Google Scholar]
- 3.Smith BD, Arthur DW, Buchholz TA, et al. Accelerated partial breast irradiation consensus statement from the American Society for Radiation Oncology (ASTRO) Int J Radiat Oncol Biol Phys. 2009;74:987–1001. doi: 10.1016/j.ijrobp.2009.02.031. [DOI] [PubMed] [Google Scholar]
- 4.Hughes KS, Schnaper LA, Berry D, et al. Lumpectomy plus tamoxifen with or without irradiation in women 70 years of age or older with early breast cancer. N Engl J Med. 2004;351:971–977. doi: 10.1056/NEJMoa040587. [DOI] [PubMed] [Google Scholar]
- 5.Hughes LL, Wang M, Page DL, et al. Local excision alone without irradiation for ductal carcinoma in situ of the breast: a trial of the Eastern Cooperative Oncology Group. J Clin Oncol. 2009;27:5319–5324. doi: 10.1200/JCO.2009.21.8560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parsons LS. Reducing bias in a propensity score matched-pair sample using greedy matching techniques. Proceedings of the Twenty-Sixth Annual SAS Users Group International Conference; Cary, NC: SAS Institute; 2001. pp. 214–226. [Google Scholar]
- 7.Albert JM, Pan IW, Shih YC, et al. Effectiveness of radiation for prevention of mastectomy in older breast cancer patients treated with conservative surgery. Cancer. 2012;118:4642–4651. doi: 10.1002/cncr.27457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soulos PR, Yu JB, Roberts KB, et al. Assessing the impact of a cooperative group trial on breast cancer care in the Medicare population. J Clin Oncol. 2012;30:1601–1607. doi: 10.1200/JCO.2011.39.4890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.King TA, Bolton JS, Kuske RR, et al. Long-term results of wide-field brachytherapy as the sole method of radiation therapy after segmental mastectomy for T(is,1,2) breast cancer. Am J Surg. 2000;180:299–304. doi: 10.1016/s0002-9610(00)00454-2. [DOI] [PubMed] [Google Scholar]
- 10.Shah C, Antonucci JV, Wilkinson JB, et al. Twelve-year clinical outcomes and patterns of failure with accelerated partial breast irradiation versus whole-breast irradiation: results of a matched-pair analysis. Radiother Oncol. 2011;100:210–214. doi: 10.1016/j.radonc.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 11.Polgar C, Fodor J, Major T, et al. Breast-conserving treatment with partial or whole breast irradiation for low-risk invasive breast carcinoma—5-year results of a randomized trial. Int J Radiat Oncol Biol Phys. 2007;69:694–702. doi: 10.1016/j.ijrobp.2007.04.022. [DOI] [PubMed] [Google Scholar]
- 12.Shaitelman SF, Vicini FA, Grills IS, et al. Differences in effective target volume between various techniques of accelerated partial breast irradiation. Int J Radiat Oncol Biol Phys. 2012;82:30–36. doi: 10.1016/j.ijrobp.2010.08.059. [DOI] [PubMed] [Google Scholar]
- 13.Leonardi MC, Maisonneuve P, Mastropasqua MG, et al. How do the ASTRO consensus statement guidelines for the application of accelerated partial breast irradiation fit intraoperative radiotherapy? A retrospective analysis of patients treated at the European Institute of Oncology. Int J Radiat Oncol Biol Phys. 2012;83:806–813. doi: 10.1016/j.ijrobp.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 14.Shah C, Vicini F, Keisch M, et al. Outcome after ipsilateral breast tumor recurrence in patients who receive accelerated partial breast irradiation. Cancer. 2012;118:4126–4131. doi: 10.1002/cncr.27400. [DOI] [PubMed] [Google Scholar]
- 15.Polgar C, Van Limbergen E, Potter R, et al. Patient selection for accelerated partial-breast irradiation (APBI) after breast-conserving surgery: recommendations of the Groupe Europeen de Curietherapie-European Society for Therapeutic Radiology and Oncology (GEC-ESTRO) breast cancer working group based on clinical evidence (2009) Radiother Oncol. 2010;94:264–273. doi: 10.1016/j.radonc.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 16.Arthur DW, Vicini FA, Kuske RR, et al. Accelerated partial breast irradiation: an updated report from the American Brachytherapy Society. Brachytherapy. 2003;2:124–130. doi: 10.1016/S1538-4721(03)00107-7. [DOI] [PubMed] [Google Scholar]
- 17. [Accessed August 31, 2012];Consensus Statement for Accelerated Partial Breast Irradiation. Available at: https://www.breastsurgeons.org/statements/PDF_Statements/APBI.pdf.
- 18.Strnad V, Hildebrandt G, Potter R, et al. Accelerated partial breast irradiation: 5-year results of the German-Austrian multicenter phase II trial using interstitial multicatheter brachytherapy alone after breast-conserving surgery. Int J Radiat Oncol Biol Phys. 2011;80:17–24. doi: 10.1016/j.ijrobp.2010.01.020. [DOI] [PubMed] [Google Scholar]
- 19.Vaidya JS, Joseph DJ, Tobias JS, et al. Targeted intraoperative radiotherapy versus whole breast radiotherapy for breast cancer (TARGIT-A trial): an international, prospective, randomised, non-inferiority phase 3 trial. Lancet. 2010;376:91–102. doi: 10.1016/S0140-6736(10)60837-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.