Abstract
Background
Hepatitis B (HBV) is prevalent in certain US populations and regular HBV disease monitoring is critical to reducing associated morbidity and mortality. Adherence to established HBV monitoring guidelines among primary care providers is unknown.
Aims
To evaluate HBV disease monitoring patterns and factors associated with adherence to HBV management guidelines in the primary care setting.
Methods
Primary providers within the San Francisco safety net healthcare system were surveyed for HBV management practices, knowledge, attitudes, and barriers to HBV care. Medical records from 1,727 HBV-infected patients were also reviewed retrospectively.
Results
Of 148 (45%) responding providers, 79% reported ALT and 44% reported HBV viral load testing every 6–12 months. Most providers were knowledgeable about HBV but 43% were unfamiliar with HBV management guidelines. Patient characteristics included: mean age 51 years; 54% male; 67% Asian. Within the past year, 75% had ALT; 24% viral load; 21% HBeAg tested, and 40% of at-risk patients had abdominal imaging for HCC. Provider familiarity with guidelines (OR 1.02, 95%CI 1.00–1.03), Asian patient race (OR 4.18, 95%CI 2.40–7.27), and patient age were associated with recommended HBV monitoring. Provider HBV knowledge and attitudes were positively associated, while provider age and perceived barriers were negatively associated with HCC surveillance.
Conclusions
Comprehensive HBV disease monitoring including HCC screening with imaging were suboptimal. While familiarity with AASLD guidelines and patient factors were associated with HBV monitoring, only provider and practice factors were associated with HCC surveillance. These findings highlight the importance of targeted provider education to improve HBV care.
Keywords: Hepatitis B, Hepatocellular carcinoma, Primary care, Provider education, Practice guidelines, Health disparities
INTRODUCTION
In the United States, an estimated 1.25 million people are chronically infected with HBV (1). Nationwide, up to 70% of HBV-infected persons are foreign born, and the prevalence is rising along with the influx of immigrants from endemic regions (2, 3). It is estimated that the majority of HBV-infected individuals are undiagnosed, and those who are aware of their infection are not receiving recommended medical care (4–6). Early identification and appropriate disease management can reduce the risk of HBV-related complications, namely hepatocellular carcinoma (HCC) and end-stage liver disease (1, 7–9), and is cost-effective in the long-term (10, 11). Despite this, recent studies highlight inadequate monitoring and treatment of at-risk HBV-infected patients (6, 12, 13), and given the negative clinical consequences of chronic infection, improving HBV disease management has been highlighted as a public health priority (14). In this country, HBV infection is a major health disparity that disproportionally affects individuals from predominantly immigrant, low income, and underinsured communities (4, 15, 16). It is well known that these patients who predominantly rely on safety net healthcare resources are prone to experiencing health disparity (17), and therefore, evaluation of HBV management practices in this setting is critical to reducing the disproportionate burden of disease in this population.
The natural course of CHB is highly variable, and understanding the dynamic nature of HBV infection is key to effective disease management and underscores the need for ongoing clinical monitoring. The American Association for the Study of Liver Disease (AASLD) has established evidence-based guidelines to assist healthcare providers in the evaluation, management, and treatment of patients with CHB (1). Depending on HBV disease phenotype, these guidelines recommend monitoring of ALT and HBV viral load every 3–12 months and HBeAg testing every 6–12 months in those with HBeAg-positive disease, though the recommendations for HBeAg testing are less clear for HBeAg-negative patients (1). Liver enzymes (ALT levels) and HBV viral load is used to determine candidacy for HBV therapy. The AASLD also recommends a regular HCC surveillance program for at-risk HBV-infected individuals, with or without cirrhosis, using biannual liver ultrasound (1, 18). Limited recent studies suggest that there is insufficient HBV monitoring and HCC surveillance in clinical practice and that treatment-eligible individuals are not being identified (6, 12, 13, 19), while patient and provider factors associated with inadequate HBV care are not well understood.
In 2010, the Institute of Medicine (IOM) highlighted the importance of improved provider education as a strategy for optimizing HBV care (14). Prior studies that focus on HBV screening have identified certain gaps in provider knowledge of risk factors for HBV acquisition and use of recommended HBV screening tests (19, 20). Less is known about provider familiarity with disease management guidelines and monitoring of those patients with established HBV infection, particularly in the primary care setting. We and others have recently reported a wide range of primary provider familiarity (38–73%) with major HBV screening and management guidelines (21, 22). In addition to these provider factors, there are likely to be specific patient and healthcare system factors that impede delivery of recommended care. In this study, the first of its kind, we sought to characterize patterns of disease monitoring in the San Francisco safety net healthcare system with a large and diverse HBV-infected patient population, both from provider self-report of their practice patterns and review of medical records in order to identify gaps in management and modifiable factors that impact HBV disease monitoring and care.
METHODS
Study Design and Subjects
Between July 2010 and December 2010, a cross-sectional survey of providers within the San Francisco Community Health Network (CHN) was conducted to evaluate provider HBV knowledge, attitudes, and perceived barriers to HBV care, including liver cancer surveillance. There are approximately 330 full and part-time practicing providers within the CHN primary care clinics. The survey was sent to these providers by mail or electronic mail, and a second mailing to non-respondents was conducted after 4 weeks. We also performed a retrospective evaluation of HBV-infected patient’ records, defined by positive hepatitis B surface antigen (HBsAg) testing, followed in the CHN primary clinics during the same study period. The San Francisco safety net health care system provides care to over 150,000 patients, including most uninsured residents in the city and county of San Francisco (23), and is comprised of the San Francisco Department of Public Health (SFDPH), the 23 different Community Consortium-affiliated clinics, and a single acute care and referral facility, San Francisco General Hospital (SFGH). The racial and ethnic distribution of patients within this system reflects the diversity of the San Francisco bay area more broadly (30% Latino, 20% non-Hispanic white, 25% Asian &Pacific Islander, 20% African American, and 5% other). The study was approved by the Committee on Human Research of the University of California, San Francisco.
Survey Design
The survey instrument was developed by study investigators with input from other primary providers, hepatologists, survey design experts, and previously published healthcare surveys. Content domains included provider and practice characteristics, HBV management practices, familiarity with management guidelines, HCC screening practices including modalities used, and provider attitudes about and perceived barriers towards HBV care. The survey was pilot-tested with 20 physicians and revised thereafter.
Data Collection & Data Analysis
De-identified patient data were extracted from electronic medical records and included patient demographics, screening and vaccination practices, liver imaging studies, and laboratory values. From this pool, 1,727 patients met criteria for chronic hepatitis B (HBsAg positive for at least 6 months or without any subsequent HBsAg negative results) and had attended at least one primary care clinic visit over the past 24 months. Based on the available data, patients at risk for HCC were defined as Asian males > 40 years old and females > 50 years old. Data were extracted from provider surveys and included provider and practice characteristics. Data were summarized using mean ±SD, median (range), and frequencies at the practice level and across practices. In order to assess overall provider knowledge, favorable attitudes, and barriers to management, composite scores were formed from responses to the questions designed to assess these factors. Specifically, the knowledge score was computed as a number of correct responses to eleven questions assessing knowledge (1 for correct, 0 for incorrect; max score 11). The attitude score was determined by summing numerical codes assigned to responses to questions designed to assess attitudes (1 for “agree” response, 0.5 for “unsure” response, and 0 for “disagree” response; max score 8). The barrier score was also determined by summing the numerical codes for questions regarding perceived barriers in their practice (1 for “agree” response, 0.5 for “unsure” response, and 0 for “disagree” response; max score 9).
Our two primary outcome variables were 1) adherence to recommended HBV management, defined as a composite of ALT and HBV viral load measured during the previous 12 months and an HBeAg measured at any time point; and 2) adherence to recommended HCC surveillance in at-risk patients, defined as abdominal imaging within the previous 12 months. Patient and provider factors associated with recommended HBV management and HCC surveillance were summarized to the practice level to retain confidentiality, and then evaluated using generalized linear mixed modeling. This approach accommodates predictors measured at either the practice or patient level and clustering of patients within practice. Multivariate logistic regression modeling included predictors identified as important a priori, as well as those determined by univariate analysis to be statistically significant, with a p value <0.05 (two-sided). All analyses were performed using STATA version 12.0 (STATA Corporation, College Station, TX).
RESULTS
Practice Patterns Based on Provider Self-Report
The response rate to the provider survey was 45% (148/329). The proportion of providers who responded to the survey from clinics with high HBV patient load was similar to those who did not respond (45% vs 44%, p=0.42). Characteristics of the 148 responding providers and the practice settings in which they deliver care are summarized in Table 1. The majority of providers were female (71%), Caucasian race (59%), and medical doctors (70%). Nearly half of providers reported that Asian and Pacific Islanders (APIs) comprised greater than 25% of the patients in their practice.
Table 1.
Provider and practice characteristics
| Characteristic | All Providers (N=148) |
|---|---|
| Age, years (N, (%)) | |
| 20–39 | 66 (45) |
| 40–59 | 67 (45.5) |
| ≥ 60 | 11 (7) |
| Not reported | 4 (2.5) |
| Female gender (N, (%)) | 105 (71) |
| Race/Ethnicity (N, (%)) | |
| Caucasian | 87 (59) |
| African-American | 4 (3) |
| Hispanic/Latino | 10 (7) |
| Asian | 35 (24) |
| Other/Not reported | 12 (7) |
| U.S. Born (N, (%)) | 123 (83) |
| Providers with Asian language proficiency (N,(%)) | 23 (16) |
| Post-Graduate Degree (N, (%)) | |
| MD | 103 (70) |
| NP/PA | 38 (26) |
| Other | 7 (4) |
| Specialty (N, (%)) | |
| Internal Medicine | 61 (41) |
| Family Medicine | 66 (45) |
| Infectious Diseases | 5 (3) |
| Other/Not reported | 16 (11) |
| Years in practice (N, (%)) | |
| 0–10 | 86 (58) |
| 11–20 | 37 (25) |
| > 20 | 22 (15) |
| Not reported | 3 (2) |
| Number of patients seen per week (N, (%)), patients | |
| 0–20 | 53 (36) |
| 21–40 | 44 (30) |
| 41–60 | 27 (18) |
| >60 | 20 (13.5) |
| Not reported | 4 (2.5) |
| Provider practice consists of more than 25% Asian patients (N, (%)) | 71 (47) |
| Provider practice consists of more than 50% of patients with limited English proficiency (N,(%)) | 59 (40) |
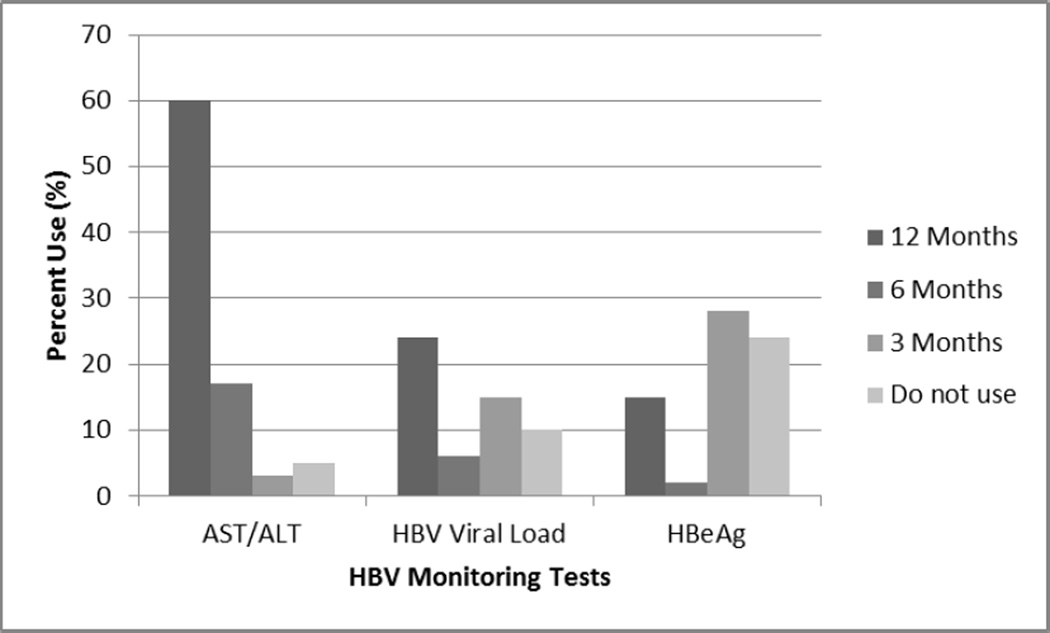
Provider self-reported management practices of HBV-infected patients are summarized in Table 2. Fifty-seven percent of providers reported administration of hepatitis A (HAV) vaccine to greater than 75% of their patients. Seventy percent reported screening more than 75% of their HBV-infected patients for hepatitis C virus (HCV) infection, and 47% for human immunodeficiency virus (HIV) co-infection. Treatment for HBV infection was offered by 19% of primary providers. In terms of HBV monitoring practices (Figure 1), 79% reported monitoring patient ALT levels every 6–12 months, and 44% monitoring HBV viral load levels every 6–12 months. There was a wide range of reported HBeAg testing, including 26% who reported never testing for HBeAg. Of those surveyed, 96% reported regular HCC surveillance in their practice, and nearly half reported that the majority of HBV-infected patients had been screened for HCC. Forty-three percent of respondents were not familiar with AASLD guidelines for HBV management.
Table 2.
Management practices by provider self-report
| HBV Management | All Providers (N=148) |
|---|---|
| Proportion of patients with CHB vaccinated against HAV (N, (%)) | |
| 1–25% | 12 (8) |
| 26–50% | 9 (6) |
| 51–75% | 29 (20) |
| 75% | 84 (57) |
| None | 1 (1) |
| Uncertain/ Not Reported | 13 (9) |
| Proportion of patients with CHB screened for HCV (N, (%)) | |
| 1–25% | 8 (5) |
| 26–50% | 10 (7) |
| 51–75% | 20 (14) |
| 75% | 103 (70) |
| None | 2 (1) |
| Uncertain/ Not Reported | 5 (4) |
| Proportion of patients with CHB screened for HIV (N, (%)) | |
| 1–25% | 16 (11) |
| 26–50% | 25 (17) |
| 51–75% | 29 (20) |
| 75% | 69 (47) |
| None | 2 (1) |
| Uncertain/ Not Reported | 7 (4) |
| Provider offers HBV treatment in their practice (N, (%)) | 28 (19) |
| Self-rated familiarity with AASLD HBV guidelines (N, (%)) | |
| Not Familiar | 64 (43) |
| Somewhat/Very Familiar | 83 (56) |
| Not reported | 1 (1) |
| Provide HCC surveillance for patients with CHB (N, (%)) | 142 (96) |
| Proportion of patients with CHB screened for HCC (N, (%)) | |
| 1–25% | 9 (6) |
| 26–50% | 18 (12) |
| 51–75% | 33 (22) |
| 75% | 68 (46) |
| None | 1 (1) |
| Uncertain/Not Reported | 19 (13) |
Figure 1. Self-reported hepatitis B monitoring tests used by providers.
The figure summarizes the responses of providers with regard to the type of test and the frequency of the test used in their practice for hepatitis B monitoring. AST aspartate aminotransferase, ALT alanine aminotransferase, HBV viral load hepatitis B viral load, HBeAg hepatitis B e antigen.
In terms of survey assessment of knowledge, attitudes, and perceived barriers (Table 3), on average, 73% of providers answered all knowledge questions correctly. Over 90% of providers knew that chronic hepatitis B is often asymptomatic, that uninfected household contacts should be vaccinated against hepatitis B, that vaccinating against hepatitis B can prevent liver cancer, and that treating hepatitis B can prevent cirrhosis. Fewer providers knew that higher levels of HBV viral load are associated with increased risk of cirrhosis (67%), and that liver cancer can occur in the absence of cirrhosis (71%). Regarding attitudes, 65% of providers reported that not screening for liver cancer among patients with hepatitis B is a malpractice risk. Over 95% indicated that good evidence regarding reduction of mortality or society recommendations influenced their decision to perform screening tests. The most common perceived barriers to HBV care were difficulty accessing specialty care resources (26%), lack of awareness of guidelines (24%), lack of clarity of guidelines (25%), and patient financial barriers (15%).
Table 3.
Provider knowledge, attitudes, and barriers towards HBV management and liver cancer screening
| Knowledge (mean score = 8.5 ± 1.76) | Correct Answers (%) |
|---|---|
| Chronic hepatitis B is often asymptomatic | 97% |
| The majority of the world’s population live in hepatitis B-endemic areas | 74% |
| Screening for liver cancer among hepatitis B patients is cost-effective | 64% |
| Vaccination against hepatitis B can prevent liver cancer | 95% |
| Treating hepatitis B can prevent cirrhosis | 90% |
| Uninfected household contacts of hepatitis B carriers do not need to receive hepatitis B vaccination | 92% |
| High levels of hepatitis B viral load are associated with increased risk of cirrhosis | 67% |
| In patients with hepatitis B, liver cancer only occurs in the setting of cirrhosis | 71% |
| All patients with hepatitis B should be treated | 62% |
| The prevalence of chronic hepatitis B remains high in this country due to high rates of acute hepatitis B | 72% |
| The balance of risks and benefits associated with liver cancer screening in patients with chronic hepatitis B are clearly known | 28% |
| Attitudes (mean score = 5.5 ± 1.71) | Percent Agree |
| What factors influence you to order screening tests? | |
| When there is good evidence that screening leads to decreased mortality | 97% |
| When it is recommended by a national organization | 92% |
| When I see an increased frequency of that disease in my population | 72% |
| When my patients ask for it | 64% |
| When it is used as a quality measure at my institution or by insurance companies | 78% |
| When it is covered by health insurance | 62% |
| Not screening for liver cancer among patients with hepatitis B is a malpractice risk | 65% |
| There are mostly other factors | 58% |
| Perceived Barriers (mean score = 1.26 ± 1.35) | Percent Agree |
| Difficulty accessing specialty (GI/Hepatology) care | 26% |
| Language barriers with patient | 9% |
| Patient financial barriers | 15% |
| Lack of blood testing resources | 1% |
| Lack of clarity of liver cancer screening guidelines | 25% |
| Uncertain or unaware of liver cancer screening guidelines | 24% |
| Discomfort with discussing liver cancer screening | 1% |
| Lack of effective treatment for liver cancer | 9% |
| Other barriers (listed by provider) | 16% |
Practice Patterns Based on Review of Electronic Medical Records
Electronic medical records of 1,727 patients with chronic HBV infection were evaluated (Table 4). Just over half were male, the mean age was 51 years, and the majority (67%) were Asian American. Sixty percent of patients were covered by public insurance, while nearly 40% were uninsured, many of whom rely on the Healthy San Francisco program for access to care. Healthy San Francisco is not medical insurance but a program operated by the San Francisco Department of Public Health designed to make healthcare more accessible and affordable to uninsured San Francisco residents (www.healthysanfrancisco.org). Testing for HAV immunity (HAV total antibody) was performed in 81% of patients, and of those tested, 84% (1,173/1,398) were immune (antibody positive). Twenty four percent of HBV-infected patients were vaccinated during this period and of these, 77% (322/420) had prior documentation of HAV immune status, while 23% (98/420) were vaccinated without prior testing. Thirty-two percent (71/225) of HAV-susceptible patients were not vaccinated, yet 14% (168/1173) of HAV-immune patients did receive HAV vaccination. HCV antibody testing was performed in 35% of patients overall, and while only one-third of Asians and Caucasians were tested (31% and 34%, respectively), nearly half of African-American and Latino patients (48% and 44%, respectively) were tested for HCV. For the 24% of patients screened for HIV, rates were again higher among African-Americans (37%) and Latinos (37%), as compared to Asians (20%) and Caucasians (29%).
Table 4.
Patient characteristics and documented HBV monitoring and management
| Characteristic | All Patients (N=1,727) |
|---|---|
| Age (mean ± SD), Years | 51 ± 12 |
| Male gender (N, (%)) | 926 (54) |
| Race/Ethnicity (N, (%)) | |
| Asian/ Pacific Islander | 1,164 (67) |
| Caucasian | 220 (13) |
| African-American | 206 (12) |
| Hispanic/Latino | 93 (5) |
| Other | 44 (3) |
| Patients Uninsured | 649 (38) |
| HAV screening performed (N, (%)) | 1,398 (81) |
| Received HAV vaccination (N, (%)) | 420 (24) |
| HCV screening performed (N, (%)) | 597 (35) |
| HIV screening performed (N, (%)) | 417 (24) |
| ALT measurement performed (N, (%)) | 1,646 (95) |
| Most recent ALT measurement within 12 months (N, (%)) | 1,291 (75) |
| ALT levels (median (min-max)) | 28 (10–193) |
| HBV viral load measurement (N, (%)) | 813 (47) |
| HBV DNA ≥2000 IU/mL | 326 (40) |
| HBV Viral Load Measurement within 12 months (N, (%)) | 413 (24) |
| HBeAg testing (N, (%)) | |
| HBeAg Negative | 1,024 (59) |
| HBeAg Positive | 207 (12) |
| Not Performed | 496 (29) |
| Most recent HBeAg test within 12 months (N, (%)) | 363 (21) |
| AFP measurement performed (N, (%)) | 1,100 (64) |
| Abdominal imaging (N, (%)) | |
| None | 1,142 (66) |
| Ultrasound | 348 (20) |
| CT | 235 (14) |
| MRI | 2 (0.1) |
| Combination of imaging studies | 410 (24) |
| Any HCC screening within 12 months (N, (%)) | 847 (51%) |
Serum ALT measurement was performed in 95% (1,646) of all patients, of which 75% (1,291/1,646) had testing within the past 12 months, and 60% (991/1,646) within the past 6 months. HBV viral load was assessed in 47% (813) of all patients, 51% (413) within the past 12 months. HBeAg serologic testing was performed in 71% (1,231) overall, but only 29% (363/1,231) were tested within the past 12 months. The majority (83%, 1,024/1,231) of those tested were HBeAg negative. In total, 369 patients or 21% of the patient population met our defined criteria for minimal recommended clinical monitoring: testing of ALT and HBV viral load within the past 12 months, and HBeAg at any time point.
HCC Surveillance
Just over half (51%) of all patients with chronic HBV infection underwent some form of HCC surveillance within the past 12 months (847/1,727). Of those screened, half (434/847) had AFP measurement alone, 36% (301/847) had combined AFP measurement and an abdominal imaging, and 13% (112/847) had imaging alone. The majority (66%) of all HBV-infected patients had no prior abdominal imaging. Among the 848 at-risk patients, defined as Asian men over 40 and Asian women over 50 years of age, 63% (531/848) had HCC surveillance over the past 12 months, and again, the most common screening modality was AFP alone in 47% (252/531) followed by abdominal imaging with or without AFP in 41% (218/531). The most common imaging modality used for screening of individuals in this at-risk population was the combination of ultrasound and a cross sectional imaging study such as CT or MRI (54%; 150/279), followed by ultrasound alone (36%; 101/279).
Patient and Provider Characteristics Associated with Recommended HBV Monitoring
On univariate analysis, patient characteristics that were positively associated with receipt of recommended HBV monitoring included male gender (OR 1.45, 95% CI 1.09–1.93, p=0.010) and Asian race (OR 4.51, 95% CI 2.53–8.05, p<0.0001). Positively associated provider and practice characteristics included provider Asian race (OR 1.01, 95% CI 1.00–1.02, p=0.015), provider fluency in an Asian language (OR 1.01, 95% CI 1.00–1.02, p=0.002), having more than 25% Asian patients in practice (OR 1.01, 95% CI 1.01–1.02, p=0.005), and provider familiarity with AASLD guidelines for HBV management (OR 1.02, 95% CI 1.01–1.03, p<0.001). Having a higher median patient load in practice was also positively associated with recommended HBV monitoring (OR 1.22, 95% CI 0.96–1.55, p=0.099), but this did not reach statistical significance. On multivariate analysis (Table 5), when controlling for certain patient, provider, and practice factors, only Asian patient race (OR 4.18, 95% CI 2.40–7.27, p<0.001) and provider familiarity with AASLD guidelines (OR 1.02, 95% CI 1.00–1.03, p=0.010) were positively associated, while older patient age was negatively associated with recommended HBV monitoring (OR 0.99 per decade, 95% CI 0.98–1.00, p=0.026).
Table 5.
Multivariable analyses of factors associated with HBV monitoring and HCC surveillance
| Chronic HBV Monitoring | ||
| Variable | Odds Ratio (95% CI) | *P-Value |
| Patient age (per decade) | 0.99 (0.98–1.00) | 0.026 |
| Patient male gender | 1.08 (0.92–1.28) | 0.358 |
| Patient race (vs. Caucasian) | ||
| Asian | 4.18 (2.40–7.27) | <0.001 |
| Black | 1.46 (0.74–2.85) | 0.273 |
| Latino | 2.13 (0.75–6.04) | 0.156 |
| Other | 3.88 (1.59–7.80) | 0.003 |
| Patient insured | 0.86 (0.69–1.07) | 0.177 |
| Provider age (per decade) | 0.87 (0.60–1.27) | 0.480 |
| Provider male gender | 0.99 (0.98–1.00) | 0.518 |
| Asian provider race (vs. non-Asian) | 1.00 (0.99–1.01) | 0.638 |
| Clinic practice comprised of > 25% Asian patients | 1.00 (0.99–1.00) | 0.444 |
| Provider knowledge score | 1.06 (0.79–1.40) | 0.712 |
| Provider attitude score | 0.99 (0.78–1.27) | 0.965 |
| Provider barrier score | 1.06 (0.78–1.43) | 0.722 |
| Provider familiarity with AASLD HBV guidelines | 1.02 (1.00–1.03) | 0.012 |
| HCC Surveillance | ||
| Variable | Odds Ratio (95% CI) | *P-Value |
| Patient male gender | 0.94 (0.74–1.19) | 0.616 |
| Patient insured | 1.32 (0.74–2.36) | 0.353 |
| Provider age (per decade) | 0.18 (0.07–0.45) | <0.001 |
| Provider male gender | 0.99 (0.98–1.04) | 0.867 |
| Asian provider race (vs. non-Asian) | 1.02 (1.01–1.03) | <0.001 |
| Clinic practice comprised of >25% Asian patients | 0.97 (0.95–0.99) | 0.006 |
| Provider knowledge score | 1.99 (1.14–3.48) | 0.016 |
| Provider attitude score | 1.48 (1.08–2.03) | 0.014 |
| Provider barrier score | 0.45 (0.24–0.85) | 0.013 |
| Provider familiarity with AASLD HBV guidelines | 0.99 (0.97–1.00) | 0.095 |
Patient and Provider Characteristics Associated with Recommended HCC Surveillance
On univariate analysis, physicians were significantly more likely to provide recommended HCC surveillance as compared to nurse practitioners and physicians assistants (OR 1.03, 95% CI 1.01–1.04, p=0.001), while older providers were less likely to do so (OR 0.57 per decade, 95% CI 0.39–0.85, p=0.005). On multivariate analysis, provider characteristics positively associated with HCC surveillance included provider Asian race (OR 1.02, 95% CI 1.01–1.03, p<0.001), provider knowledge score (OR 1.99, 95% CI 1.14–3.48, p=0.016), and provider attitudes towards screening (OR 1.48, 95% CI 1.08–2.03, p=0.014), while older provider age (OR 0.18 per decade, 95% CI 0.07–0.44, p<0.001), higher barrier score (OR 0.45, 95% CI 0.24–0.85, p=0.013), and having more than 25% Asian patients in practice (OR 0.97, 95% CI 0.95–0.99, p=0.006) were negatively associated with HCC surveillance.
DISCUSSION
To our knowledge, this is the first study to investigate patient, provider, and practice factors that impact recommended CHB management as well as patterns of HBV disease monitoring within a diverse safety net patient population. Our findings confirm that in the primary care setting, by both provider report and review of patient records, most HBV-infected patients were being monitored with at least annual ALT testing. However, the frequency of HBV viral load and HBeAg monitoring was suboptimal. Additionally, while nearly 100% of providers indicated that they perform HCC surveillance in patients with HBV infection, 60% of at-risk individuals had evidence of HCC surveillance in the past year, most often with AFP alone or in combination with liver imaging. We found that provider familiarity with AASLD guidelines and Asian patient race were independently associated with recommended HBV monitoring, whereas only provider factors including age, Asian race, knowledge, attitudes, and perceived barriers, as well as having a high proportion of Asian patients in practice, were associated with recommended HCC surveillance.
The AASLD guidelines are the most widely acknowledged evidence-based recommendations for the preferred diagnostic, therapeutic, and preventative approaches to chronic HBV care in the United States (1). It is not clearly known how these guidelines are applied in the primary practice setting, where the majority of HBV infected individuals in the US receive care. In our study, over 40% of primary care providers were unfamiliar with AASLD guidelines by self-report. While more than two-thirds of providers reported ALT and HBV viral load testing every 6–12 months, use of HBeAg testing was limited. Indeed, 75% of patients had evidence of ALT testing by review of medical records within the past 12 months, while only 25% had HBV viral load testing, and the variable frequency of HBeAg testing reflected the lack of provider clarity on the utility of this test in practice. These findings are in keeping with the recent IOM report (14) recommending provider education to improve HBV care.
It is recommended that all patients with chronic liver disease be tested for HAV and vaccinated if appropriate (24), and over 80% of patients were in fact tested for HAV. Of those tested, one-third of non-immune individuals did not go on to receive HAV vaccination. Additionally, 23% of patients were vaccinated without prior testing of immunity, and 14% of immune patients were still vaccinated against HAV. Therefore, while providers appear to be familiar with the need to screen for HAV, there are opportunities for improving provider interpretation of results and creating protocols for the systematic vaccination of at-risk patients. With respect to screening for HCV and HIV in the context of HBV infection, recommendations have been primarily based on risk factor assessment (2). African Americans and Latinos in this study were tested at a higher frequency for both HCV and HIV, which may reflect provider assessment of individual risk factors for HBV acquisition and potential for co-infection. Updated age cohort HCV screening recommendations for all individuals born between 1945–1965 (25) may increase HCV screening rates among HBV-infected Asians and Caucasians who may not have been previously tested based on traditional risk factors.
Periodic HBV disease monitoring is critical to identify those patients at risk for disease progression and adverse outcomes, as well as candidates for antiviral therapy. While ALT is an imperfect marker of viral activity, and in fact may be normal in 20% of patients with advanced fibrosis (26), in resource limited settings, regular evaluation of liver enzymes is a key component of basic disease monitoring and may prompt providers to send additional tests or refer to specialist care. However, the fact that HBV viral load was tested in 50% of patients at any time point and only 24% within the past year is concerning, given that over 40% of those tested had levels above 2,000 IU/mL. Prior studies also highlight low rates of disease monitoring among HBV-infected patients, including a chart review of a low-income patient population in Los Angeles county reporting that only 28% of HBV-infected patients had received laboratory evaluation with either HBeAg or HBV viral load (13). Lack of recommended monitoring impedes provision of appropriate disease management and likely plays a central role in the exceedingly low HBV antiviral treatment rates as highlighted by several recent studies (6, 12, 13). Targeted primary provider education aimed at increasing familiarity with monitoring guidelines is therefore likely to play a significant role in improving HBV care.
The most recent AASLD guidelines recommend semiannual HCC surveillance with abdominal ultrasound, with or without serial AFP measurement, given that the interpretation of ultrasound findings is operator dependent and access to imaging may be limited in some cases (18, 27). In the United States, the incidence of HCC has risen dramatically in recent years, and over 70% of HBV-related cases of liver cancer occur among Asian Americans (28, 29). While HCC surveillance practices in the primary care setting are not well known, insufficient screening has been highlighted by this and other studies (28, 30). Our prior study of HBV-infected Asian Americans within the San Francisco safety net system revealed that while 67% had HCC screening within the past calendar year, the rate of annual screening declined to 23% after 10 years of follow up (30). In the current study, nearly all surveyed providers reported regular HCC screening in their practices, and two-thirds felt that such screening was cost-effective. However, only half of at-risk Asian Americans were screened within the previous year, most often with AFP alone. While AFP may be the most feasible screening modality in a safety-net system, the current guidelines specify use of abdominal ultrasound as the optimal modality (1, 31). Interestingly, the utilization of cross-sectional imaging alone or use multiple imaging modalities was as frequent as ultrasound for HCC surveillance highlighting further educational opportunities to improve rates of both recommended and cost-effective care.
In this study, we identified several patient and provider factors influencing HBV management. Primary providers reported having a high proportion of Asian American patients in their practice and accordingly, were overall quite knowledgeable about HBV disease. However, familiarity with AASLD HBV disease monitoring guidelines was limited and was independently associated with delivery of recommended HBV care. While several studies have reported on suboptimal provider knowledge of HBV screening guidelines in at risk individuals (5, 19, 21, 32), less is known about provider knowledge of HBV disease monitoring practices among those chronically infected. This is the first study to show that provider knowledge of HBV management guidelines influences disease monitoring within the primary care setting. While similar to prior studies, higher provider knowledge score was associated with HCC screening (21, 38, 39), in contrast to HBV monitoring, provider familiarity with AASLD guidelines per se did not appear to play a significant role in HCC screening in this study, However, both provider attitudes and perceived barriers to HBV management were independent predictors of HCC screening practices. These findings complement a study by Nguyen et al., which found that certain provider beliefs were strongly associated with reported HCC screening and screening behaviors were influenced primarily by quality control measures and fear of malpractice (33).
With respect to patient related factors, Asian Americans were four times more likely to receive recommended HBV monitoring as compared with Caucasians. This is in contrast to a recent study that did not find Asian race to be particularly associated with HBV evaluation or treatment (13), and surprising given that overall, Asian Americans are at increased risk for inadequate preventive health care (34–36), and have low rates of HBV knowledge and disease awareness (4, 5, 37). There are several possible explanations for our findings. First, the San Francisco Bay Area has a high density of Asian immigrants with a reported HBV prevalence of 9–15% (4, 38, 39), and as such, providers may have increased familiarity with HBV management in this population. Additionally, a large grassroots campaign aimed at improving HBV awareness in the Asian American community has been ongoing in this region and an analysis of the impact of San Francisco Hepatitis B Free Campaign found that a high proportion of Asian respondents were familiar with hepatitis B (90%) and that almost half reported an increase in hepatitis B awareness within their community (40). These factors are likely to have played a role in the higher rates of HBV disease monitoring among Asian American patients in this study.
The main limitations of this study are the retrospective design and a provider response rate of 45%, while the primary strengths include large patient sample size as well as the prospective provider survey assessment. Given that there was no significant difference in the proportion of provider survey responders versus non-responders from clinics with high or low HBV patient load, it is likely that survey responses are representative of the providers within this safety net health system. While it is known that provider self-report tends to overestimate actual practice behavior (41), this study is unique in that we evaluated both practice patterns based on provider report and also through examination of patient records for the actual care received. Additionally, while a large majority of HBV-infected patients in this country are from immigrant and underserved populations, generalizability of the findings to non-safety net populations may be limited. Nevertheless, important gaps in HBV management were identified that can be targeted by future interventions directed at both providers and patients.
In summary, the majority of HBV-infected patients in this study received periodic disease monitoring with ALT measurement, but use of HBV viral load and HBeAg testing in the primary care setting was limited. Since these tests also determine criteria for treatment candidacy, it is likely that insufficient evaluation of these disease markers may underlie low HBV treatment rates (6, 12, 13). In addition, there was suboptimal utilization of imaging to screen for HCC in the at-risk Asian population. While most patients were receiving recommended preventative care in the setting of HBV, gaps in HAV vaccination practices were also identified. Importantly, we uncovered certain provider, patient, and practice factors that were associated with recommended HBV monitoring and HCC surveillance. These findings highlight the importance of targeted provider education to improve overall care for chronic HBV. Further in-depth assessment of patient-specific factors, including knowledge, attitudes, and perceived barriers to HBV disease and its management, also represents an important area of future investigation.
Acknowledgments
Financial Support: This work was in part supported by Hepatology Training Grant DK060414 (B.B.), San Francisco General Hospital Foundation Grant (M.K.), and P30 DK026743 (UCSF Liver Center).
Abbreviations
- ALT
alanine aminotransferase
- HBV
Hepatitis B virus
- HBsAg
Hepatitis B surface antigen
- HBeAg
Hepatitis B e antigen
- HAV
Hepatitis A virus
- HCV
Hepatitis C virus
- HIV
Human immunodeficiency virus
- HCC
Hepatocellular carcinoma
- AASLD
American Association for the Study of Liver Diseases
Footnotes
Potential Competing Interests: “None”
Specific Author Contributions:
Blaire E. Burman: Analysis and interpretation of data, drafting and revision of the manuscript
Nizar A. Mukhtar: Interpretation of data, critical revision of the manuscript
Brian C. Toy: Study concept design, acquisition, analysis, and interpretation of data, and critical revision of the manuscript
Tung T. Nguyen: Interpretation of results, critical revision of the manuscript
Alice Hm Chen: Interpretation of results, critical revision of the manuscript
Albert Yu: Acquisition of data, interpretation of results, critical revision of the manuscript
Peter Berman: Interpretation of results, critical revision of the manuscript
Hali Hammer: Interpretation of results, critical revision of the manuscript
Daniel Chan: Interpretation of results, critical revision of the manuscript
Charles E. McCulloch: Statistical data analysis and interpretation, critical revision of the manuscript
Mandana Khalili, MD: Study concept design, acquisition of data, analysis and interpretation of results, drafting manuscript, material support, study supervision
References
- 1.Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50:661–662. doi: 10.1002/hep.23190. [DOI] [PubMed] [Google Scholar]
- 2.Weinbaum CM, Williams I, Mast EE, et al. Recommendations for identification and public health management of persons with chronic hepatitis B virus infection. MMWR Recomm Rep. 2008;57:1–20. [PubMed] [Google Scholar]
- 3.Mitchell T, Armstrong GL, Hu DJ, et al. The increasing burden of imported chronic hepatitis B--United States, 1974–2008. PLoS One. 2011;6:e27717. doi: 10.1371/journal.pone.0027717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin SY, Chang ET, So SK. Why we should routinely screen Asian American adults for hepatitis B: a cross-sectional study of Asians in California. Hepatology. 2007;46:1034–1040. doi: 10.1002/hep.21784. [DOI] [PubMed] [Google Scholar]
- 5.Hu KQ, Pan CQ, Goodwin D. Barriers to screening for hepatitis B virus infection in Asian Americans. Dig Dis Sci. 2011;56:3163–3171. doi: 10.1007/s10620-011-1840-6. [DOI] [PubMed] [Google Scholar]
- 6.Cohen C, Holmberg SD, McMahon BJ, et al. Is chronic hepatitis B being undertreated in the United States? J Viral Hepat. 2011;18:377–383. doi: 10.1111/j.1365-2893.2010.01401.x. [DOI] [PubMed] [Google Scholar]
- 7.Chen CJ, Iloeje UH, Yang HI. Long-term outcomes in hepatitis B: the REVEAL-HBV study. Clin Liver Dis. 2007;11:797–816. viii. doi: 10.1016/j.cld.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 8.Scaglione SJ, Lok AS. Effectiveness of hepatitis B treatment in clinical practice. Gastroenterology. 2012;142:1360–1368. e1. doi: 10.1053/j.gastro.2012.01.044. [DOI] [PubMed] [Google Scholar]
- 9.Liaw YF, Sung JJ, Chow WC, et al. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med. 2004;351:1521–1531. doi: 10.1056/NEJMoa033364. [DOI] [PubMed] [Google Scholar]
- 10.Post SE, Sodhi NK, Peng CH, et al. A simulation shows that early treatment of chronic hepatitis B infection can cut deaths and be cost-effective. Health Aff (Millwood) 2011;30:340–348. doi: 10.1377/hlthaff.2008.0905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rajendra A, Wong JB. Economics of chronic hepatitis B and hepatitis C. J Hepatol. 2007;47:608–617. doi: 10.1016/j.jhep.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 12.Zhang S, Ristau JT, Trinh HN, et al. Undertreatment of Asian chronic hepatitis B patients on the basis of standard guidelines: a community-based study. Dig Dis Sci. 2012;57:1373–1383. doi: 10.1007/s10620-012-2137-0. [DOI] [PubMed] [Google Scholar]
- 13.Jung CW, Tan J, Tan N, et al. Evidence for the insufficient evaluation and undertreatment of chronic hepatitis B infection in a predominantly low-income and immigrant population. J Gastroenterol Hepatol. 2010;25:369–375. doi: 10.1111/j.1440-1746.2009.06023.x. [DOI] [PubMed] [Google Scholar]
- 14.Mitchell AE, Colvin HM, Palmer Beasley R. Institute of Medicine recommendations for the prevention and control of hepatitis B and C. Hepatology. 2010;51:729–733. doi: 10.1002/hep.23561. [DOI] [PubMed] [Google Scholar]
- 15.Pollack H, Wang S, Wyatt L, et al. A comprehensive screening and treatment model for reducing disparities in hepatitis B. Health Aff (Millwood) 2011;30:1974–1983. doi: 10.1377/hlthaff.2011.0700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.El-Serag H, McGlynn KA, Graham GN, et al. Achieving health equity to eliminate racial, ethnic, and socioeconomic disparities in HBV- and HCV-associated liver disease. J Fam Pract. 2010;59:S37–S42. [PMC free article] [PubMed] [Google Scholar]
- 17.Guy J, Yee HF., Jr Health disparities in liver disease: Time to take notice and take action. Hepatology. 2009;50:309–313. doi: 10.1002/hep.22942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bruix J, Sherman M. American Association for the Study of Liver D. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lai CJ, Nguyen TT, Hwang J, et al. Provider knowledge and practice regarding hepatitis B screening in Chinese-speaking patients. J Cancer Educ. 2007;22:37–41. doi: 10.1007/BF03174373. [DOI] [PubMed] [Google Scholar]
- 20.Ferrante JM, Winston DG, Chen PH, et al. Family physicians' knowledge and screening of chronic hepatitis and liver cancer. Fam Med. 2008;40:345–351. [PubMed] [Google Scholar]
- 21.Khalili M, Guy J, Yu A, et al. Hepatitis B and hepatocellular carcinoma screening among Asian Americans: survey of safety net healthcare providers. Dig Dis Sci. 2011;56:1516–1523. doi: 10.1007/s10620-010-1439-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Upadhyaya N, Chang R, Davis C, et al. Chronic hepatitis B: perceptions in Asian American communities and diagnosis and management practices among primary care physicians. Postgrad Med. 2010;122:165–175. doi: 10.3810/pgm.2010.09.2213. [DOI] [PubMed] [Google Scholar]
- 23.Bindman AB, Chen A, Fraser JS, et al. Healthcare reform with a safety net: lessons from San Francisco. Am J Manag Care. 2009;15:747–750. [PubMed] [Google Scholar]
- 24.Advisory Committee on Immunization P. Fiore AE, Wasley A, et al. Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2006;55:1–23. [PubMed] [Google Scholar]
- 25.Smith BD, Morgan RL, Beckett GA, et al. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945–1965. MMWR Recomm Rep. 2012;61:1–32. [PubMed] [Google Scholar]
- 26.Hu KQ, Schiff ER, Kowdley KV, et al. Histologic evidence of active liver injury in chronic hepatitis B patients with normal range or minimally elevated alanine aminotransferase levels. J Clin Gastroenterol. 2010;44:510–516. doi: 10.1097/MCG.0b013e3181d34c65. [DOI] [PubMed] [Google Scholar]
- 27.El-Serag HB, Marrero JA, Rudolph L, et al. Diagnosis and treatment of hepatocellular carcinoma. Gastroenterology. 2008;134:1752–1763. doi: 10.1053/j.gastro.2008.02.090. [DOI] [PubMed] [Google Scholar]
- 28.Nguyen VT, Law MG, Dore GJ. Hepatitis B-related hepatocellular carcinoma: epidemiological characteristics and disease burden. J Viral Hepat. 2009;16:453–463. doi: 10.1111/j.1365-2893.2009.01117.x. [DOI] [PubMed] [Google Scholar]
- 29.Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol. 2009;27:1485–1491. doi: 10.1200/JCO.2008.20.7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sarkar M, Stewart S, Yu A, et al. Hepatocellular carcinoma screening practices and impact on survival among hepatitis B-infected Asian Americans. J Viral Hepat. 2012;19:594–600. doi: 10.1111/j.1365-2893.2011.01577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bruix J, Sherman M. Practice Guidelines Committee AAftSoLD. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 32.Foster T, Hon H, Kanwal F, et al. Screening high risk individuals for hepatitis B: physician knowledge, attitudes, and beliefs. Dig Dis Sci. 2011;56:3471–3487. doi: 10.1007/s10620-011-1928-z. [DOI] [PubMed] [Google Scholar]
- 33.Nguyen TT, Gildengorin G, Truong A, et al. Factors influencing physicians' screening behavior for liver cancer among high-risk patients. J Gen Intern Med. 2007;22:523–526. doi: 10.1007/s11606-007-0128-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wong ST, Gildengorin G, Nguyen T, et al. Disparities in colorectal cancer screening rates among Asian Americans and non-Latino whites. Cancer. 2005;104:2940–2947. doi: 10.1002/cncr.21521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wen XJ, Balluz L. Racial disparities in access to health care and preventive services between Asian Americans/Pacific Islanders and Non-Hispanic Whites. Ethn Dis. 2010;20:290–295. [PubMed] [Google Scholar]
- 36.Ye J, Mack D, Fry-Johnson Y, et al. Health Care Access and Utilization Among US-Born and Foreign-Born Asian Americans. J Immigr Minor Health. 2012;14:731–737. doi: 10.1007/s10903-011-9543-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taylor VM, Yasui Y, Burke N, et al. Hepatitis B knowledge and testing among Vietnamese-American women. Ethn Dis. 2005;15:761–767. [PubMed] [Google Scholar]
- 38.Kim WR. Epidemiology of hepatitis B in the United States. Hepatology. 2009;49:S28–S34. doi: 10.1002/hep.22975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Centers for Disease C, Prevention. Screening for chronic hepatitis B among Asian/Pacific Islander populations--New York City, 2005. MMWR Morb Mortal Wkly Rep. 2006;55:505–509. [PubMed] [Google Scholar]
- 40.Shiau R, Bove F, Henne J, et al. Using survey results regarding hepatitis B knowledge, community awareness and testing behavior among Asians to improve the San Francisco Hep B Free campaign. J Community Health. 2012;37:350–364. doi: 10.1007/s10900-011-9452-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rauscher GH, Johnson TP, Cho YI, et al. Accuracy of self-reported cancer-screening histories: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2008;17:748–757. doi: 10.1158/1055-9965.EPI-07-2629. [DOI] [PubMed] [Google Scholar]