Abstract
Anemia of inflammation or chronic disease is a highly prevalent form of anemia. The inflammatory cytokine interleukin-6 (IL-6) negatively correlates with hemoglobin concentration in many disease states. The IL-6-hepcidin antimicrobial peptide axis promotes iron-restricted anemia; however the full role of IL-6 in anemia of inflammation is not well-defined. We previously reported that chronic inflammation had a negative impact on maturation of erythroid progenitors in a mouse model. We hypothesized that IL-6 may be responsible for impaired erythropoiesis, independent of iron restriction. To test the hypothesis we utilized the human erythroleukemia TF-1 cell line to model erythroid maturation and exposed them to varying doses of IL-6 over six days. At 10 ng/ml, IL-6 significantly repressed erythropoietin-dependent TF-1 erythroid maturation. While IL-6 did not decrease the expression of genes associated with hemoglobin synthesis, we observed impaired hemoglobin synthesis as demonstrated by decreased benzidine staining. We also observed that IL-6 down regulated expression of the gene SLC4a1 which is expressed late in erythropoiesis. Those findings suggested that IL-6-dependent inhibition of hemoglobin synthesis might occur. We investigated the impact of IL-6 on mitochondria. IL-6 decreased the mitochondrial membrane potential at all treatment doses, and significantly decreased mitochondrial mass at the highest dose. Our studies indicate that IL-6 may impair mitochondrial function in maturing erythroid cells resulting in impaired hemoglobin production and erythroid maturation. Our findings may indicate a novel pathway of action for IL-6 in the anemia of inflammation, and draw attention to the potential for new therapeutic targets that affect late erythroid development.
Keywords: Erythropoiesis, Inflammation, Hemoglobin, Mitochondria, Cytokine, Anemia
INTRODUCTION
Anemia of inflammation or chronic disease (AICD) is a common form of anemia [1], especially in older adults [2; 3], and associated with conditions such as cancers [1; 4; 5], autoimmune disease [6; 7], chronic kidney disease [8; 9], chronic and acute infections [4; 10], and aging [2; 3; 11; 12; 13]. Decreased hemoglobin concentration has been associated with increased mortality risk [3; 11; 14; 15; 16; 17; 18], decreased skeletal muscle strength [13], decreased mobility [19], cognitive decline [20; 21], and overall decreased quality of life [22; 23; 24] suggesting that AICD may not be an “innocent” bystander [4]. The burden of AICD on the elderly is especially notable, with 10–32% of cases of anemia in patients over 65 containing an underlying inflammatory component [2; 25; 26; 27]. Few evidence-based treatment strategies are in place for AICD because the anemia usually resolves when the underlying disease is treated. However, chronic diseases in older adults are often “managed” rather than “cured”. In order to identify the best treatment strategies for patients with anemia in various disease settings, we need to fully understand the molecular pathways connecting inflammation to decreased hemoglobin.
The mitochondrion is the site of heme biosynthesis, and thus mitochondria are necessary for generation of functional, mature erythrocytes [28; 29; 30]. Impaired mitochondrial function plays a role in anemias such as Fanconi Anemia [31; 32], sideroblastic anemia [33], and megaloblastic anemia [34]. Although mitochondrial dysfunction is thought to play a role in many of the diseases that associate with AICD [35; 36; 37; 38; 39], a direct link to impaired mitochondrial function and AICD has not been established. Inflammatory cytokines TNFα [40; 41; 42; 43], IL-1β [40; 43], IL-1α [42], IFNγ [43] and IL-6 [44] have been shown to negatively impact on mitochondrial function. It has also been suggested that mitochondrial dysfunction could modulate the inflammatory process [45; 46], which could create a cycle of increasing inflammation and further mitochondrial dysfunction.
Interleukin-6 (IL-6) is an inflammatory cytokine and a fundamental component of the acute phase response, where it is involved in the recruitment of neutrophils to the site of injury [47]. IL-6 can affect cells by binding its receptor and initiating the classical signaling pathway or through binding to a soluble receptor and initiating the trans-signaling pathway [48]. It is thought that IL-6 mediates the switch from acute to chronic inflammation via activation of trans-signaling [49]. IL-6 levels correlate negatively with hemoglobin concentration in conditions such as rheumatoid arthritis [50], lupus [51], multiple myeloma [52], ovarian cancer [53], and frailty [54]. By stimulating the production of the iron regulatory hormone, hepcidin [55], IL-6 can promote iron-restricted anemia [56]. AICD, though, is typically a normochromic, normocytic anemia [1; 57], rather than a microcytic, hypochromic anemia which would be consistent with iron-restricted anemia. The normocytic, normochromic features of AICD may indicate that inflammation does more than promote iron-restricted anemia. Patients treated with recombinant human IL-6 are known to develop a reversible, dose-dependent form of anemia, caused by an expansion of plasma volume [58; 59]. Beyond hepcidin stimulation and hemodilution, IL-6 has been shown to have direct effects on erythroid development, in vitro, by down regulating γ globin in K562 cells [60]. However, the effect of IL-6 on cells that have already committed to the erythroid lineage is not well understood. We have previously reported that, in a mouse model of chronic inflammation with sustained elevation of serum IL-6, late stage erythroid precursor production was significantly impaired [61].
In this study, we hypothesized that IL-6 has a direct, negative effect on erythroid development. To test this hypothesis, we utilized an in vitro cell culture system. We determined the effect of IL-6 on erythropoietin (Epo)-driven TF-1 cell maturation [62] by immunophenotyping with antibodies against CD235a (glycophorin A, GYPA), CD44, and CD71 (transferrin receptor) [63], as well as benzidine staining for hemoglobin. We also investigated the effect of IL-6 on the expression levels of genes marking erythroid commitment (GYPA); hemoglobin synthesis (aminolevulinate synthase 2, ALAS2; hemoglobin beta, HBB) and later stages of erythroid maturation (Band 3, SLC4A1). Because mitochondria are the site of heme biosynthesis and essential to efficient erythroid maturation, we investigated the effect of IL-6 on mitochondrial mass, membrane potential, and reactive oxygen species (ROS) production.
MATERIAL AND METHODS
Reagents
RPMI 1640 (without phenol red), Penicillin-Streptomycin, MitoTracker Green FM, 5-(and-6)-chloromethyl-2',7'-dichlorodihydrofluorescein diacetate, acetyl ester (CM-H2DCFDA), Phosphate buffered saline (PBS), Trizol Reagent, and fetal bovine serum (FBS) were obtained from Life Technologies (Grand Island, NY). Tetramethylrhodamine methyl ester perchlorate (TMRM), Bovine Serum Albumin (BSA), o-dianisidine (benzidine), acetic acid, and 30% H2O2 were obtained from Sigma-Aldrich (St. Louis, MO). αCD235a-Phycoerythrin (PE), αCD235a-PE-Cy5 conjugate, αCD44-Allophycocyanin (APC), and αCD71- Fluorescein (FITC) were obtained from BD Biosciences (San Jose, CA). Procrit Epoetin Alfa (Epo) was obtained from Amgen Pharmaceuticals (Thousand Oaks, CA). Hank’s balanced salt solution (HBSS) was obtained from Mediatech Inc. (Manassas, VA). StemSpan serum free expansion medium (SFEM) was obtained from StemCell Technologies (Vancouver, BC, CA). Human recombinant granulocyte-macrophage colony-stimulating factor (GM-CSF), human recombinant stem cell factor (SCF) and human recombinant IL-6 were obtained from Peprotech (Rocky Hill, NJ).
Cell Culture
The human erythroleukemia cell line TF-1 (ATCC, Mannassas, VA) was maintained in RPMI 1640 (without phenol red) medium supplemented with 10% FBS, 50U/ml-50μg/ml Penicillin-Streptomycin, and 2ng/ml human recombinant GM-CSF. Cells were pelleted, washed twice with PBS, and seeded at 1x105-2x105 cells/ml in RPMI 1640 supplemented with 10% FBS and 50U/ml-50μg/ml Penicillin-Streptomycin. Cells were incubated at 37°C, 5% CO2 for 24 hrs, and then pelleted and washed twice with PBS. Cells were resuspended in StemSpan SFEM supplemented with 50U/ml-50μg/ml Penicillin-Streptomycin, 5U/ml Epo, and 50ng/ml SCF. The TF-1 cells were then dosed with IL-6 at 0, 1, 10, or 100ng/ml and incubated at 37°C, 5% CO2 for six days. Cell growth and viability was determined by trypan blue staining.
Flow Cytometry
TF-1 cells were stained with αCD235a-PE or PE-Cy5, αCD44-APC, αCD71-FITC, MitoTracker Green FM, 0.1μ M TMRM, or DCF at concentrations outlined in the manufacturer’s protocol. The cells were incubated for 30 min, in the dark, at 4°C. Cells were then pelleted, resuspended in Hanks’ balanced salt solution with 1% BSA, and filtered. The immunostained cells were then assessed using a FACSCalibur (BD, Franklin Lakes, NJ). Data was acquired on 50,000 gated viable cells (based on propidium iodide signal) and analyzed using FlowJo software (Tree Star Inc, Ashland, OR).
Benzidine Staining
A staining solution of 0.49 mM H2O2 and 15.8 mM Benzidine in 12% acetic acid was prepared. TF-1 cells were pelleted and resuspended in equal parts PBS with 2.5% FBS and staining solution. Cells were incubated for 30 min, in the dark, at room temperature. Both Positive and negative benzidine stained cells were then counted. The total number of cells counted, for each sample, was 200–500.
qPCR
Total RNA was isolated from TF-1 cells by the Lowe Family Genomics Core facility using the Trizol reagent method according to the manufacturer’s directions (Invitrogen, Carlsbad, CA). The quality of total RNA samples was assessed using an Agilent 2100 Bioanalyzer (Agilent Technologies, Columbia, MD). Reverse transcription was performed by using the isolated total RNA and processing with the Applied Biosystems (Foster City, CA) High-Capacity cDNA Archive kit first-strand synthesis system for RT-PCR according to the manufacturer's protocol. QRT-PCR was performed using the TaqMan assay system from Applied Biosystems. All PCR amplifications were carried out in triplicate on an ABI Prism® 7300 Sequence Detection System, using a fluorogenic 5’ nuclease assay (TaqMan® probes). Relative gene expressions and p-values were calculated by using the 2−ΔΔCt method [64]. The ΔCt value of each sample was calculated using a total of 3 endogenous control genes [phosphoglycerate kinase 1 (Pgk1); Glyceraldehyde-3 phosphate dehydrogenase (Gapdh); Beta actin (Actb)]. Fold change values for target genes [aminolevulinic acid synthase 2 (ALAS2), hemoglobin, beta adult major chain (HBB), erythrocyte membrane protein Band 3 (SLC4a1), and glycophorin A (GYPA)] were obtained by computing 2−ΔΔCt for genes in “trial” relative to control samples. Proprietary probes and primers were designed and synthesized by Applied Biosystems.
Statistical Analyses
Immunophenotyping and benzidine staining results were analyzed using a two sample T-test, to test the effect of IL-6 at a given dose, and the nonparametric Cuzik test of the trend using STATA (StataCorp, College Station, TX), to test the dose dependent effect of IL-6. Data from mitochondrial experiments were reported as geometric mean fluorescence intensity normalized to control (MFI), and was analyzed using a one sample T-test, to test the effect of IL-6 at a given dose, and the nonparametric Cuzick test of the trend using STATA, to test the dose dependent effect of IL-6. For the qPCR data differences, for a given gene, in the average ΔCt’s between two groups were analyzed by either the two-tailed t-test (in the case of equal variance) or two-tailed Welch t-test (unequal variance), the presence of unequal variance was determined by the F-test (considered unequal if the F-test p-value < 0.2). We defined significance as a p value < 0.05.
RESULTS
Interleukin-6 Impairs TF-1 Erythroid Maturation
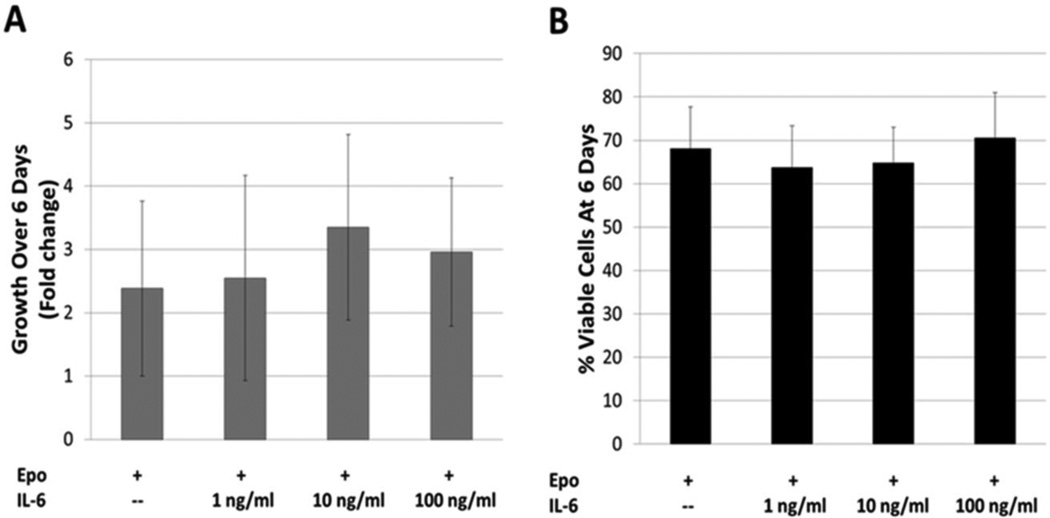
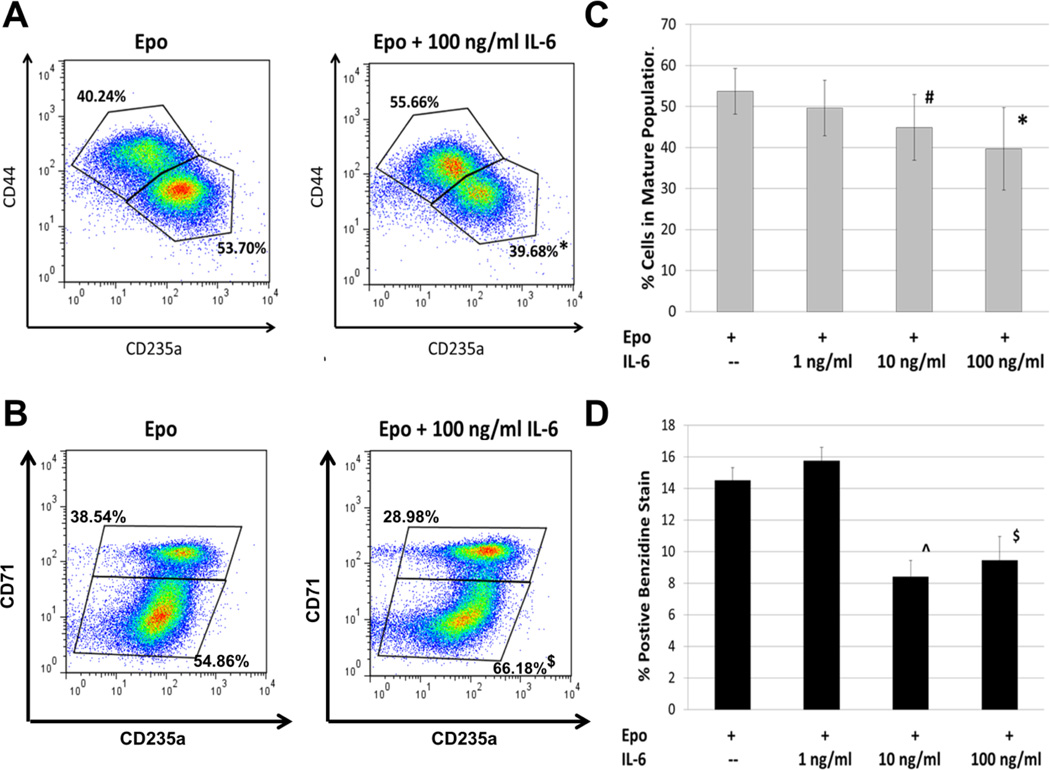
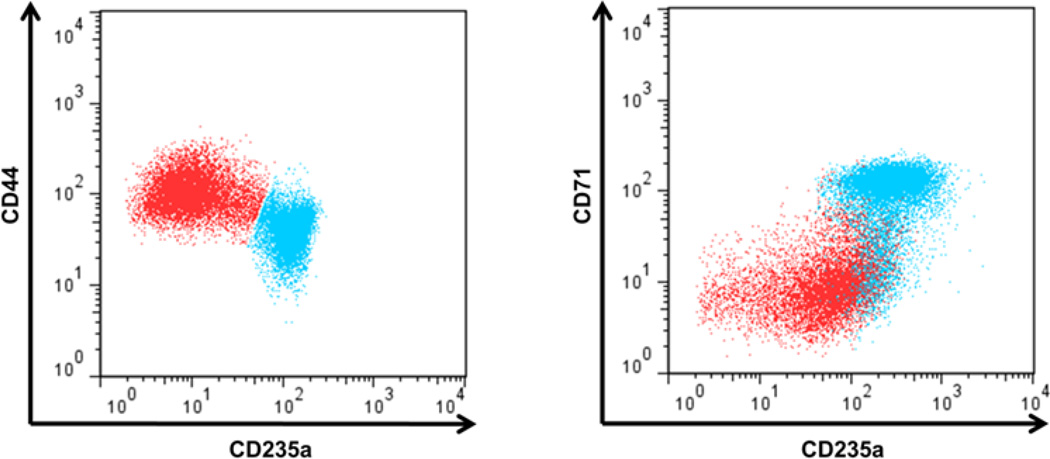
Cell counts and viability, determined by trypan blue staining, detected no change in cell growth or viability after 6 days of IL-6 treatment (Figure 1). To assess the effect of IL-6 on erythroid maturation, we utilized immunophenotyping by flow cytometry. As erythroid cells mature, expression levels of the cell surface protein CD235a and transferrin receptor CD71 increase while levels of the cell adhesion protein CD44 decrease [63]. We do note that in our system TF-1 cells cultured without GM-CSF appear to express some CD235a prior to treatment with Epo (data not shown). Stimulating human TF-1 cells with Epo results in the appearance of two distinct populations based on correlated CD235a and CD44 expression or CD235a and CD71 expression (Figures 2A and B). We defined the CD235alo CD44hi and the CD235alo CD71lo populations as “immature” cells, and the CD235ahi CD44lo and CD235ahi CD71hi populations as “mature” (though not fully mature or terminally differentiated) TF-1 cells. Although distinct “immature’ and “mature” populations emerge when using either CD44 and CD235a or CD71 and CD235a to immunophenotype, the populations do not completely overlap. As erythroid cells continue to mature, the expression of CD71 peaks and then starts to decline [61; 63; 65]. In our system this prevented successful resolution of “mature” TF-1 cells from the “immature” population (Figure 3). Therefore CD44 and CD235a were the best combination of markers tested to resolve the two populations. Addition of IL-6 to the cultures impairs this erythroid maturation, with a larger percentage of cells in the “immature” population. The resulting decrease in the percentage of “mature” cells is significant at IL-6 concentrations of 10 ng/ml and 100 ng/ml (Figure 2C). The Cuzik test indicates that the decrease in percent cells in the mature population is IL-6 dose-dependent (p=0.001).
FIGURE 1.
TF-1 cell growth and viability with IL-6 treatment. A) Cell growth was assessed by manual cell count after six days of culture. IL-6 appeared to have no significant effect on growth. Error Bars = Std Dev., n = 8–12; B) Cell viability was assessed by manual cell count and trypan blue stain. IL-6 showed no significant effect on viability over the six day time course. Error Bars = Std Dev., n = 12
FIGURE 2.
The effect of interleukin-6 on TF-1 maturation. A) TF-1 cell maturation was assessed by flow cytometry using markers for CD235a and CD44. Two distinct populations were formed with stimulation of Epo; CD235alo CD44hi (immature cells) and CD235ahi CD44lo (mature cells). Treatment with IL-6 resulted in a significant shift in cell percentage from the “mature” population to the “immature”. n = 9; B) Flow plot using markers for CD235a and CD71, showing a similar significant shift in populations. n = 5; C) The dose response of increasing IL-6 treatments on the percent of “mature” cells (defined with CD235a and CD44). IL-6 doses of 10 ng/ml and 100 ng/ml significantly reduced the percent of “mature” TF-1 cells by 6 days. Error bar = Std Dev. n = 9; D) Benzidine staining supports the results of immunophenotyping. We observed a significant decrease in positive stained cells at 6 days with IL-6 dosing of 10 ng/ml and 100 ng/ml. The results of the combined experiments suggest that IL-6 can repress the maturation of TF-1 cells. Error Bars = Std Dev. n = 3. *p<0.005, #p=0.02, ^p<0.01, $p<0.05, Student’s T-test
FIGURE 3.
Comparison of CD44/CD235a and CD71/CD235a populations. TF-1 cells treated with Epo alone were plotted by their CD44 and CD235a signal (left). The mature population (CD235ahi CD44lo, blue) and the immature population (CD235alo CD44hi red) were selected. The two populations were then plotted based on their CD71 and CD235a signal (right). When using CD71 as a marker for TF-1 cell maturation two distinct populations emerge, which are similar to the populations when using CD44 as a marker. CD71, though, is unable to distinguish the most mature TF-1 cell from the immature, since those cells lose their CD71 signal and overlap with the immature (red) population. This indicates CD44 and CD235a resolve distinct populations of erythroid precursors better than CD235a and CD71 using this time course for TF-1 cell erythroid maturation.
Since we observed IL-6-mediated inhibition of erythroid maturation based on immunophenotype, we expected hemoglobin synthesis might also be impaired by IL-6 treatment. 14.5 ± 1.2% of TF-1 cells cultured for six days with Epo stained for hemoglobin with benzidine. There were significant decreases in benzidine-stained cells cultured in 10 ng/ml and 100 ng/ml of IL-6 (Figure 2D). Using the Cuzik test, we observed that the percent of benzidine-stained cells decreased at IL-6 concentrations 10 ng/ml and above (p=0.022).
Interleukin-6 impairs late stages of erythroid development
To gain insight into the stage of erythroid development that is inhibited by IL-6, we assessed the expression of four genes representative of early, mid, and later stages of erythroid development. GYPA expression marks the earliest stage of erythroid commitment. Then ALAS2, followed by HBB, mark heme biosynthesis and globin expression. Finally, the anion transporter SLC4a1 (Band 3), which is a major site for cytoskeletal attachment and plays a crucial role in gas exchange [66], represents the latest stage of development that we tested [67]. TF-1 cells were treated with and without 100 ng/ml IL-6 and assessed for expression of these four genes by qPCR. We observed that IL-6 had no significant effect on expression of GYPA, ALAS2, or HBB (Table 1). As noted earlier, prior to treatment with Epo TF-1 cells express some level of the cell surface marker CD235a (GYPA). The TF-1 cells appear to be committed, at least partially, to the erythroid lineage without any stimulation from Epo, which may explain why we see no change in the expression level of GYPA with IL-6 treatment. While we observed a decrease in benzidine positive cells with treatment of 100 ng/ml of IL-6, we saw no change in the expression of ALAS2 or HBB. IL-6 did have a significant effect on SLC4a1 expression. We observed a two-fold decrease in the expression of SLC4a1 in TF-1 cells treated with 100 ng/ml IL-6 (p=0.005, T-test). These data indicate that IL-6 mediates its effect on TF-1 cells relatively late in the maturation process, after cells have primed themselves for hemoglobin synthesis.
TABLE 1.
Effect of interleukin-6 on genes associated with erythropoiesis
| Gene | Fold Change (100ng/ml IL-6 vs. Epo) |
p value |
|---|---|---|
| GYPA | 1.17 | 0.55^ |
| ALAS2 | −1.23 | 0.43* |
| HBB | −1.23 | 0.47^ |
| SLC4a1 | −2.04 | 0.005* |
unequal variance, Welch T-test;
equal variance, Two-tailed T-test; n = 9
Interleukin-6 Decreases Mitochondrial Membrane Potential
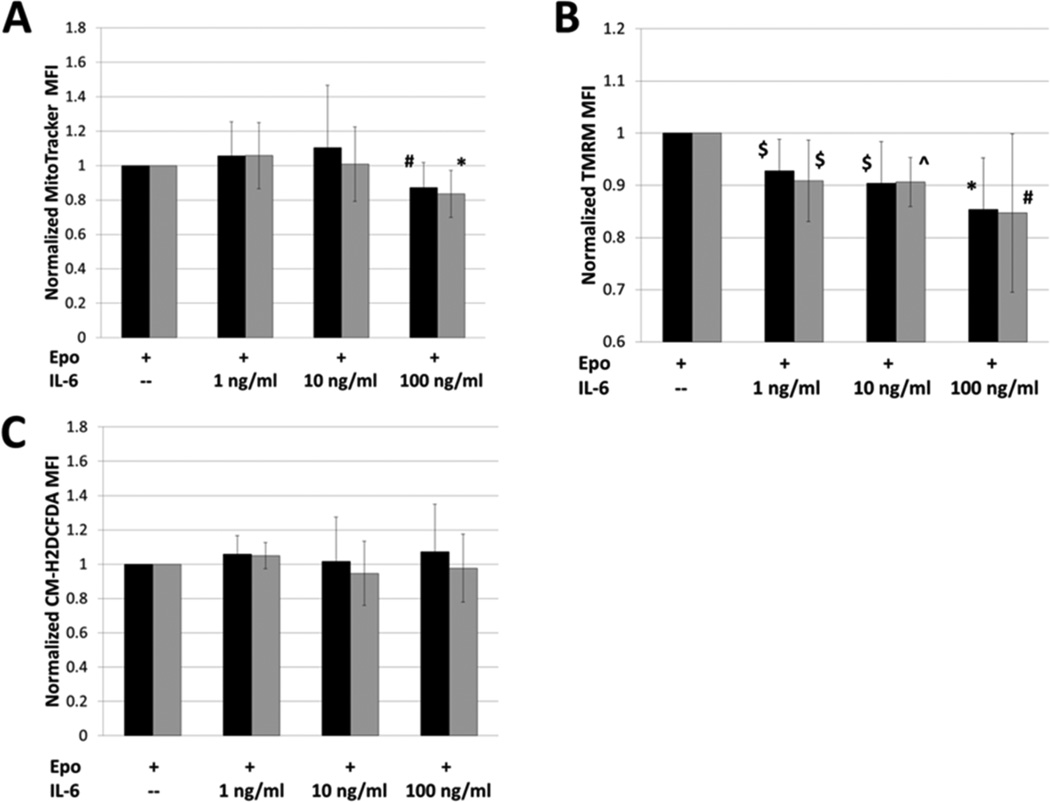
Mitochondria are central to erythroid development, as they are an integral site of hemoglobin biosynthesis [28; 29; 30]. While we observed that the percentage of benzidine-positive cells was decreased, we did not observe a decline in ALAS2 or HBB gene expression. The data may suggest that there is a post-transcriptional inhibition of hemoglobin synthesis in IL-6 treated TF-1 cells. We postulated that IL-6 may impair mitochondrial function, which could lead to an inhibition of heme biosynthesis, hemoglobin production and erythroid maturation. We first assessed whether IL-6 treatment leads to a decrease in mitochondrial mass using the cell permeant, mitochondrial selective dye, MitoTracker Green FM. MitoTracker Green FM can accumulate in the mitochondria regardless of membrane potential, allowing its signal to be used as a surrogate for mitochondrial mass [68]. We observed that mitochondrial mass was unaffected at IL-6 concentrations of 1 ng/ml and 10 ng/ml (Figure 4A). When TF-1 cells were treated with 100 ng/ml of IL-6, there was a significant reduction in mitochondrial mass in both the “immature” and “mature” populations (Figure 4A). While a reduction in mitochondria could explain impaired hemoglobin synthesis and erythroid maturation at 100 ng/ml IL-6, this did not explain the same effects observed at 10ng/mL IL-6.
FIGURE 4.
The effect of interleukin-6 on mitochondrial physiology. A) MitoTracker Green FM was used to assess changes in mitochondrial mass with treatment of TF-1 cells with IL-6. Mitochondrial mass was unchanged in both the “immature” (black bars) and “mature” (grey bars) populations at concentrations of 1 ng/ml and 10 ng/ml. There was a significant reduction in mitochondrial mass for both populations with dosing of 100 ng/ml IL-6. n = 8; B) TMRM was used to assess changes in the mitochondrial membrane potential with treatment of TF-1 cells with IL-6. Significant decreases in membrane potential were observed in both groups (“immature”-black bars"mature”-grey bars) for all doses of IL-6. n = 7; C) CM-H2DCFDA was used to assess the reactive oxygen species levels in TF-1 cells with IL-6 treatment. There was no significant change in reactive oxygen species in any of the treatment groups when compared to control. n = 7. Black bars = immature population, Grey bars = mature population, ^p<0.01, *p=0.01, $p<0.05, #p=0.05, Student’s T-test
To further probe mitochondrial function in TF-1 cells treated with IL-6, we assessed the mitochondrial membrane potential. Mitochondrial membrane potential can be used as an indicator for mitochondrial function and cell health [69]. We used the cell permeant, cationic, fluorescent dye Tetramethylrhodamine, methyl ester (TMRM), which is readily sequestered by active mitochondria. The accumulation of TMRM in the mitochondria has been shown to be driven by membrane potential and its fluorescence can be used to measure mitochondrial depolarization [70; 71]. We observed that TF-1 cells treated with IL-6 had a significant decrease in membrane potential at all concentrations, affecting both the immature and mature populations of cells (Figure 4B). Since the decrease in potential was observed in the cells treated with 1 ng/ml and 10 ng/ml of IL-6, we conclude that the reduction in fluorescence is not due to a decrease in mitochondrial mass. While we did observe significant reductions in potential at each IL-6 concentration we tested, the trend for the decrease in membrane potential did not reach statistical significance across the groups. This may be the result of larger standard deviations for this measure. Although mitochondrial membrane potential is affected in TF-1 cells treated with IL-6, we did not observe any increase in reactive oxygen species in those cells (Figure 4C).
DISCUSSION
AICD presents in various disease settings [1; 2; 3; 4; 5; 6; 7; 8; 9; 10; 11; 12; 13]. IL-6 is known to negatively associate with hemoglobin concentrations in many of the same diseases [50; 51; 52; 53; 54]. Our previous work suggests that inflammation can negatively impact on erythroid maturation [25]. In this study we tested the hypothesis that IL-6 is sufficient to inhibit erythroid maturation. While IL-6 concentrations may be higher in the bone marrow microenvironment, a normal, healthy adult typically has a serum concentration of IL-6 around 1 pg/ml [72; 73], while in patients with multiple myeloma, the concentration of circulating IL-6 is around 6 pg/ml [74; 75]. In cases of sepsis the concentration of circulating IL-6 ranges from 50 pg/mL to 4 ng/ml [72; 76; 77], and in patients experiencing septic shock the level can climb as high as 550 ng/ml [76; 78]. In using an in vitro cell culture model system, we might expect cells adapted to culture conditions to have less robust response to many growth factors and cytokines when compared to their in vivo counterparts. Additionally, without macrophages to govern the “quality control” of erythropoiesis, poorly developed erythroid progenitors may persist in a culture system that would not persist in vivo. In our system we observed that IL-6 concentrations as low as 10 ng/ml can significantly impair erythroid maturation, while concentrations as low as 1 ng/mL can impair mitochondrial membrane potential. Inhibition of TF-1 erythroid maturation was observed as a decrease in the percentage of cells with a mature (CD235ahi CD44lo or CD235ahi CD71hi) immunophenotype, and a corresponding increase in the percentage of cells with an immature (CD235alo CD44hi or CD235alo CD71lo) immunophenotype. Results from the immunophenotyping studies were supported by benzidine staining, in which we observed a significant decrease in cells containing detectable hemoglobin at IL-6 concentrations of 10 ng/ml and 100 ng/ml.
To further assess the effects of IL-6 on erythroid differentiation, we assessed mRNA expression levels for GYPA, ALAS2, HBB, and SLC4a1 in TF-1 cells cultured in 100 ng/ml IL-6 and Epo, versus Epo alone. We observed that cells cultured in IL-6 had significantly decreased SLC4a1 (Band 3) expression, while there was no effect on expression of GYPA, ALAS2, or HBB. This suggests that IL-6 mediates its effect rather late in erythroid maturation, since, of the four genes, SLC4a1 is expressed latest during erythropoiesis. Band 3 has been shown to be necessary for proper erythroid cell division [79], and although IL-6 decreased Band 3 expression we did not observe any negative effect on cell growth.
Although the genes associated with hemoglobin synthesis appear to be unaffected, benzidine staining demonstrated cellular hemoglobin is negatively impacted by IL-6. We suspected that IL-6 may be affecting the functionality of the mitochondria itself. We therefore chose to investigate whether the physiology of the mitochondria had been altered by IL-6. We observed no increase in ROS in our model, and saw a reduction in mitochondrial mass only at the highest concentration of IL-6. Cultures of cells in 100 ng/ml IL-6 also demonstrated the largest decrease in membrane potential, which may be related to the decrease in mitochondrial mass observed at that concentration. Low membrane potential in mitochondria is known to recruit Parkin and promote autophagy [80], which may result in the decrease in mitochondrial mass observed in cells treated with 100 ng/ml of IL-6.
Our results show that mitochondrial membrane potential is decreased by IL-6 at concentrations lower than those in which we can detect impaired erythroid maturation using immunophenotyping. We have shown that IL-6 treatment results in reduced expression of SLC4a1, a plasma membrane anion transporter. It is not known what affect IL-6 may have on mitochondrial membrane ion channels and transporters. While we do not yet understand the mechanism which IL-6 induces a decrease in mitochondrial membrane potential, the reduction in potential may play a part in the reduction of benzidine-positive cells and impaired erythroid maturation that we have described. In yeast, a decrease in mitochondrial membrane potential has been shown to negatively impact iron import into the organelle [81]. Any perturbation of iron import into the mitochondria would negatively affect heme biosynthesis. It is also possible that a decrease or block in heme export from the mitochondria could be responsible for both the decrease in membrane potential and cellular hemoglobin we observed. Iron overload has been shown to depolarize mitochondria [82; 83]. Additionally, mitochondria in astrocytes sequester iron in times of stress [84]. IL-6 could be acting as a stressor, which would cause the sequestration of iron in the mitochondria and lead to a repression in hemoglobin synthesis and maturation. Future studies will assess the effect of IL-6 on the molecular pathways of mitochondrial iron trafficking and metabolism.
CONCLUSION
We have shown, in an in vitro model that the presence of IL-6 in culture medium results in reduced erythroid maturation and hemoglobin synthesis. IL-6 appears to mediate its effect late in erythropoiesis, after the cells have started expressing genes for hemoglobin synthesis. We found that IL-6 also has a negative effect on mitochondria, by decreasing membrane potential. Our results make a new connection between an inflammatory cytokine and mitochondrial function in erythroid precursors, and suggest further studies into this pathway are needed to elucidate its potential clinical impact, especially in patients with anemia and elevated IL-6.
Acknowledgements
The authors would like to acknowledge the efforts of Wen-Chih Cheng and Tami J. Kingsbury for their input into early idea formation and experimental design for many of the experiments in this manuscript.
This work was supported by RO1 DK082722; BJM was supported by 5T32 AG000120. Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institute of Health under Award Number P30AR053503. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIDDK, NIA nor NIAMS. Neither NIDDK, NIA, nor NIAMS was involved in the study design; in the collection, analysis and interpretation of data; in the writing of the report; nor in the decision to submit the article for publication.
ABBREVIATIONS
- AICD
anemia of inflammation or chronic disease
- ALAS2
aminolevulinate synthase 2
- CM-H2DCFDA
chloromethyl-2',7'-dichlorodihydrofluorescein diacetate, acetyl ester
- Epo
erythropoietin/ Epoetin Alfa; GM-CSF
- GYPA
glycophorin A
- HBB
hemoglobin beta
- IFN
interferon
- IL-6
interleukin-6
- SLC4a1
Band 3
- SCF
stem cell factor
- SFEM
serum free expansion medium
- TMRM
tetramethylrhodamine methyl ester perchlorate
- TNF
tumor necrosis factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict-of-interest-disclosure - CNR held a sponsored research agreement with the Celgene Corporation in the past three years.
Contributions - CNR was the principal investigator and takes primary responsibility for the paper. CNR, BJM, MJK, CIC, and JDW designed research. BJM, MJK, NMC and CNR performed research. BJM, MJK, NMC, QLX, AEB, and CNR analyzed data. BJM, MJK, NMC, QLX, AEB, JDW, CIC, and CNR wrote the manuscript. All authors have approved the final version
REFERENCES
- 1.Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med. 2005;352:1011–1023. doi: 10.1056/NEJMra041809. [DOI] [PubMed] [Google Scholar]
- 2.Guralnik JM, Eisenstaedt RS, Ferrucci L, Klein HG, Woodman RC. Prevalence of anemia in persons 65 years and older in the United States: evidence for a high rate of unexplained anemia. Blood. 2004;104:2263–2268. doi: 10.1182/blood-2004-05-1812. [DOI] [PubMed] [Google Scholar]
- 3.Izaks GJ, Westendorp RG, Knook DL. The definition of anemia in older persons. Jama. 1999;281:1714–1717. doi: 10.1001/jama.281.18.1714. [DOI] [PubMed] [Google Scholar]
- 4.Nissenson A, Goodnough L, Dubois R. Anemia: Not just an innocent bystander? JAMA Internal Medicine. 2003;163:1400–1404. doi: 10.1001/archinte.163.12.1400. [DOI] [PubMed] [Google Scholar]
- 5.Harrison L, Shasha D, Shiaova L, et al. Prevalence of anemia in cancer patients undergoing radiation therapy. Semin Oncol. 2001;28:54–59. doi: 10.1016/s0093-7754(01)90214-3. [DOI] [PubMed] [Google Scholar]
- 6.Md Yusof M, Emery P. Targeting interleukin-6 in rheumatoid arthritis. Drugs. 2013;73:341–356. doi: 10.1007/s40265-013-0018-2. [DOI] [PubMed] [Google Scholar]
- 7.Weiss G, Schett G. Anaemia in inflammatory rheumatic diseases. Nat Rev Rheumatol. 2013;9:205–215. doi: 10.1038/nrrheum.2012.183. [DOI] [PubMed] [Google Scholar]
- 8.Stenvinkel P. Anaemia and inflammation: what are the implications for the nephrologist? Nephrol Dial Transplant 18 Suppl. 2003;8:viii17–viii22. doi: 10.1093/ndt/gfg1086. [DOI] [PubMed] [Google Scholar]
- 9.Yilmaz M, Solak Y, Covic A, Goldsmith D, Kanbay M. Renal anemia of inflammation: The name is self-explanatory. Blood Purification. 2011;32:220–225. doi: 10.1159/000328037. [DOI] [PubMed] [Google Scholar]
- 10.Sullivan PS, Hanson DL, Chu SY, Jones JL, Ward JW. Epidemiology of anemia in human immunodeficiency virus (HIV)-infected persons: results from the multistate adult and adolescent spectrum of HIV disease surveillance project. Blood. 1998;91:301–308. [PubMed] [Google Scholar]
- 11.Zakai NA, Katz R, Hirsch C, et al. A prospective study of anemia status, hemoglobin concentration, and mortality in an elderly cohort: the Cardiovascular Health Study. Arch Intern Med. 2005;165:2214–2220. doi: 10.1001/archinte.165.19.2214. [DOI] [PubMed] [Google Scholar]
- 12.Milman N, Pedersen AN, Ovesen L, Schroll M. Hemoglobin concentrations in 358 apparently healthy 80-year-old Danish men and women. Should the reference interval be adjusted for age? Aging Clin Exp Res. 2008;20:8–14. doi: 10.1007/BF03324741. [DOI] [PubMed] [Google Scholar]
- 13.Cesari M, Penninx BW, Lauretani F, et al. Hemoglobin levels and skeletal muscle: results from the InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2004;59:249–254. doi: 10.1093/gerona/59.3.m249. [DOI] [PubMed] [Google Scholar]
- 14.Culleton BF, Manns BJ, Zhang J, et al. Impact of anemia on hospitalization and mortality in older adults. Blood. 2006;107:3841–3846. doi: 10.1182/blood-2005-10-4308. [DOI] [PubMed] [Google Scholar]
- 15.Penninx BW, Pahor M, Woodman RC, Guralnik JM. Anemia in old age is associated with increased mortality and hospitalization. J Gerontol A Biol Sci Med Sci. 2006;61:474–479. doi: 10.1093/gerona/61.5.474. [DOI] [PubMed] [Google Scholar]
- 16.Arant C, Wessel T, Olson M, et al. Hemoglobin level is an independent predictor for adverse cardiovascular outcomes in women undergoing evaluation for chest pain: results from the National Heart, Lung, and Blood Institute Women's Ischemia Syndrome Evalulation Study. J Am Coll Cardiol. 2004;43:2009–2014. doi: 10.1016/j.jacc.2004.01.038. [DOI] [PubMed] [Google Scholar]
- 17.Go A, Yang J, Ackerson L, et al. Hemoglobin Level, Chronic Kidney Disease, and the Risks of Death and Hospitalization in Adults with Chronic Heart Failure. Circulation. 2006;113:2713–2723. doi: 10.1161/CIRCULATIONAHA.105.577577. [DOI] [PubMed] [Google Scholar]
- 18.Caro J, Salas M, Ward A, Goss G. Anemia as an independent prognostic factor for survival in patients with cancer. Cancer. 2001;91:2214–2221. [PubMed] [Google Scholar]
- 19.Chaves PH, Ashar B, Guralnik JM, Fried LP. Looking at the relationship between hemoglobin concentration and prevalent mobility difficulty in older women. Should the criteria currently used to define anemia in older people be reevaluated? J Am Geriatr Soc. 2002;50:1257–1264. doi: 10.1046/j.1532-5415.2002.50313.x. [DOI] [PubMed] [Google Scholar]
- 20.Chaves PH, Carlson MC, Ferrucci L, et al. Association between mild anemia and executive function impairment in community-dwelling older women: The Women's Health and Aging Study II. J Am Geriatr Soc. 2006;54:1429–1435. doi: 10.1111/j.1532-5415.2006.00863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deal JA, Carlson MC, Xue QL, Fried LP, Chaves PH. Anemia and 9-Year Domain-Specific Cognitive Decline in Community-Dwelling Older Women: The Women's Health and Aging Study II. J Am Geriatr Soc. 2009 doi: 10.1111/j.1532-5415.2009.02400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cella D, Dobrez D, Glaspy J. Control of cancer-related anemia with erythropoietic agents: a review of evidence for improved quality of life and clincal outcomes. Ann Oncol. 2003;14:511–519. doi: 10.1093/annonc/mdg167. [DOI] [PubMed] [Google Scholar]
- 23.Sabbatini P. The relationship between anemia and quality of life in cancer patients. The Oncologist. 2000;5:19–23. doi: 10.1634/theoncologist.5-suppl_2-19. [DOI] [PubMed] [Google Scholar]
- 24.Knight K, Wade S, Balducci L. Prevalence and outcomes of anemia in cancer: a systematic review of the literature. Am J Med. 2004;116:11–26. doi: 10.1016/j.amjmed.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 25.Price EA. Aging and erythropoiesis: current state of knowledge. Blood Cells Mol Dis. 2008;41:158–165. doi: 10.1016/j.bcmd.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 26.Artz A, Thirman M. Unexplained anemia predominates despite an intensive evaluation in a racially diverse cohort of older adults fro a referral anemia clinic. J Gerontol A Biol Sci Med Sci. 2011;66:925–932. doi: 10.1093/gerona/glr090. [DOI] [PubMed] [Google Scholar]
- 27.Terramanti M, Lucca U, Gandini F, et al. Prevalence, incidence and types of mild anemia in the elderly: the"Health and Anemia" population-based study. Haematologica. 2010;95:1849–1856. doi: 10.3324/haematol.2010.023101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fontenay M, Cathelin S, Amiot M, Gyan E, Solary E. Mitochondria in hematopoiesis and hematological diseases. Oncogene. 2006;25:4757–4767. doi: 10.1038/sj.onc.1209606. [DOI] [PubMed] [Google Scholar]
- 29.Yaun X, Fleming M, Hamza I. Heme transport and erythropoiesis. Curr Opin Chem Biol. 2013;17:204–211. doi: 10.1016/j.cbpa.2013.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chung J, Chen C, Paw B. Heme metabolism and erythropoiesis. Curr Opin Hematol. 2012;19:156–162. doi: 10.1097/MOH.0b013e328351c48b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kumari U, Yan Jun W, Huat Bay B, Lyakhovich A. Evidence of mitochondrial dysfunction and imparied ROS detoxifying machinery in Fanconi Anemia cells. Oncogene. 2013 doi: 10.1038/onc.2012.583. [DOI] [PubMed] [Google Scholar]
- 32.Pagano G, Aiello Talamanca A, Castello G, et al. From clinical description, to in vitro and animal studies, and backward to patients: Oxidative stress and mitochondrial dysfunction in Fanconi anemia. Free Radic Biol Med. 2013;58:118–125. doi: 10.1016/j.freeradbiomed.2013.01.015. [DOI] [PubMed] [Google Scholar]
- 33.Harigae H, Furuyama K. Hereditary sideroblastic anemia: pathophysiology and gene mutations. Int J Hematol. 2010;92:425–431. doi: 10.1007/s12185-010-0688-4. [DOI] [PubMed] [Google Scholar]
- 34.Chen M, Logan T, Hochberg M, et al. Erythriod dysplasia, megaloblastic anemia, and impaired lymphopoiesis arising from mitochondrial dysfunction. Blood. 2009;114:4045–4053. doi: 10.1182/blood-2008-08-169474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen E. Mitochondrial dysfunction and cancer metastasis. J Bioenerg Biomembr. 2012;44:619–622. doi: 10.1007/s10863-012-9465-9. [DOI] [PubMed] [Google Scholar]
- 36.Moodley D, Mody G, Patel N, Chuturgoon A. Mitochondrial depolarisation and oxidative stress in rheumatoid arthritis patients. Clin Biochem. 2008;41:1396–1401. doi: 10.1016/j.clinbiochem.2008.08.072. [DOI] [PubMed] [Google Scholar]
- 37.Granata S, Zaza G, Simone S, et al. Mitochondrial dysregulation and oxidative stress in patients with chronic kidney disease. BMC Genomics. 2009;10 doi: 10.1186/1471-2164-10-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scheibye-Knudsen M, Scheibye-Alsing K, Canugovi C, Croteau D, Bohr V. A novel diagnostic tool reveals mitochondrial pathology in human diseases and aging. Aging (Albany NY) 2013;5:192–208. doi: 10.18632/aging.100546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bratic A, Larsson N. The role of mitochondria in aging. J Clin Invest. 2013;123:951–957. doi: 10.1172/JCI64125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lopez-Armada M, Carames B, Martin M, et al. Mitochondrial activity is modulated by TNFα and IL1β in normal human chondrocyte cells. OsteoArthritis and Cartilage. 2006;14 doi: 10.1016/j.joca.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 41.Stadler J, Bentz B, Harbrecht B, et al. Tumor necrosis factor alpha inhibits hepatocyte mitochondrial respiration. Annals of Surgery. 1992;216:539–546. doi: 10.1097/00000658-199211000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zell R, Geck P, Werdan K, Boekstegers P. TNFα and IL1α inhibit both pyruvate dehydrogenase activity and mitochondrial function in cardiomyocytes: Evidence for primary impairment of mitochondrial function. Molecular and Cellular Biochemistry. 1997;177:61–67. doi: 10.1023/a:1006896832582. [DOI] [PubMed] [Google Scholar]
- 43.Zhang Z, Ding Y, Dai X, Wang J, Li Y. Epigallocatechin-3-gallate protects pro-inflammatory cytokine induced injuries in insulin-producing cells through the mitochondrial pathway. Eur J Pharmacol. 2011;670:311–316. doi: 10.1016/j.ejphar.2011.08.033. [DOI] [PubMed] [Google Scholar]
- 44.Ji C, Chen X, Gao C, et al. IL-6 induces lipolysis and mitochondrial dysfunction, but does not affect insulin-mediated glucose transport in 3T3-L1 adipocytes. J Bioenerg Biomembr. 2011;43:367–375. doi: 10.1007/s10863-011-9361-8. [DOI] [PubMed] [Google Scholar]
- 45.Tschopp J. Mitochondria: Sovereign of inflammation? Eur J Immunol. 2011;41:1196–1202. doi: 10.1002/eji.201141436. [DOI] [PubMed] [Google Scholar]
- 46.Lopez-Armada M, Riveiro-Naveira R, Vaamonde-Garcia C, Valcarcel-Ares M. Mitochondrial dysfunction and the inflammatory response. Mitochondrion. 2013;13:106–118. doi: 10.1016/j.mito.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 47.Mihara M, Hashizume M, Yoshida H, Suzuki M, Shiina M. IL-6/IL-6 receptor system and its role in physiological and pathological condtions. Clin Sci (Lond.) 2012;122:143–159. doi: 10.1042/CS20110340. [DOI] [PubMed] [Google Scholar]
- 48.Gabay C. Interleukin-6 and chronic inflammation. Arthritis Res Ther 8 Suppl. 2006;2:S3. doi: 10.1186/ar1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kaplanski G, Marin V, Montero-Julian F, Mantovani A, Farnarier C. IL-6: a regulator of the transition from neutrophil to monocyte recruitment during inflammation. Trends in Immunology. 2003;24:25–29. doi: 10.1016/s1471-4906(02)00013-3. [DOI] [PubMed] [Google Scholar]
- 50.Nikolaisen C, Figenschau Y, Nossent JC. Anemia in early rheumatoid arthritis is associated with interleukin 6-mediated bone marrow suppression, but has no effect on disease course or mortality. J Rheumatol. 2008;35:380–386. [PubMed] [Google Scholar]
- 51.Ripley BJ, Goncalves B, Isenberg DA, Latchman DS, Rahman A. Raised levels of interleukin 6 in systemic lupus erythematosus correlate with anaemia. Ann Rheum Dis. 2005;64:849–853. doi: 10.1136/ard.2004.022681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nachbaur D, Herold M, Maneschg A, Huber H. Serum levels of interleukin-6 in multiple myeloma and other hematological disorders: correlation with disease activity and other prognostic parameters. Ann Hematol. 1991;62:54–58. doi: 10.1007/BF01714900. [DOI] [PubMed] [Google Scholar]
- 53.Maccio A, Madeddu C, Massa D, et al. Hemoglobin levels correlate with interleukin-6 levels in patients with advanced untreated epithelial ovarian cancer: role of inflammation in cancer-related anemia. Blood. 2005;106:362–367. doi: 10.1182/blood-2005-01-0160. [DOI] [PubMed] [Google Scholar]
- 54.Leng S, Chaves P, Koenig K, Walston J. Serum interleukin-6 and hemoglobin as physiological correlates in the geriatric syndrome of frailty: a pilot study. J Am Geriatr Soc. 2002;50:1268–1271. doi: 10.1046/j.1532-5415.2002.50315.x. [DOI] [PubMed] [Google Scholar]
- 55.Nemeth E, Valore EV, Territo M, et al. Hepcidin, a putative mediator of anemia of inflammation, is a type II acute-phase protein. Blood. 2003;101:2461–2463. doi: 10.1182/blood-2002-10-3235. [DOI] [PubMed] [Google Scholar]
- 56.Nemeth E, Rivera S, Gabayan V, et al. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest. 2004;113:1271–1276. doi: 10.1172/JCI20945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Roy C. Anemia of inflammation. Hematology Am Soc Hematol Educ Program. 2010;2010:276–280. doi: 10.1182/asheducation-2010.1.276. [DOI] [PubMed] [Google Scholar]
- 58.Nieken J, Mulder NH, Buter J, et al. Recombinant human interleukin-6 induces a rapid and reversible anemia in cancer patients. Blood. 1995;86:900–905. [PubMed] [Google Scholar]
- 59.Atkins MB, Kappler K, Mier JW, Isaacs RE, Berkman EM. Interleukin-6-associated anemia: determination of the underlying mechanism. Blood. 1995;86:1288–1291. [PubMed] [Google Scholar]
- 60.Ferry AE, Baliga SB, Monteiro C, Pace BS. Globin gene silencing in primary erythroid cultures. An inhibitory role for interleukin-6. J Biol Chem. 1997;272:20030–20037. doi: 10.1074/jbc.272.32.20030. [DOI] [PubMed] [Google Scholar]
- 61.Prince OD, Langdon JM, Layman AJ, et al. Late stage erythroid precursor production is impaired in mice with chronic inflammation. Haematologica. 2012;97:1648–1656. doi: 10.3324/haematol.2011.053397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kitamura T, Tange T, Terasawa T, et al. Establishment and characterization of a unique human cell line that proliferates dependently on GM-CSF, IL-3, or erythropoietin. J Cell Physiol. 1989;140:323–334. doi: 10.1002/jcp.1041400219. [DOI] [PubMed] [Google Scholar]
- 63.Chen K, Liu J, Heck S, et al. Resolving the distinct stages in erythroid differentiation based on dynamic changes in membrane protein expression during erythropoiesis. Proc Natl Acad Sci U S A. 2009;106:17413–17418. doi: 10.1073/pnas.0909296106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yuan JS, Reed A, Chen F, Stewart CN., Jr Statistical analysis of real-time PCR data. BMC Bioinformatics. 2006;7:85. doi: 10.1186/1471-2105-7-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Socolovsky M, Nam H, Fleming MD, et al. Ineffective erythropoiesis in Stat5a(-/-)5b(-/-) mice due to decreased survival of early erythroblasts. Blood. 2001;98:3261–3273. doi: 10.1182/blood.v98.12.3261. [DOI] [PubMed] [Google Scholar]
- 66.Wu F, Satchwell TJ, Toye AM. Anion exchanger 1 in red blood cells and kidney: Band 3's in a pod. Biochem Cell Biol. 2011;89:106–114. doi: 10.1139/o10-146. [DOI] [PubMed] [Google Scholar]
- 67.Southcott M, Tanner M, Anstee D. The expression of human blood group antigens during erythropoiesis in a cell culture system. Blood. 1999;93:4425–4435. [PubMed] [Google Scholar]
- 68.Pendergrass W, Wolf N, Poot M. Efficacy of MitoTracker Green and CMXrosamine to measure changes in mitochondrial membrane potentials in living cells and tissues. Cytometry A. 2004;61:162–169. doi: 10.1002/cyto.a.20033. [DOI] [PubMed] [Google Scholar]
- 69.Duchen M. Roles of mitocondria in health and disease. Diabetes. 2004;53:S96–S102. doi: 10.2337/diabetes.53.2007.s96. [DOI] [PubMed] [Google Scholar]
- 70.Ehrenberg B, Montana V, Wei M, Wuskell J, Loew L. Membrane potential can be determined in individual cells from the nernstian distribution of cationic dyes. Biophys J. 1988;53:785–794. doi: 10.1016/S0006-3495(88)83158-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Loew L, Carrington W, Tuft R, Fay F. Physiological cytosolic Ca2+ transients evoke concurrent mitochondrial depolarizations. PNAS. 1994;91:12579–12583. doi: 10.1073/pnas.91.26.12579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tsai WH, Shih CH, Yu YB, Hsu HC. Plasma levels in sepsis patients of annexin A1, lipoxin A4, macrophage inflammatory protein-3a, and neutrophil gelatinase-associated lipocalin. J Clin Med Assoc. 2013 doi: 10.1016/j.jcma.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 73.Kado S, Nagase T, Nagata N. Circulating levels of interleukin-6, its soluble receptor and interleukin-6/interleukin-6 receptor complexes in patients with type 2 diabetes mellitus. Acta Diabetol. 1999;36:67–72. doi: 10.1007/s005920050147. [DOI] [PubMed] [Google Scholar]
- 74.Pappa CA, Tsirakis G, Psarakis FE, et al. Lack of correlation between angiogenic cytokines and serum insulin-like growth factor-1 in patients with multiple myeloma. Med Oncol. 2013;30:363. doi: 10.1007/s12032-012-0363-0. [DOI] [PubMed] [Google Scholar]
- 75.Fraqioudaki M, Boula A, Tsirakis G, et al. B cell-activating factor: its clinical significance in multiple myeloma patients. Ann Hematol. 2012;91:1413–1418. doi: 10.1007/s00277-012-1470-x. [DOI] [PubMed] [Google Scholar]
- 76.Endo S, Aikawa N, Fujishima S, et al. Usefulness of procalcitonin serum level for the discrimination of severe sepsis from sepsis: a multicenter prospective study. J Infect Chemother. 2008;14:244–249. doi: 10.1007/s10156-008-0608-1. [DOI] [PubMed] [Google Scholar]
- 77.Ando K, Kato H, Kotani T, et al. Plasma leukocyte cell-derived chemotaxin 2 is associated with the severity of systemic inflammation in patients with sepsis. Microbiol Immunol. 2012;56:708–718. doi: 10.1111/j.1348-0421.2012.00488.x. [DOI] [PubMed] [Google Scholar]
- 78.Oda S, Hirasawa H, Shiqa H, et al. Sequential measurement of IL-6 blood levels in patients with systmeic inflammatory response syndrome (SIRS)/sepsis. Cytokine. 2005;29:169–175. doi: 10.1016/j.cyto.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 79.Paw BH, Davidson AJ, Zhou Y, et al. Cell-specific mitotic defect and dyserythropoiesis associated with erythriod band 3 deficiency. Nat Genet. 2003;34:59–64. doi: 10.1038/ng1137. [DOI] [PubMed] [Google Scholar]
- 80.Narendra D, Tanaka A, Suen D, Youle R. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J Cell Biol. 2008;183:795–803. doi: 10.1083/jcb.200809125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lange H, Kispal G, Lill R. Mechanism of iron transport to the site of heme synthesis inside yeast mitochondria. J Biol Chem. 1999;274:18989–18996. doi: 10.1074/jbc.274.27.18989. [DOI] [PubMed] [Google Scholar]
- 82.Santos N, Castilho R, Meinicke A, Hermes-Lima M. The iron chelator pyridoxal isonicotinoyl hydrazone inhibits mitochondrial lipid peroxidation induced by Fe(II)-citrate. Eur J Pharmacol. 2001;428:37–44. doi: 10.1016/s0014-2999(01)01291-2. [DOI] [PubMed] [Google Scholar]
- 83.Uchiyama A, Kim J, Kon K, et al. Translocation or iron from lysosomes into mitochondria is a key event during oxidative stress-induced hepatocellular injury. Hepatology. 2008;48:1644–1654. doi: 10.1002/hep.22498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schipper H, Bernier L, Mehindate K, Frankel D. Mitochondrial iron sequestration in dopaminechallenged astroglia: role of heme oxygenase-1 and the permeability transition pore. J Neurochem. 1999;72:1802–1811. doi: 10.1046/j.1471-4159.1999.0721802.x. [DOI] [PubMed] [Google Scholar]